Abstract
Clubroot, caused by Plasmodiophora brassicae, is an important soilborne disease of canola (Brassica napus) in Alberta, Canada. Genetic resistance is the most effective clubroot management tool, and resistant cultivars are grown extensively in affected regions. In 2013, relatively severe symptoms of clubroot were observed in some fields of resistant canola. In greenhouse tests, four populations of P. brassicae from two of these fields caused significantly increased levels of clubroot on the cultivars from which they had been first recovered; these included three populations (L-G1, L-G2 and L-G3) recovered from the cultivar ‘L135C’, and one population (D-G3) recovered from ‘D3152’. Further testing showed that L-G1, L-G2 and L-G3 were highly virulent on a suite of six resistant canola cultivars (‘45H29’, ‘D3152’, ‘74–47CR’, ‘1960’, ‘L135C’ and ‘6056CR’) representing a cross-section of products available in Canada, while a seventh cultivar (‘9558c’) was moderately resistant to moderately susceptible. Bioassays of field soil with a dozen clubroot-resistant host genotypes confirmed that in most cases, resistance was no longer effective. Host responses to the population D-G3 were more variable, with most cultivars developing intermediate levels of disease. All four P. brassicae populations were classified as pathotypes 5, P3 and 16/6/8 on the differentials of Williams, Somé et al., and the European Clubroot Differential set. The pathotype classifications, however, do not reflect the increased virulence of these populations on clubroot-resistant canola. The identification of new virulence phenotypes of P. brassicae capable of overcoming genetic resistance underscores the need for increased stewardship of resistance sources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clubroot, caused by the obligate parasite Plasmodiophora brassicae Woronin, is an important soilborne disease of canola (Brassica napus L.) and other crucifers. Disease development is associated with the formation of large galls or ‘clubs’ on the roots of susceptible hosts. When gall development is severe, normal intake of water and nutrients by the plant is impeded, resulting in aboveground symptoms that include stunting, yellowing and premature senesce. These effects on plant growth can significantly impact crop yield and quality, with the disease estimated to cause losses of 10 % - 15 % worldwide (Dixon 2006, 2009). Clubroot is a relatively new problem in the canola-producing prairie region of western Canada, and was first identified on canola in the province of Alberta in 2003 (Tewari et al. 2005). The number of P. brassicae-infested fields has increased rapidly since this discovery, from just 12 confirmed infestations in 2003 to over 1800 infestations in Alberta by 2014 (Strelkov et al. 2015). In addition, isolated instances of clubroot on canola have been identified in the provinces of Manitoba and Saskatchewan (Cao et al. 2009; Dokken-Bouchard et al. 2012), as well as in the adjacent American state of North Dakota (Chittem et al. 2014). The spread of clubroot has resulted primarily from the movement of P. brassicae-infested soil on farm and other machinery (Cao et al. 2009), although other mechanisms of dispersal, including the movement of pathogen resting spores in windborne dust, also have been implicated (Rennie et al. 2015).
The occurrence and spread of clubroot represents a major challenge to the Canadian canola industry, which is worth an estimated C$15.4 billion annually to the national economy (Rempel et al. 2014). Management of the disease is difficult, since resting spores of P. brassicae can remain viable in the soil for more than 15 years (Wallenhammar 1996), and soil inoculum loads can increase quickly in the presence of susceptible hosts (Hwang et al. 2013). A number of strategies have been recommended for the management of clubroot. These include the manipulation of seeding dates to escape infection (Gossen et al. 2012), liming of affected soils to increase the soil pH (Donald and Porter 2014; Murakami et al. 2002; Webster and Dixon 1991), and the application of fungicides to suppress the disease (reviewed in Donald and Porter 2014 and Peng et al. 2014). While some of these strategies have proven to be helpful in controlling clubroot in cruciferous vegetables, they are not practical or cost-effective in canola, which is grown on a very large scale in fields that are typically about 65 ha in size (Howard et al. 2010; Hwang et al. 2014). Further challenges to the management of clubroot on canola include limited cropping options in western Canada and the reduced economic returns associated with pulses and cereals, which lead many farmers to grow canola in very short rotation (Strelkov et al. 2011), thereby contributing to increases in soil inoculum levels. Moreover, the sanitization of farm machinery to remove P. brassicae-infested soil and slow down the spread of clubroot is not widely practiced by farmers, who often view this task as prohibitively onerous and time-consuming (Hwang et al. 2014; Strelkov and Hwang 2014).
The lack of practical cultural or chemical strategies for managing clubroot on canola led to significant interest in the development of cultivars with genetic resistance to P. brassicae (Rahman et al. 2014). The cultivation of resistant varieties is one of the most effective and environmentally friendly ways to manage plant diseases, and P. brassicae-resistant cultivars of numerous Brassica crops have been developed successfully in the past (Diederichsen et al. 2009; Gowers 1982; Hirai 2006). In Canada, the first clubroot resistant canola cultivar became available to farmers in 2009, and was followed by other resistant cultivars from various seed companies starting in 2010. These cultivars exhibit strong resistance to the predominant pathotypes of P. brassicae reported from Canada (Strelkov and Hwang 2014), and have enabled the production of high yielding canola crops even in fields where pathogen inoculum levels are very high. Indeed, resistant cultivars have become the most important tool for the management of clubroot on canola (Peng et al. 2014), and sometimes are grown in very short rotations in heavily infested fields. Clubroot disease resistance can erode quickly, however, in response to the selection pressure imposed by resistant cultivars (Diederichsen et al. 2014; Hatakeyama et al. 2006; Seaman et al. 1963), and the repeated cropping of a resistant cultivar may increase this risk (LeBoldus et al. 2012). In this context, it is important to monitor the performance of clubroot-resistant genotypes as a proactive measure to identify shifts in the virulence of P. brassicae populations.
