Abstract
The frequency and incidence of fungi, as well as their interdependence, on rachis and grain of 14 wheat cultivars grown under 19 different agroecological conditions in Serbia, were studied. Out of the 23 identified fungal genera, a significantly higher number of species was isolated and identified from rachides (22) than from kernels of wheat (9). Fusarium and Alternaria species were the most frequent (up to 100 %) species on both, rachides and kernels, but the incidence of these fungi were higher on rachides than on kernels. The most frequent of the 14 Fusarium species were F. graminearum (96.8 % on both, rachides and kernels) and F. poae (93.8 % on rachides and 51.6 % on kernels). The frequency of F. verticillioides was significantly higher on rachides (64.5 %) than on kernels (19.4 %). A positive correlation (r = 0.5356 **) was established between the frequency of F. graminearum on rachides and on kernels. Furthermore, the frequency of Alternaria spp. was also statistically higher on rachides than on kernels, but the correlation was not statistically significant (r = 0.1729). The incidence of F. graminearum was negatively correlated with the incidence of Alternaria species in both, rachides (r = −0.3783 *) and kernels (r = −0.4863 **). These are the first data on the frequency and incidence of fungi on wheat rachides in Serbia, and they support the few data presented in the world literature. Results of this research could be useful for better understanding of pathways in a fungal infection and the improvement of wheat breeding for resistance, as well as, a proper application of fungicides in the wheat head protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, there are more studies and results on mycobiota of wheat kernels than on rachides. This is not in accordance with the importance of a rachis pathogen from the aspect of both resistance of small grains to Fusarium species (Jansen et al. 2005) and the biosynthesis of some fusariotoxins (Voigt et al. 2007). According to Mesterházy (2002), seven components (as stated by the author) or types (the term previously used) of resistance can be identified according to: pathogen invasion, pathogen distribution, grain infection, plant tolerance, toxin (deoxynivalenol) accumulation, late infection and death of a spike segment above the infection point. The component of the first type of resistance (I) is the infection distribution from the spikelet into the rachis, while the component of the second type of resistance (II) is the infection distribution from the rachis into other spikelets (Bai and Shaner 1996).
Genera of Fusarium and Alternaria are the dominant constituents of mycobiota isolated from wheat kernels worldwide (Trigo-Stockli et al. 1995; Krysinska-Traczyk et al. 2001; Bakutis et al. 2006; González et al. 2008). The other fungal species belonging to genera Absidia, Aspergillus, Bipolaris/Drechslera, Cladosporium, Cephalosporium, Chaetomium, Epicoccum, Oidiodendron, Penicillium, Rhizoctonia, Rhizopus, Stemphyllium and Trichothecium have not been always determined, and even if they have occurred they used to have a different status, because sometimes they have been dominant and sometimes they have occurred occasionally (Trigo-Stockli et al. 1995; Krysinska-Traczyk et al. 2001; Bakutis et al. 2006; Fakhrunnisa et al. 2006).
Many species of the genus Fusarium have been isolated worldwide from wheat kernels (Muthomi et al. 2008; González et al. 2008; Oerkea et al. 2010; Spanic et al. 2010). F. graminearum (Trigo-Stockli et al. 1995) or the combination of this species with F. poae (Ioos et al. 2004; Xu et al. 2005; González et al. 2008; Oerkea et al. 2010; Spanic et al. 2010) have been most often identified. Ioos et al. (2004) have clearly identified the increasing importance of F. graminearum and F. poae in the FEB complex in Europe.
In Serbia, the most important pathogenic species associated with wheat, barley and maize is F. graminearum. Furthermore, F. poae also is the most important pathogenic species for wheat and barely, while species of the section Liseola (F. verticillioides, F. subglutinans and F. proliferatum) are more important for maize and sorghum (Lević et al. 2009). The precipitation in Serbia during the wheat flowering time in recent decades has been of a local character and has had a significant effect on a variation of the intensity of the spike infestation with fusarioses and Fusarium head blight (FHB). In such a way, the grain yield reduction varied from 3.5 to 38.3 %, depending on agroecological conditions of the cultivation and resistance of wheat varieties (Lević et al. 2008a).
