Abstract
Rhizoctonia solani is the causal agent of stem canker and black scurf in potato, resulting in significant yield and economic losses. To understand the infection process of R. solani and potato responses to the pathogen, histological observation of inoculated potato was performed under light and electron microscopes, under laboratory conditions. R. solani was observed at the invasion site, for the initial infection time, and for the presence of infection structures (appressoria, infection cushions). These attributes were compared when R. solani affected different parts of the host: aboveground stems, underground stems, tubers; as well as in different potato cultivars: ‘Desiree’ (resistant to R. solani) and ‘Atlantic’ (susceptible). In aboveground stems of potato, the invasion of R. solani was mainly limited to intercellular spaces. In underground stems and tubers, the primary invasion sites were epidermal cracks and lenticels. Initial infection time was 8 to 12 h post inoculation (HPI) in aboveground stems, 8 HPI in underground stems, and 4 HPI in tubers. The hyphae of R. solani produced various infection structures, with a few infection cushions, and a large number of appressoria with different morphologies. In aboveground stems, more infection structures were found than in underground stems and tubers. ‘Desiree’ had fewer numbers and smaller sizes of lenticels, thicker cuticle and periderm, and fewer epidermal cracks compared to ‘Atlantic’. Fewer numbers of infection structure of R. solani were observed in ‘Desiree’ than in ‘Atlantic’. It was suggested that the epidermal and perithelial structures of potato were related to disease resistance, such as number and size of lenticels, thickness of cuticle and periderm, and epidermal cracks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rhizoctonia solani Kühn is a common pathogen of potato (Solanum tuberosum L.), causes stem canker and black scurf (Bakali and Martín 2006; Campion et al. 2003; Carling et al. 1986). The pathogen can infect all belowground portions of potato plants, including seed tubers, sprouts, roots, stolons, and stems (Woodhall et al. 2007; Atkinson et al. 2010; Carling et al. 1989; Simons and Gilligan 1997; Woodhall et al. 2008). Black scurf generally occurs on superficial tissues of potato tubers, where sclerotia are formed. China is the largest potato-producing country, and its potato production has been threatened by R. solani since 2008. In 2009, a black scurf incidence rate of 100 % on tubers and 85 % in stem canker was observed in the city of Wulanchabu in Inner Mongolia (Zhang et al. 2012).
When it comes to controlling R. solani, chemical fungicides are not fully effective (Bautista et al. 2007; Grosch et al. 2005; Lahlali and Hijri 2010; Brewer and Larkin 2005; Jiang et al. 2005), and the cultivation of resistant cultivars is a long-term process (Naz et al. 2008; Djébali and Belhassen 2010; Khandaker et al. 2011; Leach and Webb 1993). There are two types of disease resistance: vertical and horizontal. Vertical resistance is determined by one to a few genes of the host, but this type of resistance germplasm may not be available. Alternatively, attributes of horizontal resistance can be used in plant breeding and disease management, which include physical, physiological and biological characteristics (Xu 2009).
Rhizoctonia solani not only has a broad host range, infecting at least 200 plant species (Gvozdeva et al. 2006), but also has a diverse genetic background (Dowley 1972). Due to lack of observable fruiting structures, the genetics of this fungus is based on anastomosis. Currently 14 anastomosis groups (AGs) have been reported (Jin and Korpradiskul 1998), with AG3 and AG2-1 being the most common groups on potato (Campion et al. 2003; Carling et al. 1986; Woodhall et al. 2007; Chand and Logan 1983).
Regardless of AGs, the infection process of R. solani has seldom been visually observed in R. solani-potato interaction. Plant resistance can be judged by examining the pathogen behavior, for example, whether infection structures, such as infection cushions or lobate appressoria, are produced (Amita et al. 2003; Matsuura 1986; Marshall and Rush 1980a; Marshall and Rush 1980b; Tao and Tan 1995; Demirci and Döken 1998), and how disease severity correlates with the number and the size of infection structures (Marshall and Rush 1980b; Tao 1992; Kousik et al. 1994; Hofman and Jongebloed 1988). A detailed examination of potato responses to the pathogen at tissue level, such as sites for pathogen entry and penetration, and surface structures of the host plant, will enhance understanding of the pathogen-host interaction.
The anatomy of the potato is such that the outermost layer of both stem and tuber is epidermis, which contains cuticles, an important component in forming the first barrier against pathogen infection (Jin 2006). Under the epidermis of the tuber, lies the periderm and cortex, while the stem contains cortex only. The periderm consists of many layers of cells, which can block the invasion of pathogens and prevent the leakage of water and nutrients (Jin 2006). The hypothesis of the current study was that differences of the epidermal and peridermal structures lead to different levels of resistance within the potato plant. It was further hypothesized that the thickness of the cell layers can also affect resistance in potato hosts.
