Abstract
Thirty-one loquat orchards (Eriobotrya japonica ‘Algerie’) with plants exhibiting decline symptoms were surveyed between 2004 and 2007 in the province of Alicante, Spain. Twenty-eight representative isolates with Cylindrocarpon-like asexual morphs recovered from affected roots were included in this study, with the objective to characterize them by means of phenotypical characterization, DNA analysis and pathogenicity tests. Dactylonectria alcacerensis, D. torresensis and Ilyonectria robusta were identified based on morphological and cultural characteristics as well as DNA sequence data for part of histone H3, with D. torresensis the most frequent species. All of them are reported for the first time on loquat, and I. robusta is reported for the first time in Spain. In addition, one species is newly described, Cylindrodendrum alicantinum. Pathogenicity tests with representative isolates showed that these species were able to induce typical root rot disease symptoms, affecting plant development or even leading to plant death. This research demonstrates the association of species belonging to the genera Cylindrodendrum, Dactylonectria and Ilyonectria with root rot of loquat and loquat decline in the province of Alicante (eastern Spain). This information should be considered for the improvement of the current management strategies against these soil-borne pathogens when establishing new loquat plantations or introducing new susceptible fruit crops in the region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Loquat (Eriobotrya japonica) is a subtropical evergreen fruit tree species native to southern China that, under Mediterranean climatic conditions, flowers in autumn, develops fruits in winter, and ripens in spring (Janick 2011; Reig et al. 2012). It is mainly cultivated in Asia and the Mediterranean basin, being Spain, Israel, Italy and Turkey the main producing countries in this area (Calabrese 2006; Reig et al. 2012). Although there are no FAO statistics available for world loquat fruit production, Lin (2007) reported 550,000 tons for 2006, with a crop area exceeding 130,000 ha, with China and Spain being the main producing countries. In Spain, one of the most important areas of loquat production is located in the province of Alicante (eastern Spain), in the valleys of the rivers Algar and Guadalest, which accounts for nearly 60 % of total Spanish loquat production. The crop is mostly located in family orchards, in high-density plantations, using drip irrigation. The cultivar Algerie and its mutations represent 98 % of the total production (Soler et al. 2007).
Loquat scab caused by Fusicladium eriobotryae is the main disease affecting this crop in Spain as well as in the whole Mediterranean basin (Soler et al. 2007; Sánchez-Torres et al. 2009; González-Domínguez et al. 2013, 2014). Nevertheless, since the late 1990s, an emerging problem of decline and death of loquat trees associated with Armillaria mellea, Rosellinia necatrix and Phytophthora spp. has been observed in the province of Alicante (González-Domínguez et al. 2008, 2009). Loquat trees infected by these soil-borne pathogens manifest two types of symptoms; those of the root system, and those of the aerial part of the plant arising as a consequence of the damaged roots. In some cases, very limited external symptoms of infections at ground level are observed, but in others, cankers are very noticeable. In this latter case, affected areas often include the lower trunk, root collar and large roots. As a consequence, affected trees present reduced plant vigour, chlorosis, small leaves and fruits, early leaf drop and dieback of twigs and branches. Dieback of young shoots has been observed when the crown area is severely diseased, presumably due to invasion by secondary pathogens. This condition is often referred as “tree decline”, because it is a gradual loss of vigor, and the trees eventually die (González-Domínguez et al. 2008, 2009).
In addition to A. mellea, R. necatrix and Phytophthora spp., surveys of loquats showing the common symptomatology described before, conducted from 2004 to 2007 in the province of Alicante, lead to the recovery of abundant fungal isolates with Cylindrocarpon-like asexual morphs from rotted roots. It is well known that these are soil-borne fungi, which are generally regarded as pathogens and/or saprobes of various hosts and substrates, associated with a variety of disease symptoms that include rot of roots, stems and cuttings of agricultural, forestry and horticultural crops (Halleen et al. 2006; Schroers et al. 2008; Chaverri et al. 2011; Cabral et al. 2012b; Agustí-Brisach and Armengol 2013; Lombard et al. 2014). Recently, species belonging to this group of fungi have been isolated from fruit trees showing root rot symptoms, such as avocado (Persea americana) (Vitale et al. 2012), kiwifruit (Actinidia chinensis) (Erper et al. 2013) and Arecaceae palms (Aiello et al. 2014). However, species with Cylindrocarpon-like asexual morphs have never been described associated with root rot of loquat. Thus, the aim of this study was to characterize a collection of Cylindrocarpon-like isolates, which were obtained from the roots of loquat trees showing symptoms of decline in the province of Alicante, by means of phenotypical characterization, DNA analysis and pathogenicity tests.
Materials and methods
Fungal isolation
Thirty-one loquat orchards (‘Algerie’) exhibiting decline symptoms were surveyed between 2004 and 2007 in the province of Alicante. In each orchard, at least three loquat trees were examined carefully. Affected trees showed symptoms at ground level which included necrotic lesions on roots, with a reduction in root biomass and root hairs.
