Abstract
The literature on the entomopathogenic fungi in the genus Cordyceps describing its use in the agricultural area as a biocontrol agent is limited. In this study, a total of 47 isolates of entomopathogenic fungi were isolated from dead cicada nymphs obtained from various locations in the northeast of Thailand. These isolates were primarily screened for antagonistic activity to inhibit the mycelial growth of one isolate of Colletotrichum gloeosporioides and one isolate of C. capsici. The screen revealed that five isolates of entomopathogenic fungi showed good inhibitory effects on the fungal mycelial growth and were chosen for further confirmation of their antagonistic effects against five isolates of C. gloeosporioides and five isolates of C. capsici by the dual culture method. After investigation, the isolate Cod-NB1302 had the best inhibitory effect. Moreover, the mycelium extract and culture filtrate of isolate Cod-NB1302 also had inhibitory effects on the mycelial growth and conidial germination of all isolates of plant pathogenic Colletotrichum spp. under in vitro conditions. Interestingly, the mycelium extract and culture filtrate effectively reduced the size of the disease lesion and disease severity on chili fruits after inoculation with the plant pathogenic fungi. However, the mycelium extract exhibited greater antifungal activity than the culture filtrate. Finally, the isolate Cod-NB1302 was identified as Ophiocordyceps sobolifera based on the sequence of three ribosomal nuclear DNA genes and two protein-coding genes. These findings suggest that the isolate Cod-NB1302 is a potential candidate, with antagonistic activity, for use as a source of antifungal agents to control anthracnose disease caused by the plant pathogenic Colletotrichum spp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chili (Capsicum annuum L.) is an important crop in tropical and subtropical areas (Ratanacherdchai et al. 2007). During the process of production, chili is attacked by many plant diseases and caused there yield lose (Pakdeevaraporn et al. 2005; Poonpolgul and Kumphai 2007; Than et al. 2008). One major disease in chili production areas is anthracnose (Shin et al. 2000; Sharma et al. 2005). In Thailand, anthracnose of chili is caused by the fungi Colletotrichum gloeosporioides (Penz.) Sacc. and C. capsici (H.Syd.) E. Butl. & Bisby, which are the most serious fungal pathogens (Sangchote et al. 1998; Oanh et al. 2004). These fungal pathogens can produce disease symptoms on leaves, stems and both young and mature fruits (Hong and Hwang 1998; Kim et al. 1999), and it reduces the chili yield from 10 % to 80 % in some developing countries (Poonpolgul and Kumphai 2007).
The method to protect against these fungal pathogens is usually done through the integration of cultural management practices and chemical treatments (Asthana et al. 1989), but these ways posses a risk to human health and the environment (Montri et al. 2009). Therefore, an alternative and effective method to control the anthracnose pathogen is the use of biological control agents (Harman et al. 2004), as alternative methods that could potentially be less harmful to human health and the environment as chemical pesticides. For example, Trichoderma spp. are ubiquitous in the soil and has been known for many years as potential biological control agents (Bae and Knudsen 2005). T. harzianum (Th-F, Th-G, Th-I and Th-N) has been applied to control C. capsici in pepper (Ekefan et al. 2009). Interestingly, some entomopathogenic fungi, such as Cordyceps sobolifera, have been reported for use as a biocontrol agent against C. gloeosporioides and C. miyabeanus (Imtiaj and Lee 2007).
Cordyceps species are an entomopathogenic macrofungi that are well known for their use in traditional Chinese medicine (Cha et al. 2006; Wang et al. 2011). The various species in this genus produce many bioactive compounds that have only been used in medical and pharmacological areas. For example, Imtiaj and Lee (2007) found that a bioactive compound from C. sobolifera could inhibit the growth of the human pathogenic bacteria Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus under in vitro conditions. Wong et al. (2011) reported the anti-microbial properties of the peptide ‘cordymin’ isolated from C. militaris that could inhibit the mycelial growth of Candida albicans. Meanwhile, the literature describing agricultural uses of the biocontrol agents is limited. However, previous reports indicated that Cordyceps species are a potential candidate with broad antagonistic activity that could be used as a biocontrol agent. Therefore, the present study aimed to isolate the entomopathogenic fungi from dead cicada nymphs in the northeast of Thailand and to investigate their potential to inhibit the growth of the plant pathogenic fungi Colletotrichum spp., which is the causal agent of chili anthracnose disease under in vitro and in vivo conditions. The potential isolate was identified by its particular genome using the PCR sequencing technique.
Materials and methods
Isolation of entomopathogenic fungus
The entomopathogenic fungus was isolated from dead cicada nymphs collected from the mixed deciduous forest in the northeast of Thailand using the tissue transplanting technique as described by Sangdee and Sangdee (2013). The mycelium growing out from the cicada larvae tissue was sub-cultured on potato dextrose agar (PDA) and incubated at 28 °C for further study.
