Abstract
Sixty two rhizospheric and endophytic bacterial strains were evaluated for their biocontrol effect on two aggressive Fusarium culmorum isolates (Fc2 and Fc3). We observed that 35 % and 23 % of the tested strains inhibited the in vitro growth of Fc2 and Fc3 respectively. The observed antagonism was due to inhibition by contact (13–19 % of the strains) or at distance (10–16 % of the strains) for both fungal isolates. Some of the antagonistic bacteria showed the ability to produce diffuse and/or volatile compounds that inhibit the growth, the sporulation and macroconidia germination of F. culmorum. None of the tested antagonistic bacteria showed chitinase activity on synthetic medium. The sequencing of the 16S rDNA genes of some antagonistic bacteria showed that they belong to the genera Bacillus, Pseudomonas and Microbacterium. The double inoculation of durum wheat seeds by the antagonistic bacterial strains (B13, B18, BSE1, BSE3 and B16E) and the two F. culmorum isolates showed that germination and seedling vigor were generally improved in vitro. The percentage of infected seeds was also reduced. In greenhouse trials, the biocontrol effectiveness of F. culmorum was dependant from the virulence of the fungal strain and the specificity of the antagonistic interaction between bacterial and fungal strains. The bacterial strains B18 and B16E reduced F. culmorum infection on durum wheat plants probably due to their antagonistic and plant growth promoting activities and they may be used in a mixture as seed biopriming inoculum for plant growth bio-promoting and Fusarium wheat diseases biocontrol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat production provides a staple food for people and an important component of the national agriculture system (Latiri et al. 2010). The northern regions of Tunisia, where wheat is predominantly cultivated, are characterized by a hot and humid climate which promotes the development of several diseases that decrease the production of this crop (Gargouri-Kammoun et al. 2010). These diseases are mainly caused by pathogenic fungi, of which Fusarium head blight (FHB) is caused by a complex of Fusarium species (Rotter et al. 1996). This disease is a serious problem causing significant reduction in wheat grain yield and quality in most production areas of this crop (Bai and Shaner 1994). Several Fusarium species have been described as causing Fusarium head blight (FHB) including Fusarium graminearum, Fusarium culmorum, Fusarium poae, Fusarium avenaceum, Fusarium sporotrichioides and Microdochium nivale (Rotter et al. 1996). These pathogens can attack different plant organs (Parry et al. 1995) at different stages of growth (Nicholson et al. 2004). In recent years, there were serious attacks of wheat by Fusarium in northern Tunisia, with Fusarium culmorum reported as the dominant pathogen species (Gargouri-Kammoun et al. 2009). Infection of seeds may decrease seed germination, reduce emergence, and cause a post emergence blight of seedlings thus leading to a less dense plant stand (Reddy et al. 1999). Fusarium attack is often accompanied by contamination of grains with trichothecene toxins which are harmful to human and animal health (Gargouri-Kammoun et al. 2010). The control of these fungal diseases is mainly through chemical fungicides. However, this method is limited in some cases by the emergence of resistance in the pathogen, the lack of effective fungicides against the target fungal agent and the adverse effects on the environment. Therefore, the search for alternative control methods is important for controlling crop pathogens (Gerhardson 2002). Biopriming, i.e. coating seed with a biocontrol agent, is a promising technique which can potentially replace chemical seed treatments. It can fight against seed- and soil-borne pathogens and also offers the advantage to stimulate plant defense against aggressors during the later stages of development (El-Mohamedy and Abd El-Baky 2008). Biocontrol agents act by different types of antagonism such as competition for nutrients, antibiosis and parasitism. Strains of Pseudomonas fluorescens have been shown capable of producing antibiotics, volatile substances, enzymes, phytohormones and siderophores (Gupta et al. 2001).
In order to select potential biocontrol agents against Fusarium head blight disease of durum wheat, we studied the antagonistic effect of some rhizospheric and endophytic bacteria on the in vitro growth and sporulation of F. culmorum. In addition, the efficacy of the antagonistic bacterial strains applied as seed coating against F. culmorum root infection was studied.
Materials and methods
Plant material
The seeds of durum wheat (Triticum durum L. cv. Karim) used in this study were free from chemicals and were stored at room temperature (20–24 °C). The cv. Karim is one of the most used and productive durum wheat cultivars in Tunisia (Rezgui et al. 2000), but it is known for its susceptibility to several foliar and root diseases, especially F. culmorum (Chekali et al. 2011).
Fungal material
Two F. culmorum strains Fc2 and Fc3 isolated from infected durum wheat plants in northern Tunisia were kindly provided by Dr. Samia Gargouri (National Institute of Agronomic Research of Tunisia) (Gargouri-Kammoun et al. 2009). For macroconidia production the fungal isolates were cultivated on Joffe’s medium (Dhingra and Sinclar 1995) at 25 °C in the dark for 21 days. The conidial suspensions were prepared by flooding the plates with autoclaved distilled water and manual disruption of the culture. The conidial suspensions used for inoculations were prepared from 21-day-old cultures, and were applied at a concentration of 105 macroconidia ml−1. The fungal strains were stored on Potato Dextrose Agar (PDA) slants at 4 °C in the dark.
Bacterial material
The rhizospheric and endophytic bacterial strains were isolated from roots of healthy wheat and barley plants collected from three sites in northern Tunisia (governorate of Beja). For the isolation of rhizospheric bacteria, root pieces (3–5 cm in long) were dropped in 100 ml of autoclaved distilled water and shaken at 120 rpm for one hour. After drying on autoclaved filter paper, root pieces were placed in Petri dishes on Luria Broth (LB) agar medium and incubated at 28 °C in the dark. For the isolation of the endophytic bacteria, root pieces were rinsed 5 times in sterile distilled water and surface sterilized with sodium hypochlorite (2 %) containing 0.1 % Tween20 for 1 min and then in 70 % ethanol for 1 min. Disinfected roots were washed five times in sterile water to remove disinfectants and finally dried on sterile paper towels under aseptic conditions. The dried roots were cut longitudinally and placed in Petri dishes on LB agar medium and incubated at 28 °C in the dark. To ensure that surface disinfection of roots was successful and no microorganism survived on them, the final wash water was poured on LB agar medium and incubated in the same conditions as above. Endophytic strains are indicated with the letter “E” in their names. From each plate, colonies representing different morphological types were picked and streaked for purity on LB agar medium. Bacterial strains were maintained on LB agar medium at 4 °C or stored at −20 °C in LB broth supplemented with 25 % (v/v) glycerol for long term storage.
