Abstract
Three hundred and fifty six isolates of Rhizoctonia solani were obtained from potato stems and tubers in the main potato growing regions of China between 2011 and 2014. On the basis of the hyphal anastomosis and sequence analysis of the internal transcribed spacers of the ribosomal DNA, 249 isolates (69.94 %) were identified to anastomosis group (AG)-3 PT, 44 (12.36 %) to AG-5, 39 (10.96 %) to AG-4-HGI, 12 (3.37 %) to AG-4-HGII, seven (1.97 %) to AG-4-HGIII, three (0.84 %) to AG-2-1, one (0.28 %) to AG-1-IB, and one (0.28 %) to AG-11. All these AGs could be isolated from potato cankered subterranean stems, whereas isolates obtained from sclerotia on tubers just belonged to AG-3 PT, AG-4-HGI, and AG-5. The selected 56 isolates representing different AGs and subgroups were able to cause brown, dry lesions on potato subterranean stems with disease incidences ranging from 21.43 to 92.87 %. The lesions caused by AG-3 PT isolates were deeply sunken, while those caused by isolates of other AGs were slightly sunken. Taken together, AG-3 PT of R. solani was the predominant pathogen causing stem canker and black scurf on potatoes in China. To our knowledge, this is the first detailed report of the AG composition of R. solani populations causing potato disease in China.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rhizoctonia solani Kühn [teleomorph Thanatephorus cucumeris (Frank) Donk] is an important soil-borne pathogen associated with severe damage on many agricultural crops (Ogoshi 1996). On potato (Solanum tuberosum), this fungus causes stem canker and black scurf disease in all potato-growing areas worldwide (Lehtonen et al. 2008). The pathogen attacks subterranean parts of potato plants causing brown canker lesions on sprouts, stems, stolons, and roots (Banville 1978). Black scurf refers to the presence of sclerotia on tubers, which is the most conspicuous sign of Rhizoctonia disease (Tsror 2010). Besides the typical symptoms of stem canker and black scurf on tuber, the fungus can also lead to delayed emergence, cracked or malformed tubers, and sometimes development of green aerial tubers on stems above the soil (Tsror 2010). The disease can cause both quantitative and qualitative damage to potato crops, leading marketable yield losses up to 30 % (Banville 1989; Platt et al. 1993). Infected subterranean parts are responsible for quantitative losses since it affects tuber size and number (Carling et al. 1989; Platt 1989), whereas qualitative losses occur mainly due to the production of misshapen tubers and the development of sclerotia on the tuber surface (James and McKenzie 1972; Escande and Echandi 1991).
R. solani is a species complex composed of 13 anastomosis groups, from AG-1 to AG-13 (Carling et al. 2002a). Some AGs have been further divided into subgroups based on colony morphology, pathogenicity, host range, biochemical and genetic properties (Carling 1996; Cubeta and Vilgalys 1997). For example, AG-3 was divided into three subgroups: AG-3 PT (potato type), AG-3 TB (tobacco type) and AG-3 TM (tomato type) (Bartz et al. 2010). It is acknowledged that R. solani AG-3 PT is the predominant and the most aggressive AG to potato plants (Bandy et al. 1988; Balali et al. 1995; Virgen-Calleros et al. 2000; Ceresini et al. 2002; Campion et al. 2003; Tsror 2010; Woodhall et al. 2013). Isolates belonging to AG-2 (Campion et al. 2003; Woodhall et al. 2007, 2008, 2012a; Das et al. 2014), AG-4 (Balali et al. 1995; Virgen-Calleros et al. 2000; Woodhall et al. 2012b), and AG-5 (Balali et al. 1995; Woodhall et al. 2007) are often associated with potato diseases in different parts of the world. The aggressiveness of isolates within AG-2-1 seems to be very different. Some AG-2-1 isolates were able to cause severe lesions on potato plants and induce malformed tubers (Woodhall et al. 2007; Das et al. 2014), while some AG-2-1 isolates only caused slight lesions or even were not pathogenic (Woodhall et al. 2007; Lehtonen et al. 2008). AG-4 and AG-5 are more common in warm environments and they were mainly found to cause canker symptoms on potato plants but have little or no role in causing black scurf on tubers (Anguiz and Martin 1989; Carling and Leiner 1990; Balali et al. 1995). Additionally, in Mexico AG-4 was found only during the flowering stage of potato growth, whereas AG-3 was present on plants at every stage of development (Virgen-Calleros et al. 2000). Other AGs are occasionally found to infect potato, such as AG-1(Yanar et al. 2005), AG-7 (Carling et al. 1998), AG-8 (Balali et al. 1995; Yanar et al. 2005; Woodhall et al. 2008), AG-9 (Carling et al. 1987; Yanar et al. 2005), and AG-10 (Yanar et al. 2005). These AGs were limited in their ability to infect different parts of the potato plants. For instance, it was reported that AG-7 infected stems, stolons, and tubers, but didn’t cause symptoms on roots (Carling et al. 1998). In contrast, AG-8 was only capable of infecting potato roots (Hide and Firmager 1990; Balali et al. 1995).