A large survey for clubroot conducted in Alberta in 2013 revealed six fields of resistant canola in which disease severity, as evaluated by the extent of root galling, was greater than expected for clubroot-resistant hosts (Strelkov et al. 2014). This study was undertaken to characterize the P. brassicae populations recovered from these fields in order to: (1) determine if the increased levels of clubroot represented an erosion of resistance or some other confounding factor, and (2) assess the virulence patterns of the pathogen populations on a suite of clubroot-resistant canola genotypes and host differential sets. Knowledge of the effectiveness of resistance in a particular region and of changes in the composition of P. brassicae pathotypes is important to help guide canola breeding activities, and to ensure proper stewardship of genetic resistance.
Materials and methods
Collection of field samples
Six fields of clubroot resistant canola with higher than expected levels of the disease were identified in a 2013 survey conducted in Alberta (Strelkov et al. 2014). These fields were located in four counties (Lac Ste. Anne, Strathcona, Sturgeon, and Westlock) in central Alberta, where the clubroot outbreak is most severe (Strelkov and Hwang 2014). A total of four different canola cultivars, all classified as clubroot resistant, had been grown in the fields in question, including ‘6056 CR’ (2 fields; Lac Ste. Anne and Strathcona), ‘D3152’ (2 fields; Strathcona and Westlock), ‘45H29’ (1 field; Sturgeon), and ‘L135C’ (1 field; Westlock). Symptomatic canola root samples were collected from each field (50–100 roots per field) and allowed to air dry at room temperature in the laboratory. After swathing of the crop, soil (150–200 kg) was collected from areas in each field where clubroot symptoms had been observed, and transported back to the laboratory. For comparison purposes, soil also was collected from a field nursery located in a rural area in the northeast part of the City of Edmonton, Alberta, in which clubroot resistance still appeared to be effective.
Virulence on hosts from which pathogen populations were first recovered
Resting spores of P. brassicae were extracted as described by Strelkov et al. (2006) from three randomly selected galls (each on a different root sample) from each field. Briefly, a single dry gall was placed in a mortar and ground to a coarse powder with a pestle. Approximately half of the material was removed from the mortar and stored at −20 °C for additional analysis (see below). Twenty ml of sterile distilled water was added to the remaining half, mixed with the ground root material, and filtered through six layers of cheesecloth (American Fiber & Finishing Inc., Albemarle, NC). Resting spores in the filtrate were quantified under a microscope with a haemocytometer (VWR, Mississauga, ON) and used immediately or stored at 4 °C for a maximum of 2 d. Galls were processed individually, with each pathogen population that was characterized corresponding to a single original gall.
In order to determine whether or not the increased levels of clubroot observed in the field represented increased virulence in the pathogen populations, as opposed to confounding factors such as the presence of large numbers of volunteer canola plants or off-types, each of the three pathogen populations from each field were inoculated on to the same canola genotypes from which they had been first recovered, and evaluated for disease development under controlled conditions. Suspensions of P. brassicae resting spores were adjusted to a concentration of 1 × 107 resting spores/ml with sterile distilled water and used to inoculate the host seedlings (Strelkov et al. 2006). One-week-old seedlings of the host genotypes, which had been germinated on moistened filter paper in Petri dishes, were inoculated by dipping the entire root system in the resting spore suspension for 10 s. The inoculated seedlings were then immediately planted in 6 cm × 6 cm × 6 cm plastic pots filled with Sunshine LA4 potting mixture (Sunshine Growers, Vancouver, BC) at a density of one seedling per pot. The pots were watered thoroughly and transferred to a greenhouse maintained at 20 °C ± 2 °C with a 16 h photoperiod under natural light supplemented by artificial lighting. The potting mixture was kept saturated with water (pH 6.5) for the first week after inoculation by placing the pots on water-filled trays; subsequently, the pots were watered and fertilized (20 N:20P:20 K) from above as needed.
The reaction of the clubroot resistant canola genotypes to each pathogen population was compared with that of a susceptible canola cultivar (‘45H26’). In addition, the host genotypes were inoculated with the P. brassicae population SACAN03-1, which was derived from a field collection made prior to the introduction of clubroot resistant canola in Canada and which had not been exposed to any sources of resistance (Strelkov et al. 2006). The population is classified as pathotype 3 on the differentials of Williams (1966), which is predominant in Alberta and highly virulent on clubroot susceptible canola (Howard et al. 2010; Strelkov and Hwang 2014). In this initial assessment of the virulence of the P. brassicae populations, three replicates (each consisting of 12 seedlings) were evaluated for each host × pathogen combination.