The bacterial strains were identified by 16S rDNA gene sequencing.The 16S rDNA genes were amplified using the universal primers fD1 (AGAGTTTGATCCTGGCTCAG) and rD1 (AAGGAGGTGATCCAGCC) (Weisburg et al. 1991) as previously described by Mhamdi et al. (2002). PCR products were purified from agarose gels using the Wizard SV Gel and PCR-clean up system from Promega (Madison, Wisconsin USA). The amplified fragments were sequenced using the same primers. DNA sequences were assembled using the CAP program available on the NCBI website (http://www.ncbi.nlm.njh.gov/blast). The nucleotide sequences were deposited in GenBank and the accession numbers were obtained.
Dual culture assay
A fungal plug (6 mm in diameter) from a one-week-old PDA culture was placed in the centre of a 90-mm-diameter Petri dish containing PDA medium. The bacterial strain was applied in two opposite streaks at approximately 25 mm from the fungal plug. The ability of the bacterial strain to inhibit the fungal growth was assessed by measuring the radius of the fungal colony in front of the bacterium (R1) after six days of incubation at 28 °C. The radius of the fungal colony cultivated by itself on PDA medium was taken as a control (R2). Two independent experiments were performed with two plates for each bacterial strain. The percentage of inhibition of fungal growth (PI) was determined according to the following formula (Sivan et al. 1987):
In order to evaluate the antagonistic effect of the bacterial strains on F. culmorum macroconidia germination, in vitro assays using special slides for microculture were performed (Ben Slimene et al. 2012). Aliquots of the bacterial strains (45 μl) suspended in sterile 0.9 % NaCl solution and corresponding to 108cells ml−1 were deposited on the slide and 45 μl of the fungal macroconidia suspension (105 macroconidia ml−1) were immediately added. Controls were made with 45 μl of sterile 0.9 % NaCl solution and 45 μl of the fungal macroconidia suspension. Each treatment was conducted in triplicate. Slides were incubated in a high humidity chamber at 28 °C for 24 h and then observed with an optical microscope (Olympus BX41). Germination of macroconidia and swelling of the fungal structures were assessed in nine replicates (three slides per treatment and three regions per slide).
Bacterial volatile compound activity
The effect of bacterial volatile compounds (BVC) on F. culmorum growth and sporulation was done according to the method described by Alimi et al. (2012). A 24 h old fungal culture was put facing in proximity to but not touching a 24 h old bacterial culture on LB agar medium. For control plates the fungal culture was put facing a plate with LB agar medium. To test the effect of BVC on the fungal growth, F. culmorum was cultivated on PDA medium, whereas for the effect on sporulation the pathogen was cultivated on Joffe medium. The two plates were sealed to avoid the dispersion of BVC and preventing any physical contact between the fungal pathogen and the bacterial culture. The plates were incubated at 28 °C. The fungal growth was measured at 3 days and the macroconidia production at 20 days of co-culture of the two microorganisms.
Colloidal chitin preparation and bacterial chitinolytic activity assay
A colloidal chitin solution was prepared from commercial chitin powder (chitin from shrimp shells, Sigma-Aldrich) according to Hsu and Lockwood (1975), and chitin content was determined after drying and weighing an aliquot from the solution. Storage was performed for several weeks in the dark below 4 °C. For chitinolytic activity assay, bacterial cells were seeded onto plates with semi-minimal medium (SM) consisting of a mixture of SM and nutrient broth (Difco, USA) (3:1) supplemented with colloidal chitin (0.2 % [wt/vol]) and solidified with 1.5 % agar (Chernin et al. 1995). The plates were incubated at 28 °C for 72 to 96 h and the chitinolytic activity was detected by a clear zone of the chitin around the colonies. This experiment was repeated once with two plates per bacterium strain.
In vitro biocontrol assay
In order to determine the effect of the antagonistic bacteria on wheat seed germination and early development of plantlets prior and post infection with F. culmorum, a bioassay on seeds was set up. Seeds (cv. Karim) were soaked in 5 ml of a 24 h bacterial culture (108 cells ml−1) supplemented with 0.05 % of Tween20 for 3.5 h (Djébali 2012). Three controls were considered in this assay: (i) seeds soaked in LB medium, (ii) seeds soaked in sterile distilled water (pH = 7), and (iii) untreated seeds. After treatment, 35 seeds were put in square Petri dishes (12 cm × 12 cm) on two filter papers soaked with sterile distilled water. The plates were sealed to keep humidity nearing saturation and incubated at 25 °C in the dark (Essemine et al. 2007). In the case of infection, seeds were soaked first in a bacterial suspension for 3.5 h then in a macroconidia suspension (2 × 106 macroconidia ml−1) of F. culmorum for 2 h. Three controls were considered in this experiment: (i) seeds soaked in LB medium and then in the macroconidia suspension, (ii) seeds soaked in sterile distilled water (pH = 7) and then in the macroconidia suspension, and (iii) untreated seeds soaked in the macroconidia suspension. The germination test was done in the same way as before. Monitoring of seed germination was done for 3 days by counting the number of seeds that showed the emergence of their radicle, coleoptile and lateral roots. At the end of the test, the length of the coleoptile (± the first leaf) and the longest root and fresh weight of 10 seedlings were measured. The final germination percentage was calculated using the formula of Belcher and Miller (1974):
where n is the number of germinated seeds and N is the number of tested seeds. The average germination time (MGT) was calculated according to the formula of Ellis and Roberts (1981):
where n is the number of seeds germinated on day D and D is the number of days counted from the beginning of the germination test. The vigor index (VI) was estimated using the formula of Abdul-Baki and Anderson (1973):
where SL is the length of the seedling (coleoptile + roots length) in cm.