AG-1-IB, AG-2-1, AG-3, AG-4 (including all three homogeneous groups: HGI, HGII and HGIII), AG-5, and AG-11 were previously isolated from potato crops in northern China, such as Hebei Province, Heilongjiang Province, and Inner Mongolia Autonomous Region (Tian et al. 2011; Yang and Wu 2012, 2013; Wang et al. 2013; Li et al. 2014). However, the pathogenicity tests of AG-1-IB, AG-2-1, and AG-11 weren’t conducted on potatoes in these reports. In addition, no data exist on the incidence of individual AGs associated with potato crops in main production areas throughout China. From 2011 to 2014, 425 isolates of Rhizoctonia spp. were obtained from cankered potato stems and tubers with sclerotia in 21 provinces, autonomous regions, and municipalities of China. Among them, 69 were classified into AG-A, AG-F, AG-I, AG-K, and AG-U of binucleate Rhizoctonia (Yang et al. 2014). The aim of this study was to determine the AG identities of the remaining 356 R. solani isolates and their pathogenicity on potato plants.
Materials and methods
Sample collection and fungal isolation
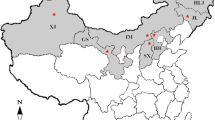
From 2011 to 2014, potato subterraneous stems with canker lesions and tubers with black scurf were collected from the main potato production areas in twenty one provinces, municipalities, and autonomous regions throughout China (Fig. 1). The potato production in these areas occupied over 90 % of total yield in China (Editorial Board of China Agriculture Yearbook 2013). The samples were gently washed under running tap water to remove adhering soil and debris. Diseased stems were cut into pieces (about 5 × 5 mm) from the margins of the healthy and diseased tissues. Surface-disinfection were performed with 70 % ethanol for 30 s, followed by soaking in 0.5 % sodium hypochlorite for 3 min, and then rinsed three times with sterile distilled water. After drying on sterilized filter paper, the fragments were placed on water agar (WA) containing 50 μg/ml streptomycin sulphate and incubated at 25 °C for 24–72 h. For tuber samples, individual sclerotia were removed from tubers and directly placed on WA. The resulted fungi were initially identified as Rhizoctonia by visual observation of right angle branching with a septum near the branch and a slight constriction at the branch base. The hyphal tips of Rhizoctonia spp. were transferred onto potato dextrose agar (PDA) plates for purification. Following sufficient growth, cultured plates were kept at 4 °C for short-term storage. For long-term preservation, these isolates were colonized on sterile barley grains and maintained at 4 °C.
Geographic origins where the Rhizoctonia solani isolates were collected in China. Red markers indicate the collection areas. AH Anhui Province, BJ Beijing Municipality, CQ: Chongqing Municipality, GS Gansu Province, GX Guangxi Zhuang Autonomous Region, GZ Guizhou Province, HeB Hebei Province, HeN Henan Province, HL Heilongjiang Province, HuB Hubei Province, HuN Hunan Province, JL Jilin Province, LN Liaoning Province, IM Inner Mongolia Autonomous Region, NX Ningxia Hui Autonomous Region, QH Qinghai Province, SC Sichuan Province, SD Shandong Province, SX Shaanxi Province, XJ Xinjiang Uygur Autonomous Region, YN Yunnan Province
Nuclear condition
Unknown Rhizoctonia isolates were characterized as either binucleate or multinucleate and only those multinucleate isolates were used for further study. Nuclear condition was determined by using the clean slide method modified from Kronland and Stanghellini (1988). Briefly, a 7-mm-diameter disk of PDA was removed from the margin of an actively growing colony, and placed on sterilized microscope slides overlaying WA in 9 cm diameter Petri dishes and incubated at 20 °C in the dark for 24–48 h. Developed mycelia were stained with 1 μg/ml 4′-6-diamidino-2-phenylindole (DAPI; cat. No. D9542, Sigma) for 10 min in the dark and the slides subsequently rinsed with sterile distilled water. Nuclei of at least 20 cells of more than one hypha were counted under a fluorescence microscope at 400× magnification in order to determine the nuclear status of each isolate.
Hyphal anastomosis
Hyphal anastomosis with reference strains was used to determine the AG identities of unknown R. solani isolates. Reference isolates including Cs-Ka (AG-1-IA), B-19 (AG-1-IB), PS-4 (AG-2-1), ST4-1 (AG-3), UHBC (AG-4-HGII), ST2-1 (AG-5), OHT1-1 (AG-6), and H10-155G (AG-7) were kindly provided by N. Kondo (Graduate School of Agriculture, Hokkaido University, Sapporo, Japan).
Clean-slide method was used for observing hyphal interactions (Kronland and Stanghellini 1988). Briefly, 7-mm-diameter disk of tested isolates were placed on microscope slide with reference strains 2–3 cm away. When the hyphae from two sides overlapped, the fungi disks were slightly removed and observed at 40× magnification to search for potential fusion events, which were confirmed at higher magnification (×100 and ×400). All pairings were repeated twice and at least ten fusion events were analyzed. Anastomosis reactions were classified according to Carling (1996) as C0, no interaction; C1, hyphal contact without fusion; C2, cell wall fused while anastomosing and adjacent cells die; C3, fusion of cell wall and membrane, with no cell death.