Affected roots were washed under running tap water, surface disinfested for 1 min in a 1.5 % sodium hypochlorite solution, and washed twice with sterile distilled water. Small pieces of discoloured tissues were placed on potato dextrose agar (PDA) (Biokar-Diagnostics, Zac de Ther, France) amended with 0.5 g l−1 of streptomycin sulfate (Sigma-Aldrich, St. Louis, MO, USA) (PDAS). Plates were incubated for 5–10 days at 25 °C in the dark.
Twenty-eight representative isolates with Cylindrocarpon-like asexual morphs, obtained from nine orchards, were selected for further analysis (Table 1). These isolates were single-spored prior to morphological and molecular identification with the serial dilution method (Dhingra and Sinclair 1995). For long-term storage, cultures were transferred to Whatman no. 1 filter papers (Whatman International Ltd., Maidstone, England) overlaid on PDA, and after colonization, the filters were dried and stored at −20 °C (Petit and Gubler 2005).
Fungal identification
Morphological characterization
For morphological identification, single conidial cultures were grown for up to 5 weeks at 20 °C on synthetic nutrient-poor agar (SNA; Nirenberg 1976) with or without the addition of two 1 × 1 cm pieces of filter paper to the medium surface, PDA, and oatmeal agar (OA; Crous et al. 2009) under continuous near-UV light (NUV; 400–315 nm; Sylvania Blacklight-Blue, The Netherlands). To induce perithecial formation, isolates were crossed as described by Cabral et al. (2012a).
Fungal structures were measured at a 1000× magnification using a Leica DM2500 and images were captured using a Leica DFC295 digital camera with the Leica Application Suite. For this purpose, an agar square was removed and placed on a microscope slide, to which a drop of water and a cover slip were added. For each isolate, 30 measurements were obtained for each structure. For conidial measurements, the 95 % confidence intervals were determined for the new species and the averages were calculated for the previously known species. The extremes of the conidial measurements are shown inside parenthesis. For the other structures only the extremes are presented. Detailed measurements were conducted for three isolates per species (once identified following morphological examination and DNA analyses), with the exception of I. robusta for which only one isolate was available for the study.
Culture characteristics (texture, density, colour, growth front, transparency and zonation) were described on PDA and OA after incubation at 20 °C in the dark for 14 days. Colour (surface and reverse) was described using the colour chart of Rayner (1970).
Cardinal temperatures for growth were assessed by inoculating 90 mm diam. PDA dishes with a 3 mm diam. plug cut from the edge of an actively growing colony. Growth was determined after 7 days in two orthogonal directions. Trials were conducted at 4 °C, 18–22 °C, 25° and 35 °C, with three replicate plates per strain at each temperature.
DNA isolation, sequencing and phylogenetic analysis
For DNA extraction, fungal mycelium and conidia from pure cultures grown on PDA for 2–3 weeks at 25 °C in the dark were scraped and mechanically disrupted by grinding to a fine powder under liquid nitrogen using a mortar and pestle. Total DNA was extracted using the E.Z.N.A. Plant Miniprep Kit (Omega Bio-tek, Doraville, USA) following manufacturer’s instructions. DNA was visualized after electrophoresis on 0.7 % agarose gels stained with ethidium bromide and was stored at −20 °C
In order to identify the species involved, DNA of all isolates was amplified and sequenced for part of the histone H3 gene (HIS), that previously showed to be a very informative locus (Cabral et al. 2012a). Four isolates (Cyl-3, Cyl-8, Cyl-10 and Cyl-11) were additionally sequenced for the Internal Transcribed Spacer (ITS) region, and partial β-tubulin (TUB) and translation elongation factor 1-α (TEF) genes to better resolve their phylogenetic position. PCR amplifications were carried out using 1× PCR buffer, 1.25 mM MgCl2, 80 μM of each dNTP, 0.2 μM of each primer, 0.7 U of Taq polymerase (Dominion MBL, Córdoba, Spain), and 1 μl of template DNA (20 ng μl−1). The PCR reaction mix was adjusted to a final volume of 25 μl with ultrapure sterile water (Chromasolv Plus, Sigma-Aldrich, Steinheim, Germany). The cycle conditions in a Peltier Thermal Cycler-200 (MJ Research) were 94 °C for 3 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, elongation at 72 °C for 45 s, and a final extension at 72 °C for 10 min. Primers were CYLH3F and CYLH3R (Crous et al. 2004b) for HIS, ITS1F and ITS4 (Gardes and Bruns 1993) for ITS, T1 (O’Donnell and Cigelnik 1997) and Bt-2b (Glass and Donaldson 1995) for TUB, and CylEF-1 (5′- ATG GGT AAG GAV GAV AAG AC-3′; J.Z. Groenewald, unpublished) and CylEF-R2 (Crous et al. 2004b) for TEF. After confirmation by agarose gel electrophoresis, PCR products were purified with the High Pure PCR Product Purification Kit (Roche Diagnostics, Mannheim, Germany) and sequenced in both directions by Macrogen Inc., Sequencing Center (Seoul, South Korea). The products were analyzed using Sequencer software v. 5.3 (Gene Codes Corporation, Ann Arbor, MI, USA). Sequences were assembled and edited to resolve ambiguities using the program DNAman (Version 4.03, Lynnon BioSoft, Quebec, Canada), and consensus sequences for all isolates were compiled into a single file (Fasta format).