Isolation of Colletotrichum spp. causal agent of chili anthracnose disease
The plant pathogenic fungi Colletotrichum spp. were isolated from infected chili with typical disease symptoms using the tissue transplantation technique as described by Sangdee et al. (2011). The mycelium growing out of the plant tissue were sub-cultured to PDA medium, and incubated at 25–28 °C. After confirming as Colletotrichum species by microscope examination based on conidia morphological characteristics, a pathogenicity test was done strictly with Koch’s postulates before being used in this study.
Primary screening of antagonistic entomopathogenic fungus
Forty-seven isolates of the entomopathogenic fungi were initially screened for their antagonistic activity against two isolates of Colletotrichum spp. by the dual culture method in 90 mm Petri dishes containing 20 mL of PDA. Fungal hypha tips of all isolates of the entomopathogenic fungi were cut with a 7 mm diameter cork borer and placed on PDA at a 10 mm from the plate periphery, while a mycelium plug of the pathogen was placed on the opposite side at a 70 mm distance. The dual culture plates were incubated at 28 °C for 14 days. The control plates consisted of individual cultures of the pathogen. After 14 days, the dual culture plates were evaluated for antagonistic activity that reduced pathogen colony expansion. Each dual culture had three replicates. The top five isolates that inhibited the mycelial growth of the plant pathogenic fungi and produced inhibition zones were selected for further confirmation of their antagonistic activity.
Confirmation of antagonistic activity against the plant pathogenic Colletotrichum spp.
The selected isolates of antagonistic entomopathogenic fungi were tested for their antagonistic activity against five isolates of C. gloeosporioides (CgC) and five isolates of C. capsici (CcC) using the dual culture method as described in the primary screening test. After 14 days, the dual culture plates were evaluated for antagonistic activity that reduced the pathogen colony expansion. The percentage of mycelial growth reduction (PGI-1) was calculated using the formula:
where KR represents the fungal growth radius (mm) of the control culture and R1 represents the fungal growth radius distance (mm) in the direction of the entomopathogenic fungal growth (Korsten et al. 1995). The data from each experiment were analyzed with an analysis of variance, and means were compared by Duncan’s Multiple Range Test (DMRT) (at P = 0.05). The best isolates that inhibited the mycelial growth of the plant pathogenic fungi were selected as candidate antagonistic biocontrol agents for further investigation of their antagonistic activity.
Effect of culture filtrate and cultured mycelium extract on mycelial growth of Colletotrichum spp.
Preparation of culture filtrate and cultured mycelium extract
A 20 day old disk of the mycelium of the best selected antagonistic biocontrol agent was inoculated in 25 mL of induced medium (Huang et al. 2009) without shaking at 28 °C for 20 days. The mycelium on the surface of the induced culture was collected and dried at 50 °C for 2–3 days, while the culture filtrate was also collected and then filtrated through a 0.2 μm filter before being used. The dried mycelium was powdered using a pestle and mortar. Then the mycelium powder was suspended in 50 % ethanol with the final concentration at 100 mg/mL (w/v). The mycelium suspension was sonicated with a High Intensity Ultrasonic Processor (Model VCX 750, Newtown, CT, USA) on ice for a total of 5 min in 10 s bursts with 2 s gaps for cooling. The sonicated solutions were centrifuged (Tomy MX-301, Tokyo, Japan) at 9100×g for 5 min and filtered through a 0.2 μm filter before being used.
Tube dilution assay
The sterile culture filtrate, mycelium extract and 50 % ethanol were diluted with two fold dilutions in PDB medium for a 5 mL total volume. The diluted culture filtrate, mycelium extract and 50 % ethanol were incubated with a mycelial disk of the plant pathogenic fungi Colletotrichum spp. for seven days at 28 °C under a static condition. The control tube contained 5 mL PDB that was incubated with the individual plant pathogenic fungi. The mycelial growth was categorized on a scale of 0 to 2, where 0 = no mycelial growth, 1 = growth limited around mycelial disk and 2 = mycelia overgrows into liquid medium (Alvindia and Natsuaki 2008). The experiment was done with three replications.
Pour plate technique
Five milliliter of the sterile culture filtrate, mycelium extract and 50 % ethanol were mixed in 100 mL PDA medium before being plated to 90 mm Petri dishes. Seven-day-old mycelial discs of each pathogen were cut with a sterilized cork borer to a diameter of 7 mm under aseptic conditions and placed onto the 25 mL PDA plates containing sterile culture filtrate, mycelium extract and 50 % ethanol. The plates were incubated at 28 °C. The mycelium growth was determined at 14 days. The fungus grew on the PDA plate that was used as a control plate. The experiment was done with three replications. The percentage of mycelial growth reduction (PGI-2) was calculated using the formula:
where R represents the fungal growth radius (mm) of the control culture and R1 represents the fungal growth radius (mm) in the treatment culture (Kumer et al. 2007). The PGI-2 was categorized from 0 to 4, where 0 = no growth inhibition; 1 = 1–25 % growth inhibition; 2 = 26–50 % growth inhibition; 3 = 51–75 % growth inhibition; and 4 = 76–100 % growth inhibition (Korsten et al. 1995). The data from each experiment was analyzed with an analysis of variance and means were compared by DMRT (at P = 0.05).