Virulence on a suite of clubroot-resistant canola cultivars
Populations of P. brassicae causing clubroot symptoms significantly greater than those caused by SACAN03-1 on the host cultivars from which they first were collected were characterized further by inoculation onto a suite of seven clubroot resistant canola cultivars (‘45H29’, ‘9558c’, ‘D3152’, ‘74–47CR’, ‘1960’, ‘L135C’ and ‘6056CR’). This enabled a comparison of the infectivity of these populations across multiple resistant genotypes. Each pathogen population also was inoculated onto the universally susceptible Chinese cabbage (Brassica rapa var. pekinensis) ‘Granaat’ (European Clubroot Differential (ECD) 05; Buczacki et al. 1975). To avoid the possibility of further shifts in the virulence of the pathogen populations as a result of repeated cycling on resistant hosts under greenhouse conditions (LeBoldus et al. 2012), inoculum was prepared from the portions of each gall that had been stored at −20 °C prior to the initial assessment of virulence (see above). Assessments were conducted in the greenhouse as described above. Each run of this experiment consisted of three replicates (each consisting of 12 seedlings) per treatment, and the entire experiment was run twice (Exp. 1 and Exp. 2).
Soil assays
Soil collected from one of the fields (no. 359–13) in Weslock County and from the Edmonton field nursery was tested in greenhouse bioassays. Two experiments were set up separately with each of the two soil collections. The field soil was mixed thoroughly with a commercial potting mix (Sunshine Mix LA7, Sunshine Growers) in a 1:1 (vol:vol) ratio. For each experimental unit, 8 L of the soil mixture was placed in a plastic container (35 cm × 24 cm × 13 cm) with six holes in the bottom. Treatments consisted of different canola genotypes, including the clubroot resistant cultivars ‘45H29’, ‘6056CR’, ‘73-67RR’, ‘73-77RR’, ‘74–47CR’, ‘9558C’, ‘D3152’ and ‘L135C’, the resistant lines TC72439-10, TC72447-10, TC72451-10 and 08 N8234, and the susceptible cultivar ‘45H26’. The containers were arranged on a greenhouse bench in a randomized complete block design with eight replications. To assess the reaction of the canola genotypes, 120 seeds of each cultivar or line were planted in four rows per container (replicate) for each soil collection. The containers were placed on water-filled trays (water pH = 6.5) for the first 2 weeks after seeding, in order to ensure high soil moisture levels for infection, and were then watered from above with a sprinkler as needed. The plants were fertilized with a 0.1 % solution of NPK (20–20-20) once a week (commencing at 2 weeks after seeding) until the end of the experiment.
Pathotype classification
The pathotype classification of P. brassicae populations showing increased virulence on clubroot resistant canola cultivars was determined on the differential hosts of Williams (1966), the ECD set (Buczacki et al. 1975), and Somé et al. (1996). Pathotype nomenclature is presented as proposed for each of those systems, except that the term ‘pathotype’ is substituted for ‘race’ (Strelkov et al. 2006). Seeds of the ECD set were obtained from the Genetic Resources Unit, Warkwick Crop Centre, The University of Warwick, Wellesbourne, UK, while the differentials of Williams (1966) were obtained from Wisconsin Fast Plants, University of Wisconsin, Madison, WI, USA. Two of the differentials of Somé et al. (1996) are shared in common with the ECD set, while a third, the spring oilseed rape (B. napus) ‘Brutor’, was obtained from the Canola Breeding Program, University of Alberta. The winter oilseed rape ‘Mendel’ also was included along with the differential hosts in all of the inoculations. Inoculum preparation, inoculations and plant growth conditions were as described above for the virulence assessments on the resistant canola genotypes. Three replicates (each consisting of 12 seedlings) of each differential were included in inoculations of the differential sets.
Disease assessment
Six weeks after inoculation (or, in the case of the soil assays, 5 weeks after planting), the seedlings were gently uprooted, washed with water, and evaluated for clubroot symptom development. The severity of root infection was assessed on a 0-to-3 scale modified from Kuginuki et al. (1999), where: 0 = no galling, 1 = a few small galls (small galls on less than one-third of the roots), 2 = moderate galling (small to medium galls on one-third to two-thirds of the roots), and 3 = severe galling (medium to large galls on more than two-thirds of the roots). Severity ratings for each experimental unit were converted to an index of disease (ID) according to the formula of Horiuchi and Hori (1980) as modified by Strelkov et al. (2006): ID (%) = {[∑ (n × 0) + (n × 1) + (n × 2) + (n × 3)]/N × 3} × 100 %, where n is the number of plants in a class, N is the total number of plants, and 0, 1, 2 and 3 are the symptom severity classes.
Analysis
Mean IDs and 95 % confidence intervals were calculated with Microsoft Excel 2010 (Microsoft Corp., Redmond, USA). Host genotypes were considered resistant to a particular P. brassicae population if the mean ID was <50 % and its associated 95 % confidence interval (CI) did not overlap 50 % (LeBoldus et al. 2012). For nonparametric data analysis (i.e., disease rating data), the CATMOD Procedure (SAS Institute Inc. 2014) was used to make pairwise contrasts among host genotypes or P. brassicae populations. For analysis of the plant growth parameters in the soil assays, ANOVA was performed using the GLM Procedure (SAS Institute Inc. 2014), with mean separation conducted according to Duncan’s Multiple Range test. Differences were considered significant at P = 0.05 unless otherwise stated.
