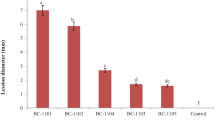
Abstract
In Botrytis cinerea, multidrug resistant (MDR) strains collected in French and German vineyards were tested in vitro, at the germ-tube elongation stage, towards a wide range of fungicides. Whatever the MDR phenotype, resistance was recorded to anilinopyrimidines, diethofencarb, iprodione, fludioxonil, tolnaftate and several respiratory inhibitors (e.g., penthiopyrad, pyraclostrobin). In MDR1 strains, overproducing the ABC transporter BcatrB, resistance extended to carbendazim and the uncouplers fluazinam and malonoben. In MDR2 strains, overproducing the MFS transporter BcmfsM2, resistance extended to cycloheximide, fenhexamid and sterol 14α-demethylation inhibitors (DMIs). MDR3 strains combined the overexpression of both transporters and exhibited the widest spectrum of cross resistance and the highest resistance levels. The four transport modulators, amitriptyline, chlorpromazine, diethylstilbestrol, and verapamil, known to affect some ABC transporters, were tested in B. cinerea. In our experimental conditions, the activity of several fungicides was only enhanced by verapamil. Interestingly, synergism was only recorded in MDR2 and/or MDR3 isolates treated with tolnaftate, fenhexamid, fludioxonil or pyrimethanil, suggesting that verapamil may inhibit the MFS transporter BcmfsM2. This is the first report indicating that a known modulator of ABC transporters could also block MFS transporters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Botrytis cinerea Pers ex Fr, the anamorph of Botryotinia fuckeliana (de Barry) Whetzel is a ubiquitous fungus that causes grey mould on a wide range of crop plants, including grapevine (Elmer and Michailides 2004). Recent studies in French vineyards have shown that this disease is caused by a complex of two cryptic species living in sympatry: B. cinerea (the most abundant species at harvest) and B. pseudocinerea (which is well established before flowering) (Walker et al. 2011). Furthermore, B. pseudocinerea, previously referred to as HydR1 strains, is naturally more tolerant to fenhexamid and more susceptible to fenpropidin and fenpropimorph than B. cinerea (Leroux et al. 1999).
Chemical control remains the principal means of reducing the incidence of grey mould on major crops and the available botryticides can be classified according to their modes of action (Leroux 2004). They affect microtubules (i.e., benzimidazoles, thiophanates, phenylcarbamates), osmoregulation (i.e., dicarboximides, phenylpyrroles), methionine biosynthesis (i.e., anilinopyrimidines), sterol biosynthesis (i.e., 14α-demethylation inhibitors (DMIs), hydroxyanilides), respiration (i.e., carboxamides or succinate dehydrogenase inhibitors (SDHIs), quinone outside inhibitors (QoIs), dinitroaniline uncouplers) or have multisite activity (i.e., dithiocarbamates, phthalimides, phthalonitriles, sulphamides,) (Tables 1 and 2; FRAC Code list http://www.frac.info/frac/index.htm).
Despite a large number of compounds active against grey mould, strains resistant to most of them have emerged in many countries (Leroux 2004). This acquired resistance principally concerns B. cinerea and is generally determined by mutations in the genes encoding the target site protein of the fungicide. They often lead to “specific resistance” to a single molecule or class of fungicides, and generally result in moderate to high levels of resistance. Such a phenomenon was firstly identified for antimicrotubule fungicides but it now also concerns dicarboximides, SDHIs, strobilurins (i.e., the main group of QoIs), the hydroxyanilide fenhexamid and probably anilinopyrimidines (Fillinger et al. 2008; Leroux et al. 2002, 2010). Besides specific resistances, multidrug resistant (MDR) strains have been detected in French and German vineyards. They exhibit low resistance levels towards several classes of botryticides and are mediated by a single gene (Chapeland et al. 1999; Kretschmer et al. 2009).