Effect of mycelium extract and culture filtrate on conidial germination of Colletotrichum spp.
Inoculum of the Colletotrichum spp. was prepared by culturing on PDA medium until sporulation. Conidia were harvested by flooding the cultures with distilled water. The concentrations of the propagules in suspension were standardized with the aid of a hemocytometer to 1 × 104 conidia mL−1 for each fungus. The conidial germination test was determined on PDA plates containing 10 % sterile mycelium extract, 10 % culture filtrate and 10 % of 50 % ethanol. The plates were incubated at 28 °C. Then 100 spores of all treatments were determined within 24 h under light microscope. The inhibition rate of conidial germination (ICG) was calculated using the formula:
where T represents the germination rate of the treatment and C represents the germination rate of the control (Kwak et al. 2012). The experiment was done with five replicates.
Control of anthracnose disease on chili fruit by detached fruit bioassay
Mature chili fruits were surface sterilized in 70 % alcohol for five minutes before being washed several times with sterile distilled water and then blotted dry on sterilized filter paper. The sterilized chili fruits were pin pricked gently with a sterilized needle prior to inoculation. Inoculums of the Colletotrichum spp. were prepared by culturing on PDA medium until sporulation. The mycelium extract and culture filtrate of the best selected antagonistic isolates were applied to a pin point wound on the mature chili fruits one day before the conidia suspensions of the pathogen were applied. The inoculated fruits were incubated in a moist chamber and kept at room temperature. The disease severity was observed daily for seven days. The severity index (SI) of the anthracnose symptoms was categorized based on the lesion diameter, SL = no lesion or symptomless, HR = highly resistant (1.0–4.9 mm), MR = moderately resistant (5.0–9.9 mm), MS = moderately susceptible (10.0–19.9) and HS = highly susceptible (>20.0 mm) (Hartman and Wang 1992).
Molecular identification of entomopathogenic fungal isolate Cod-NB1302
The entomopathogenic fungus was cultured on PDB medium at 28 °C for 20 days. Mycelia were harvested from the PDB medium before being homogenized with liquid nitrogen. The mycelium powder was transferred to a microcentrifuge tube and genomic DNA was extracted using the DNA extraction kit (Vivantis, Malaysia). The DNA samples were analyzed by 1 % agarose gel electrophoresis and stored at −20 °C.
Three regions of ribosomal nuclear DNA, the internal transcribed spacers of nuclear ribosomal DNA repeats (ITS), the partial small subunit of (nrSSU) rDNA, the partial large subunit of (nrLSU) rDNA, two protein-coding regions, the elongation factor 1α (EF-1α) and the largest subunit of the RNA polymerase II (rpb1) gene were used for fungal identification. The ITS region was amplified using the primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC −3′) (White et al. 1990). The partial nrSSU rDNA was amplified using the primers NS1 (5′-GTAGTCATATGCTTGTCTC-3′) and NS2 (5′-GGCTGCTGGCACCAGACTTGC-3′) (White et al. 1990). The partial nrLSU rDNA was amplified by PCR using the primers LROR (5′-ACCCGCTGAACTTAAGC-3′) and LR7 (5′-TACTACCACCAAGATCT-3′) (Vilgalys and Hester 1990). The EF-1α was amplified using the primers EF-983F (5′-GCYCCYGGHCAYGGTGAYTTYAT-3′) and EF-2218R (5′-GACTTGACTTCRGTVGTGAC-3′) (Currie et al. 2003), and the rpb1 gene was amplified using the primers CRPB1 (5′-CCWGGYTTYATCAAGAARGT-3′) and RPB1Cr (5′-CCNGCDATNTCRTTRTCCATRTA-3′) (Castlebury et al. 2004). The PCR reactions of all genes were performed as described by Sangdee et al. (2015).
The PCR products obtained were purified with a Gel/PCR DNA Fragments extraction kit (Geneaid, USA). Sequencing was performed by Macrogen Advancing through Genomics (Macrogen Inc., Korea). The sequence data of the partial ITS, nrSSU, nrLSU, EF-1α and rpb1 were compared with sequences in the National Center for Biotechnology Information data bank using the BLAST program (www.ncbi.nih.gov/blast). The novel partial sequences were deposited in the GenBank nucleotide sequence database, and reference sequences of related species were downloaded and aligned using ClustalW (www.genome.jp/tools/clustalw/). Phylogenetic analyses of the ITS and combined data sets of the ITS, nrSSU, nrLSU, EF-1α and rpb1 were performed using Mega 6 (Tamura et al. 2013) and a Neighbor Joining tree (NJ tree) was constructed (bootstrap replicates =1000) using the Kimura 2 parameter method for pairwise deletion at uniform rates. Bionectria ochroleuca was used as the outgroup.