Results
Virulence on hosts from which pathogen populations were first recovered
When inoculated under greenhouse conditions, all three P. brassicae populations tested from field no. 359–13 in Westlock County caused severe clubroot on the resistant canola cultivar ‘L135C’, from which they had been first recovered (Table 1). Indeed, clubroot severity following inoculation of ‘L135C’ with resting spores from Galls 1 (ID =99.1 %), 2 (ID =100 %), or 3 (ID =100 %) was not significantly different from clubroot severity on the susceptible check ‘45H26’ (ID =100 %). By comparison, the population SACAN03-1, which had not been exposed to any resistance source, caused only trace amounts of disease on ‘L135C’ (ID =1.9 %). Similarly, one of the three populations (Gall 3) tested from another field in Westlock County (field no. 360–13), which had been cropped to the clubroot resistant canola ‘D3152’, also caused significantly more severe clubroot (ID =69.4 %) on this cultivar than did SACAN03-1 (ID =32.4 %) (Table 1). However, clubroot severity was milder than on the susceptible check ‘45H26’ (ID =100 %). The four P. brassicae populations (Galls 1, 2 and 3 from field no. 359–13, and Gall 3 from field no. 360–13) exhibiting increased virulence on the clubroot-resistant cultivars ‘L135C’ and ‘D3152’ were renamed L-G1, L-G2, L-G3 and D-G3, respectively, and characterized further as described below. None of the other pathogen populations tested, namely Galls 1 and 2 from field no. 360–13 and the 12 populations recovered from Lac Ste. Anne (field no. 356–13), Strathcona (field nos. 358-13 A and 358-13B) and Sturgeon (field no. 357–13) counties, induced significantly increased levels of clubroot on the resistant cultivars (Table 1).
Virulence on a suite of clubroot-resistant canola cultivars
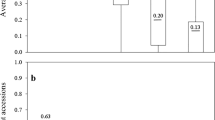
The P. brassicae populations L-G1, L-G2, L-G3 and D-G3, which in the initial assessment caused significantly increased levels of clubroot on the resistant genotypes ‘L135C’ or ‘D3152’ (Table 1), were evaluated for their virulence on seven canola cultivars representing a cross-section of clubroot resistant products available on the Canadian market (Table 2). Very similar trends were observed in both runs of this experiment, as reflected in a highly significant positive correlation (r2 = 0.8592, P < 0.0001) between Exp. 1 and Exp. 2. As expected, all four populations of the pathogen caused severe disease in the Chinese cabbage ‘Granaat’, which was included as a susceptible check (IDs = 94.4 % - 100 %). Moreover, the populations recovered from ‘L135C’ (L-G1, L-G2, L-G3) also were highly virulent on ‘45H29’, ‘D3152’, ‘74–47 CR’, ‘1960’, ‘6056 CR’, as well as on ‘L135C’, causing IDs ranging from 76.9 % to 100 % (Table 2). The lowest levels of clubroot were observed to occur consistently on the cultivar ‘9558C’, although for L-G1 and L-G2 the severity of disease on this genotype was significantly greater in Exp. 2 than in Exp. 1.
The reaction of the host genotypes to inoculation with population D-G3, collected from the resistant canola cultivar ‘D3152’, was more variable (Table 2). For instance, while D-G3 caused an ID of nearly 70 % on ‘D3152’ in the initial evaluation of virulence (Table 1), in the subsequent experiment, ID on this cultivar averaged 37.0 % (Exp. 1) and 45.5 % (Exp. 2) (Table 2). There also was significant fluctuation in the reactions of ‘74–47 CR’, ‘1960’ and ‘L135C’ in between the two runs of the experiment, with significant increases in ID observed in Exp. 2. Nonetheless, clear trends did emerge with respect to inoculation with D-G3. The cultivar ‘D3152’ was always among the most susceptible to this population, while resistance to D-G3 in ‘45H29’, ‘9558C’ and ‘6056 CR’ appeared to hold up well in both runs of the experiment (Table 2).
Soil assays
In soil from field no. 359–13 in Westlock County, plant establishment was similar for most of the host genotypes except ‘73-67RR’, ‘9558C’ and TC72451-10. Root weight was greater for ‘45H29’ and ‘74 -47CR’ relative to the other cultivars (Table 3). Gall weight was lowest for line 08N823R and greatest on ‘45H29’, on which galls formed that were larger than those on the known susceptible cultivar ‘45H26’. Plant height was greatest for line 08N823R and ‘74–47CR’. Clubroot severity (ID) was lowest on 08N823R, followed by ‘9558C’, with the other genotypes developing similar levels of disease (Table 3). In the Westlock soil, only line 08N823R had an ID <50 % and could be regarded as resistant, while all of the other lines and cultivars were susceptible.
In soil collected from a field nursery located in Edmonton, plant establishment was generally poor compared with the Westlock soil. Establishment was greatest for line 08N823R and lowest for ‘73-77RR’ and TC72451-10. The average root weight was greatest for the susceptible cultivar ‘45H26’ followed by 08N823R, and lowest for ‘73-67RR’ (Table 4). Gall weight also was greatest on ‘45H26’ and lowest on the genotypes TC72451-10 and ‘73-67RR’. The greatest plant heights were observed for ‘9558C’ and ‘L135C’. Clubroot severity was greatest on ‘45H26’ and 08N823R, which were the only two genotypes in which the ID >50 % (Table 4). All of the other lines and cultivars exhibited resistant reactions in the soil from Edmonton.