DNA extraction and polymerase chain reaction (PCR) amplification
The isolates were grown on a cellophane disc placed on PDA media. After 3–5 days, mycelia were collected, freeze-dried, and ground to fine powder in liquid nitrogen. Then the mycelium powder was suspended with 700 μl CTAB extraction buffer (100 mM TrisHCl pH 8.0, 20 mM EDTA pH 8.0, 1.4 M NaCl, 20 mM β-mercaptoethanol, and 2 % w/v Cetyltrimethylammonium bromide) in a 2-ml microcentrifuge tube and incubated at 65 °C for 30 min (with intermittent mixing of tube contents). The solution was extracted with an equal volume of chloroform: isoamyl alcohol (24:1, v/v) and centrifuged at 12,000 rpm for 10 min at room temperature. The resulting supernatant was incubated with 40 μg/ml of RNase A at 37 °C for 30 min. The solution was re-extracted with an equal volume of chloroform: isoamyl alcohol (24:1, v/v) and centrifuged at 12,000 rpm for 10 min. The supernatant was mixed with an equal volume of isopropanol and 1/10 volume of 3 M sodium acetate (pH 4.8) to precipitate genomic DNA. After centrifuged at 12,000 rpm for 10 min at room temperature, the resulting pellet was washed twice with 70 % ethanol. Then the pellet was dried under vacuum and dissolved in 50 μl ddH2O. To ensure that each DNA sample was of adequate quality for polymerase chain reaction (PCR), UV spectrophotometry (Nanodrop 2000, Thermo) was used to determine the concentration and purity of DNA.
The internal transcribed spacer (ITS) region of ribosomal DNA (rDNA), including ITS1, ITS2, and the 5.8S ribosomal DNA, were amplified from genomic DNA with primers ITS1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) (White et al. 1990). The 25 μl PCR mixture consisted of 11 μl ddH2O, 12.5 μl Premix Ex Taq (Version 2.0, TaKaRa, containing 0.625 U DNA polymerase, 200 μM dNTP and 1.5 mM Mg2+), 0.5 μl each of primer (10 μM), and 0.5 μl DNA (100 μg/ml). Negative control was samples containing the same reagents but without DNA.
Amplification was performed in an Eppendorf Mastercycler gradient thermal cycler (Eppendorf AG, Hamburg, Germany) using the following program: initial denaturation at 94 °C for 3 min; followed by 30 cycles of denaturing at 94 °C for 40 s, annealing at 56 °C for 40 s, and extension at 72 °C for 1 min; one cycle of extension at 72 °C for 10 min and final incubation at 4 °C.
DNA sequencing and phylogenic analysis
The PCR products of rDNA-ITS region were purified with AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Hangzhou, China) and cloned into the vector pMD19-T (TaKaRa Biotechnology, Dalian, China) according to the manufacturer’s instructions. The cloning reaction mixture was transformed into competent cells of Escherichia coli MC1022 by heat shock at 42 °C for 90 s, which were then cultured on Luria-Bertani broth (LB) medium containing ampicillin, 5-bromo-4-chloro-3-indolyl-β-D-galactoside and isopropyl-β-D-thiogalactopyranoside. White colonies verified by PCR were sent to Beijing Sunbiotech Co., Ltd (Beijing, China) for bidirectional sequencing. The resulting sequences were edited with DANMAN (Version 7).
The rDNA-ITS sequences of all potato isolates from this study were used for phylogenetic analysis with those of reference strains retrieved from GenBank database. The Athelia rolfsii isolate (FSR-052) was used as the outgroup (Sharon et al. 2008). All sequences were aligned by MEGA 5 (version 5.2.2) software program using the Clustal W algorithm and obvious errors were adjusted manually (Tamura et al. 2011). Maximum Likelihood (ML) and Neighbor-Joining (NJ) analyses both used the Tamura 3-parameter model with Gamma distribution as determined using model test function in MEGA 5 (Tamura et al. 2011). Bootstrap support was estimated based on 1000 pseudoreplicates and only bootstrap values ≥ 70 % are shown in the phylogenetic tree. Representative sequences of each AG from each geographic origin were deposited in GenBank, and the accession numbers are shown in the phylogenetic tree.
Pathogenicity tests
R. solani isolates that were used for phylogenetic analysis were also tested for their pathogenicity on potato plants according to revised procedures described by Lehtonen et al. (2008). The amount of inoculum was standardized using fungus-infested wheat seeds as a source of infection. Wheat seeds were moistened with sterile distilled water (60 % v/w) in 250-ml flasks and sterilized by autoclaving twice at 121 °C for 1 h, with a 24 h interval. The seeds were cooled at room temperature and spread on the 4-day-old actively growing mycelium of R. solani isolates on PDA. The fungus was allowed to colonize the seeds for 3 days at room temperature in the dark.
Disease-free potato seed tubers (cv. Favorita) sprouted at room temperature in the dark until the sprouts were about 3 mm long. One sprouted tuber was placed in a sterile plastic pot (1-l) filled with 3 cm depth of sand and sawdust mixture (1:2 v/v, dry heat sterilization at 161 °C for 4 h before use). A single wheat seed colonized with each isolate was placed 10 mm above the uppermost sprout tip and then the pot was filled with the mixture. The test was performed on 20 plants for each treatment including controls which were inoculated with non-infested autoclaved wheat seeds. The potato plants were incubated in a glasshouse maintained at 25 to 27 °C and watered whenever the surface soil appeared dry. After 3 weeks, all potato plants were harvested and assessed for disease incidence and disease index.
Based on the relative size of necrotic area on subterraneous stems, the disease rating scale was as follows: 0 = no disease, l = less than 10 %, 2 = 10–50 %, 3 = 50–100 %, and 4 = plants dead. The disease incidence and the disease index were calculated as follows: disease incidence = 100 % × (n 1 + n 2 + n 3 + n 4) / N and disease index = 100 × (0n 0 + 1n 1 + 2n 2 + 3n 3 + 4n 4) / (4N), where n 0–4 is the number of plants in each degree and N is the total number of inoculated plants (Yang et al. 2014). All the potato subterraneous stems were used to re-isolate Rhizoctonia fungus and the resulting Rhizoctonia isolates were identified by morphological and molecular methods described above to fulfill Koch’s postulates. The experiments were repeated once.