Results
Isolation and primary screening of antagonistic entomopathogenic fungi
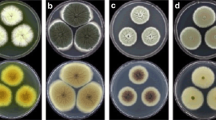
Within 7–14 days, a total of 47 isolates of the entomopathogenic fungi produced cottony colonies with white cream synnemata on cicada nymph tissues, were isolated and used to evaluate their antagonistic activity against the plant pathogenic fungi C. gloeosporioides and C. capsici. The results showed that the interaction between the mycelial growth of the entomopathogenic fungi and plant pathogenic Colletotrichum spp. were categorized into two groups. The first group, the mycelium of the entomopathogenic fungi and plant pathogenic fungi grow well and inhibited each other without an inhibition zone. The second group, the mycelial growth of plant pathogenic Colletotrichum spp. was inhibited by the mycelium of the entomopathogenic fungi with an inhibition zone (Fig. 1). The high percentage of mycelial growth reduction and large inhibition zone were used as criteria for selection of the antagonistic strain. Therefore, the top five isolates of the entomopathogenic fungi consisted of the isolates Cod-NB1302, Cod-NB1305, Cod-NN1307, Cod-MK1208 and Cod-Loei1301, which showed a high percentage of mycelium reduction in the range from 16.67 to 54.55 % and produced inhibition zones of at least 3 mm, and they were selected for further studies.
Interaction between mycelial growth of entomopathogenic fungi and plant pathogenic Colletotrichum spp. (C. capsici a-e and k-o; C. gloeosporioides f-j and p-t) by dual culture method. Entomopathogenic fungi group 1 consisted of isolates Cod-MK1301 (a, f), Cod-MK1311 (b, g), Cod-MK1321 (c, h), Cod-NN1303 (d, i) and Cod-NB1307 (e, j). Group 2 consisted of Cod-NB1302 (k, p), Cod-NB1305 (l, q), Cod-NN1307 (m, r), Cod-MK1208 (n, s) and Cod- Loei1301 (o, t)
Confirmation of antagonistic activity against the plant pathogenic Colletotrichum spp.
The top five isolates of entomopathogenic fungi were assayed against the five isolates of C. gloeosporioides and five isolates of C. capsici. The results showed that all the selected isolates inhibited the growth of C. gloeosporioides and C. capsici in the range from 18.00 to 34.45 % and 31.11 to 43.55 %, respectively. The percentage of fungal mycelial growth reduction varied depending on the isolate of entomopathogenic fungi and plant pathogenic Colletotrichum spp. However, the selected entomopathogenic fungi isolate Cod-NB1302 showed the highest percentage of mycelial growth reduction in all the tested fungi, which was significantly greater than with the other isolates (Table 1). The inhibition zone produced by the isolate Cod-NB1302 varied from 12 to 19 mm and 16 to 21 mm for C. gloeosporioides and C. capsici, respectively (Fig. 2). Therefore, isolate Cod-NB1302 was chosen for further evaluation of the antagonistic activity.
Effect of culture filtrate and mycelium extract on mycelial growth of Colletotrichum spp.
The mycelium extract of isolate Cod-NB1302 exhibited greater antifungal activity against all 10 isolates of the tested plant pathogenic fungi than the culture filtrate and 50 % ethanol (Table 2). The mycelium extract completely controlled the mycelial growth of C. gloeosporioides and C. capsici at the dilutions of 1:1 up to 1:8, whereas the culture filtrate showed partial effects on the growth of all 10 isolates of the tested fungi at the dilutions of 1:1 to 1:4. The 50 % ethanol also completely inhibited the fungal mycelial growth at the dilutions of 1:1 and 1:2, while at the dilution of 1:4 it inhibited the mycelial growth of some isolates (Table 2). Similar results were obtained with the pour plate technique. The percentage of mycelial growth reductions ranged from 50.4 to 71.09 % (PGI-2 = 3) for all 10 isolates of the tested fungi, and they were more sensitive to the mycelium extract than the other treatments with culture filtrate and 50 % ethanol. Interestingly, the mycelium extract exhibited greater antifungal activity than the 50 % ethanol in the range from 1.0 to 2.0-fold; whereas, the culture filtrate had no effect on the mycelial growth (Table 3).
Effect of mycelium extract and culture filtrate on conidial germination of Colletotrichum spp.
The effect of the mycelium extract and culture filtrate of the selected entomopathogenic fungal isolate Cod-NB1302 on conidial germination was investigated. The results showed that the culture filtrate suppressed conidial germination of all 10 isolates of the tested fungi ranging from 20.16 to 32.33 %, while the mycelium extract and 50 % ethanol exhibited suppression of the conidial germination that ranged from 38.63 to 48.25 % and 33.98 to 43.94 %, respectively (Table 3). Moreover, the effect of the mycelium extract and culture filtrate on the length of conidial germ tube were observed in this study. The mycelium extract had a greater effect on the conidial germ tube than the other treatments with culture filtrate and 50 % ethanol. The length of the germ tube on PDA plus mycelium extract ranged from 90 to 200 μm. While, germ tube lengths of 170 to 400 μm, 100 to 230 μm and 350 to 620 μm were observed from PDA plus culture filtrate, 50 % ethanol and control group, respectively. In addition, abnormally shaped mycelium was observed from the mycelium extract and culture filtrate treatments.