Phylogenetic analysis was first conducted on the HIS single-locus alignment for all isolates, and successively, the combined alignment of the four loci (HIS, ITS, TUB and TEF) was analyzed for inferring organismal phylogeny of Cyl-3, Cyl-8, Cyl-10 and Cyl-11 isolates. GenBank sequences (Table 1) from different species of Cylindrodendrum, Dactylonectria and Ilyonectria were selected based on their high similarity with our query sequences using MegaBLAST. These were added to the sequences obtained and aligned using CLUSTAL W v. 2.0.11 (Larkin et al. 2007). Phylogenetic analyses consisting of Maximum Parsimony were performed in MEGA 6.06 (Tamura et al. 2013) with the subtree-pruning-regrafting algorithm, where gaps and missing data were treated as complete deletions. The robustness of the topology was evaluated by 1000 bootstrap replications (Felsenstein 1985). Measures for the maximum parsimony as tree length (TL), consistency index (CI), retention index (RI) and rescaled consistency index (RC) were also calculated
Sequences derived in this study were lodged at GenBank, the alignment in TreeBASE (www.treebase.org), and taxonomic novelties in MycoBank (www.MycoBank.org) (Crous et al. 2004a). GenBank accession numbers of the strains collected during this study are listed in Table 1.
Pathogenicity tests
Thirteen fungal isolates representative of the different genera and species determined by phenotypical studies and HIS phylogeny, were selected to complete Koch’s postulates on loquat (Tables 1 and 2).
Inoculum was produced on wheat (Triticum aestivum) seeds (Brayford 1993). Seeds were soaked for 12 h in distilled water; air dried, and transferred to 300 ml flasks. Each flask was autoclaved three times on 3 successive days at 120 °C during 1 h. Two fungal disks of a 2-week old culture of each isolate grown on PDA at 25 °C were placed aseptically in separate flasks. The flasks were incubated at 25 °C for 4 weeks, and shaken once a week to avoid clustering of inoculum.
Plastic pots (220 ml) were filled with a mixture of sterilized peat moss and 10 g of inoculum per pot. Seedlings of loquat ‘Algerie’ were planted individually in each pot at the two-true-leaf stage. Controls were inoculated with sterile uninoculated wheat seeds. Six replicates (each one in individual pots) for each isolate were used, with an equal number of control plants. After inoculation, plants were placed in a greenhouse at 25–30 °C in a completely randomized design and watered every 3 days or as needed.
Three months after inoculation, plants were observed for the development of foliar symptoms, and evaluated using a 0 to 4 rating scale: 0 = no symptoms, 1 = 1 to 25 %, 2 = 26 to 50 %, 3 = 51 to 75 %, and 4 = 76 to 100 % chlorotic and necrotic leaves (the latter including plant death). Plants were gently uprooted and washed free of soil. Root symptoms of individual plants were evaluated on the following scale: 0 = healthy with no lesions, 1 = slight discoloration with 0 to 25 % of root mass reduction, 2 = discoloration with 26 to 50 % of root mass reduction, 3 = moderate discoloration with 51 to 75 % of root mass reduction, and 4 = severe discoloration with >75 % of root mass reduction. In addition, dry weights of shoot and root were recorded for each plant. Symptomatic roots were aseptically plated on PDAS in an attempt to re-isolate Cylindrodendrum, Dactylonectria or Ilyonectria and complete Koch’s postulates. The experiment was repeated.
For all fungal isolates, analysis of variance (ANOVA) indicated that the data between the two repetitions were similar (P > 0.05). Thus, data from both experiments were combined. ANOVA was performed on plant growth data (shoot and root dry weights), and disease severity and dry weight values were compared with those from control plants by the Dunnett’s test. Data were analyzed using STATISTIX 9 (Analytical Software, Tallahassee, FL, USA).
Results
Morphological characterization and phylogenetic analyses
All isolates showed floccose to felted aerial mycelium which colour varied from white to yellow or light to dark brown on PDA. Colony margins were entire, slightly lobulated, or lobulated. In general, the isolates produced both microconidia and macroconidia, and chlamydospores were also present, generally intercalary, globose, single or in chains.
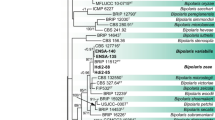
All isolates were amplified with the primers CYLH3F and CYLH3R. A PCR fragment of about 500 bp was obtained for all of them. Phylogenetic analysis on the HIS single-locus alignment contained a total of 49 ingroup taxa and two outgroup taxa (Campylocarpon fasciculare and Campyl. pseudofasciculare) resulting in a dataset of 531 characters, including alignment gaps, of which 346 were constant, 163 parsimony-informative, and 22 parsimony-uninformative. Parsimony analysis of 371 characters yielded three most parsimonious trees (TL = 287 steps; CI = 0.635; RI = 0.918; and RC = 0.583) which the first is shown in Fig. 1.