In the experiment with fungal infection the number of infected seeds was also determined. Two independent experiments were performed with two plates per treatment.
Greenhouse biocontrol assay
The plants were cultivated inside a controlled polycarbonate greenhouse (temperature maintained at 19 °C). Ten seeds previously inoculated with bacteria and F. culmorum as described above were deposited on the surface of double autoclaved sandy soil in 500 ml pots and covered by the same amount of sand. Irrigation was done according to the plants’ need with Hoagland solution (Hoagland and Arnon 1950). The plants were irrigated for 30 days, and then in the last 20 days of the experiment, alternation between wet and dry periods was applied to improve F. culmorum infection on plants as previously described by Gargouri-Kammoun et al. (2009). The percentage of emergence, the number of leaves per plant, the length of the shoot and root parts and the fresh and dry weights of the shoot and root parts were measured 50 days post inoculation. Symptoms of infection (degree of infection) were measured according to the scale established by Miedaner and Schilling (1996) and by measuring the length of the stem and root browning. In addition, diurnal changes in some photosynthetic parameters (net photosynthesis, transpiration and stomatal conductance) were measured at harvest (growth stage 6–7 leaves) using a portable gas-exchange system (Li-Cor 6200, Li-Cor Lincoln, NE). The ratio of net photosynthetic rate to stomatal conductance was taken as an estimate of intrinsic water use efficiency (transpiration efficiency of water) (Mediavilla et al. 2002). Measurements were carried out between 10:00 am and 12:00 am on10 fully expanded mature leaves (third leaf from the top) per treatment. Data were automatically collected every minute for 10 min after photosynthesis rate was stabilized. This experiment was repeated twice with three pots and 10 plants per pot for each treatment.
Statistical analysis
Statistical analysis was performed using Statistica software version 5.1 (www.statsoft.com) and a statistics package (Microsoft Excel 2007). In all experiments, One-Way ANOVAs, specifically Fisher’s least significant difference (LSD) test with a P value 0.05, were used to analyze significant differences between each treatment group.
Results
Effect of bacteria on F. culmorum growth in the dual culture assay
Forty three rhizospheric and 19 endophytic bacterial strains were isolated from wheat and barley roots. Screening for antifungal activity of the isolated bacteria showed strain differences (Fig. 1). The majority (60–74 %) of the isolated bacteria did not show any antagonistic effect against the two fungal strains (Table 1). Around 35 % and 23 % of the bacterial strains tested showed an inhibitory effect on the in vitro growth of the fungal strains Fc2 and Fc3, respectively. At 6 days of dual culture, 19 % and 13 % of bacterial strains showed an inhibition by direct contact of Fc2 and Fc3 growth in vitro (Table 1). The subculture of these antagonistic bacteria on agar medium supplemented with colloidal chitin showed that none of the tested strains produced clear halos around colonies, suggesting the absence of chitinase activity in these bacteria (data not shown). Sixteen and 10 % of the isolated bacterial strains induced an inhibitory effect at distance on Fc2 and Fc3 colony growth at 6 days (Table 1). We also noted that bacterial strains had a specific or general inhibitory effect against fungal strains. At 6 days of dual culture, Fc2 and Fc3 fungal strains were specifically inhibited by 26 % (e.g. B16E strain) and 13 % (e.g. B18 strain) of the bacterial strains, respectively (Table 1). Five per cent of the isolated bacterial strains showed an inhibitory activity against both fungal strains (e.g. B5E, B13E, B23, B24, B25 and B29 strains). Nevertheless, between 3 % and 5 % of the tested bacterial strains increased the fungal growth (e.g. B19, B21, B22, B6E and B11E strains) (Table 1). The fungal strain Fc2 was inhibited by 30 % of the rhizospheric bacteria and 47 % of the endophytic bacteria, whereas the Fc3 was inhibited by 23 % of rhizospheric and 21 % of endophytic bacterial strains (Table 1). Regardless of the fungal strain, we noted that the percentage of growth inhibition was higher for the endophytic bacterial strains which ranged between 20 % and 40 %, in comparison to the percentage of inhibition of the rhizospheric bacterial strains which ranged between 15 % and 33 % (Table 1).
Screening of antifungal activity of rhizospheric and endophytic bacteria isolated from wheat and barley roots against two strains of Fusarium culmorum (Fc2 and Fc3) in vitro at 6 days of dual culture. Error bars represent standard deviation of four replicates of two independent experiments. Bars annotated with the same letters are not significantly different at P ≤ 0.05 (LSD test)
The 16SrDNA genes of some bacterial strains were partially sequenced and submitted to GenBank. Blast analysis showed that strains B12 (KT326322), B13 (KT326323), B18 (KT326324), B19 (KT326325), BSE1, and BSE3 (KT326326) were affiliated with the genus Bacillus. However, strain B16E (KU647163) and strain B31 (KT326327) were assigned to the genera Pseudomonas and Microbacterium, respectively.