Control of anthracnose disease on chili fruit by detached fruit bioassay
The potential of the selected entomopathogenic fungus isolate Cod-NB1302 to inhibit C. gloeosporioides and C. capsici was successfully evaluated by the detached fruit technique. The mycelium extract showed generally lower disease severity on the inoculated chili fruits than the culture filtrate, 50 % ethanol and positive control. The disease lesion sizes for the mycelium extract ranged from 6.8 ± 2.7 to 11.2 ± 4.9 mm and 3.0 ± 1.2 to 5.8 ± 1.6 mm for C. gloeosporioides and C. capsici, respectively. While, the culture filtrate generally had larger disease lesion sizes than the mycelium extract. The disease lesion sizes for the culture filtrate ranged from 12.4 ± 2.5 to 15.6 ± 2.8 mm and 3.6 ± 0.9 to 8.0 ± 2.1 mm for C. gloeosporioides and C. capsici, respectively. In the 50 % ethanol treatment, C. gloeosporioides and C. capsici induced disease lesion sizes that ranged from 13.8 ± 2.8 to 16.0 ± 4.2 mm and 14.6 ± 1.1 to 16.4 ± 2.2 mm, respectively. While, the disease lesion sizes ranging from 15.6 ± 3.3 to 17.6 ± 3.7 mm and 13.8 ± 3.9 to 18.8 ± 2.3 mm were observed in the positive control after inoculation of chili fruits with C. gloeosporioides and C. capsici, respectively (Table 4 and Fig. 3).
Effect of mycelium extract (c and g) and culture filtrate (d and h) of the entomopathogenic fungal isolate Cod-NB1302 and 50 % ethanol (b and f) on chili fruits after inoculation with plant pathogenic C. gloeosporioides isolate CgC12 (a-d) and C. capsici isolate CcC6 (e-h) compared with control group (a and e)
Molecular identification of entomopathogenic fungal isolate Cod-NB1302
The ITS, nrSSU, nrLSU, EF-1α and rpb1 regions of the selected entomopathogenic fungal isolate Cod-NB1302 were amplified using universal primers. The DNA fragments were purified and sequenced. The DNA sequences of the ITS, nrSSU, nrLSU, EF-1α and rpb1 regions, which consisted of 508, 510, 1001, 437 and 662 nucleotides, respectively, were submitted to GenBank (Table 5). A BLAST search in NCBI (www.ncbi.nih.gov/blast) showed that the ITS sequence was most similar to Ophiocordyceps sobolifera (=Cordyceps sobolifera) with 91 % homology. A phylogenetic tree of the ITS region was generated from 27 aligned sequences with similar characters that indicated the isolate Cod-NB1302 was located in the same clade as O. sobolifera with a 6–7 % genetic distance and 93 % NJ bootstrap support (Fig. 4). These similar data findings were obtained with alignment of the combined data set consisting of ITS, nrSSU, nrLSU, EF-1α and rpb1 sequences (3096 nucleotides) for 28 taxa. The NJ tree of the combined data set confirmed that the isolate Cod-NB1302 was also placed within the same clade as O. sobolifera with 3–8 % genetic distance and 79 % NJ bootstrap support (Fig. 5). Based on the molecular data we suggest that the isolate Cod-NB1302 be designated O. sobolifera isolate Cod-NB1302.