The aim of this study was to examine mycobiota, especially species of the genus Fusarium, and to determine whether there was interdependence in their occurrence on rachides and kernels of wheat.
Materials and methods
Field sampling
Thirty one field sites in 19 growing locations where 14 bread wheat varieties were grown were chosen mostly in the northern part of Serbia. Mature heads were hand collected from four sites each of 0.25 m2 from each field. The samples were packed in paper bags and immediately sent to the laboratory. The heads were manually threshed. Samples contained, on the average, 630 rachides and 725.1 g kernels. The kernel moisture ranged from 11.1 to 13.2 %. The samples were stored at 5 ± 1°C until analysed. Field sampling was done in cooperation with the Extension Services across Serbia.
Analysis of field samples
The rachis and kernel samples were rinsed under tap water for an hour and then were surface-sterilised by incubating for 1 min in 1 % (v/v) sodium hypochlorite solution and rinsed three times with distilled water. They were then dried, and one rachis or eight kernels were transferred onto 2 % water agar (WA) and incubated under ambient conditions, 25 ± 2°C and daylight. Six to seven days after incubation, samples were examined under a low magnification stereomicroscope. In order to determine species such as Chaetomium spp., Sordaria spp. and Thielaviopsis spp., incubated samples were once again examined after 5–7 days.
The mycelia of Fusarium species growing out of the rachides or kernels were transferred to potato dextrose agar (PDA) and carnation leaf agar (CLA) under sterile conditions to obtain pure cultures for the morphological identification of species according to Burgess et al. (1994). Subcultures on PDA were incubated in the dark at 25°C, while those on CLA were incubated at 12 h light (combined fluorescent and NUV light)/dark regime. All mentioned media were prepared according to procedures described by Burgess et al. (1994). Forty rachides and 128 kernels of each sample were analysed in four replications.
Statistical analysis
The prevalence of fungi was expressed as the percentage of infected samples (isolation frequency) and the infection levels as the percentage of infected kernels (isolation incidence).
The isolation frequency (F) and the incidence (I) of fungus were estimated as follows (Ghiasian et al. 2004): F (%) = [Number of samples in which a species occurred/Total number of samples] x 100; and I (%) = [Number of rachides/kernels in which a species occurred/Total number of rachides or kernels] x 100. The distribution of the infection levels in five frequency classes was performed according to Ioos et al. (2004).
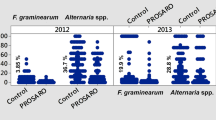
The interrelation of the fungal species on rachides and kernels were determined by the Pearson Product–moment Correlation. The median value of the incidence of F. graminearum and Alternaria spp. in rachides and kernels of different wheat varieties in various environments in Serbia was calculated using Microsoft Office Excel 2007.
Results
Fungal enumeration
Out of 31 surface-disinfected wheat samples, 30 and nine fungal genera were isolated and identified from rachides and from kernels, respectively (Table 1). Phoma spp. was the only species that was not isolated from the rachides, but was isolated from kernels.
Species of the genera Fusarium, Alternaria, Chaetomium and Bipolaris were determined in 100 %, 96.8 %, 90.3 % and 61.3 % rachis samples, respectively, with the incidence of 39.0 (±21.7), 87.0 (±8.8), 18.1 (±18.3) and 6.7 (±6.8) percentages, respectively (Table 1). The most often identified species of the genus Bipolaris was B. sorokiniana, and much less B. tetramera and others. The distribution of the infection levels of other fungal species was within the frequency range of >5 to ≤10 (Epicoccum spp., Aspergillus spp., Paecylomices spp., Sordaria spp., Sporotrix spp., Thielaviopsis spp., Microdochium spp., Mucor spp. and Papulospora spp.), and of >10 to ≤25 (Acremoniella atra, Gonatobotrys spp., Nigrospora oryzae, Rhizopus spp., Trichoderama spp., Cladosporium spp., Cephalosporium spp. and Penicillium spp.).
Out of fungi identified on wheat kernels, species of the genera Fusarium and Alternaria were the most frequent (100 %) with the incidence of 22.0 (±18.2) and 48.5 (±21.5) percentages, respectively (Table 1). One species was in the class within a frequency range of >5 % to ≤10 % (Phoma spp.), five species within the range of >10 % to ≤25 % (Cephalosporium spp., Bipolaris spp., Cladosporium spp., Epicoccum spp. and Nigrospora oryzae), while the frequency of Chaetomium spp. was over 25 %.