Effect of the bacterial volatile compounds on F. culmorum growth and macroconidia production in vitro
The volatile compound assay showed that 37 % and 30 % of the isolated bacteria had an inhibitory effect on the growth of Fc2 and Fc3, respectively. The Fc2 strain was more susceptible to the bacterial volatile compounds, showing a percentage of growth inhibition between 62 % and 75 %. However, the percentage of growth inhibition for Fc3 did not exceed 52 % at 3 days of dual culture (Fig. 2). The bacterial strains B16, B18, B16E showed the maximum inhibitory effect against Fc2 fungal strain; while the bacterial strains B14 and B25 showed the maximum inhibition effect against the Fc2 fungal strain (Fig. 2). The monitoring of the inhibitory effect of the bacterial volatile compounds on fungal growth at 3, 6 and 16 days showed that the majority of antagonistic strains (> 95 %) had maximum activity between 3 and 6 days (data not shown). Twenty days after confrontation some bacterial strains were able to reduce F. culmorum macroconidia production. The strains B13, B16E and BSE3 significantly decreased (P < 0.05) the Fc2 macroconidia production, whereas the strains BSE1and B16E significantly decreased (P < 0.05) the Fc3 macroconidia production (Table 2). The strain B16E caused 100 % and 97 % reduction in macroconidia production of Fc2 and Fc3 fungal strains, respectively (Table 2).
Screening of antifungal activity of volatile compounds produced by rhizospheric and endophytic bacteria isolated from wheat and barley roots against two strains of Fusarium culmorum (Fc2 and Fc3) in vitro at 3 days of dual culture. Error bars represent standard deviation of four replicates of two independent experiments. Bars annotated with the same letters are not significantly different at P ≤ 0.05 (LSD test)
Effect of the antagonistic bacteria on germination and development of fungal macroconidia
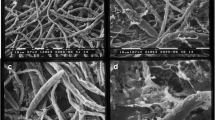
Several alterations in macroconidia of Fc3 were observed at 24 h of co-culture with the two antagonistic strains B18 and B16E. The germination percentage of macroconidia treated with the bacteria was reduced in comparison to the control (treated with water) (Fig. 3a, b). In addition, the few germinated macroconidia in the presence of strain B16E produced less branched germ tubes in comparison to the control (Fig. 3c). Swelling of Fc3 macroconidia was observed in the presence of strains B16E and B18 (black arrow in Fig. 3c, d). In comparison with the control fungal germ tubes (red arrow in Fig. 3e), strain B18 cells agglomerated around germ tubes causing the swelling of the apical parts and consequently their alteration (red arrow in Fig. 3f).
Effect of the antagonistic bacteria strains B18 and B16E on germination of macroconidia of Fusarium culmorum strain Fc3 after 24 h of co-culture. (a and c) Fc3 macroconidia and germ tube treated with water. (b and c) Fc3 macroconidia treated with B16E. (d and f) Fc3 macroconidia treated with B18, note the swelling of macroconidia (black arrow) and the end of germ tube (red arrow). Bars equal to 10 μm in (a, b, c and d) and 2 μm in (e and f)
The antagonistic bacteria effect on durum wheat seed germination in vitro
The effect of antagonistic bacteria on the germination of durum wheat seeds was assessed pre and post infection with both F. culmorum strains. The analysis of variance of the parameters of germination and early seedling growth pre and post infection with F. culmorum showed that they depended on the bacterial strain, Fusarium isolate and their interactions (data not shown). Bioprimed seeds with strains B13, B14, B29, BSE3 and B16E and infected with strain Fc2 showed a significant increase in the percentage of emergence of the radicle, coleoptile and roots at 3 days post infection (dpi) in comparison to infected and non-primed seeds (Table 3). However, in the case of the seeds infected with isolate Fc3, priming with all tested strains did not show any positive effect (Table 3). The studied antagonistic bacterial strains significantly increased the length of coleoptile and roots, the fresh weight and the vigor of Karim seedlings after infection with the two F. culmorum strains Fc2 and Fc3 in comparison to seedlings infected only with the pathogen at 3 dpi (Table 3). The coleoptile and root lengths were improved on average by 2.3 fold when seeds were primed with the bacteria B13, B14, B29, BSE3 and B16E and subsequently infected with Fc2 strain in comparison to seeds infected with the pathogen only (Table 3). The coleoptile and root lengths were improved on average by1.3 when seeds were primed with the bacteria B9, B18, B29, BSE1 and B16E and subsequently infected with Fc3 strain in comparison to seeds infected with the pathogen only (Table 3). All antagonistic bacteria tested induced a significant improvement in seedling vigor (Table 3). For example, the bacteria B13, B14 and B16E increased the vigor index about 2.8 fold in the case of Fc2 infected seedlings and the bacteria B18 and B29 increased the vigor index about 1.5 fold in the case of the seedlings infected with Fc3 (Table 3). None of the antagonistic bacteria tested affected the average germination time (Table 3). All the studied bacterial strains significantly reduced the natural contamination of seeds with Alternaria sp. and the percentage of seed infection by Fc2 and Fc3 fungal strains (Table 3). In general, the percentage of seed infection was reduced by about 90 % and 70 % when seeds were primed with the antagonistic bacteria and subsequently infected by Fc2 and Fc3 fungal strains, respectively (Table 3).
Greenhouse assay
Both of the used Fc2 and Fc3 strains of F. culmorum reduced plant growth parameters in infected versus non infected durum wheat plants with the greatest negative effect observed for the Fc2 strain in comparison to Fc3 strain (Table 4). The selected antagonistic bacteria that improved seed germination, seedling growth and reduced seed infection (strains B13, B16E and BSE3 against Fc2 and strains B18, B16E and BSE1 against Fc3) were used for biocontrol of F. culmorum in a greenhouse assay. The results showed that in general the inoculation with the selected antagonistic bacteria improved seedling development and reduced fungal pathogenicity (Table 4). In the case of infection with the isolate Fc2, the results showed that after inoculation with the strains B13, BSE3 and B16E the percentage of plant emergence per pot increased by 11 %, 17 % and 33 % respectively (Table 4). The inoculation with B16E strain increased significantly the length of shoot (aerial part of plant) and to a less extent the fresh and dry weights of shoots and roots, whereas the severity of infection was decrease significantly by half in comparison to plants infected only by Fc2 fungal strain (Table 4). In the case of infection with Fc3, the inoculation with the strain B18 increased the percentage of emerged plants per pot by 12 % and the number of leaves per plant by 38 % and decreased the level of infection by 3 fold (Table 4). The inoculation with strain B16E did not affect significantly the growth of plants but reduced the level of infection and length of browning on stems and roots in comparison to plants infected only by Fc3 fungal strain (Table 4).