Phylogenetic relationship of entomopathogenic fungal isolate Cod-NB1302, the 25 related Cordyceps species and one out group based on partial ITS gene sequences. Neighbor Joining (NJ) tree was constructed using Mega 6. Percentages expressed above the branches are frequencies with which a given branch appeared in 1000 bootstrap replications when using the NJ method (branches corresponding to partitions reproduced in <50 % were collapsed)
Phylogenetic relationship of entomopathogenic fungal isolate Cod-NB1302 and 27 related species based on partial ITS, nrSSU, nrLSU, EF-1α and rpb1 sequences. Neighbor Joining (NJ) tree was constructed using Mega 6. Percentages expressed above the branches are frequencies with which a given branch appeared in 1000 bootstrap replications by NJ method (branches corresponding to partitions reproduced in <50 % were collapsed)
Discussion
The entomopathogenic macrofungi in the genus Cordyceps have been known as important ingredients in Chinese medicine for thousands of years (Zhu et al. 1998; Cha et al. 2006). Information on the bioactivities of these macrofungi have been reported in the medical and pharmaceutical areas, including antitumor, antioxidant, immunomodulatory, anti-inflammatory and antimicrobial activities (Tuli et al. 2014). While, the information on the bioactive compounds that use in the agricultural area, such as a biocontrol agent, is limited. In the present study, antifungal activity against the plant pathogenic fungus Colletotrichum spp. by an entomopathogenic macrofungus isolated from a dead cicada nymph was observed. Five of 47 isolates from the entomopathogenic fungi showed good inhibition of the mycelial growth of the plant pathogenic fungi. However, the antifungal effects of the entomopathogenic fungi depended on the strain and species of the entomopathogenic fungi and the plant pathogenic Colletotrichum. For example, entomopathogenic fungal isolate Cod-NB1302 showed the highest percentage of mycelial growth reduction of C. capsici isolate CcC4 at 43.55 %, while the mycelial growth reduction of C. gloeosporioides isolate CgC10 was 25.02 %. In another case, a different isolate of entomopathogenic fungi showed variations in the percentage of mycelial growth reduction when tested with the same isolates of the plant pathogenic Colletotrichum spp. These findings correlate with those of Chen and Huang (2010) who found that the antimicrobial effect depended on the strains and species of mushroom and the species of test microorganism. Moreover, these suggest that different antagonistic strains from the same species produce different amounts of antimicrobial compounds (Suay et al. 2000).
In this study, the entomopathogenic fungal isolate Cod-NB1302 was identified as O. sobolifera based on three ribosomal nuclear DNA genes and two protein-coding genes, and this showed the best inhibitory effect against the mycelial growth of all the test fungi using the dual culture method, as this isolate was chosen for confirmation of the antagonistic activity. The mycelium extract appeared to completely inhibit the mycelial growth of all test fungi using the tube dilution assay (Table 2) and there were partially inhibited by the pour plate assay (Table 3). Unfortunately, the inhibitory effect of the mycelium extract was interfering by 50 % ethanol that used as an organic solvent which is shown in Tables 2 and 3. A similar inhibition effect was found in the conidial germination test. However, we suggest that the mycelium extract has some bioactive compounds that affect the mycelial growth because the mycelium extract exhibited greater antifungal activity than the 50 % ethanol. Interestingly, the fungal effect on mycelial growth and conidial germination were also found in the culture filtrate treatments. These findings correlate with those of Imtiaj and Lee (2007) who reported that the culture filtrate from O. sobolifera showed good inhibitory effects against the mycelial growth of three plant pathogenic fungi: Botrytis cinerea, C. gloeosporioides and C. miyabeanus. Vesely and Koubova (1994) found that the entomopathogenic fungi Beauveria bassiana could inhibit the mycelial growth of the plant pathogenic Pythium ultimum, P. debaryanum and Septoria nodorum by a cell lysis mechanism. Chen and Huang (2010) also found that the culture filtrate of the mushroom Lentinula edodes could inhibit the mycelial growth and zoospore germination of the plant pathogenic fungi Phytophtora capsici. Therefore, the mycelium extract and culture filtrate that contained some bioactive compounds, such as chitinase (Lee and Min 2003) and β-1-3, glucanase enzyme, may degrade the cell and lead to the lysis of the hypha of the pathogen (Wu et al. 1986). These possible reasons indicate that the isolate Cod-NB1302 had bioactive compounds that effected the mycelial growth and conidial germination of the plant pathogenic Colletotrichum spp. under in vitro conditions.
For the detached chili fruit bioassay, the chili fruits treated with the mycelium extract and culture filtrate of the isolate Cod-NB1302 effectively reduced the size of the disease lesion and disease severity of all test plant pathogenic Colletotrichum. These results indicated that the mycelium extract could reduce the disease severity of the pathogen without interference from the 50 % ethanol (Table 4). These findings correlate with those of Chen and Huang (2010) who found that pepper leaves treated with the culture filtrate of the mushroom Clitocybe nuda (LA82) and C. aureus effectively reduced the disease severity of phytophtora blight caused by P. capsici. Ownley et al. (2010) reported that the endophytic fungal entomopathogen B. bassiana could produce bioactive metabolites to reduce the disease severity caused by soil borne plant pathogens. To our knowledge, we suggest that the O. sobolifera isolate Cod-NB1302 could be used as a source of antifungal agents to control anthracnose disease caused by the plant pathogenic Colletotrichum spp.
References
Alvindia, D. G., & Natsuaki, K. T. (2008). Evaluation of fungal epiphytes isolated from banana fruit surfaces for biocontrol of banana crown rot disease. Crop Protection, 27, 1200–1207.
Asthana, A., Dixit, K., Tripathi, N. N., & Dixit, S. N. (1989). Efficacy of ocimum oil against fungi attacking chili seed during storage. Tropical Science, 49, 15–20.
Bae, Y. S., & Knudsen, G. R. (2005). Soil microbial biomass influence on growth and biocontrol efficacy of Trichoderma harzianum. Biological Control, 32, 236–242.