The objective of this study was to investigate the interaction between R. solani and potato cultivars with different levels of resistance. This would provide visual evidence of structures that may be involved in resistance, and assist in cultivar selection and breeding.
Materials and methods
Potato and pathogen
Potato cultivars, ‘Desiree’ (resistant to R. solani) and ‘Atlantic’ (susceptible) were used in this study (Zhang et al. 2014). The plant materials were virus free and maintained at the Research Center of Potato Breeding of Inner Mongolia Agricultural University. R. solani AG2-1 (WC-16) was isolated from infected potato tubers grown in Wuchuan of Inner Mongolia, which was confirmed to be highly pathogenic to ‘Atlantic’. The culture was stored on potato sucrose agar (PSA) at 4 °C for later use.
Inoculation and observation of the infection process
Potato seed tubers of ‘Desiree’ and ‘Atlantic’ were sown at the farm of Inner Mongolia Agricultural University. Standard operation was practiced during the growing season. The plants were removed 70 days after planting, and thirty healthy plants of each cultivar were chosen for evaluation. The stems were washed for 10 min with running tap water, sterilized in 1 % sodium hypochlorite for 10 min, and rinsed three times with sterile distilled water. The aboveground and underground stems of two cultivars were cut into 5-cm-long sections for each plant, and placed in a container (45 × 35 × 8 cm) with 500 mL 0.8 % water agar (having approximately 88 % relative humidity). A total of sixty sections, thirty of each cultivar on aboveground or underground stems were placed in two separate dishes. For inoculation, a mycelial culture disc (5 mm in diameter) obtained from 3-day-old cultures on PSA were placed on each stem section with the mycelial side facing the stem. The dish was then sealed with Parafilm and incubated at 25 °C in the dark.
After harvesting, 30 healthy and mature potato tubers of each cultivar were chosen and washed as described above, which was considered as one replication. The washed tubers were cut into disks with 2.5-cm height and 6-cm diameter, keeping one side of the disk having skin for inoculation. Sixty disks from two cultivars were placed in a same container as described above, with the cut surface (no skin) facing the bottom. For inoculation, culture plugs of R. solani were placed on tuber pieces with the mycelial side facing the periderm, then were incubated at 25 °C in the dark.
The stem sections and tuber disks were removed 4, 8, 12, 24, 36, 48 and 60 h post incubation (HPI), and manually cut into thin slices horizontally and vertically along the edge of the inoculated mycelial disc. The specimens were immersed in a mixture of equivalent 95 % alcohol and glacial acetic acid for 5 min, stained with 0.05 % of medan lactophenol oil for 10 min. Infection site, initial infection time, and infection structures were observed, and the size of infection cushion was measured with crossing method under an optical microscope (Olympus BX51, Olympus, Japan). The experiment was conducted three times (replications) in three independent samplings from the same planted stems and harvested tubers.
At 48 HPI, thirty underground stems and tubers of each cultivar were cut into 1.0 cm2 pieces. The underground stem sections were immersed in 2.5 % glutaraldehyde and the tuber pieces in 4 % glutaraldehyde; both were dehydrated in an ethanol gradient, critical-point dried, sprayed with platinum, then subjected to scanning electron microscopy (Hitachi Limited S-530, Hitachi Limited, Japan). The experiments were repeated twice.
Field-infected tubers
At 48 HPI, celloidin was applied over the hyphae on the inoculated stem sections and tuber pieces, followed by air-drying. After removing the celloidin, the surface of potato pieces was observed for invasion holes under cushions and appressoria using both optical and scanning electron microscopy. To observe hyphae in infected tuber tissue, ‘Atlantic’ tubers exhibiting R. solani infection with sclerotia were collected from fields. Tubers were cut vertically into slices through sclerotia and observed using optical microscopy to determine if hyphae penetrated the periderm and cortex cells.