Statistical analysis
The data of disease incidence and disease index were analyzed by one-way ANOVA using SPSS software version 20.0 (SPSS Inc., Chicago, USA). The isolates were classified into three groups, Group I (<50 %), Group II (50–90 %), and Group III (>90 %), based on threshold values of 50 and 90 % of disease incidence. On the basis of threshold values of 10 and 40 of disease index, the isolates were classified into low aggressiveness (<10), moderate aggressiveness (10–40) and high aggressiveness (>40). Differences among the means of those categories were evaluated by Fisher’s Least Significant Difference (LSD) test at P = 0.05.
Results
Determination of anastomosis groups
Rhizoctonia sp. was preliminarily identified by the hyphal characteristics as right angle branching with a septum near the branch and a slight constriction at the branch base. Since multiple Rhizoctonia isolates were obtained from each location, only those with different cultural morphology were selected for further study. Nuclear staining revealed that 356 multinucleate Rhizoctonia isolates were obtained from 21 provinces, municipalities, and autonomous regions across China (Table 1). Among them, 191 isolates were obtained from cankered subterraneous stems, while the remaining 165 isolates were recovered from sclerotia on tubers.
When paired with reference strains, 249 isolates gave C2 or C3 reaction with AG-3, 58 isolates with AG-4, 44 isolates with AG-5, three isolates with AG-2-1, one isolate with AG-1-IB, and one isolate designated as NM-17-2 didn’t anastomose with any of the reference strains from AG-1 to AG-7 (Fig. 2).
In order to confirm the AG identities determined on the basis of hyphal anastomosis, the rDNA-ITS sequences of all potato isolates from this study were used for phylogenetic analysis with those of reference strains retrieved from GenBank database. PCR amplification with primers ITS1 and ITS4 produced a single DNA fragment about 700 bp for each isolate. Phylogenetic analysis of tested isolates and reference strains on the basis of ITS1-5.8S rDNA-ITS2 sequences assigned 249 (69.94 %) isolates to AG-3 PT, 44 (12.36 %) to AG-5, 39 (10.96 %) to AG-4-HGI, 12 (3.37 %) to AG-4-HGII, seven (1.97 %) to AG-4-HGIII, three (0.84 %) to AG-2-1, one (0.28 %) to AG-1-IB, and 1 (0.28 %) to AG-11. Of the isolates recovered from sclerotia on tubers, 161 (97.57 %) were AG-3 PT, three (1.82 %) belonged to AG-4-HGI, and one (0.61 %) belonged to AG-5. For the selected sequences, ML and NJ methods found the same tree topology (Fig. 3). AG-2-1 isolates designated as HL-27 and LN-3 clustered with the subset of Japanese-2-1 plus Dutch-2t, whereas the isolate designated as XJ-8 grouped into the subset of Alaskan & Australian 2-1.
Phylogenetic analysis of the rDNA-ITS sequences of Rhizoctonia solani isolates from this study and GenBank. Tree was constructed using Maximum Likelihood (ML) and Neighbor-Joining (NJ) analysis. Numbers at nodes indicate bootstrap values from ML/NJ analysis, respectively. Only bootstrap values ≥ 70 % are shown. Scale bar represents a genetic distance of 0.05 for horizontal branch lengths. The reference sequences retrieved from GenBank are shown in bold type of isolate’s name followed by accession number and reference paper in the parentheses. The isolates from this study are shown as their name followed by GenBank accession number in the parentheses
Pathogenicity of R. solani isolates on potato plants
The isolates used for phylogenetic analysis were tested for their pathogenicity to potato plants. The results indicated that all tested R. solani isolates were capable of causing brown, dry lesions on potato stems, while the control plants inoculated with non-infested wheat seeds remained healthy (Fig. 4). The symptoms of stem canker caused by AG-3 isolates were deeply sunken. In some diseased stems, parts of phloem were completely destroyed and the xylem was exposed. Potato plants inoculated with other AGs only exhibited slight sunken lesions. No Rhizoctonia sp. was isolated from healthy plants, whereas R. solani isolates were consistently re-isolated from symptomatic stems of the inoculated plants. Their identities were confirmed by morphological and molecular characteristics as described above, fulfilling Koch’s postulates.
Disease incidences of stem canker caused by tested isolates ranged from 21.43 to 92.87 % (Fig. 5). On the basis of threshold values of 50 % and 90 %, five isolates (8.93 %), 42 isolates (75.00 %) and nine isolates (16.07 %) were classified into Group I, Group II and Group III, respectively. The differences of disease incidence among the three groups were statistically significant (F 2, 53 = 154.37, P < 0.001).
Variation of disease incidence caused by selected isolates of Rhizoctonia solani in this study. Thin bars indicate standard deviation. Based on threshold values of 50 % and 90 %, the isolates were classified into three groups: Group I (<50 %), Group II (50–90 %) and Group III (>90 %). Different letters followed with parentheses indicate significant differences among three groups (F 2, 53 = 154.37, P < 0.001)
Disease indexes of stem canker caused by tested isolates ranged from 5.36 to 47.73 (Fig. 6). Based on threshold values of 10 and 40, 43 isolates (76.78 %) were classified into moderate aggressiveness, while 8 (14.28 %) isolates were of high aggressiveness and five isolates (8.93 %) were of low aggressiveness. The differences of disease index among the ‘High’, ‘Moderate’, and ‘Low’ levels of aggressiveness were statistically significant (F 2, 53 = 96.46, P < 0.001). Among the eight high aggressiveness isolates, five belonged to AG-3 PT, two belonged to AG-5, and one was AG-2-1. Five low aggressiveness isolates consisted of two AG-4-HGI, one AG-HGII, one AG-5, and one AG-2-1. AG-1-IB and AG-11 isolates in this study were regarded as moderately aggressive to potato plants, since the disease indexes caused by them were 31.67 and 29.69, respectively.