Castlebury, L. A., Rossman, A. Y., Sung, G. H., Hyten, A. S., & Spatafora, J. W. (2004). Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycological Research, 108(8), 864–872.
Cha, S. H., Kim, J. C., Lim, J. S., Yoon, C. S., Koh, J. H., Chang, H. I., et al. (2006). Morphological characteristics of Cordyceps sinensis 16 and production of mycelia and exo-biopolymer from molasses in submerged culture. Journal of Industrial and Engineering Chemistry, 12(1), 115–120.
Chen, J. T., & Huang, J. W. (2010). Antimicrobial activity of edible mushroom culture filtrates on plant pathogens. Plant Pathology Bulletin, 19, 261–270.
Currie, C. R., Wong, B., Stuart, A. E., Schultz, T. R., Rehner, S. A., Mueller, U. G., et al. (2003). Ancient tripartite coevolution in the attine ant-microbe symbiosis. Science, 299, 386–388.
Ekefan, E. J., Jama, A., & Gowen, S. R. (2009). Potential of Trichoderma harzianum isolates in biocontrol of Colletotrichum capsici causing anthracnose of pepper (Capsicum spp.) in Nigeria. Journal of Applied Biosciences, 20, 1138–1145.
Harman, G. E., Howell, C. R., Viterbo, A., Chet, I., & Lorito, M. (2004). Trichoderma species opportunistic, avirulent plant symbionts. Nature Reviews Microbiology, 2(1), 43–56.
Hartman, G.L., & Wang, T.C. (1992). Characteristics of two Colletotrichum species and evaluation of resistance to anthracnose in pepper. Proc. 3rd Intl. Conf. Plant Protection in the Tropics, vol 6. Malaysian Plant Protection Society, Kuala Lumpur, pp 202–205.
Hong, J. K., & Hwang, B. K. (1998). Influence of inoculum density, wetness duration, plant age, inoculation method, and cultivar resistance on infection of pepper plants by Colletotrichum coccodes. Plant Disease, 82, 1079–1083.
Huang, L., Li, Q., Chen, Y., Wang, X., & Zhou, X. (2009). Determination and analysis of cordycepin and adenosine in the products of Cordyceps spp. African Journal of Microbiology Research, 3(12), 957–961.
Imtiaj, A., & Lee, T. S. (2007). Screening of antibacterial and antifungal activities from Korean wild mushrooms. World Journal of Agricultural Science, 3(3), 316–321.
Kim, K. D., Oh, B. J., & Yang, J. (1999). Differential interactions of a Colletotrichum gloeosporioides isolate with green and red pepper fruits. Phytoparasitica, 27(2), 97–106.
Korsten, L., De-Jager, E. S., De-Villers, E. E., Lourens, A., Kotzé, J. M., & Wehner, F. C. (1995). Evaluation of bacterial epiphytes isolated from avocado leaf and fruit surfaces for biocontrol of avocado postharvest diseases. Plant Disease, 79, 1149–1156.
Kumer, A. S., Eswara Reddy, N. P., Hariprasad Reddy, K., & Charitha Devi, M. (2007). Evaluation of fungicidal resistance among Colletotrichum gloeosporioides isolates causing mango anthracnose in agri export zone of Andhra Pradesh, India. Plant Pathology Bulletin, 16, 157–160.
Kwak, Y. K., Kim, I. S., Cho, M. C., Lee, S. C., & Kim, S. (2012). Growth inhibition effect of environment-friendly farm materials in Colletotrichum acutatum in vitro. Journal of Bio-Environment Control, 21, 127–133.
Lee, K. H., & Min, T. J. (2003). Purification and characterization of a chitinase in culture media of Cordyceps militaris (L.) link. The Korean Journal of Mycology, 31, 168–174.
Montri, P., Taylor, P. W. J., & Mongkolporn, O. (2009). Pathotypes of Colletotrichum capsici, the causal agent of chili anthracnose, in Thailand. Plant Disease, 93, 17–20.
Oanh, L. T. K., Korpraditskul, V., & Rattanakreetakul, C. (2004). A pathogenicity of anthracnose fungus. Colletotrichum capsici on various Thai chilli varieties. Kasetsart Journal (Nat. Sci.), 38, 103–108.
Ownley, B. H., Gwinn, K. D., & Vega, F. E. (2010). Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution. BioControl, 55, 113–128.
Pakdeevaraporn, P., Wasee, S., Taylor, P. W. J., & Mongkolporn, O. (2005). Inheritance of resistance to anthracnose caused by Colletotrichum capsici in Capsicum. Plant Breeding, 124(2), 206–208.
Poonpolgul, S., & Kumphai, S. (2007). Chilli pepper anthracnose in Thailand. Country report. In D. G. Oh & K. T. Kim (Eds.), Abstracts of the first international symposium on chilli anthracnose (p. 23). National Horticultural Research Institute: Rural Development of Administration, Republic of Korea.