Structural differences of potato cultivars related to Rhizoctonia solani resistance
To determine the size and number of lenticels, the underground stems and tubers of two cultivars were washed with tap water, and rinsed three times with sterile distilled water. Each stem was cut into 10-cm sections, and the tuber pieces cut in half. A total of 30 stem sections and tuber pieces from 30 plants and tubers for each cultivar were prepared. The cut pieces were placed in separate containers as described above and sealed with plastic film until the lenticels opened to the maximal size. Eight days later, whole lenticels in each stem section and tuber piece were counted, and the sizes of lenticels were measured under an optical microscopy. Thirty lenticels were randomly chosen and evaluated from 30 stem sections and tuber piecesfor each cultivar. To measure the cuticle thickness in underground stems and tubers, 30 randomly chosen views from 30 plants and tubers were observed for each cultivar using electronic microscopy. To measure periderm thickness of potato tubers, thirty mature healthy tubers of each cultivar were washed to remove soil from the surface, and then cut into pieces vertically from the surface. The peridermal cells were observed and their thicknesses were measured using optical microscopy. A total of 30 tuber pieces from 30 tubers were measured for each cultivar. To compare epidermal cracks on potato underground stems and tubers, 30 randomly chosen electron microscopic views of 30 plants and tubers were investigated for each cultivar.
Statistical analysis
Data were analyzed using the statistical program SAS 9.1 (Cary, NC, USA). Analysis of variance was performed with general linear model procedure. Mean values were compared between the two cultivars with Student’s t-test or multiple range test with LSD.
Results
Infection process of Rhizoctonia solani in aboveground stems of potato
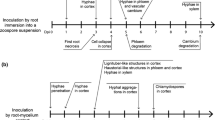
Similar infection processes by R. solani were observed on the two potato cultivars. At 4 HPI, the runner hyphae mainly grew longitudinally on the plant epidermis, with the hyphal tip being thin and sharp (Fig. 1-a). At 8 HPI, the hyphae continued to spread; a few hyphae produced short and thick lateral branches with coarse ends (Fig. 1b), and some hyphae appeared tangled, fused, and to form irregular infection structures or early infection cushions. At 12 HPI, the hyphae continued to branch, appeared tangled and fused, and a few hyphae formed infection cushions. Most of the hyphae were deformed and had various shapes of appressoria (Fig. 1c). The infection cushions varied in shape, including loose round (Fig. 1d), and irregular shapes (Fig. 1e). The morphology of appressoria varied from lobate (Fig. 1f and g), trident (Fig. 1h), foot (Fig. 1i), spherical (Fig. 1j), to irregular shapes (Fig. 1k). Sizes of infection cushions varied from 491 to 11,737 μm2 based on the average of 21 views of microscope, but there was no significant difference in infection cushion size between ‘Desiree’ (3377.9 μm2) and ‘Atlantic’ (3658.7 μm2) (P > 0.05). Some appressoria appeared to form infection pegs (Fig. 1l), and minimal viscous material was found surrounding these structures (Fig. 1m). No invasion occurred until 12 HPI (Figs. 1n and o). At 24 HPI, the hyphae appeared more tightly tangled, and the number of infection structures increased (Fig. 1p), and included a few infection cushions and many appressoria. The number of infection cushions (P < 0.05) and appressoria (P < 0.01) in ‘Atlantic’ were significantly higher than ‘Desiree’ (Table 1).
Infection of Rhizoctonia solani on the aboveground stem of potato: (a) at 4 h post infection (HPI) (cv. ‘Desiree’); (b) at 8 HPI (‘Desiree’); (c) at 12 HPI (‘Atlantic’); (d, e) infection cushion (cv. ‘Atlantic’); (f, g) lobate appressoria (‘Atlantic’); (h) trident-form appressorium (‘Desiree’); (i) foot-form appressoria (‘Atlantic’); (j) globular appressoria (‘Desiree’); (k) irregular-form appressoria (‘Atlantic’); and (l, m) infection peg (‘Atlantic’ and ‘Desiree’). (n) infecting hyphae of Rhizoctonia solani in the aboveground stem tissues of ‘Desiree’; (o) infecting hyphae of Rhizoctonia solani in the aboveground stem tissues of ‘Atlantic’; (p) at 24 HPI (‘Atlantic’). Arrows show deformed hyphae and infection cushions
Hyphae invaded the host tissues via the epidermal intercellular space (Figs. 2a and b) and stomata (Figs. 2c and d), and appeared to directly penetrated into epidermal cells (Figs. 2e and f). The primary invasion sites were intercellular spaces, regardless of the production of infection peg-like structures (Fig. 1l). Some holes resulting from penetration, ranging from 2.56 μm to 6.78 μm diameter, were found under cushions (Fig. 2f), suggesting direct hyphal invasion. Only a few such holes were observed, suggesting that this invasion mechanism was not the main mode of infection (Fig. 2f). Hyphae also entered through the stomata (Figs. 2c, and d), but the stomata were not the main invading sites. Among the 50 stomata observed, hyphae were only found in eight. Even hyphae very close to stomata, appeared to bypassed them (Fig. 2g).