Range, quartiles, medians and outliers of the disease indexes caused by Rhizoctonia solani isolates belonging to different anastomosis groups or subgroups. The tested isolates were classified into low (<10), moderate (10–40) and high (>40) aggressiveness based on threshold values of 10 and 40. The differences of mean disease index among three levels were statistically significant (F 2, 53 = 96.46, P < 0.001)
Discussion
This is the first detailed study to examine the AG composition and aggressiveness of R. solani from the major potato-producing regions in China. The results indicated that AG-3 PT was the predominant R. solani infecting potato in China, which was concordant with previous reports from other parts of the world, including France (Campion et al. 2003), Finland (Lehtonen et al. 2008), the UK (Woodhall et al. 2007), the USA (Bandy et al. 1988; Ceresini et al. 2002), Mexico (Virgen-Calleros et al. 2000), Colombia (Ferrucho et al. 2013), Australia (Balali et al. 1995), New Zealand (Das et al. 2014), and Japan (Abe and Tsuboki 1978). Besides AG-3, isolates belonged to AG-1-IB, AG-2-1, AG-4, AG-5, and AG-11 were also found to infect potato plants in China. Similarly, potato stem canker caused by AG-2, AG-4, and AG-5 were also reported in Japan (Abe and Tsuboki 1978) and Pakistan (Rauf et al. 2007), the neighbor countries of China.
The anastomosis groups of all tested isolates in this study were determined by phylogenetic analysis with reference strains based on rDNA-ITS sequences. Although identity of the isolate designated as NM-17-2 was not confirmed by hyphal anastomosis, BLASTn on the National Center for Biotechnology Information (NCBI) showed that its rDNA-ITS sequence was closely related to that of AG-11 isolate PT321 (KC146421) with identities of 99 %. Very recently, three AG-11 isolates were reported to have been isolated from diseased potatoes in Inner Mongolia Autonomous Region. However, pathogenicity tests of these three isolates were not carried out on potato plants (Li et al. 2014). Therefore, to our knowledge, this is the first report of the AG-11 isolate causing potato disease of stem canker in China.
Environmental conditions may influence the prevalence of certain AGs (Virgen-Calleros et al. 2000). In Peru, for example, AG-4 and AG-5 are more common in warm environments, whereas AG-3 prefers cooler and drier conditions (Anguiz and Martin 1989). In our research, the geographical distribution of AG-1-IB, AG-2-1, AG-4-HGII, AG-4-HGIII, and AG-11 on potato plants was quite distinct. Since they were only isolated from north China, it seems that they prefer cool conditions. Except for Beijing Municipality, AG-3 isolates were recovered from the remaining 20 provinces. The reason for not obtaining AG-3 might be that only one potato sample was collected in this site. AG-4-HGI and AG-5 isolates in this study were obtained from potato plants in 13 and 11 provinces, respectively, which located from northern to southern China. This may indicate that isolates in these AGs could adapt to various environmental conditions.
Sclerotia on tubers are considered to be a very important inoculum because they mainly contribute to the long distance dispersal and infect potato plants more rapidly due to the close proximity to the host (Balali et al. 1995; Banville et al. 1996). The AGs diversity on potato tubers was much lower than those on potato stems in this study. For the isolates originating from tubers, 161 belonged to AG-3. Three isolates and one isolate belonged to AG-4-HGI and AG-5, respectively. These results confirmed previous observations that AG-3 was the predominant AG on potato tubers, while the presence of AG-2-1, AG-4, and AG-5 was a small proportion (Balali et al. 1995; Banville et al. 1996). In other parts of the world, AG-2-1 isolates were mainly collected from potato tubers (Campion et al. 2003; Das et al. 2014). However, no AG-2-1 isolate was recovered from tubers in this research. One reason might be that we only collected tuber samples with typical symptoms of black scurf, whereas AG-2-1 survives on tubers as hyphae or as atypical tuber blemishes, such as elephant hide (Woodhall et al. 2013). Another possible reason is that AG-2-1 does not produce sclerotia as abundantly as AG-3 and the sclerotia produced by AG-2-1 had poor survival on tubers after harvest (Campion et al. 2003).
AG-2-1 is a heterogenous group and has been separated into three subsets: i) Japanese -2-1 plus Dutch-2t, ii) Alaskan and Australian 2-1, and iii) Italian Nt (AG-2Nt) (Kuninaga et al. 2000; Carling et al. 2002b; Woodhall et al. 2007; Das et al. 2014). It was considered that the subset of AG-2Nt was particularly aggressive to cause malformed tubers, whereas other isolates of AG-2-1 do not cause misshapen tubers (Das et al. 2014). Although AG-2-1 isolates have been recovered from potato crops in China previously, the subsets of them were not clear (Wang et al. 2013). In the present study, two AG-2-1 isolates were classified into the subset of Japanese-2-1 plus Dutch-2t. One was high aggressive to potato crops, while the other was low aggressive. One AG-2-1 isolate designated as XJ-8 was classified into the subset of Alaskan and Australian 2-1 and its aggressiveness to potato was moderate.