Twelve and 11 species of the genus Fusarium were identified on rachides and kernels, respectively (Table 2). On the average, Fusarium species were more frequent on rachides (mean 24.0 %) than on kernels (mean 15.2 %). F. chlamydosporum, F. oxysporum and F. solani were detected only on rachides, while F. arthrosporioides and F. avenaceum were determined only on kernels. The remaining Fusarium species were recorded on both, rachides and kernels.
F. graminearum was detected in 30 out of 31 samples (in 96.8 % cases) on both, rachides and kernels. The incidence of this species on rachides and kernels were almost the same (24.0 % vs. 22.0 %).
F. poae was the second most prevalent Fusarium species on both, rachides (93.5 %) and kernels (51.6 %). However, the difference in the incidence of this species on rachides and kernels was significant: 11.6 (±9.1) and 1.8 (± 1.4) percentages, respectively (Table 2). Furthermore, F. sporotrichiodes was more frequent on rachides (16.1 %) than on kernels (6.5 %).
A very high frequency of species of the section Liseola was determined on rachides. This was particularly attributed to F. verticillioides (64.5 %). F. proliferatum and F. subglutinans were detected on rachides in 12.9 % and 9.8 % of samples, respectively (Table 2). The most often isolated species of this section from grain was F. verticillioides (19.4 %), while F. proliferatum and F. subglutinans were detected in 9.7 % of samples. The incidence of these fungi on kernels amounted to 1.7 (±1.1), 3.1 (±4.0) and 1.4 (±0.7) percentages, respectively.
The distribution of the infection levels of Fusarium species on rachis samples was in the classes within a frequency range of >5 to ≤10 (F. chlamydosporum, F. equiseti, F. semitectum, F. solani, F. subglutinans and F. tricinctum), and >10 to ≤25 (F. oxysporum and F. sporotrichiodes), while on kernels they were distributed only in one frequency class (>5 to ≤10).
Fungal relationships
The positive correlation was determined for the frequency and incidence of fungi in both, rachides (r = 0.6191**) and kernels (r = 0.8524**). Furthermore, there was also a positive correlation (r = 0.8074**) between the incidences of fungi on rachides and kernels, as well as, between the incidences of fungi in all samples (r = 0.9584**).
The isolation incidence of F. graminearum and Alternaria spp. in rachides and kernels of different wheat varieties in diverse environments in Serbia is presented in Table 3. The positive correlation was established between frequencies of F. graminearum on rachides and kernels (r = 0.5356**), while this correlation, although positive, was not statistically significant for Alternaria spp. (r = 0.1729). The correlation between frequencies of F. graminearum and Alternaria spp. was negative on both, rachides (r = −0.3783*) and kernels (r = −0.4863**).
Discussion
Generally, greater abundance of different fungal species was determined in rachis samples than in kernel samples of the 14 wheat cultivars grown under diverse agroecological conditions (19 locations). However, differences between rachis and kernel mycobiota related to the frequency and incidence of certain species of the genus Fusarium are much smaller.
The positive correlation established between infections of rachides and kernels caused by F. graminearum, has a scientific explanation. During the plant infection, F. graminearum spreads by systemic growth through the rachis from one spike to another (Ribichich et al. 2000; Wanjiru et al. 2002). Histopathological studies showed that the colonisation of wheat heads by F. graminearum occurred in two ways, either by a horizontal or by a vertical path (Ribichich et al. 2000). In the horizontal path, the fungus invaded anthers and bracts of contiguous florets in the first spikelet invaded, then moved through the rachis and the rachilla to the contiguous spikelet. In the vertical path, the fungus moved through vascular bundles and parenchyma to invade spikelets above and below the originally infected spikelet. At later stages of infection, the pathogen switched from predominately vertical to lateral growth and accumulated below the surface of the rachis (Brown et al. 2010). Delayed hyphal colonisation of the vascular bundles in the rachis was observed in the type II resistant genotypes (Foroud and Eudes 2009). Transmission electron microscope studies showed that at 5 days after inoculation, the hyphae had reached the rachis from the infected ovary, lemma and palea (Wanjiru et al. 2002). The fungus extended inter- and intracellularly in the cortical tissue and vascular bundles of the rachis.