Infection sites of Rhizoctonia solani in aboveground stems of potato: (a, b) hyphal infection of intercellular space (cv. ‘Atlantic’ and ‘Desiree’); (c, d) Invasion from the stoma (‘Atlantic’ and ‘Desiree’); (e) direct hyphal invasion (‘Atlantic’); (f) penetration holes under cushions (‘Desiree’); (g) hyphae around the stoma (‘Desiree’)
Infection process of Rhizoctonia solani in underground stems of potato
The pattern of infection structure formation in underground stems was similar to that in aboveground stems (Figs. 3a and b), having a few infection cushions, many appressoria, and some structures that appeared to be infection pegs (Fig. 3c). However, the infection cushions and appressoria in both potato cultivars were significantly fewer than those in aboveground stems (P < 0.05) (Table 1). The initial infection time also occurred earlier than in aboveground stems. At 8 HPI, hyphal invasion occurred. The hyphae invaded via epidermal cracks (Fig. 3d), intercellular spaces (Fig. 3e), lenticels (Fig. 3f), wounds (Fig. 3g), or appeared to directly penetrate into the epidermal cells (Fig. 3h). The primary infection sites were epidermal cracks and lenticels. The lenticels had strong attraction to hypha. Among the 50 lenticels observed, 37 were invaded by hypha. Many of the invading hypha were in bundles inside, but not found on the outer surface of the eyes (Fig. 3f). Few invaded holes were observed (Fig. 3i), suggesting direct hyphal penetration was not the primary mode of infection. The infecting hyphae could extend into intercellular spaces and deep into the intracellular areas (Fig. 3j). More infection cushions (P < 0.05) and appressoria (P < 0.01) were observed on the underground stem surface of ‘Atlantic’ than on ‘Desiree’ (Table 1).
Infection structures and sites of Rhizoctonia solani infection in underground stems of potato: a infection cushion (‘Desiree’); b appressorium (‘Desiree’); c infection peg (‘Atlantic’); d infection through epidermal crack (‘Desiree’); e infection into intercellular space (‘Desiree’); f infection in stomata (‘Desiree’); g infection through wound (‘Desiree’); h, i direct invasion (‘Desiree’); j hyphal extension at the intercellular space and intracellular region (‘Desiree’)
Infection process of rhizoctonia solani on potato tubers
The infection structures, infection cushions and appressoria on tubers of the two potato cultivars were significantly fewer than on underground stems (P < 0.05) (Table 1). Some apparently tangled and fused hyphae were observed, but were fewer than on underground stems. At the initial infection time, 4 HPI, a few hyphae infected the plant tissue via the epidermal cracks; at 8 HPI, most of the hypha successfully infected the plant tissue. After infection, the hyphae extended longitudinally and horizontally into the epidermis (Figs. 4a and b) and appeared to partially damage the peridermal cells (Fig. 4c). However, most hyphae did not penetrate the periderm into the cortex. When ‘Atlantic’ tubers were cut vertically through the sclerotia, a few hyphae were found in the intercellular and intracellular regions, indicating that the hyphae could penetrate the periderm and internal cortex tissue (Fig. 4d). The penetration depth was approximately 400 μm. The hyphae could infect via epidermal cracks, lenticels, and intercellular spaces, and penetrate directly into the epidermal cells. The majority of hyphe infected through epidermal cracks. The lenticels were also a primary infection site. Almost every lenticel in each inoculation area was invaded by bundled hypha (Figs. 4e and f). Moreover, after tubers were inoculated with R. solani, small sclerotia were formed in almost every lenticel (Fig. 4g). A few hyphae appeared to directly infect the epidermal cells with the hyphal tip, starting off thinner and then apparently restored back to the original hyphal shape (Fig. 4h). Throughout the infection process on tubers, more infection cushions (P < 0.05) and appressoria (P < 0.01) were found on the surface of ‘Atlantic’ than on ‘Desiree’ (Table 1).
Infection structures and sites of Rhizoctonia solani on potato tubers: a hyphal infection at epidermal cracks (cv. ‘Desiree’); b hyphae extension within epidermal cracks (‘Desiree’); c damage of peridermal cells by hyphae (‘Atlantic’); d hyphal extension within the internal cortex tissue of potato (‘Atlantic’); e, f, g hyphal infection through lenticels (‘Atlantic’, ‘Atlantic’ and ‘Desiree’); and h hyphal invasion, showing it becoming thin (‘Desiree’).