The first of three maximum parsimony trees obtained from the alignment of partial sequences of the histone H3 gene (HIS) of all isolates collected from loquat (Cyl isolates), and additional sequences of Cylindrodendrum album (KM231484 and KM231485), C. hubeiense (KR909093, KM231486 and KP639560), Dactylonectria. alcacerensis (JF735629 and JF735630), D. macrodidyma (JF735647 and JF735656), D. novozelandica (JF735632 and JF735633), D. torresensis (JF735661 and JF735681), Ilyonectria europaea (JF735567 and JF735569), I. liriodendri (JF735507 and JF735508), I. pseudodestructans (JF735562 and JF735563) and I. robusta (JF735518 and JF735522) obtained from GenBank. The tree was rooted using Campylocarpon isolates as outgroup sequences and bootstrap support values are indicated near the nodes. Ex-type strains are indicated in bold. New species is indicated by yellow boxes. Scale bar shows 10 changes
HIS region sequences of the isolates included in this study clustered into four groups with sequences from Cylindrodendrum (one group), Dactyonectria (two groups) and Ilyonectria (one group) species obtained from Genbank (Fig. 1).
The first group, comprising seven isolates, clustered with high bootstrap support (99 %) with the ex-type culture of D. alcacerensis CBS 129087. These isolates produced straight, hyaline macroconidia with one-septum (14.25−) 21.48 (−34.45) × (2.92−) 4.41 (−6.33) μm, two-septa (31.16−) 36.74 (−41.74) × (4.82−) 6.14 (−7.36) μm and three-septa (29.39−) 40.26 (−48.62) × (4.26−) 6.13 (−7.45) μm; and oval to ellipsoidal microconidia with 0–septa measuring (7.44−) 11.14 (−14.85) × (2.55−) 3.54 (−5.38) μm.
The second group, comprising sixteen isolates, clustered (89 % bootstrap support) with the ex-type culture of D. torresensis CBS 129086. These isolates produced straight to slightly curved hyaline macroconidia with one-septum (17.37−) 28.16 (−38.78) × (3.22−) 5.44 (−7.89) μm, two-septa (31.32−) 37.12 (−42.67) × (4.71−) 6.48 (−8.41) μm and three-septa (35.59−) 39.43 (−42.98) × (5.04−) 6.52 (−8.22) μm; and oval to ellipsoidal microconidia with 0–septa measuring (6.39−) 10.78 (−15.33) × (2.83−) 3.69 (−5.58) μm.
The third group, including only one isolate, formed a highly supported clade (99 % bootstrap support) with the ex-type culture of I. robusta CBS 129084. This isolate produced straight hyaline macroconidia with one-septum (11.33−) 15.86 (−24.12) × (3.2−) 4.66 (−6.16) μm; and oval to ellipsoidal microconidia with 0–septa measuring (5.56−) 9.03 (−11.73) × (2.51−) 3.59 (−4.77) μm.
The fourth group, comprising four isolates, formed a monophyletic clade with 99 % bootstrap support that is closely related with Cylindrodendrum species such as C. album or C. hubeiense (46 % bootstrap support). Therefore, this group was indicated as a novel phylogenetic species (yellow box in Fig. 1).
The combined alignment of ITS, TUB, HIS and TEF analyzed for inferring organismal phylogeny of the unknown species group (Cylindrodendrum sp. isolates Cyl-3, Cyl-8, Cyl-10 and Cyl-11) contained 57 taxa (including the two outgroups) and 2846 characters, including alignment gaps, of which 752 were constant, 857 parsimony-informative, and 147 parsimony-uninformative. Parsimony analysis of 1639 characters yielded seven most parsimonious trees (TL = 1471 steps; CI = 0.573; RI = 0.877; and RC = 0.503) the first of which is shown in Fig. 2. In this phylogenetic tree, combined sequences of the genera Cylindrodendrum (bootstrap = 94), Ilyonectria (BS = 100) and Dactylonectria (BS = 100) formed three well-supported clades. The Cylindrodendrum clade incorporated representatives of C. album (CBS 110655 and ex-type CBS 301.83) and of C. hubeiense (CBS 949.70, CBS 129.97 and ex-type CBS 124071). Moreover, this clade also includes our unknown isolates previously classified inside Cylindrodendrum genus. These four strains (Cyl-3, Cyl-8, Cyl-10 and Cyl-11) grouped together in a monophyletic clade with 100 % bootstrap support (green box in Fig. 2), basal to the clades containing C. album and C. hubeiense, with no other closely related species, confirming them as a new Cylindrodendrum species.

Dactylonectria alcacerensis, D. torresensis and I. robusta were found in five, eight and one of the nine orchards where Cylindrocarpon-like asexual morphs were isolated, respectively. The new Cylindrodendrum species was found in only one orchard.
Taxonomy
Based on the DNA sequence analyses and morphological characters, one species of Cylindrodendrum proved to be distinct from all known species, and is newly described below. Sexual compatibility tests failed to induce perithecia among isolates.
Cylindrodendrum alicantinum C. Agustí-Brisach, J. Armengol & A. Cabral, sp. nov. — MycoBank MB 811663; Fig. 3.