In conclusion, this work has provided a better knowledge about R. solani AGs associated with potato in different locations in China. The results have confirmed that potato stem canker and black scurf was predominantly caused by AG-3 PT isolates, whereas AG-1-IB, AG-2-1, AG-4 (including subgroups HGI, HGII and HGIII), AG-5, and AG-11 were also pathogenic to potato crops. Based on these findings, further work should be undertaken to investigate the sensitivity of AGs to different fungicides. In addition, some of these AGs have been reported to infect bean crops (Yang et al. 2005), cotton (Maimaiti et al. 2007; Jing et al. 2012), sugar beet (Zhao et al. 2002), and corn (Li et al. 2011; Zhou et al. 2012a, b) in China. Since these crops are grown very commonly in China, they should be avoided for use in crop rotation when considering integrated management strategies for potato Rhizoctonia disease.
References
Abe, H., & Tsuboki, K. (1978). Anastomosis groups of isolates of Rhizoctonia solani Kühn from potatoes. Bulletin of Hokkaido Prefectural Agricultural Experiment Stations, 40, 61–70.
Anguiz, R., & Martin, C. (1989). Anastomosis groups, pathogenicity, and other characteristics of Rhizoctonia solani isolated from potatoes in Peru. Plant Disease, 73, 199–201.
Balali, G. R., Neate, S. M., Scott, E. S., Whisson, D. L., & Wicks, T. J. (1995). Anastomosis group and pathogenicity of isolates of Rhizoctonia solani from potato crops in South Australia. Plant Pathology, 44, 1050–1057.
Bandy, B. P., Leach, S. S., & Tavantzis, S. M. (1988). Anastomosis group 3 is the major cause of Rhizoctonia disease of potato in Maine. Plant Disease, 72, 596–598.
Banville, G. J. (1978). Studies on the Rhizoctonia disease of potatoes. American Journal of Potato Research, 55, 56.
Banville, G. J. (1989). Yield losses and damage to potato plants caused by Rhizoctonia solani Kühn. American Potato Journal, 66, 821–834.
Banville, G. J., Carling, D. E., & Otrysko, B. E. (1996). Rhizoctonia diseases on potato. In B. Sneh, S. Jabaji-Hare, S. Neate, & G. Dijst (Eds.), Rhizoctonia species: Taxonomy, molecular biology, ecology, pathology and disease control (pp. 321–330). Dordrecht: Kluwer Academic Publishers.
Bartz, F. E., Cubeta, M. A., Toda, T., Naito, S., & Ivors, K. L. (2010). An in planta method for assessing the role of Basidiospores in Rhizoctonia foliar disease of tomato. Plant Disease, 94, 515–520.
Budge, G. E., Shaw, M. W., Lambourne, C., Jennings, P., Clayburn, R., Boonham, N., & McPherson, M. (2009). Characterization and origin of infection of Rhizoctonia solani associated with Brassica oleracea crops in the UK. Plant Pathology, 58, 1059–1070.
Campion, C., Chatot, C., Perraton, B., & Andrivon, D. (2003). Anastomosis groups, pathogenicity and sensitivity to fungicides of Rhizoctonia solani isolates collected on potato crops in France. European Journal of Plant Pathology, 109, 983–992.
Carling, D. E. (1996). Grouping in Rhizoctonia solani by the anastomosis reaction. In B. Sneh, S. Jabaji-Hare, S. Neate, & G. Dijst (Eds.), Rhizoctonia species: Taxonomy, molecular biology, ecology, pathology and disease control (pp. 37–47). Dordrecht: Kluwer Academic Publishers.
Carling, D. E., & Leiner, R. H. (1990). Effect of temperature on virulence of Rhizoctonia solani and other Rhizoctonia on potato. Phytopathology, 80, 930–934.
Carling, D. E., Leiner, R. H., & Kebler, K. M. (1987). Characterization of a new anastomosis group (AG-9) of Rhizoctonia solani. Phytopathology, 77, 1609–1612.
Carling, D. E., Leiner, R. H., & Westphale, P. C. (1989). Symptoms, signs and yield reduction associated with Rhizoctonia disease of potato induced by tuber-borne inoculum of Rhizoctonia solani AG-3. American Potato Journal, 66, 693–701.
Carling, D. E., Brainard, K. A., Virgen-Calleros, G., & Olalde-Portugal, V. (1998). First report of Rhizoctonia solani AG-7 on potato in Mexico. Plant Disease, 82, 127.
Carling, D. E., Baird, R. E., Gitaitis, R. D., Brainard, K. A., & Kuninaga, S. (2002a). Characterization of AG-13, a newly reported anastomosis group of Rhizoctonia solani. Phytopathology, 92, 893–899.
Carling, D. E., Kuninaga, S., & Brainard, K. A. (2002b). Hyphal anastomosis reactions, rDNA-internal transcribed spacer sequences, and virulence levels among subsets of Rhizoctonia solani anastomosis group-2 (AG-2) and AG-BI. Phytopathology, 92, 43–50.
Ceresini, P. C., Shew, H. D., Vilgalys, R. J., & Cubeta, M. A. (2002). Genetic diversity of Rhizoctonia solani AG-3 from potato and tobacco in North Carolina. Mycologia, 94, 437–449.
Cubeta, M. A., & Vilgalys, R. (1997). Population biology of the Rhizoctonia solani complex. Phytopathology, 87, 480–484.