Jansen et al. (2005) found that hyphae of F. graminearum reaching the rachis proceeded to apically located developing kernels. In the absence of trichothecenes, the fungus is blocked by the development of heavy cell wall thickenings in the rachis node of wheat, a defence inhibited by the mycotoxin. In barley, hyphae of both wild-type and trichothecene knockout mutant are inhibited at the rachis node and rachilla, limiting infection of adjacent florets through the phloem and along the surface of the rachis.
According to the literature more studies have been conducted on kernel mycobiota than on rachis mycobiota. Brandfass and Karlovsky (2006) have stated that the only Fusarium species found apart from F. graminearum and F. culmorum was F. poae, occurring just in three instances in rachides, which also contained F. graminearum or F. culmorum, and in four instances in rachides where none of the other two Fusarium spp. were detected. Other fungal species occasionally recovered from the rachides were Alternaria spp., Rhizoctonia cerealis and Epicoccum spp. In comparison with these results, our results show that a significantly higher number of fungi (22 fungal genera) were determined on wheat rachides and that the genera Fusarium, Alternaria, Chaetomium and Bipolaris were dominant. Out of 11 species of the genus Fusarium, F. graminearum (96.8 %) and F. poae (93.5 %) had the highest incidence, while F. culmorum was not detected.
It should be mentioned that the frequency of the Fusarium species of the section Liseola (F. verticillioides, F. proliferatum and F. subglutinans) was relatively high on rachides and kernels of wheat. Recent literature data has been indicating the importance of these species in the aetiology of diseases of wheat kernels and contamination by mycotoxins. Desjardins et al. (2006) characterised nine F. proliferatum strains from wheat from Nepal for ability to cause wheat kernel black point under greenhouse conditions, and for fumonisin contamination of infected kernels. According to Stanković et al. (2012) a large number of stored wheat kernel samples (53.1–64.0 %) contained FB1 at levels higher than 1,000 mg kg−1.
Our studies confirmed that in Serbia, as well as, in Europe (Ioos et al. 2004), F. graminearum and F. poae were the most frequent species in the FEB complex. Our previous studies show that under agroecological conditions of Serbia, F. graminearum mainly developed along the rachides (Lević et al. 2008a). These points out that cultivated varieties of wheat are not the type II resistant or resistant to spreading of pathogens on spikes. On the other hand, F. poae was the most often developed on the rachis tips, and although it was very frequent it did not express pathogenic properties against wheat seedlings (Lević et al. 2008b).
Unlike F. graminearum and F. poae, other species of the genus Fusarium did not spread beyond kernels (F. arthrosporioides and F. avenaceum) or rachides (F. chlamydosporum, F. oxysporum and F. solani). These results point out to smaller effects of these Fusarium species on the development of FHB.
Different incidences of F. graminearum on wheat kernels were established in dependence on the location in Serbia in which the varieties were grown. For example, the incidence of this species in kernels of the variety cv-10 varied from 0 % to 71.9 % over seven locations. These differences can be explained by different impacts of agrometeorological conditions on the development of spike infection, as well as, by the application of fungicides. We have previously determined that precipitation during the stage of wheat flowering varied over locations and significantly affected the variation of the incidence of Fusarium head blight over the environments in Serbia (Lević et al. 2008a). For the particular years, the climatic conditions reported by Ioos et al. (2004) were quite favourable for FHB development with high levels of moisture at the cereal flowering stage.
Our results are in accordance with results obtained by other researchers who have studied FHB and determined a great variation in the species composition and in dominant species in the fungal complex that caused FHB. This variation related not only to their year-to-year variation (Ioos et al. 2004) or variations in previous decade (Tomczak et al. 2002), but also to region-to-region variation (Xu et al. 2005) or zone-to-zone variation (Muthomi et al. 2008), and also to their field-to-field variation or even within-field variation (Birzele et al. 2002; Oerkea et al. 2010). Results obtained in eastern Croatia, located eastwards from the northern part of Serbia, differed in frequencies of certain Fusarium species in comparison with our results, although the spatial distance between the two countries is small.