Cylindrodendrum alicantinum (A) Fourteen-day-old colony grown on Potato Dextrose Agar at 20 °C in a 90 mm petri dish (B-D) Simple, sparsely branched conidiophores of the aerial mycelium (E) Phialides bearing microconidia in false heads (F-K) Micro- and macroconidia (L) Anastomosis in fungal hyphae (M-P) Chlamydospores. Scale bars: D, M, N - 50 μm; B, E, O - 20 μm; C-D, F-L and P - 10 μm; A, B, D, F and H-P from CBS 139518 = Cyl-3 and C, E and G from Cyl-8
Etymology. Named after the province of Alicante (Eastern Spain) where this fungus was first collected.
Conidiophores simple, branched or unbranched, bearing up to five phialides, 1-5- septate, frequently 3-septate, 70–170 μm long; phialides monophialidic, cylindrical to slightly subulate, 20–50 μm long, 1.5 − 3.3 μm wide at the base, 1.7 − 3.4 μm at widest point, 0.8 − 1.8 μm near the aperture. No sporodochial conidiophores were observed.
Microconidia (0-)1-septate, ellipsoid to subcylindrical, more or less straight, mostly without a visible hilum; 0-septate (5.8−)8.1 − 9.4(−13.1) × (1.9−)2.5 − 2.8(−4.0) μm (av. = 8.8 × 2.7 μm), with a length:width ratio of 2.0 − 4.9; 1-septate (7.7−)11 − 11.5(−15.9) × (2.1−)2.9 − 3(−4.2) μm (av. = 11.3 × 3.0 μm), with a length:width ratio of 2.4 − 5.6.
Macroconidia formed on simple conidiophores or agar surface, on SNA formed in flat domes of slimy masses, 1(−3)-septate, straight, cylindrical with both ends broadly rounded, mostly without a visible hilum; 1-septate, (12.0−)15.0 − 15.5(−19.9) × (1.7−)2.8 − 2.9(−3.7) μm (av. = 15.2 × 2.8 μm), with a length:width ratio of 3.9 − 8.3; 2-septate, (12.8−)17 − 18.5(−20.9) × (2.2−)2.9 − 3.2(−4.5) μm (av. = 17.7 × 3 μm), with a length:width ratio of 3.7 − 8.5; 3-septate, (14.6−)18.7 − 19.8(−29.7) × (2.4−)3.3 − 3.4(−4.3) μm (av. = 19.2 × 3.4 μm), with a length:width ratio of 3.9 − 9.0.
Chlamydospores subglobose to ellipsoidal, 8–19 × 6–10 μm, smooth but often appearing rough due to deposits, thick-walled, mainly in chains or in clumps, hyaline, becoming slightly brown in the outer wall.
Holotype: Spain: Alicante, Eriobotrya japonica ‘Algerie’ showing decline symptoms, coll./isol. J. Armengol CBS H-22113, culture ex-type CBS 139518 = Cyl-3.
Culture characteristics: Mycelium felty with average to strong density. Surface on PDA umber, with aerial mycelium with dark saffron to cinnamon tufts in the centre, isabelline to buff towards the margin. Surface on OA sepia, with aerial mycelium buff to saffron, luteous to umber towards the margin. Reverse similar, except in colour, chestnut on PDA with luteous to orange margin, and umber on OA. Zonation absent, transparency homogeneous; entire margins. Colonies on PDA grow poorly (4–7 mm diam) at 4 °C after 7 days. Optimum temperature between 18 and 22 °C, when colonies reach 20–22 mm and 22–26 mm diam, respectively, after 7 days. Colony diam. was 21–25 mm at 25 ºC after 7 days. No growth was observed at 35 °C.
Isolates studied: Cyl-3, Cyl-8, Cyl-10 and Cyl-11.
Host and distribution: Eriobotrya japonica (province of Alicante, Eastern Spain).
Notes: Cylindrodendrum alicantinum is the closest phylogenetic neighbour of Cylindrodendrum hubeiense (W.Y. Zhuang, Y. Nong & J. Luo) L. Lombard & Crous based on the phylogenetic analysis in this study. The phialides of C. alicantinum (20–50 μm long) are shorter than those of C. hubeiense (38–75 μm; Zhuang et al. 2007; Lombard et al. 2014). Also, the macroconidia (considering 1 to 3 septate, 12.1 − 29.7 × 1.7 − 4.5 μm) are wider than those of C. hubeiense (15–30 × 1.8 − 2.7 μm; Zhuang et al. 2007; Lombard et al. 2014). No reference is made for chlamydospores for C. hubeiense while they are abundant in C. alicantinum. Anastomoses were observed between hyphae (Read et al. 2009).
Pathogenicity tests
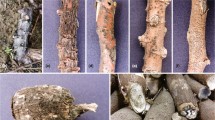
Symptoms developed in inoculated loquat seedlings after 3 months of inoculation, and consisted in reduced vigour, leaves with interveinal chlorosis and necrosis, and necrotic root lesions with a reduction in root biomass (Fig. 4).