Pathotype classification
The P. brassicae populations L-G1, L-G2, L-G3 and D-G3 were evaluated for pathotype designation based on the most widely used sets of differential hosts (Table 5). All four populations were classified as pathotype 16/6/8 on the ECD set (Buczacki et al. 1975), pathotype 5 on the differentials of Williams (1966), and pathotype P3 on the differentials of Somé et al. (1996). The pathogen populations were highly virulent on the universally susceptible ‘Granaat’ (ECD 05), but all other hosts of the B. rapa subset (ECD 01 – ECD 04) of the ECD set were resistant. The B. napus differentials ECD 07 and ECD 08 were susceptible, but ECD 06, ECD 09 and ECD 10 were resistant (Table 5). In the Brassica oleracea L. subset of the ECD set (ECD 11 – ECD 15), only the cabbage (B. oleracea var. capitata L.) ‘Septa’ was susceptible. The pathogen populations induced very mild or no symptoms on the differentials of Williams (1966), which included ECD 11, ECD 13, and the rutabagas (B. napus var. rapifera L.) ‘Wilhemsburger’ and ‘Laurentian.’ Among the differentials of Somé et al. (1996), which consisted of ECD 06, ECD 10 and the spring oilseed rape ‘Brutor’, only the latter was susceptible (Table 5). The reaction of the winter oilseed rape ‘Mendel’, which is not a member of any of these differential sets but was included for comparison, varied in response to the P. brassicae populations. While D-G3 appeared to be largely avirulent on this host (ID =10.2 %), ‘Mendel’ was susceptible to L-G1, L-G2 and L-G3, since the IDs obtained ranged between 43.5 % and 51.9 %, but the 95 % CI overlapped 50 % in each case (Table 5).
Discussion
Genetically resistant cultivars represent the most important clubroot management tool available to canola farmers in western Canada (Peng et al. 2014; Strelkov and Hwang 2014). In recent years, these cultivars have been grown extensively in clubroot-affected regions, enabling the continued production of canola in fields where inoculum levels are very high. Therefore, the identification in Alberta of six fields of clubroot-resistant canola with relatively severe clubroot was a cause for concern (Strelkov et al. 2014), since it suggested reductions in the efficacy of genetic resistance. In order to gain a better understanding of the situation and identify possible shifts in the virulence of P. brassicae populations, three pathogen populations from each of these fields were evaluated for their virulence profiles on clubroot-resistant canola, and for their pathotype designations on three sets of differential hosts.
An initial assessment of the virulence of these populations on the cultivars from which they were first isolated indicated that none of the populations tested from four of the six fields in question (356–13, 357–13, 358-13 A and 358-13B) caused significantly increased levels of clubroot. This suggests that the relatively high levels of clubroot that were observed in those fields did not reflect shifts in the virulence of the P. brassicae populations, but rather other factors, such as the persistence of susceptible volunteer canola (Gulden et al. 2003), and (or) the presence of off-types of the hybrid cultivars that did not carry the clubroot resistance trait. In contrast, four pathogen populations (L-G1, L-G2, L-G3 and D-G3) from the other two fields in question (359–13 and 360–13) did cause significantly increased clubroot severity on the resistant varieties from which they were collected, as well as on a suite of additional clubroot resistant cultivars produced by various seed companies. Consultations with the agronomists involved in each case indicated that in those fields, clubroot-resistant cultivars had been grown two to three times in a span of 5 years. The increased virulence of these populations on the resistant cultivars, the continued effectiveness of the resistance in these cultivars against a population of P. brassicae (SACAN03-1) collected prior to the introduction of the resistance trait, and the cropping history of the fields strongly suggest that resistance to the clubroot pathogen was overcome. Moreover, soil assays to evaluate the clubroot response of a dozen resistant hosts showed that most genotypes yielded a susceptible reaction when grown in soil from one of these fields (359–13), but were still resistant when grown in soil from a clubroot nursery where no resistance issues had been reported.
The erosion or defeat of clubroot resistance in Brassica genotypes has been observed in the past. For example, clubroot resistance has been overcome in cabbage (Seaman et al. 1963), Chinese cabbage (Hatakeyama et al. 2006; Osaki et al. 2004 cited in Tanaka and Ito 2013), Dutch stubble turnip (B. rapa subsp. rapifera) (G.R. Dixon, personal communication) and, perhaps most relevant to this study, in the oilseed rape ‘Mendel’ (Diederichsen et al. 2014; Oxley 2007). Repeated exposure of a P. brassicae population to a particular resistance source can result in a loss in the effectiveness of that resistance, as virulent components of the pathogen population are preferentially selected (Howard et al. 2010; Tanaka and Ito 2013). In experiments under controlled conditions, single-spore isolates and populations of P. brassicae were found to adapt rapidly to Brassica host genotypes, resulting in significant increases in clubroot disease severity after just a few cycles of continuous cropping (LeBoldus et al. 2012). In the present study, some host genotypes developed varying levels of disease, particularly in response to inoculation with the population D-G3. This suggests that there was a significant amount of heterogeneity in this population of the pathogen. Heterogeneity in P. brassicae populations has been reported previously (Xue et al. 2008 and references therein), and its occurrence would not be surprising in galls recovered from a field where the virulence of the pathogen appears to be in flux. The purification and characterization of single-spore derived isolates from P. brassicae populations exhibiting novel virulence profiles will help to determine the extent of pathogen diversity in individual fields, and will be the focus of subsequent studies.