Structural differences of potato cultivars related to rhizoctonia solani resistance
‘Desiree’ had fewer and smaller lenticels than ‘Atlantic’ (P < 0.01) on both underground stems and tubers (Table 2). ‘Desiree’ also had thicker cuticle layers (10.5 ± 0.43μm) (Mean ± SE) (Fig. 5a) than ‘Atlantic’ (7.2 ± 0.19 μm) (Mean ± SE) (Fig. 5b) (P < 0.01) on the underground stems, as well as on tubers [(33.6 ± 0.16μm) (Mean ± SE) for ‘Desiree’, and 12.7 ± 0.53 μm (Mean ± SE) for ‘Atlantic’], (Figs. 5c, and d) (P < 0.01). In addition, the cuticle of ‘Atlantic’ was thinner than ‘Desiree’ (Figs. 5e and f). ‘Desiree’ had 11 to 14 layers of peridermal cells, 195.1 ± 8.73 μm (Mean ± SE) in thickness, which were higher than ‘Atlantic’ [7 to 12 layers of peridermal cells in 151.6 ± 3.88 μm (Mean ± SE)] (P < 0.01) (Figs. 5g and h). On underground stems and tubers, ‘Desiree’ had fewer epidermal cracks and a smoother surface than ‘Atlantic’ (Figs. 5e and f, Figs. 5i to l).
Cuticle thickness of underground stems of a ‘Desiree’ and b ‘Atlantic’; tubers of c ‘Desiree’ and d ‘Atlantic’; and epidermis of tubers of e ‘Desiree’ and f ‘Atlantic’; peridermal thickness of tubers of g ‘Desiree’ and h ‘Atlantic’ potatoes; cracks in epidermis of the underground stems of i cv. ‘Desiree’ and j Atlantic; and tubers of k ‘Desiree’ and l ‘Atlantic’ potatoes
Discussion
The anatomy of the host plant epidermis is an important factor affecting response to R. solani infection. The inhibition of infection structure formation is an important defense mechanism to disease. Infection structures can attack the surface of host plants and overcome the physical barrier of the epidermis (Liu and Xiao 1999).
We have demonstrated that R. solani infects potato via various ways. Intercellular space was the main port of entry on aboveground stems, whereas epidermal cracks and lenticels played an important role on underground stems and tubers. The entry pathway can vary depending on the host. For example, R. solani can infect rice leaves via intercellular spaces, stomata, and anywhere of on the epidermis (Zhang et al. 2010), but infects maize leaf sheaths though stomata (Liu et al. 2011). In the present study, the time of infection varied depending on the tissue of potato, with the order from long to short time being: aboveground stem, underground stem, and tuber. The infection time for aboveground stem infection was similar to that observed in maize (10 to 12 h) as reported by Tao (1992), or shorter than the 12 to 24 h reported by Chen et al. in maize (2000).
The formation of infection cushions may be stimulated by physical factors (Liu and Xiao 1999; Armentrout and Downer 1987; Murray 1982), host exudates (Marshall and Rush 1980a; Kousik et al. 1994; Dodman and Flentje 1970; Stockwell and Hanchey 1983), or both (Downer and Armentrout 1983). We have found that infection structures of R. solani may be induced by contact with the potato surface. More cushions and appressoria were observed on the aboveground stems of potato, compared with underground stems and tubers, possibly due to differences in physical factors. However, infection cushions may not be critical for R. solani infection in potato. Potato tubers were the most heavily infected by R. solani but no or few infection cushions were observed.
The formation of infection structures can be affected by environmental conditions such as moisture. Our studies indicated relative humidity at 85 % to 90 % was the most suitable for infection structures; at 100 % relative humidity, hyphe grew rapidly but fewer infection structures were formed, while lower relative humidity resulted in both slower hyphal growth and fewer infection structures. Kousik and Snow (1991) reported that temperature had also affected the number of infection cushions formed by R. solani on soybean leaves.
Rhizoctonia solani can be observed on aboveground stems of potato. If infection occurs on this part of stem, it is most likely via intercellular spaces of the epidermis and stomata. In addition, we have observed the sexual stage (basidia) under field conditions (Zhang, unpublished). Infection of the aboveground stem may only partially reflect natural infection, because the stem can be infected by basidiospores if the sexual stage occurs. The role of the sexual stage needs to be further studied.
Epidermal cracks and larger and more numerous lenticels are likely to increase the host’s susceptibility to diseases. Similar conclusions were made by Bakali and Martin (2006), who showed that during maturity of potato, the number of sclerotia on daughter tubers increased rapidly, which may have been due to the sharp increase in epidermis cracks.