One well documented mechanism of MDR involves an increase in drug efflux activity due to the constitutive overexpression of ATP-binding cassette (ABC) or major facilitator superfamily (MFS) transporters (Stergiopoulos et al. 2002). MDR plays a major role in the simultaneous loss of activity of unrelated anti-cancer drugs (Gottesman 2002). Moreover, clinical isolates of the human pathogenic yeast Candida albicans with MDR phenotypes have been selected by prolonged use of antimycotic DMIs (e.g., fluconazole, itraconazole). These isolates constitutively overproduce the ABC transporters CaCDR1 and CaCDR2 or the MFS transporter CaMDR1, due to mutations in the genes encoding the transcription factors CaTac1 and CaMrr1 (Morschhäuser 2010). In phytopathogenic fungi, B. cinerea provides the best documented case of MDR. Three MDR phenotypes, defined on the basis of cross-resistance patterns, have been characterised in B. cinerea populations. MDR1 strains (previously known as AniR2 strains), displaying resistance to anilinopyrimidines and phenylpyrroles, harbour activating mutations in the gene encoding the transcription factor BcMrr1, which controls the transcription of the gene encoding the ABC transporter BcatrB. In MDR2 strains (previously known as AniR3 strains), simultaneous resistance to anilinopyrimidines and fenhexamid results from overproduction of the MFS transporter BcmfsM2, due to the insertion of a retroelement-derived sequence into the BcmfsM2 promoter. Finally, MDR3 strains, which display resistance to many botryticides are genetic recombinants between MDR1 and MDR2 strains (Chapeland et al. 1999; Kretschmer et al. 2009).
Compounds able to modulate the activity of ABC or MFS transporters can reverse MDR, as they inhibit efflux of toxicants from cells. Such compounds have been described in medical publications as “modulators”, “reverters”, “inhibitors” or “chemosensitisers” for the treatment of MDR due to ABC transporters in human cancer cells (Robert and Jarry 2003). They include antipsychotic drugs (e.g., amitriptyline, chlorpromazine, thioridazine), coronary vasodilatators (e.g., nifedipine, verapamil), immune-suppressive agents (e.g., cyclosporine A, tacrolimus), steroids or hormone analogues (e.g., progesterone, diethylstilbestrol), and various plant metabolites (e.g., curcumin, flavavone, quercetin)(Roohparvar et al. 2007b). Several of these modulators have been shown to increase the toxicity of DMIs in fungi, especially in strains overexpressing ABC transporters (Hayashi et al. 2003; Schuetzer-Muehlbauer et al. 2003; Sharma et al. 2009).
In this study, we aimed to establish the pattern of cross-resistance to a wide range of fungicides for the various MDR phenotypes recorded in field strains of B. cinerea collected in French and German vineyards. We also studied the interaction of the four ABC transporter modulators, amitriptyline, chlorpromazine, diethylstilbestrol and verapamil, with several fungicides.
Materials and methods
Fungal strains
The field strains were isolated from diseased grape berries collected, from vineyards in the Champagne (France) and Palatinate (Germany) regions. This collection was previously described (Kretschmer et al. 2009; Leroux et al. 2010). They were maintained on MYA medium (20 g malt, 5 g yeast extract, 12.5 g agar in 1 l of deionised water) and sporulation occurred after 1 week of culture under white light, at 19 °C.
Fungicides and chemicals
The fungicides tested were of technical grade and were kindly donated by the manufacturers. Amitriptyline, chlorpromazine, cycloheximide, diethylstilbestrol, salicylhydroxamic acid (SHAM) and verapamil were purchased from Sigma Aldrich. Stock solutions of these compounds in ethanol were stored at 4 °C in the dark. For dilution series, fungicide stock solutions were adjusted such that the final concentration of solvent in the culture medium was 0.5 % (vol/vol).