Ratanacherdchai, K., Wang, H. K., Lin, F. C., & Soytong, K. (2007). RAPD analysis of Colletotrichum species causing chilli anthracnose disease in Thailand. Journal of Agricultural Technology, 3(2), 211–219.
Sangchote, S., Pongpisutta, R., Kongsamai, B., Taweechai, N., & Sukprakarn, S. (1998). Resistance of pepper to Colletotrichum spp. In The first announcement and international conference on Periurban vegetable production in the 21st century, 29th September-1st October 1998. Bangkok: Kasetsart University.
Sangdee, A., Sachan, S., & Khankhum, S. (2011). Morphological, pathological and molecular variability of Colletotrichum capsici causing anthracnose of chilli in the north-east of Thailand. African Journal of Microbiology Research, 5(25), 4368–4372.
Sangdee, A., & Sangdee, K. (2013). Isolation, identification, culture and production of adenosine and cordycepin from cicada larva infected with entomopathogenic fungi in Thailand. African Journal of Microbiology Research, 7(2), 137–146.
Sangdee, K., Nakbanpote, W., & Sangdee, A. (2015). Isolation of the entomopathogenic fungal strain Cod-MK1201 from a cicada nymph and assessment of its antibacterial activities. International journal of Medicinal Mushrooms, 17(1), 51–63.
Sharma, P. N., Kaur, M., Sharma, O. P., Sharma, P., & Pathania, A. (2005). Morphological, pathological and molecular variability in Colletotrichum capsici, the cause of fruit rot of chilies in the subtropical region of North-Western India. Journal of Phytopathology, 153, 232–237.
Shin, H. J., Xu, T., Zhang, C. L., & Chen, Z. J. (2000). The comparative study of capsicum anthracnose pathogens from Korea with that of China. Journal of Zhejiang University Agriculture and Life Sciences, 26, 629–634.
Suay, I., Arenal, F., Asensio, F. J., Basilio, A., Cabello, M. A., Díez, M. T., et al. (2000). Screening of basidiomycetes for antimicrobial activities. Antonie Van Leeuwenhoek, 78, 129–139.
Tamura K, Stecher, G., Peterson, D., Filipski, A. & Kumar S (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution. doi:10.1093/molbev/mst197.
Than, P. P., Prihastuti, H., Phoulivong, S., Taylor, P. W. J., & Hyde, K. D. (2008). Chilli anthracnose disease caused by Colletotrichum species. Journal of Zhejiang University SCIENCE B, 9(10), 764–778.
Tuli, H.S., Sandhu, S.S. & Sharma, A.K. (2014). Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin. 3 Biotech, 4, 1–12.
Vesely, D., & Koubova, D. (1994). In vitro effect of the entomopathogenic fungi Beauveria bassiana (Bals.-Criv.) Vuill. And B. brongniartii (Sacc.) Petch on Phytopathogenic fungi. Ochrana Rostlin, 30, 113–120.
Vilgalys, R., & Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology, 172, 4238–4246.
Wang, X. L., Liu, G. Q., Zhu, C. Y., Zhou, G. Y., & Kuang, S. M. (2011). Enhanced production of mycelial biomass and extracellular polysaccharides in caterpillar-shaped medicinal mushroom Cordyceps sinensis CS001 by the addition of palmitic acid. Journal of Medicinal Plants Research, 5, 2873–2878.
White, T. J., Bruns, T., Lee, S., & Taylor, J. W. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, Pp. 315–322 In: PCR Protocols: A Guide to Methods and Applications, eds. Innis, M.A., Gelfand, D.H., Sninsky, J.J., and White, T.J. New York: Academic Press, Inc..
Wong, J. H., Ng, T. B., Wang, H., Wing Sze, S. C., Zhang, K. Y., Li, Q., et al. (2011). Cordymin, an antifungal peptide from the medicinal fungus Cordyceps militaris. Phytomedicine, 18, 387–392.
Wu, W. S., Liu, S. D., Chung, Y. C., & Tschen, S. (1986). Hyperparasitic relationship between antagonists and Rhizoctonia solani. Plant Protection Bulletin, 28, 91–100.
Zhu, J. S., Halpern, G. M., & Jones, K. (1998). The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis. Part 1. The Journal of Alternative and Complementary Medicine, 4(3), 289–303.
Acknowledgments
The authors express their thanks to the Mahasarakham University for providing financial support for this study (grant no. 5601010/2556), as well as the Mahasarakham University Faculty of Science for providing equipment. P. Jaihan gratefully thanks the Human Resource Development in Science Project (Science Achievement Scholarship of Thailand; SAST). Finally, we thank Dr. Jolyon Dodgson for language editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Jaihan, P., Sangdee, K. & Sangdee, A. Selection of entomopathogenic fungus for biological control of chili anthracnose disease caused by Colletotrichum spp.. Eur J Plant Pathol 146, 551–564 (2016). https://doi.org/10.1007/s10658-016-0941-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-0941-7