No significant effects of the antagonistic bacteria were observed on parameters related to photosynthesis, transpiration, transpiration efficiency and stomatal conductance compared to plants infected by Fc2 only (Table 5). However, significant improvements in physiological parameters were observed using strains B18, BSE1and B16E in plants infected with Fc3. The inoculation with the bacterial strains B18, BSE1 and B16E increased net photosynthesis by 49 %, 55 % and 71 % respectively in comparison to the plants infected only by Fc3 fungal strain (Table 5). In general, the used bacterial strains against Fc3 fungal strain improved the transpiration efficiency of water and to less extent the transpiration and stomatal conductance (Table 5).
Discussion
Fusarium foot and root rot of wheat is a disease caused by a complex of fungal species of the genera Fusarium and Microdochium. Among the Fusarium species involved, F. culmorum is the most dominant species in northern Tunisia that infects wheat, especially in sub-humid and semi-arid bioclimatic regions (Gargouri-Kammoun et al. 2009). The seeds infected with Fusarium may induce a loss of seed germination, a reduced emergence, and a post emergence blight of seedling, thus causing less dense plant stand. The biological control through seed biopriming is a promising method that can be used as part of integrated management against Fusarium disease of wheat. The biopriming is a process of surface seed treatment with useful microorganisms to protect plants against future attacks by pathogens. In addition, seed biopriming can improve the rate and uniformity of seed germination and seedling emergence and growth which will have a positive effect on crop stand and production (Khan et al. 2008). Few studies were conducted in Tunisia on the biocontrol of Fusarium diseases of wheat (Hmissi et al. 2011; Rebib et al. 2012); nevertheless this study constitutes a first case of a global analysis of the effect of rhizospheric and endophytic bacteria isolated from cereal root and of testing seed biopriming technique for the biocontrol of F. culmorum.
Molecular characterization by 16S rDNA sequencing showed that the isolated bacterial strains mainly belonged to the genus Bacillus with two strains belonging to the genera Pseudomonas and Microbacterium. This result is in agreement with previous reports (Palazzini et al. 2007; Yang et al. 2009) showing that the majority of bacteria isolated from the roots of wheat belonged to the genus Bacillus. The predominance of this bacterial genus is due to its rapid growth and competitiveness in the soil for nutrients compared to other bacterial genera (Garbeva et al. 2003). This dominance can be explained by the attraction of these bacteria to root exudates of plants (Bais et al. 2004). The genus Bacillus contains the most antagonistic strains to plant pathogens (Liu et al. 2009; Elkahoui et al. 2012) providing effective protection for plants against Fusarium diseases (Kurek and Ściseł 2003). In our studied bacteria collection, 35 % and 23 % of the strains showed antagonistic activity against F. culmorum isolates Fc3 and Fc2, respectively. Depending on the fungal strain used, 13 % to 19 % of the bacterial strains showed inhibition of F. culmorum growth by contact. These antagonistic bacteria did not show any chitinase activity during four day incubation. However, longer incubation may have revealed some chitinase activity and may be examined in the future. Nevertheless, these bacteria may act by producing other enzymes or antifungal substances secreted in contact with the pathogen. The study done by Mamarabadi et al. (2009) showed that the bacterium Clonostachys rosea produces cell wall degrading enzymes such as chitinase, cellulase and glucanase. Chitinase has been implicated in biological control against F. culmorum in this later study. In addition, we observed that 10–16 % of the studied bacterial strains showed inhibition of F. culmorum growth at distance, probably due to the production of volatile compounds. Bacillus cereus strains isolated from wheat exhibited capabilities of producing volatile antagonistic compounds against F. graminearum (Alimi et al. 2012). Several volatile substances with antifungal activity produced by bacteria were identified such as acetamide, benzaldehyde, benzothiazole, 1-butanamine, methanamine and phenylacetaldehyde (Zou et al. 2007). Yuan et al. (2012) showed that Bacillus amyloliquefaciens produces volatile compounds (NJN-6) that inhibit the growth and conidial germination of Fusarium oxysporum f. sp. cubense. The implication of soluble antifungal compounds in the phenomena of inhibition at distance can not be excluded since we observed the production of these compounds in some bacterial strains (data not shown). In fact, bacteria of the genus Bacillus are well known for their production of diffuse substances against phytopathogenic fungi. Elkahoui et al. (2012) showed that B. subtilis strain SE14 has an inhibitory action on the growth of Rhizoctonia solani that persists beyond one month. Ben Slimene et al. (2012) showed that endospores of B. subtilis strain L194 produced lipopeptides belonging to the families of iturins, fengycines and surfactins causing the collapse of Phoma medicaginis conidia.
The sporulation of F. culmorum was inhibited by volatile compounds of strains BSE1, BSE3, B13 and B16E, which is an interesting behavior for the biocontrol of pathogens in the field and during postharvest storage of seeds. The conidial germination of F. culmorum was reduced by the vegetative cells of B18 and B16E strains. In addition, we observed the swelling of conidia and the apex of germ tubes of F. culmorum by these strains which are probably due to an alteration in membrane permeability. Similarly, Mauch et al. (2010) showed that Lactobacillus brevis PS1 affected both mycelial growth and germination of macroconidia of F. culmorum. Ben Slimene et al. (2012) found that vegetative cells (107 cells ml−1) and endospores of B. subtilis strain L194 reduce the germination of conidia of P. medicaginis following their swelling and bursting. According to these authors, these changes in the morphology of conidia are due to the production of different lipopeptides such as iturins, fengycines and mycosubtilines that can form transmembrane channels (Hsieh et al. 2008).