Das, S., Shah, F. A., Butler, R. C., Falloon, R. E., Stewart, A., Raikar, S., & Pitman, A. R. (2014). Genetic variability and pathogenicity of Rhizoctonia solani associated with black scurf of potato in New Zealand. Plant Pathology, 63, 651–666.
Editorial Board of China Agriculture Yearbook. (2013). China agriculture yearbook (p. 214). Beijing: China Agriculture Press (In Chinese).
Escande, A. R., & Echandi, E. (1991). Protection of potato from Rhizoctonia canker with binucleate Rhizoctonia fungi. Plant Pathology, 40, 197–202.
Ferrucho, R. L., Ceresini, P. C., Ramirez-Escobar, U. M., McDonald, B. A., Cubeta, M. A., & García-Domínguez, C. (2013). The population genetic structure of Rhizoctonia solani AG-3PT from potato in the Colombian Andes. Phytopathology, 103, 862–869.
Gonzalez, D., Carling, D. E., Kuninaga, S., Vilgalys, R., & Cubeta, M. A. (2001). Ribosomal DNA systematics of Ceratobasidium and Thanatephorus with Rhizoctonia anamorphs. Mycologia, 93, 1138–1150.
Grosch, R., Schneider, J. H. M., Peth, A., Waschke, A., Franken, P., Kofoet, A., & Jabaji-Hare, S. H. (2007). Development of a specific PCR assay for the detection of Rhizoctonia solani AG 1-IB using SCAR primers. Journal of Applied Microbiology, 102, 806–819.
Hide, G. A., & Firmager, J. P. (1990). Effects of an isolate of Rhizoctonia solani Kühn AG8 from diseased barley on the growth and infection of potatoes (Solanum tuberosum). Potato Research, 33, 229–234.
James, W. C., & McKenzie, A. R. (1972). The effect of tuberborne sclerotia of Rhizoctonia solani Kuhn on the potato crop. American Journal of Potato Research, 49, 296–301.
Jing, Y., Li, X. N., & Yu, J. F. (2012). Anastomosis groups of Rhizoctonia solani from cotton in northern China. Mycosystema, 31, 540–547 (In Chinese).
Kronland, W. C., & Stanghellini, M. E. (1988). Clean slide technique for the observation of anastomosis and nuclear condition of Rhizoctonia solani. Phytopathology, 78, 820–822.
Kuninaga, S., Natsuaki, T., Takeuchi, T., & Yokosawa, R. (1997). Sequence variation of the rDNA ITS regions within and between anastomosis groups in Rhizoctonia solani. Current Genetics, 32, 237–243.
Kuninaga, S., Nicoletti, R., Lahoz, E., & Naito, S. (2000). Ascription of Nt-isolates of Rhizoctonia solani to anastomosis group 2-1 (AG-2-1) on account of rDNA-ITS sequence similarity. Journal of Plant Pathology, 82, 61–64.
Kuramae, E. E., Buzeto, A. L., Ciampi, M. B., & Souza, N. L. (2003). Identification of Rhizoctonia solani AG 1-IB in lettuce, AG 4 HG-I in tomato and melon, and AG 4 HG-III in broccoli and spinach, in Brazil. European Journal of Plant Pathology, 109, 391–395.
Lehtonen, M. J., Ahvenniemi, P., Wilson, P. S., German-Kinnari, M., & Valkonen, J. P. T. (2008). Biological diversity of Rhizoctonia solani (AG-3) in a northern potato-cultivation environment in Finland. Plant Pathology, 57, 141–151.
Li, J., Xia, H. B., & Yu, J. F. (2011). The anastomosis groups of the corn sheath blight pathogen Rhizoctonia spp. in northeastern China. Mycosystema, 30, 392–399 (In Chinese).
Li, X. N., Xu, N. N., & Yu, J. F. (2014). Anastomosis groups of Rhizoctonia solani from black scurf of potato in northern China. Mycosystema, 33, 584–593 (In Chinese).
Maimaiti, Y., Guo, Q. Y., & Fulati, D. (2007). Anastomosis groups of Rhizoctonia solani Kuhn isolated from cotton and their vegetative compatibility groups in the south of Xinjiang. Journal of Xinjiang Agricultural University, 30, 10–13 (In Chinese).
Mathew, F. M., Lamppa, R. S., Chittem, K., Chang, Y. W., Botschner, M., Kinzer, K., & Markell, S. G. (2012). Characterization and pathogenicity of Rhizoctonia solani isolates affecting Pisum sativum in North Dakota. Plant Disease, 96, 666–672.
Ogoshi, A. (1996). Introduction—the genus Rhizoctonia. In B. Sneh, S. Jabaji-Hare, S. Neate, & G. Dijst (Eds.), Rhizoctonia species: Taxonomy, molecular biology, ecology, pathology and disease control (pp. 1–9). Dordrecht: Kluwer Academic Publishers.
Platt, H. W. (1989). Potato growth and tuber production as affected by inoculation of cut and whole seed with Rhizoctonia solani (AG 3) and the use of seed treatment fungicides. American Journal of Potato Research, 66, 365–378.
Platt, H. W., Canale, F., & Gimenez, G. (1993). Effects of tuber-borne inoculum of Rhizoctonia solani and fungicidal seed potato treatment of plant growth and Rhizoctonia disease in Canada and Uruguay. American Journal of Potato Research, 70, 553–559.
Rauf, C. A., Ahmad, I., & Ashraf, M. (2007). Anastomosis groups of Rhizoctonia solani Kuhn isolates from potato in Pakistan. Pakistan Journal of Botany, 39, 1335–1340.