We have demonstrated that on the surface of the resistant cultivar ‘Desiree’, fewer infection structures and hyphae of R. solani were observed compared to that in the susceptible cultivar ‘Atlantic’. This result is supported by many other observations in hosts of R. solani (Marshall and Rush 1980a; Tao 1992; Kousik et al.1994; Marshall and Rush 1980b). For example, in rice, infection cushions are formed on the sheath surface in susceptible cultivars, but none or fewer infection cushions or appressoria were found in resistant cultivars (Marshall and Rush 1980a; Zhang et al. 1990). In soybean, similar observations were made (Kousik et al.1994). A thicker layer of cuticle may contribute to resistance in plants (Jin 2006) This is supported in the present study with the finding that the resistant cultivar ‘Desiree’ had a thicker cuticle layer than the susceptible cultivar ‘Atlantic’ in underground stems and tubers, there were similar findings in rice studies (Tong et al. 2000).
Potato periderm forms a barrier to protect the tuber from pathogen infection and dehydration; a thicker periderm structure enhances the defense of the tuber (Jin 2006; Barel and Ginzberg 2008; Sabba and Lulai 2005). The formation of sclerotial initials on the tuber surface is the characteristic sign and symptom, and results in quality reduction. The tuber periderm plays an important role to block the pathogen (Barel and Ginzberg 2008; Sabba and Lulai 2005). As shown in the current study, the hyphae of R. solani penetrated into the epidermis but could appear to only partially damage the peridermal cells. Underground stems without periderm may be easily infected by the pathogen and develop necrosis and possibly result in shoot death. Although black scurf is considered a superficial pathogen on mature tubers, hyphe were observed in the internal cortex of the susceptible ‘Atlantic’ tubers, which may have resulted from the thinner periderm. The periderm of tubers of the resistant cultivar ‘Desiree’ was thicker than that of the susceptible cultivar ‘Atlantic’. In addition, Leach and Webb (1993) observed that most of brown potato cultivars are more resistant than white cultivars, which may be due to thicker periderm.
In conclusion, infection structure formation by R. solani was associated with potato response/resistance. Fewer numbers and smaller sizes of lenticels, fewer epidermal cracks, thicker cuticle and periderms may all be relevant to resistance of potato against R. solani. The anatomic evidence on the resistance described in this study is useful to assist in cultivar selection and breeding, as well as cultural practices that may affect the structures of epidermis and periderm in potato.
References
Amita, S., Rashmi, R., Savary, S., Willocquet, L., & Singh, U. S. (2003). Infection process in sheath blight of rice caused by Rhizoctonia solani. Indian Phytopathology, 56(4), 434–438.
Armentrout, V. N., & Downer, A. J. (1987). Factors affecting infection cushion development by Rhizoctonia solani on cotton. Phytopathology, 77, 623–630.
Atkinson, D., Thornton, M. K., & Miller, J. S. (2010). Development of Rhizoctonia solani on stems, stolons and tubers of potatoes I. effect of inoculum source. American Journal of Potato Research, 87, 374–381.
Bains, P. S., Bennypaul, H. S., Lynch, D. R., Kawchuk, L. M., & Schaupmeyer, C. A. (2002). Rhizoctonia disease of potatoes (Rhizoctonia solani): fungicidal efficacy and cultivar susceptibility. American Journal of Potato Research, 79, 99–106.
Bakali, E. L. A. M., & Martín, M. P. (2006). Black scurf of potato. Mycologist, 20, 130–132.
Barel, G., & Ginzberg, I. (2008). Potato skin proteome is enriched with plant defence components. Journal of Experimental Botany, 59(12), 3347–3357.
Bautista, G., Mendoza, H., & Uribe, D. (2007). Biocontrol of Rhizoctonia solani in native potato (solanum phureja) plants using native Pseudomonas fluorescens. Acta Biologica Colombiana, 12(1), 19–32.
Brewer, M. T., & Larkin, R. P. (2005). Efficacy of several potential biocontrol organisms against Rhizoctonia solani on potato. Crop Protection, 24, 939–950.
Campion, C., Chatot, C., Perraton, B., & Andrivon, D. (2003). Anastomosis groups, pathogenicity and sensitivity to fungicides of Rhizoctonia solani isolates collected on potato crops in France. European Journal of Plant Pathology, 109, 983–992.
Carling, D. E., Leiner, R. H., & Kebler, K. M. (1986). Characterization of Rhizoctonia solani and binucleate Rhizoctonia-like fungi collected from Alaskan soils with varied crop histories. Canadian Journal of Plant Pathology, 8(3), 305–310.