All Tunisian wheat varieties showed high sensitivity to F. culmorum attack (Gargouri-Kammoun et al. 2009). Therefore, the control of this pathogen by the selection of resistant varieties is limited by the lack of resistance genes in plant germplasm. Improving the quality of seeds by the technique of biopriming may be a promising way to increase the level of resistance of wheat varieties to this disease. In vitro antagonism tests allowed us to select several strains (e.g. B13, B16E, B18, BSE1 and BSE3) with an inhibitor effect on F. culmorum growth and sporulation. In order to use these bacteria in a biological control program against F. culmorum in wheat, it is mandatory to test their effect on wheat seed germination and subsequent development stages in order to eliminate deleterious bacterial strains. The inoculation of durum wheat seeds showed the existence of three types of bacteria. The first type includes bacteria that do not affect seed germination (most strains), the second type includes bacteria that improve seed germination and subsequent seedling development called plant growth promoting (PGP) bacteria (e.g. B13, B18 and B16E) and the third type of bacteria reduces the germination and seedling growth (deleterious bacteria like B19). So, this simple and rapid test allowed us to select among the antagonistic bacteria those with PGP activity. The bacteria B18 and B16E improved the length and fresh weight of coleoptile and roots of seedlings, the percentage of germination and seedling vigor index in vitro. Similarly, Somova et al. (2001) showed that some rhizospheric bacteria were able to improve seed germination and seedling growth of wheat. We also noted that the studied bacterial strains reduced the percentage of natural seed contamination by Alternaria sp. Begum et al. (2010) also showed that inoculation of carrot seed with the bacterium Clonostachys rosea significantly reduced mortality caused by Alternaria species transmitted by seeds such as A. dauci and A. radicina. All tested rhizospheric and endophytic antagonistic bacteria induced a decrease in the percentage of seed infection by F. culmorum in vitro which can be due to the direct pathogen antagonism of the bacteria and/or the improvement of seedling growth (Wang et al. 2009).
The biocontrol of F. culmorum in greenhouse using different antagonistic bacteria showed a better control results against Fc3 strain in comparison to Fc2 strain which is probably due to the difference in virulence between to two fungal strains. Indeed, in vitro and greenhouse tests showed that Fc2 strain was more virulent than Fc3 strain on durum wheat seeds and plants. This variability in the biocontrol of pathogens can be avoided by developing inoculums containing a mixture of different antagonistic bacterial strains or by selecting bacterial strains that induce systemic resistance in plants. The bacterial strains B16E and B18 showed specific antagonistic effect toward Fc2 and Fc3 fungal strains, respectively and when applied as seed coating agents they were able to reduce significantly seed and plant infections by F. culmorum. This specificity of action was also observed in seed and plant infection tests especially for B16E bacterial strain, which was more effectively in reducing infection caused by Fc2 strain in comparison to Fc3 strain. These two bacterial strains were able significantly to increase plant emergence, which is an important parameters that influence positively plant stand and production in the field (Khan et al. 2008). In addition, these bacterial strains promoted shoot length and number of leaves in infected plants in greenhouse conditions, probably indicating their capacity to produce hormones like auxin as reported for others PGP bacteria (Kakar et al. 2013). In addition to their complementary action against the two fungal strains, these two bacterial strains colonize different habitats i.e. B16E is an endophytic Pseudomonas strain and B18 is a rhizospheric Bacillus strain, so they may be used in a mixture to develop a broad spectrum antagonistic inoculum against F. culmorum. A mixture of strains of B. amyloliquefaciens and B. pumilus was effectively used for the protection of wheat against F. culmorum by reducing the length of root browning (Jetiyanon and Flowler 2003). Bacillus sp. strains have been also used in several studies to induce systemic resistance against plant diseases (Kumar et al. 2007). Both of the bacterial strains B18 and B16E improved significantly net photosynthesis in plants infected with the Fc3 fungal strain. This result indicates that PGP activity of B18 and B16E strains is due in part to the increase of CO2 assimilation in bioprimed plants. Somova et al. (2001) showed that the CO2 photo-assimilation was dependent on the amount of bacteria on the roots of plants. The development of a broad-spectrum inoculum with multiple PGP and antagonistic activities represent an excellent option to be used as either for plant growth bio-promoting or disease biocontrol in durum wheat crop. This application may help to reduce dependence on pesticides leading to a sustainable agriculture system.
Conclusion
This study revealed the presence of different kinds of rhizospheric and endophytic pathogen-antagonistic bacteria that colonize the roots of cereals with a predominance of the genus Bacillus. Some antagonistic bacteria were capable of inhibiting the mycelial growth, sporulation and conidia germination of F. culmorum. The inhibition effect of these bacteria was likely due in part to the production of volatile antifungal compounds. The results of antifungal activity showed that cereal roots harbors bacteria with a good potential in biocontrol of F. culmorum pathogen. Durum wheat seed biopriming with some antagonistic bacteria pre and post infection with F. culmorum increased the length of coleoptile and roots, fresh weight and vigor of seedlings and reduced the percentage of seed infection with two used F. culmorum strains. Under greenhouse conditions the effectiveness of the biocontrol of F. culmorum was dependant from the virulence of the fungal strain and the specificity of the antagonistic interaction between bacterial and fungal strains. The bacterial strains B18 and B16E reduced F. culmorum infection on durum wheat plants at 50 dpi probably due to their antagonistic and PGP activities and they may be used in a mixture as seed biopriming agents for plant growth bio-promoting and biocontrol of Fusarium wheat diseases.
References
Abdul-Baki, A. A., & Anderson, J. D. (1973). Vigor determination in soybean seed by multiple criteria. Crop Science, 13, 630–633.
Alimi, M., Soleimani, M. J., & Darzi, M. T. (2012). Characterization and application of microbial antagonists for control of Fusarium head blight of wheat caused by Fusarium graminearum using single and mixture strain of antagonistic bacteria on resistance and susceptible cultivars. African Journal of Microbiology Research, 6, 326–334.