In order to determine whether L-G1, L-G2, L-G3 and D-G3 represented novel pathotypes of P. brassicae in Canada, which may have risen to preeminence as a result of the selection pressure imposed by resistant cultivars, the pathotype designations of these populations were determined on the most commonly used sets of differentials hosts. All four pathogen populations were classified as pathotypes 5, P3 and 16/6/8, respectively, on the differential systems of Williams (1966), Somé et al. (1996), and the ECD set (Buczacki et al. 1975). These pathotypes have been reported from Alberta previously but are distinct from the most commonly identified pathotypes, which include pathotypes 3, P2 and 16/15/8 (Cao et al. 2009; Strelkov et al. 2006; Strelkov and Hwang 2014; Xue et al. 2008). Nonetheless, the pathotype classifications of the novel populations are somewhat misleading, at least on the differentials of Williams (1966) and Somé et al. (1996), because previously characterized strains of pathotype 5/P3 from Alberta were not virulent on any clubroot-resistant canola genotypes (S.E. Strelkov, unpublished data), whereas L-G1, L-G2, L-G3 and D-G3 are virulent on all or some of these hosts. Thus, the identification of these populations serves to highlight the limitations of the differentials, which may not reflect the full virulence spectrum of P. brassicae on B. napus canola (Strelkov and Hwang 2014). Pathotype designations using the complete ECD set have been obtained less frequently for P. brassicae populations from Canada, but the designations of L-G1, L-G2, L-G3 and D-G3 on the B. napus and B. oleracea subsets (/6/and /8/, respectively) also have been detected occasionally (Cao et al. 2009). It is worth noting, however, that despite the identical classification of these four populations on the differentials of Williams (1966); Somé et al. (1996) and the ECD set (Buczacki et al. 1975), D-G3 was much less virulent on ‘Mendel’ than were L-G1, L-G2 and L-G3. Hence, if this genotype had been included as a differential host, the pathotype designations of the P. brassicae populations from fields 359–13 and 360–13 would have been distinct. Notwithstanding the pathotype designations on the differential sets, it is clear that these populations represent novel virulence phenotypes of P. brassicae in Canada, with the ability to overcome the commonly used source(s) of resistance.
The nature of clubroot resistance in the commercial canola cultivars is not publicly known because of proprietary considerations. In the absence of genetic data or knowledge of the pedigree of these cultivars, it is difficult to draw conclusions regarding the relationships between the source(s) of resistance in the different hosts. Nevertheless, the severe clubroot caused by L-G1, L-G2 and L-G3 on nearly all of the resistant canola cultivars evaluated suggests that these hosts share a similar basis for resistance. In contrast, the milder clubroot observed on ‘9558C’ would seem to indicate certain differences in the resistance in this variety, which ranged from moderately resistant to moderately susceptible. Regardless of the exact nature of the resistance in the current cultivars, the identification of multiple differential hosts with strong resistance to the novel strains of P. brassicae indicates that effective sources of resistance do exist and could be introgressed into canola. The oilseed rape ‘Mendel’, which in recent years has been used as a source of clubroot resistance in spring canola (Rahman et al. 2014), would not seem to be a good choice for developing cultivars with resistance to the novel strains of the pathogen, given its increased susceptibility. On the other hand, breeders should not focus their activities exclusively on the new virulence phenotypes of P. brassicae, since these are still rare and the current set of resistant cultivars are still effective in the vast majority of fields. The observation that line 08N823R exhibited a resistant reaction in field soil infested with the new strains of the pathogen, yet was susceptible in soil from a nursery infested with the predominant pathotypes, serves to highlight this point.
Fields no. 359–13 and 360–13 are both located in Westlock County, within 2 km of each other. It is possible, therefore, that the novel strains of P. brassicae originated in one of the fields and were transported to the other on farm machinery (Cao et al. 2009) or in windborne dust (Rennie et al. 2015). An alternative possibility is that these novel virulence phenotypes arose independently of each other via the selection pressure imposed in each field, resulting in the distinct virulence profiles observed in the P. brassicae populations from fields 359–13 (L-G1, L-G2 and L-G3) and 360–13 (D-G3). Additional surveillance and testing of pathogen populations from clubroot-resistant canola crops may help to clarify the issue. Indeed, a survey in 2014 revealed more cases of clubroot resistant canola with unusually high levels of clubroot (Strelkov et al. 2015). These cases were distributed over a larger geographical region than those identified in 2013 and, although they are still under investigation, could indicate that the loss of resistance is a more widespread issue. As a precautionary measure in the meantime, the province of Alberta has taken steps to restrict the planting of canola in, and the movement of soil from, the fields in question.
The emergence of new virulence phenotypes of P. brassicae capable of overcoming the resistance in Canadian canola cultivars indicates that the canola industry is still highly vulnerable to clubroot. The loss of genetic resistance would represent a significant challenge to clubroot management efforts, hindering the ability of farmers to mitigate the impact of this disease. As such, strategies aimed at clubroot resistance stewardship are increasingly important. Longer rotations out of canola, especially in fields where P. brassicae infestation is an issue, along with the use of tactics such as the sanitization of farm machinery to reduce spread of infested soil, will contribute to the sustainable management of clubroot disease.