Carling, D. E., Leiner, R. H., & Westphale, P. C. (1989). Symptoms, signs and yield reduction associated with Rhizoctonia disease of potato induced by tuberborne inoculum of Rhizoctonia solani AG-31. American Potato Journal, 66, 693–701.
Carling, D. E., Baird, R. E., Gitaitis, R. D., Brainard, K. A., & Kuninaga, S. (2002). Characterization of AG-13, a newly reported anastomosis group of Rhizoctonia soalani. Phytopathology, 92, 893–899.
Chand, T., & Logan, C. (1983). Cultural and pathogenic variation in potato isolates of Rhizoctonia solani in Northern Ireland. Transactions of the British Mycological Society, 81, 585–589.
Chen, J., Tang, C. R., Gao, Z. G., Xue, C. S., Niu, X. F., & Song, Z. H. (2000). On penetration process of sheath blight pathogen in maize. Journal of Shenyang Agricultural University, 31(5), 503–506.
Demirci, E., & Döken, M. T. (1998). Host penetration and infection by the anastomosis groups of Rhizoctonia solani kühn isolated from potatoes*. Turkish Journal of Agriculture and Forestry, 22, 609–613.
Djébali, N., & Belhassen, T. (2010). Field study of the relative susceptibility of eleven potato (Solanum tuberosum L.) cultivars and the efficacy of two fungicides against Rhizoctonia solani attack. Crop Protection, 29, 998–1002.
Dodman, R. L., & Flentje, N. T. (1970). The mechanism and physiology by Rhizoctonia solani, Biology and Pathology Uni (pp. 149–160). Berkeley: California Press.
Dowley, L. J. (1972). Varietal susceptibility of potato tubers to Rhizoctonia solani in Ireland. Iranian Journal of Agricultural Research, 11(3), 281–285.
Downer, A. J., & Armentrout, V. N. (1983). The effect of host exudates on infection cushion morphogenesis of Rhizoctonia solani. Phytopathology, 73, 958.
Grosch, R., Faltin, F., Lottmann, J., Kofoet, A., & Berg, G. (2005). Effectiveness of 3 antagonistic bacterial isolates to control Rhizoctonia solani kühn on lettuce and potato. Canadian Journal of Microbiology, 51, 345–353.
Gvozdeva, E. L., Volotskaya, A. V., Sof'in, A. V., Kudryavtseva, N. N., Revina, T. A., & Valueva, T. A. (2006). Interaction of proteinases secreted by fungal pathogen Rhizoctonia solani with natural proteinase inhibitors produced by plants. Applied Biochemistry and Microbiology, 42, 502–507.
Hofman, T. W., & Jongebloed, P. H. J. (1988). Infection process of Rhizoctonia solani on solarium tuberosum and effects of granular nematicides. Netherlands Journal of Plant Pathology, 94, 243–252.
Jiang, J. Z., Wu, S. Y., & Zhao, L. K. (2005). Resistance of potato tuber slices against Rhizoctonia solani induced by abiotic factors. Journal of Hebei University (Natural Science Edition), 25(2), 167–172.
Jin, Y. G. (2006). Botany (pp. 250–251). Beijing: Science Press.
Jin, M. S., & Korpradiskul, V. (1998). Isozyme analysis of genetic diversity among Rhizoctonia solani manastomosis group1-IA (AG1-IA). Mycosystem, 17(4), 331–338.
Khandaker, M. M., Khair, A., & Bhuiyan, M. K. A. (2011). Disease reaction of potato germplasms and true potato seeds against Rhizoctonia solani Kuhn. Bangladesh Journal Botany, 40(2), 193–196.
Kousik, C. S., & Snow, J. P. (1991). Effect of temperature on aggressiveness of Rhizoctonia solani Kuhn on soybean leaves and seedlings. Phytopathology, 81, 1205.
Kousik, C. S., Snow, J. P., & Berggren, G. T. (1994). Factors affecting infection cushion development by Rhizoctonia solani AG-1 IA and IB on soybean leaves. Plant Pathology, 43, 237–244.
Lahlali, R., & Hijri, M. (2010). Screening, identification and evaluation of potential biocontrol fungal endophytes against Rhizoctonia solani AG3 on potato plants. FEMS Microbiology Letters, 311, 152–159.
Leach, S. S., & Webb, R. E. (1993). Evaluation of potato cultivars, clones and a true seed population for resistance to Rhizoctonia solani. American Potato Journal, 70, 317–328.
Liu, X. M., & Xiao, J. G. (1999). Histopathological studies on infection process of wheat sheath blight. Rhizoctonia Cerealis. Mycosystema, 18(3), 288–293.