Rating scale used for pathogenicity tests evaluation. a Foliar symptoms of individual plants were evaluated using a 0 to 4 rating scale: 0 = no symptoms, 1 = 1 to 25 %, 2 = 26 to 50 %, 3 = 51 to 75 %, and 4 = 76 to 100 % chlorotic and necrotic leaves with, eventually plant death. b Root symptoms of individual plants were evaluated using a 0 to 4 rating scale: 0 = healthy with no lesions, 1 = slight discoloration with 0 to 25 % of root mass reduction, 2 = discoloration with 26 to 50 % of root mass reduction, 3 = moderate discoloration with 51 to 75 % of root mass reduction, and 4 = severe discoloration with >75 % of root mass reduction
The statistical analysis indicated significant differences from the control in root disease severity (P < 0.001) and root dry weight (P < 0.001), whereas shoot disease severity (P = 0.076) and shoot dry weight (P = 0.088) did not show significant differences. All isolates caused a significant increase of root disease severity and a significant reduction of root dry weight when compared to the uninoculated controls, with the exception of isolate Cyl-10, which was not significantly different for root dry weight (Table 2). All the isolates were re-isolated from root fragments of affected plants on PDAS (80 % to 100 % of isolation), confirming Koch’s postulates.
Discussion
This research demonstrates the association of species belonging to the genera Cylindrodendrum, Dactylonectria and Ilyonectria with root rot of loquat and loquat decline in eastern Spain. To date, only A. mellea, R. necatrix and Phytophthora spp. had been described as causal agents of loquat decline in this region (González-Domínguez et al. 2008, 2009).
In our work, a collection of isolates with Cylindrocarpon-like asexual morphs obtained from diseased roots of loquat trees showing decline symptoms were characterized. Among them, two Dactylonectria species (D. alcacerensis and D. torresensis) and one Ilyonectria species (I. robusta) were identified based on the analysis of phenotypical characters and HIS data, with D. torresensis being the most frequent species. These three species are reported here for the first time on loquat and I. robusta is reported for the first time in Spain.
In addition, a group of four unidentified Ilyonectria-like isolates (Cyl-3, Cyl-8, Cyl-10 and Cyl-11) were also evaluated. According to their morphological characteristics, we hypothesized that they belonged to Ilyonectria or Dactylonectria genera (Booth 1966; Samuels and Brayford 1990; Halleen et al. 2004; Schroers et al. 2008; Chaverri et al. 2011; Cabral et al. 2012a, b; Lombard et al. 2014). Nevertheless, based on the multigene DNA analysis conducted of known species, these four isolates grouped together in a monophyletic clade closely to Cylindrodendrum spp., with no other closely Ilyonectria spp. or Dactylonectria spp. Thus, our results demonstrated that Cylindrodendrum isolates obtained from loquat orchards in Spain represent a novel species, described here as C. alicantinum.
Our study confirmed the pathogenicity of C. alicantinum, D. alcacerensis, D. torresensis and I. robusta to loquat. Root rot symptoms were reproduced on loquat seedlings and, although leaf yellowing and a shoot dry weight reduction were noticed, the statistical analysis showed that only root disease severity and root dry weight variables were significant when compared to the uninoculated controls. This could be due to the controlled conditions of incubation and the short period from inoculation to evaluation that were not enough to induce severe symptoms in the aerial part of loquat seedlings, which emerge as a consequence of root damage.
It has been demonstrated that Cylindrodendrum, Dactylonectria and Ilyonectria spp. cause root rot diseases on a range of diverse hosts worldwide (Halleen et al. 2006; Chaverri et al. 2011; Cabral et al. 2012a, b; Lombard et al. 2014). In fact, some Dactylonectria spp. such as D. alcacerensis and D. torresensis, which have been characterized in this study, were also isolated from grapevines in Spain (Agustí-Brisach et al. 2013a, b). In addition, Vitale et al. (2012) isolated fungal colonies belonging to D. macrodidyma-complex from avocado in Italy. Recently, Erper et al. (2013) also demonstrated the pathogenicity of D. torresensis, I. europaea, I. liriodendri and I. robusta to kiwifruit.
The simultaneous presence of A. mellea, Phytophthora spp., R. necatrix and the Cylindrodendrum, Dactylonectria and Ilyonectria species reported here in loquat orchards threatens the production of this fruit crop in eastern Spain. These pathogens severely affect mature and new plantations, as well as replanting (González-Domínguez et al. 2008, 2009). A similar scenario was reported in olive plantations (Olea europaea ssp. europaea) in southern Spain, where Sánchez-Hernández et al. (1998), reported the simultaneous presence of several soil-borne pathogens such as Cylindrocarpon spp., Phytophthora spp., Pythium spp. etc., inducing root and crown rot and/or dieback of twigs, resulting in severe economic losses, mainly in new plantations. In South Africa, I. liriodendri, D. pauciseptata and species belonging to the D. macrodidyma-complex were also associated with apple roots as causal agents of apple replant disease (Tewoldemedhin et al. 2011).