Sharon, M., Kuninaga, S., Hyakumachi, M., Naito, S., & Sneh, B. (2008). Classification of Rhizoctonia spp. using rDNA-ITS sequence analysis supports the genetic basis of the classical anastomosis grouping. Mycoscience, 49, 93–114.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731–2739.
Tian, X. Y., Meng, M. L., Zhang, X. Y., Dong, R. L., & Hu, J. (2011). Identification of anastomosis groups of Rhizoctonia solani on potato plant. Chinese Potato, 25, 298–301 (In Chinese).
Toda, T., Mghalu, J. M., Priyatomojo, A., & Hyakumachi, M. (2004). Comparison of sequences for the internal transcribed spacer region in Rhizoctonia solani AG 1-ID and other subgroups of AG 1. Journal of General Plant Pathology, 70, 270–272.
Tsror, L. (2010). Biology, epidemiology and management of Rhizoctonia solani on potato. Journal of Phytopathology, 158, 649–658.
Virgen-Calleros, G., Olalde-Portugal, V., & Carling, D. E. (2000). Anastomosis groups of Rhizoctonia solani on potato in central Mexico and potential for biological and chemical control. American Journal of Potato Research, 77, 219–224.
Wang, Y., Yang, Z. H., Qin, Y. X., & Zhu, J. H. (2013). Analysis on anastomosis groups of Rhizoctonia solani AG2-1 and AG3 from potato (Solanum tuberosum). Journal of Agricultural Biotechnology, 21, 230–237 (In Chinese).
White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. J. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR protocols: A guide to methods and applications (pp. 315–322). New York: Academic.
Woodhall, J. W., Lees, A. K., Edwards, S. G., & Jenkinson, P. (2007). Characterization of Rhizoctonia solani from potato in Great Britain. Plant Pathology, 56, 286–295.
Woodhall, J. W., Lees, A. K., Edwards, S. G., & Jenkinson, P. (2008). Infection of potato by Rhizoctonia solani: effect of anastomosis group. Plant Pathology, 57, 897–905.
Woodhall, J. W., Belcher, A. R., Peters, J. C., Kirk, W. W., & Wharton, P. S. (2012a). First report of Rhizoctonia solani AG2-2IIIB infecting potato stems and stolons in the United States. Plant Disease, 96, 460.
Woodhall, J. W., Wharton, P. S., & Peters, J. C. (2012b). First report of Rhizoctonia solani AG4 HG-II infecting potato stems in Idaho. Plant Disease, 96, 1701.
Woodhall, J. W., Adams, I. P., Peters, J. C., Harper, G., & Boonham, N. (2013). A new quantitative real-time PCR assay for Rhizoctonia solani AG3-PT and the detection of AGs of Rhizoctonia solani associated with potato in soil and tuber samples in Great Britain. European Journal of Plant Pathology, 136, 273–280.
Yanar, Y., Yllmaz, G., Cesmeli, I., & Coskun, S. (2005). Characterization of Rhizoctonia solani isolates from potatoes in Turkey and screening potato cultivars for resistance to AG-3 isolates. Phytoparasitica, 33, 370–376.
Yang, Y. G., & Wu, X. H. (2012). First report of potato stem canker caused by Rhizoctonia solani AG-5 in China. Plant Disease, 96, 1579.
Yang, Y. G., & Wu, X. H. (2013). First report of potato stem canker caused by Rhizoctonia solani AG4 HGII in Gansu Province, China. Plant Disease, 97, 840.
Yang, J. H., Guo, Q. Y., & Ji, L. (2005). Study on anastomosis groups Rhizoctonia solani isolated from six Leguminaceous crops in Xinjiang. Xinjiang Agricultural Sciences, 42, 382–385 (In Chinese).
Yang, Y. G., Zhao, C., Guo, Z. J., & Wu, X. H. (2014). Anastomosis groups and pathogenicity of binucleate Rhizoctonia isolates associated with stem canker of potato in China. European Journal of Plant Pathology, 139, 535–544.
Zhao, S. F., Li, G. Y., & Du, Y. J. (2002). Beet root rot disease and prevention and control. Xinjiang Agricultural Sciences, 39, 150–152 (in Chinese).
Zhou, S. Y., Ji, Z., Zhao, C. D., Liu, Y., & Li, B. D. (2012a). Anastomosis grouping and genetic diversity of maize pathogen Rhizoctonia solani in Shandong. Mycosystema, 31, 31–39 (In Chinese).
Zhou, X. K., Gao, Z. G., Zhang, X. X., Zhang, S., Li, Z. Z., & Gao, J. X. (2012b). Construction of UP-PCR system and genetic diversity analysis of Rhizoctonia solani. Journal of Shenyang Agricultural University, 43, 33–38 (In Chinese).
Acknowledgments
We are grateful to N. Kondo (Graduate School of Agriculture, Hokkaido University, Sapporo, Japan) for kindly providing reference strains. This work was supported by Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1042). Mention of trade names or commercial products in this report is solely for the purpose of providing specific information and does not imply recommendation or endorsement.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table
(DOC 532 kb)
Rights and permissions
About this article
Cite this article
Yang, Y., Zhao, C., Guo, Z. et al. Anastomosis group and pathogenicity of Rhizoctonia solani associated with stem canker and black scurf of potato in China. Eur J Plant Pathol 143, 99–111 (2015). https://doi.org/10.1007/s10658-015-0668-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0668-x