The results of Kosaik et al. (2004) indicated a negative interaction between F. graminearum and Alternaria spp. in Norwegian grains, as well as, between F. graminearum and other Fusarium spp. Our results, related to a negative interaction between F. graminearum and Alternaria spp. (r = −0.4863**), are in accordance with results gained by these authors. Moreover, we have also determined a negative interaction between these two species in rachides (r = −0.3783*).
In conclusion, the majority of rachis samples contained more notable quantities of fungal species than kernel samples of wheat collected from 19 locations in Serbia characterised with different agroecological conditions. But, the number of Fusarium species was approximately the same on rachides and kernels. The high frequency and incidence of F. graminearum, F. poae and Alternaria species in wheat is a matter of concern for wheat production.
References
Bai, G.-H., & Shaner, G. (1996). Variation in Fusarium graminearum and cultivar resistance to wheat scab. Plant Disease, 80, 975–979.
Bakutis, B., Baliukonienė, V., & Lugauskas, A. (2006). Factors predetermining the abundance of fungi and mycotoxins in grain from organic and conventional farms. Ekologija, 3, 122–127.
Birzele, B., Meier, A., Hindorf, H., Krämer, J., & Dehne, H.-W. (2002). Epidemiology of Fusarium infection and deoxynivalenol content in winter wheat in the Rhineland, Germany. European Journal of Plant Pathology, 108, 667–673.
Brandfass, C., & Karlovsky, P. (2006). Simultaneous detection of Fusarium culmorum and F. graminearum in plant material by duplex PCR with melting curve analysis. BMC Microbiology, 6, 4. doi:10.1186/1471-2180-6-4.
Brown, N. A., Urban, M., van De Meene, A. M. L., & Hammond-Kosack, K. E. (2010). The infection biology of Fusarium graminearum: Defining the pathways of spikelet to spikelet colonisation in wheat ears. Fungal Biology, 114, 555–571.
Burgess, L. W., Summerell, B. A., Bullock, S., Gott, K. P., & Backhouse, D. (1994). Laboratory Manual for Fusarium Research. Sydney: University of Sydney an Royal Botanic Gardens. 133 pp.
Desjardins, A. E., Busman, M., Proctor, R., & Stessman, R. J. (2006). Wheat kernel black point and fumonisin contamination by Fusarium proliferatum. Food Additives and Contaminants, 24, 1131–1137.
Fakhrunnisa, Hashmi, M. H., & Ghaffar, A. (2006). Seed-borne mycoflora of wheat, sorghum and barley. Pakistan Journal of Botany, 38, 185–192.
Foroud, N. A., & Eudes, F. (2009). Trichothecenes in cereal grains. International Journal of Molecular Sciences, 10, 147–173.
Ghiasian, S.-A., Kork-Bacheh, P., Rezayat, S. M., Maghsood, A. H., & Teherkhan, H. (2004). Mycoflora of Iranian maize harvested in the main production aerias in 2000. Mycopathologia, 158, 113–121.
González, H. H. L., Moltó, G. A., Pacin, A., Resnik, S. L., Zelaya, M. J., Masana, M., & Martínez, E. J. (2008). Trichothecenes and mycoflora in wheat harvested in nine locations in Buenos Aires Province, Argentina. Mycopathologia, 65, 105–114.
Ioos, R., Belhadj, A., & Menez, M. (2004). Occurrence and distribution of Microdochium nivale and Fusarium species isolated from barley, durum and soft wheat grains in France from 2000 to 2002. Mycopathologia, 158, 351–362.
Jansen, C., von Wettstein, D., Schäfer, W., Kogel, K.-H., Felk, A., & Maier, F. J. (2005). Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthese gene disrupted Fusarium graminearum. Proceedings of the National Academy of Sciences of the United States of America, 102, 16892–16897.
Kosaik, B., Torp, M., Skjerve, E., & Anderson, B. (2004). Alternaria and Fusarium in Norwegian grains of reduced quality – a matched pair sample study. International Journal of Food Microbiology, 93, 51–62.
Krysinska-Traczyk, E., Perkowski, J., & Dutkiewicz, J. (2001). Levels of fungi and mycotoxins in samples of grain and grain dust collected on farms in eastern Poland. Annals of Agricultural and Environmental Medicine, 8, 269–274.