This information should be considered when establishing new loquat plantations or new susceptible fruit crops. In the area in which this research has been performed loquat and citrus are the main fruit crops. Currently, the production of citrus in this area is declining, due to the low price of this fruit. Thus, the farmers are encouraged to look for alternative fruits crops such as avocado, persimmon or kiwifruit. In this context, the susceptibility of these crops to the pathogens related here should be taken into account. Moreover, new research is needed focused on the improvement of the current management strategies against these soil-borne pathogens. This includes an evaluation of the different rootstocks proposed for loquat cultivation, such as Cydonia oblonga, Eriobotrya deflexa or Photinia serrulata (Lin 2007; Soler et al. 2007).
References
Agustí-Brisach, C., & Armengol, J. (2013). Black-foot disease of grapevine: an update on taxonomy, epidemiology and management strategies. Phytopathologia Mediterranea, 52, 245–261.
Agustí-Brisach, C., Gramaje, D., García-Jiménez, J., & Armengol, J. (2013a). Detection of Ilyonectria spp. in the grapevine nursery propagation process in Spain. European Journal of Plant Pathology, 137, 103–112.
Agustí-Brisach, C., Gramaje, D., García-Jiménez, J., & Armengol, J. (2013b). Detection of black-foot and Petri disease pathogens in natural soils of grapevine nurseries and vineyards using bait plants. Plant and Soil, 364, 5–13.
Aiello, D., Guarnaccia, V., Vitale, A., Cirvilleri, G., Granata, G., Epifani, F., Perrone, G., Polizzi, G., Groenewald, J. Z., & Crous, P. W. (2014). Ilyonectria palmarum sp. nov. causing dry basal stem rot of Arecaceae. European Journal of Plant Pathology, 138, 347–359.
Booth, C. D. (1966). The genus Cylindrocarpon. Mycological Papers (CMI), 104, 1–56.
Brayford, D. (1993). Cylindrocarpon. In L. L. Singleton, J. D. Mihail, & C. M. Rush (Eds.), Methods for research on soilborne phytopathogenic fungi (pp. 103–106). St. Paul: APS Press.
Cabral, A., Groenewald, J. Z., Rego, C., Oliveira, H., & Crous, P. W. (2012a). Cylindrocarpon root rot: multi-gene analysis reveals novel species within the Ilyonectria radicicola species complex. Mycological Progress, 11, 655–688.
Cabral, A., Rego, C., Nascimento, T., Oliveira, H., Groenewald, J. Z., & Crous, P. W. (2012b). Multi-gene analysis and morphology reveal novel Ilyonectria species associated with black foot disease of grapevines. Fungal Biology, 116, 62–80.
Calabrese, F. (2006). Origen de la especie. In M. Agustí, C. Reig, & P. Undurraga (Eds.), El cultivo del níspero japonés. España: Pontificia Universidad Católica de Valparaíso, Chile and Universidad Politécnica de Valencia.
Chaverri, P., Salgado, C., Hirooka, Y., Rossman, A. Y., & Samuels, G. J. (2011). Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon-like anamorphs. Studies in Mycology, 68, 57–78.
Crous, P. W., Gams, W., Stalpers, J. A., Robert, V., & Stegehuis, G. (2004a). MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology, 50, 19–22.
Crous, P. W., Groenewald, J. Z., Risede, J. M., & Hywel-Jones, N. L. (2004b). Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology, 50, 415–429.
Crous, P.W., Verkleij, G.J.M., Groenewald, J.Z., Samson, R.A. (Eds.) (2009). Fungal biodiversity. CBS laboratory manual series 1. Centraalbureau voor Schimmelcultures, Utrecht.
Dhingra, O. D., & Sinclair, J. B. (1995). Basic plant pathology methods (2nd ed.). Boca Raton: CRC Press.
Erper, I., Agustí-Brisach, C., Tunali, B., & Armengol, J. (2013). Characterization of root rot disease of kiwifruit in the Black Sea region of Turkey. European Journal of Plant Pathology, 136, 291–300.
Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution, 39, 783–791.
Gardes, M., & Bruns, T. D. (1993). ITS primers with enhanced specificity for basiodiomycetes-applications to the identification of mycorrhizae and rusts. Molecular Ecology, 2, 113–118.
Glass, N. L., & Donaldson, G. (1995). Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology, 61, 1323–1330.
González-Domínguez, E., Pérez-Sierra, A., Álvarez, L. A., Abad-Campos, P., Armengol, J., & García-Jiménez, J. (2008). Ethiology of decline of loquat (Eriobotrya japonica) in eastern Spain. Journal of Plant Pathology, 90(2, supplement), 179.
González-Domínguez, E., Pérez-Sierra, A., Álvarez, L. A., León, M., Abad-Campos, P., Armengol, J., & García-Jiménez, J. (2009). Agentes fúngicos presentes en plantaciones de nísperos (Eriobotrya japonica Lindl.) con síntomas de decaimiento en la provincia de Alicante. Boletín Sanidad Vegetal Plagas, 35, 453–467.
González-Domínguez, E., Rossi, V., Armengol, J., & García-Jiménez, J. (2013). Effect of environmental factors on mycelial growth and conidial germination of Fusicladium eriobotryae, and the infection of loquat leaves. Plant Disease, 97, 1331–1338.