Liu, L., Zhang, Z. M., Li, D. B., Wang, J., Gao, J., Zhao, M. J., et al. (2011). Analysis of infection process and methylation-sensitive amplified polymorphism in Zea mays genome stressed by Rhizoctonia solani. Journal of Agricultural Biotechnology, 19(2), 243–249.
Marshall, D. S., & Rush, M. C. (1980a). Infection cushion formation on rice sheaths by Rhizoctonia solani. Phytopathology, 70, 947–950.
Marshall, D. S., & Rush, M. C. (1980b). Relation between infection by Rhizoctonia solani and R. oryzae and disease severity in rice. Phytopathology, 70, 941–946.
Matsuura, K. (1986). Scanning electron microscopy of the infection process of Rhizoctonia solani in leaf sheaths of rice plants. Phytopathology, 76(8), 811–814.
Murray, D. I. L. (1982). Penetration of barley root and coleoptile surfaces by Rhizoctonia solani. Transactions of the British Mycological Society, 79(2), 354–360.
Naz, F., Rauf, C. A., Abbasi, N. A., Irfan, U. H., & Ahmad, I. (2008). Influence of inoculum levels of Rhizoctonia solani and susceptibility on new potato germplasm. Pakistan Journal of Botany, 40(5), 2199–2209.
Sabba, R. P., & Lulai, E. C. (2005). Immunocytological analysis of potato tuber periderm and changes in pectin and extensin epitopes associated with periderm maturation. Journal of the American Society for Horticultural Science, 130(6), 936–942.
Simons, S. A., & Gilligan, C. A. (1997). Relationships between stem canker, stolon canker, black scurf (Rhizoctonia solani) and yield of potato (Solanum tuberosum) under different agronomic conditions. Plant Pathology, 46, 651–658.
Stockwell, V., & Hanchey, P. (1983). The role of the cuticle in resistance of beans to Rhizoctonia solani. Phytopathology, 73, 1640–1642.
Tao, J. F. (1992). Studies on the infection process of Rhizoctonia solani in rice. Journal Sichuan Agricultural University, 10(3), 471–477.
Tao, J. F., & Tan, F. H. (1995). Studies on the infection by Rhizoctonia solani on maize. Acta Physica Sinica, 25(3), 253–257.
Tong, Y. H., Xu, J. Y., Pan, X. B., Chen, X. J., & Zhang, B. S. (2000). A primary study on the resistant mechanism of rice plants to the infection of Rhizoctonia solani. Jiangsu Agricultural Research, 21(4), 45–47.
Woodhall, J. W., Lees, A. K., Edwards, S. G., & Jenkinson, P. (2007). Characterization of Rhizoctonia solani from potato in Great Britain. Plant Pathology, 56, 286–295.
Woodhall, J. W., Lees, A. K., Edwards, S. G., & Jenkinson, P. (2008). Infection of potato by Rhizoctonia solani, effect of anastomosis group. Plant Pathology, 57, 897–905.
Xu, Z. G. (2009). General plant pathology (pp. 295–296). Beijing: Higher Education Press.
Zhang, H. S., Zhu, L. H., & Sha, X. Y. (1990). Primary study on resistant mechanism in rice by Rhizoctonia solani (pp. 153–164). Nan Jing: Science and Technology Press.
Zhang, G. L., Yan, D. W., & He, Z. H. (2010). Cytological characteristics of infection process by Rhizoctonia solani in rice. Chinese Journal of Cell Biology, 32(3), 451–455.
Zhang, X. Y., Yu, X. X., Yu, Z., Zhang, W. Q., Ju, L. L., & Xue, Y. F. (2012). Changes on activity of defensive enzyme after inoculating with toxin of Rhizoctonia solani in potato. Acta Agriculturae Boreali-Sinica, 27(4), 153–157.
Zhang, X. Y., Yu, X. X., Yu, Z., Xue, Y. F., & Qi, L. P. (2014). A simple method based on laboratory inoculum and field inoculum for evaluating potato to resistance to black scurf caused by Rhizoctonia solani. Breeding Science, 64, 156–163.
Acknowledgments
The research was supported by The Twelfth Five-year Plan, State Science and Technology Support Program (2012BAD02B05), Major Project of Inner Mongolia Natural Science Foundation (2013ZD03), Integrated Innovation and Demonstration Project of Potato Industry Development in Inner Mongolia (20131706), and Chinese Natural Science Foundation (31460468).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, X.Y., Huo, H.L., Xi, X.M. et al. Histological observation of potato in response to Rhizoctonia solani infection. Eur J Plant Pathol 145, 289–303 (2016). https://doi.org/10.1007/s10658-015-0842-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0842-1