Lević, J., Stanković, S., Krnjaja, V., Kovačević, T., Tančić, S., & Bočarov-Stančić, S. (2008a). Fusarium head blight and grain yield losses of Serbian wheat. Cereal Research Communications, 26(Suppl. B), 513–514.
Lević, J., Stanković, S., Krnjaja, V., Kovačević, T., Tančić, S., & Bočarov-Stančić, S. (2008b). Pathogenicity and phytotoxicity of Fusarium langsethiae on wheat seedlings. Cereal Research Communications, 26(Suppl. B), 515–516.
Lević, T. J., Stanković, S. Ž., Krnjaja, V. S., & Bočarov-Stančić, S. A. (2009). Fusarium species: The occurrence and the importance in agriculture of Serbia. Matica Srpska Proceedings for Nature Sciences, 116, 33–48.
Mesterházy, A. (2002). Theory and practice of the breeding for Fusarium head blight resistance in wheat. Journal of Applied Genetics, 43A, 289–302.
Muthomi, J. W., Ndung’u, J. K., Gathumbi, J. K., Mutitu, E. W., & Wagacha, J. M. (2008). The occurrence of Fusarium species and mycotoxins in Kenyan wheat. Crop Protection, 27, 1215–1219.
Oerkea, E.-C., Meier, A., Dehne, H.-W., Sulyok, M., Krska, R., & Steiner, U. (2010). Spatial variability of Fusarium head blight pathogens and associated mycotoxins in wheat crops. Plant Pathology, 59, 671–682.
Ribichich, K. F., Lopez, S. E., & Vegetti, A. C. (2000). Histopathological spikelet changes produced by Fusarium graminearum in susceptible and resistant wheat cultivars. Plant Disease, 84, 794–802.
Spanic, V., Lemmens, M., & Drezner, G. (2010). Morphological and molecular identification of Fusarium species associated with head blight on wheat in East Croatia. European Journal of Plant Pathology, 128, 511–516.
Stanković, S., Lević, J., Ivanović, D., Krnjaja, V., Stanković, G., & Tančć, S. (2012). Fumonisin B1 and its co-occurrence with other fusariotoxins in naturally-contaminated wheat grain. Food Control, 23, 384–388.
Tomczak, M., Wiśśniewska, H., Stępién, Ł., Kostecki, M., Chełkowski, J., & Goliński, P. (2002). Deoxynivalenol, nivalenol and moniliformin in wheat samples with head blight (scab) symptoms in Poland (1998–2000). European Journal of Plant Pathology, 108, 625–630.
Trigo-Stockli, D. M., Curran, S. P., & Pedersen, J. R. (1995). Distribution and occurrence of mycotoxins in 1993 Kansas wheat. Cereal Chemistry, 72, 470–474.
Voigt, C. A., von Scheidt, B., Gácser, A., Kassner, H., Lieberei, R., Schäfer, W., & Siegfried, S. (2007). Enhanced mycotoxin production of a lipase-deficient Fusarium graminearum mutant correlates to toxin-related gene expression. European Journal of Plant Pathology, 117, 1–12.
Wanjiru, W. M., Zhensheng, K., & Buchenauer, H. (2002). Importance of cell wall degrading enzymes produced by Fusarium graminearum during infection of wheat heads. European Journal of Plant Pathology, 108, 803–810.
Xu, X.-M., Parry, D. W., Nicholson, P., Thomsett, M. A., Simpson, D., Edwards, S. G., Cooke, B. M., Doohan, F. M., Brennan, J. M., Moretti, A., Tocco, G., Mule, G., Hornok, L., Giczey, G., & Tatnell, J. (2005). Predominance and association of pathogenic fungi causing Fusarium ear blight in wheat in four European countries. European Journal of Plant Pathology, 112, 143–154.
Acknowledgments
The study was a part of investigations realised within the scope of the Project No. TR-31023 financially supported by the Ministry of Science and Technological Development of Republic of Serbia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lević, J., Stanković, S., Krnjaja, V. et al. Relationships of mycobiota on rachides and kernels of wheat. Eur J Plant Pathol 134, 249–256 (2012). https://doi.org/10.1007/s10658-012-9982-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-012-9982-8