González-Domínguez, E., Armengol, J., & Rossi, V. (2014). Development and validation of a weather-based model for predicting infection of loquat fruit by Fusicladium eriobotryae. Plos One, 9, e107547.
Halleen, F., Schroers, H. J., Groenewald, J. Z., & Crous, P. W. (2004). Novel species of Cylindrocarpon (Neonectria) and Campylocarpon gen. nov. associated with black-foot disease of grapevines (Vitis spp). Studies in Mycology, 50, 431–455.
Halleen, F., Fourie, P. H., & Crous, P. W. (2006). A review of black-foot disease of grapevine. Phytopathologia Mediterranea, 45, S55–S67.
Janick, J. (2011). Predictions for loquat improvement in the next decade. Acta Horticulturae, 887, 25–30.
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGettigan, P. A., McWilliam, H., Valentin, F., Wallace, I. M., Wilm, A., Lopez, R., Thompson, J. D., Gibson, T. J., & Higgins, D. G. (2007). Clustal W and Clustal X version 2.0. Bioinformatics, 23, 2947–2948.
Lin, S. Q. (2007). World loquat production and research with special reference to China. Acta Horticulturae, 750, 37–44.
Lombard, L., Van der Merwe, N. A., Groenewald, J. Z., & Crous, P. W. (2014). Lineages in Nectriaceae: Re-evaluating the generic status of Ilyonectria and allied genera. Phytopathologia Mediterranea, 53, 515–532.
Nirenberg, H. (1976). Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft, 169, 1–117.
O’Donnell, K., & Cigelnik, E. (1997). Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution, 7, 103–116.
Petit, E., & Gubler, W. D. (2005). Characterization of Cylindrocarpon species, the cause of black foot disease of grapevine in California. Plant Disease, 89, 1051–1059.
Rayner, R. W. (1970). A mycological colour chart. Kew: British Mycological Society and CAB International Mycological Institute.
Read, N. D., Lichius, A., Shoji, J. Y., & Goryachev, A. B. (2009). Self-signalling and self-fusion in filamentous fungi. Current Opinion in Microbiology, 12, 608–615.
Reig, C., Farina, V., Volpe, G., Mesejo, C., Martínez-Fuentes, A., Barone, F., Calabrese, F., & Agustí, M. (2012). Giberellic acid and flower bud development in loquat (Eriobotrya japonica Lindl.). Scientia Horticulturae, 129, 27–31.
Samuels, G. J., & Brayford, D. (1990). Variation in Nectria radicicola and its anamorph, Cylindrocarpon destructans. Mycological Research, 94, 433–442.
Sánchez-Hernández, M. E., Ruiz-Dávila, A., Pérez de Algaba, A., Blanco-López, M. A., & Trapero-Casas, A. (1998). Occurrence and etiology of death of young olive tres in southern Spain. European Journal of Plant Pathology, 104, 347–357.
Sánchez-Torres, P., Hinarejos, R., & Tuset, J. J. (2009). Characterization and pathogenicity of Fusicladium eriobotryae, the fungal pathogen responsible for loquat scab. Plant Disease, 93, 1151–1157.
Schroers, H. J., Zerjav, M., Munda, A., Halleen, F., & Crous, P. W. (2008). Cylindrocarpon pauciseptatum sp. nov., with notes on Cylindrocarpon species with wide, predominantly 3-septate macroconidia. Mycological Research, 112, 82–92.
Soler, E., Martínez-Calvo, J., Llácer, G., & Badenes, M. L. (2007). Loquat in Spain: production and marketing. Acta Horticulturae, 750, 45–47.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: molecular evolutionary 16 genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729.
Tewoldemedhin, Y. T., Mazzola, M., Mostert, L., & McLeod, A. (2011). Cylindrocarpon species associated with apple tree roots in South Africa and their quantification using real-time PCR. European Journal of Plant Pathology, 129, 637–651.
Vitale, A., Aiello, D., Guarnaccia, V., Perrone, G., Stea, G., & Polizzi, G. (2012). First report of root rot caused by Ilyonectria (=Neonectria) macrodidyma on avocado (Persea americana) in Italy. Journal of Phytopathology, 160, 156–159.
Zhuang, W. Y., Nong, Y., & Luo, J. (2007). New species and new Chinese records of Bionectriaceae and Nectriaceae (Hypocreales, Ascomycetes) from Hubei, China. Fungal Diversity, 24, 347–357.
Acknowledgments
We acknowledge Dr. L. Lombard and Prof. Dr. P.W. Crous (CBS-KNAW Fungal Biodiversity Centre, The Netherlands) for valuable discussions and data sharing. This work was funded by the Cooperativa Agrícola de Callosa d’En Sarrià (Alicante, Spain). We would like to thank E. Soler for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agustí-Brisach, C., Cabral, A., González-Domínguez, E. et al. Characterization of Cylindrodendrum, Dactylonectria and Ilyonectria isolates associated with loquat decline in Spain, with description of Cylindrodendrum alicantinum sp. nov.. Eur J Plant Pathol 145, 103–118 (2016). https://doi.org/10.1007/s10658-015-0820-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0820-7