References
Buczacki, S. T., Toxopeus, H., Johnston, T. D., Dixon, G. R., & Holboth, L. A. (1975). Study of physiologic specialization in Plasmodiophora brassicae: proposals for attempted rationalization through an international approach. Transactions of the British Mycological Society, 65, 295–303.
Cao, T., Manolii, V. P., Hwang, S. F., Howard, R. J., & Strelkov, S. E. (2009). Virulence and spread of Plasmodiophora brassicae [clubroot] in Alberta, Canada. Canadian Journal of Plant Pathology, 31, 321–329.
Chittem, K., Mansouripour, S. M., & del Río Mendoza, L. E. (2014). First report of clubroot on canola caused by Plasmodiophora brassicae in North Dakota. Plant Disease, 98, 1438.
Diederichsen, E., Frauen, M., Linders, E. G. A., Hatakeyama, K., & Hirai, M. (2009). Status and perspectives of clubroot resistance breeding in crucifer crops. Journal of Plant Growth Regulation, 28, 265–281.
Diederichsen, E., Frauen, M., & Ludwig-Müller (2014). Clubroot disease management challenges from a German perspective. Canadian Journal of Plant Pathology, 36(S1), 85–98.
Dixon, G. R. (2006). The biology of Plasmodiophora brassicae Wor. – A review of recent advances. Acta Horticulturae, 706, 271–282.
Dixon, G. R. (2009). The occurrence and economic impact of Plasmodiophora brassicae and clubroot disease. Journal of Plant Growth Regulation, 28, 194–202.
Dokken-Bouchard, F. L., Anderson, K., Bassendowski, K. A., Bouchard, A., Brown, B., et al. (2012). Survey of canola diseases in Saskatchewan, 2011. Canadian Plant Disease Survey, 92, 125–129.
Donald, E. C. & Porter, I. J. (2014). Clubroot in Australia: the history and impact of Plasmodiophora brassicae in Brassica crops and research efforts directed towards its control. Canadian Journal of Plant Pathology, 36(S1), 66–84.
Gossen, B. D., Adhikari, K. K. C., & McDonald, M. R. (2012). Effect of seeding date on development of clubroot in vegetable Brassica crops. Canadian Journal of Plant Pathology, 34, 516–523.
Gowers, S. (1982). The transfer of characters from Brassica campestris L. to Brassica napus L.: production of clubroot-resistant oilseed rape (B. napus ssp. oleifera). Euphytica, 31, 971–976.
Gulden, R. H., Shirtliffe, S. J., & Thomas, A. G. (2003). Secondary seed dormancy prolongs persistence of volunteer canola in western Canada. Weed Science, 51, 904–913.
Hatakeyama, K., Fujimura, M., Ishida, M., Suzuki, T., & Sato, T. (2006). Classification of pathogenicity of Plasmodiophora brassicae field isolates in Japan based on resistance of F1 cultivars of Chinese cabbage (Brassica rapa L.) to clubroot. Acta Horticulturae, 706, 323–328.
Hirai, M. (2006). Genetic analysis of clubroot resistance in Brassica crops. Breeding Science, 56, 223–229.
Horiuchi, S. & Hori, M. (1980). A simple greenhouse technique for obtaining high levels of clubroot incidence. Bulletin of the Chugoku National Agricultural Experiment Station: Series E, 17, 33–55.
Howard, R. J., Strelkov, S. E., & Harding, M. W. (2010). Clubroot of cruciferous crops – new perspectives on an old disease. Canadian Journal of Plant Pathology, 32, 43–57.
Hwang, S. F., Ahmed, H. U., Zhou, Q., Rashi, A., Strelkov, S. E., et al. (2013). Effect of susceptible and resistant canola plants on Plasmodiophora brassicae resting spore populations in the soil. Plant Pathology, 62, 404–412.
Hwang, S. F., Howard, R. J., Strelkov, S. E., Gossen, B. D., & Peng, G. (2014). Management of clubroot (Plasmodiophora brassicae) on canola (Brassica napus) in western Canada. Canadian Journal of Plant Pathology, 36(S1), 49–65.
Kuginuki, Y., Hiroaki, Y., & Hirai, M. (1999). Variation in virulence of Plasmodiophora brassicae in Japan tested with clubroot-resistant cultivars of Chinese cabbage (Brassica rapa L. spp. pekinensis). European Journal of Plant Pathology, 105, 327–332.
LeBoldus, J. M., Manolii, V. P., Turkington, T. K., & Strelkov, S. E. (2012). Adaptation to Brassica host genotypes by a single-spore isolate and population of Plasmodiophora brassicae [clubroot]. Plant Disease, 96, 833–838.
Murakami, H., Tshushima, S., Kuroyagani, Y., & Shishido, Y. (2002). Reduction in resting spore density of Plasmodiophora brassicae and clubroot severity by liming. Soil Science & Plant Nutrition, 48, 685–691.
Osaki, K., Tanaka, S., & Ito, S. (2004). A case of breakdown in clubroot-resistant cultivars of Chinese cabbage. Japanese Journal of Phytopathology, 70, 66.