Bai, G. H., & Shaner, G. E. (1994). Scab of wheat: prospects for control. Plant Disease, 78, 760–766.
Bais, H. P., Park, S. W., Weir, T. L., Callaway, R. M., & Vivanco, J. M. (2004). How plants communicate using the underground information superhighway. Trends in Plant Science, 9, 26–32.
Begum, M. M., Sariah, M., Puteh, A. B., Zainal-Abidin, M. A., Rahman, M. A., & Siddiqui, Y. (2010). Field performance of bio-primed seeds to suppress Colletotrichum truncatum causing damping-off and seedling stand of soybean. Biological Control, 53, 18–23.
Belcher, E. W., & Miller, L. (1974). Influence of substrate moisture level on the germination of sweetgum and pine seed. Proceedings of the association of official seed analysts, 65, 88–89.
Ben Slimene, I., Tabbene, O., Djébali, N., Cosette, P., Schmitter, J. M., Jouenne, T., Urdaci, M. C., & Limam, F. (2012). Putative use of a Bacillus subtilis L194 strain for biocontrol of Phoma medicaginis in Medicago truncatula seedlings. Research in Microbiology, 163, 388–397.
Chekali, S., Gargouri, S., Paulitz, T., Nicol, J. M., Rezgui, M., & Nasraoui, B. (2011). Effects of Fusarium culmorum and water stress on durum wheat in Tunisia. Crop Protection, 30, 718–725.
Chernin, L., Ismailov, Z., Haran, S., & Chet, I. (1995). Chitinolytic Enterobacter agglomerans antagonistic to fungal plant pathogens. Applied and Environmental Microbiology, 6, 1720–1726.
Dhingra, O.D., & Sinclar, J.B. (1995). Basic plant pathology methods, 2nd edition, CRC Press, Boca Raton, London,UK.
Djébali, N. (2012). Seed hydropriming effect on Triticum durum and Hordeum vulgare germination, seedling growth and resistance to Fusarium culmorum. Plant Pathology Journal, 11, 77–86.
Elkahoui, S., Djébali, N., Tabbene, O., Hadjbrahim, A., Mnasri, B., Mhamdi, R., Shaaban, M., & Limam, F. (2012). Evaluation of antifungal activity from Bacillus strains against Rhizoctonia solani. African Journal of Biotechnology, 11, 4196–4201.
Ellis, R. A., & Roberts, E. H. (1981). The quantification of ageing and survival in orthodox seeds. Seed Science Technology, 9, 373–409.
El-Mohamedy, R. S. R., & Abd El-Baky, M. M. H. (2008). Evaluation of different types of seed treatment on control of root rot disease, improvement growth and yield quality of pea plant in Nobaria province. Research Journal of Agriculture and Biological Sciences, 4, 611–622.
Essemine, J. E., Ammar, S., Jbir, N., & Bouzid, S. (2007). Sensitivity of two wheat species (Triticum durum, variety Karim and Triticum aestivum variety Salambô) to heat constraint during germination. Pakistan Journal of Biology Science, 10, 3762–3768.
Garbeva, P., van Veen, J. A., & van Elsas, J. D. (2003). Predominant Bacillus spp. in agricultural soil under different management regimes detected via PCR-DGGE. Microbial Ecology, 45, 302–316.
Gargouri-Kammoun, L., Gargouri, S., Hajlaoui, M. R., & Marrakchi, M. (2009). Occurrence and distribution of Microdochium and Fusarium species isolated from durum wheat in northern Tunisia and detection of mycotoxins in naturally infested grain. Journal of Phytopathology, 157, 546–551.
Gargouri-Kammoun, L., Gargouri, S., Barreau, C., Richard-Forget, F., & Hajlaoui, M. R. (2010). Trichothecene chemotypes of Fusarium culmorum infecting wheat in Tunisia. International Journal of Food Microbiology, 140, 84–89.
Gerhardson, B. (2002). Biological substitute for pesticides. Trends in Biotechnology, 20, 338–343.
Gupta, C. D., Dubey, R. C., Kang, S. C., & Maheshwari, D. K. (2001). Antibiotic mediated necrotrophic effect on Pseudomonas GRC2 against two fungal plant pathogens. Current Science, 81, 91–94.
Hmissi, I., Gargouri, S., & Sifi, B. (2011). Attempt of wheat protection against Fusarium culmorum using Rhizobium isolates. Tunisian Journal of Plant Protection, 6, 75–86.
Hoagland, D. R., & Arnon, D. I. (1950). The water culture method for growing plants without soil, Circular 347, p. 32. Berkley: University of California Agricultural Experiment Station.
Hsieh, F. C., Lin, T. C., Meng, M., & Kao, S. S. (2008). Comparing methods for identifying Bacillus strains capable of producing the antifungal lipopeptide Iturin a. Current Microbiology, 56, 1–5.
Hsu, S. C., & Lockwood, J. L. (1975). Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Applied Microbiology, 29, 422–426.
Jetiyanon, K., & Flowler, J. W. (2003). Broad spectrum protection against several pathogens by PGPR mixtures under field conditions. Plant Disease, 87, 1390–1394.
Kakar, K. U., Duan, Y. P., Nawaz, Z., Sun, G., Almoneafy, A. A., Hassan, M. A., Elshakh, A., Li, B., & Xie, G. L. (2013). A novel rhizobacterium Bk7 for biological control of brown sheath rot of rice caused by Pseudomonas fuscovaginae and its mode of action. European Journal of Plant Pathology, 137, 1–16.
Khan, A., Khalil, S. K., Khan, A. Z., Marwat, K. B., & Afzal, A. (2008). The role of seed priming in semi-arid area for mung bean phenology and yield. Pakistan Journal of Botany, 40, 2471–2480.
Kumar, B., Trivedi, P., & Pandey, A. (2007). Pseudomonas corrugata: a suitable bacterial inoculant for maize grown under rained conditions of Himalayan region. Soil Biology and Biochemistry, 12, 3093–3100.