Oxley, S. (2007). Clubroot disease of oilseed rape and other brassica crops. Technical Note 602. Edinburgh: Scottish Agricultural College.
Peng, G., Lahlali, R., Hwang, S. F., Pageau, D., Hynes, R. K., et al. (2014). Crop rotation, cultivar resistance, and fungicides/biofungicides for managing clubroot (Plasmodiophora brassicae) on canola. Canadian Journal of Plant Pathology, 36(S1), 99–112.
Rahman, H., Peng, G., Yu, F., Falk, K. C., Kulkarni, M., & Selvaraj, G. (2014). Genetics and breeding for clubroot resistance in Canadian spring canola (Brassica napus L.). Canadian Journal of Plant Pathology, 36(S1), 122–134.
Rempel, C. B., Hutton, S. N., & Jurke, C. J. (2014). Clubroot and the importance of canola in Canada. Canadian Journal of Plant Pathology, 36(S1), 19–26.
Rennie, D. C., Holtz, M. D., Turkington, T. K., Leboldus, J. M., et al. (2015). Movement of Plasmodiophora brassicae resting spores in windblown dust. Canadian Journal of Plant Pathology, 37, 188–196.
Seaman, W. L., Walker, J. C., & Larson, R. (1963). A new race of Plasmodiophora brassicae affecting Badger shipper cabbage. Phytopathology, 53, 1426–1429.
Somé, A., Manzanares, M. J., Laurens, F., Baron, F., Thomas, G., & Rouxel, F. (1996). Variation for virulence on Brassica napus L. amongst Plasmodiophora brassicae collections from France and derived single-spore isolates. Plant Pathology, 45, 432–439.
Strelkov, S. E. & Hwang, S. F. (2014). Clubroot in the Canadian canola crop: 10 years into the outbreak. Canadian Journal of Plant Pathology, 36(S1), 27–36.
Strelkov, S. E., Tewari, J. P., & Smith-Degenhardt, E. (2006). Characterization of Plasmodiophora brassicae populations from Alberta, Canada. Canadian Journal of Plant Pathology, 28, 467–474.
Strelkov, S. E., Hwang, S. F., Howard, R. J., Hartman, M., & Turkington, T. K. (2011). Progress towards the sustainable management of clubroot [Plasmodiophora brassicae] of canola on the Canadian prairies. Prairie Soils and Crops, 4, 114–121.
Strelkov, S. E., Manolii, V. P., Harding, M. W., Hwang, S. F., Poscente, N., et al. (2014). The occurrence of clubroot on canola in Alberta in 2013. Canadian Plant Disease Survey, 94, 158–161.
Strelkov, S. E., Manolii, V. P., Harding, M. W., Hwang, S. F., Fei, W., et al. (2015). The spread of clubroot on canola in Alberta in 2014. Canadian Plant Disease Survey, 95, 155–158.
Tanaka, S. & Ito, S. (2013). Pathogenic and genetic diversity in Plasmodiophora brassicae (clubroot) from Japan. Journal of General Plant Pathology, 79, 297–306.
Tewari, J. P., Strelkov, S. E., Orchard, D., Hartman, M., Lange, R. M., & Turkington, T. K. (2005). Identification of clubroot disease of crucifers on canola (Brassica napus) in Alberta. Canadian Journal of Plant Pathology, 27, 143–144.
Wallenhammar, A. C. (1996). Prevalence of Plasmodiophora brassicae in a spring oilseed rape growing area in central Sweden and factors influencing soil infestation levels. Plant Pathology, 45, 710–719.
Webster, M. A. & Dixon, G. R. (1991). Calcium, pH and inoculum concentration influencing colonization by Plasmodiophora brassicae. Mycological Research, 95, 64–73.
Williams, P. H. (1966). A system for the determination of races of Plasmodiophora brassicae that infect cabbage and rutabaga. Phytopathology, 56, 624–626.
Xue, S., Cao, T., Howard, R. J., Hwang, S. F., & Strelkov, S. E. (2008). Isolation and variation in virulence of single spore isolates of Plasmodiophora brassicae from Canada. Plant Disease, 92, 456–462.
Acknowledgments
The authors gratefully acknowledge the financial support provided by the Alberta Crop Industry Development Fund Ltd., the Canola Agronomic Research Program, the Growing Forward 2 Program (Agriculture and Agri-Food Canada/Canola Council of Canada), and the provincial canola grower groups including the Alberta Canola Producers Commission, SaskCanola, and the Manitoba Canola Growers Association. The assistance of Canola Council of Canada agronomists and cooperating farmers also is gratefully acknowledged, as is the technical assistance of G. Turnbull, Q. Zhou (Alberta Agriculture and Forestry), and several summer students.
Author information
Authors and Affiliations
Corresponding author
Additional information
Submitted for Inclusion in the Special Issue on Clubroot Disease of Crucifers
Rights and permissions
About this article
Cite this article
Strelkov, S.E., Hwang, SF., Manolii, V.P. et al. Emergence of new virulence phenotypes of Plasmodiophora brassicae on canola (Brassica napus) in Alberta, Canada. Eur J Plant Pathol 145, 517–529 (2016). https://doi.org/10.1007/s10658-016-0888-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-0888-8