Kurek, E., & Ściseł, J. J. (2003). Rye (Secale cereale) growth promotion by Pseudomonas fluorescens strains and their interactions with Fusarium culmorum under various soil conditions. Biological Control, 1, 48–56.
Latiri, K., Lhommeb, J. P., Annabia, M., & Setterc, T. L. (2010). Wheat production in Tunisia: progress, inter-annual variability and relation to rainfall. European Journal of Agronomy, 33, 33–42.
Liu, B., Qiao, H., Huang, L., Buchenauer, H., Han, Q., Kang, Z., & Gong, Y. (2009). Biological control of take- all in wheat by endophytic Bacillus subtilis ER-j and potential mode of action. Biological Control, 49, 277–285.
Mamarabadi, M., Dan Funck, J., & Mette, L. (2009). An N-acetyl-β-d-glucosaminidase gene, cr-nag1, from the biocontrol agent Clonostachysrosea is up-regulated in antagonistic interactions with Fusarium culmorum. Mycology Research, 1, 33–43.
Mauch, A., Dal Bello, F., Coffey, A., & Arendt, E. K. (2010). The use of Lactobacillus brevis PS1 to in vitro inhibit the outgrowth of Fusarium culmorum and other common Fusarium species found on barley. International Journal of Food Microbiology, 12, 116–121.
Mediavilla, S., Santiago, H., & Escudero, A. (2002). Stomatal and mesophyll limitations to photosynthesis in one evergreen and one deciduous mediterranean oak species. Photosynthetica, 40, 553–559.
Mhamdi, R., Laguerre, G., Aouani, M. E., Mars, M., & Amarger, N. (2002). Different species and symbiotic genotypes of field rhizobia can nodulate Phaseolus vulgaris in Tunisian soils. FEMS Microbiology and Ecology, 41, 77–84.
Miedaner, T., & Schilling, A. G. (1996). Genetic variation of aggressiveness in individual field populations of Fusarium graminearum and Fusarium culmorum tested on young plants of winter rye. European Journal of Plant Pathology, 102, 823–830.
Nicholson, P., Simpson, D. R., Wilson, A. H., Chandler, E., & Thomsett, M. (2004). Detection and differentiation of trichothecene and enniatin- producing Fusarium species on small- grain cereals. European Journal of Plant Pathology, 110, 503–514.
Palazzini, J. M., Maria, L., Ramirez, M. L., Torres, A. A. M., & Chulze, S. N. (2007). Potential biocontrol agents for Fusarium head blight and deoxynivalenol production in wheat. Crop Protection, 11, 1702–1710.
Parry, D. W., Jenkinson, P., & McLeod, L. (1995). Fusarium ear blight (scab) in small grain cereals. Plant Pathology, 44, 207–238.
Rebib, H., Hedi, A., Rousset, M., Boudabous, A., Limam, F., & Sadfi-Zouaoui, N. (2012). Biological control of Fusarium foot rot of wheat using fengycin-producing Bacillus subtilis isolated from salty soil. African Journal of Biotechnology, 11, 8464–8475.
Reddy, R. M., Reddy, P. G., & Seenayya, G. (1999). Enhanced production of thermostable β-amylaseand pullanase in the presence of surfactants by Clostridium thermosulfurogenes SV2. Process Biochemistry, 34, 87–92.
Rezgui, M., Ben Mechlia, N., Bizid, E., Kalboussi, R., & Hayouni, R. (2000). Etude de la stabilité du rendement de blé dur dans différentes régions de la Tunisie. L’amélioration du blé dur dans la région méditerranéenne: nouveaux défis. Cahier Option Méditerranéenne, 40, 167–172.
Rotter, B. A., Prelusky, D. B., & Pestka, J. J. (1996). Toxicology of deoxynivalenol (vomitoxin). Journal of Toxicology and Environmental Health, 48, 1–34.
Sivan, A., Ucko, O., & Chet, I. (1987). Biological control of Fusarium crown rot of tomato by Trichoderma harzianum under field condition. Plant Disease, 71, 587–595.
Somova, L. A., Pechurkin, N. S., Sarangova, A. B., & Pisman, T. I. (2001). Effect of bacterial population density on germination of wheat seeds and dynamics of simple artificial ecosystems. Advances in Space Research, 9, 1611–1615.
Wang, H., Wen, K., Zhao, X., Wang, X., Li, A., & Hong, H. (2009). The inhibitory activity of endophytic Bacillus sp. strain CHM1 against plant pathogenic fungi and its plant growth-promoting effect. Crop Protection, 8, 634–639.
Weisburg, W. G., Barns, S. M., Pelletier, D. A., & Lane, D. J. (1991). 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology, 173, 697–703.
Yang, J., Kloepper, J. W., & Choong-Min, R. (2009). Rhizosphere bacteria help plants tolerate abiotic stress. Trends in Plant Sciences, 14, 1–4.
Yuan, J., Raza, W., Shen, O., & Huang, Q. (2012). Plant microbiology: antifungal activity of Bacillus amyloliquefaciens NJN- 6 volatile compounds against Fusarium oxysporum f. sp. cubense. Applied Environmental Microbiology, 78, 5942–5944.
Zou, C. S., Mo, M. H., Gu, Y. Q., Zhou, J. P., & Zhang, K. Q. (2007). Possible contributions of volatile- producing bacteria to soil fungistases. Soil Biology and Biochemistry, 39, 2371–2379.
Acknowledgments
Financial support of this work was obtained from the Tunisian Ministry of High Education and Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mnasri, N., Chennaoui, C., Gargouri, S. et al. Efficacy of some rhizospheric and endophytic bacteria in vitro and as seed coating for the control of Fusarium culmorum infecting durum wheat in Tunisia. Eur J Plant Pathol 147, 501–515 (2017). https://doi.org/10.1007/s10658-016-1018-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-1018-3