Fungicide sensitivity tests
We assessed the effect of fungicides on germ tube elongation in B. cinerea, as previously described (Leroux et al. 1999, 2010). For all antifungal compounds other than SDHIs, the medium contained 10 g glucose, 2 g K2HPO4, 2 g KH2PO4 and 12.5 g agar in 1 l of deionised water. For SDHIs, the glucose was replaced by 4 g of the dibasic hexahydrate of sodium succinate. Moreover, as B. cinerea has an active alternative oxidase, we had to add SHAM (0.5 mM) for the testing of strobilurins (Leroux et al. 2010). Media supplemented with various concentrations of fungicide were poured into 5.5 cm-diameter Petri dishes. We then dispensed 0.3 ml of a conidial suspension (200,000 conidia ml−1) over the surface of the agar with a pipette. Plates were incubated for 24 h, at 19 °C, in the dark, and germ tube lengths (25–50 germinated conidia for each treatment) were estimated under a microscope, with a micrometer.
For each fungicide, we tested 5 to 10 concentrations, according to a geometric progression, with dose increments of ×2 or ×2.5. For each strain/fungicide combination, the concentration causing a 50 % decrease in germ tube elongation (EC50) was identified by linear regression analysis of germ tube length (as percentage of control values) against log10 fungicide concentrations. We calculated the mean EC50 value for each phenotype tested (four to six strains) and a mean resistance factor (RF) was then estimated as the EC50 of the resistant phenotype/EC50 of the sensitive phenotype (wild type). Phenotypes were classified as resistant if they had a resistance factor greater than 2 (Leroux et al. 1999).
Interactions between fungicides and drug transporter modulators
We assessed germ-tube elongation in B. cinerea treated with 5 mg l−1 amitryptiline, 5 mg l−1 chlorpromazine, 2 mg l−1 diethylstilbestrol or 30 mg l−1 verapamil. The concentrations of the putative modulators were chosen so as to limit their intrinsic toxicity (see Table 2). For each of the fungicides tested, we chose at least two concentrations close to the EC50 values for wild-type and MDR strains. The bioassays were carried out as described for the fungicide sensitivity tests and mean germ tube length was determined in each set of conditions.
Interactions between fungicides and modulators were analysed as previously described (Colby 1967). XF and XM represent growth as a percentage of the control with fungicide F at concentration p and modulator M at concentration q, respectively. The expected growth (Exp.) was calculated as a percentage of the control for the mixture of F + M according to the formula \( \mathrm{Exp}.={{{{{\mathrm{X}}_{\mathrm{F}}}.{{\mathrm{Y}}_{\mathrm{M}}}}} \left/ {100 } \right.} \). The observed response (Obs.) was obtained experimentally by estimating growth in the presence of the mixture F + M. The level of interaction was then calculated as the ratio \( \mathrm{iR}={{{\mathrm{Exp}.\,\mathrm{growth}}} \left/ {{\mathrm{Obs}.\,\mathrm{growth}}} \right.} \). Additive interactions occur if iR = 1; synergy occurs if iR > 1 and antagonism occurs if iR < 1. Owing to the biological variability of the test systems, synergy was considered significant if iR ≥ 1.5, and antagonism was considered significant if iR ≤ 0.5; additive interactions were considered to occur when 0.5 < iR < 1.5 (Stergiopoulos and De Waard 2002).
Results
Different patterns of cross-resistance between MDR1 and MDR2 phenotypes
Several classes of fungicides affecting respiration have been tested (Table 1). The highest activity towards wild-type strains (EC50 values below 0.2 mg l−1) was recorded with strobilurins, novel SDHIs with a wide spectrum of activity (i.e., boscalid, isopyrazam, penthiopyrad) (Avenot and Michailides 2010), compounds affecting oxidative phosphorylation (i.e., fentin acetate, fluazinam, malonoben) and a few multisite toxicants (i.e., captafol, chlorothalonil, thiram, tolylfluanid). On the other hand, the less effective molecules (EC50 values greater than 0.4 mg l−1) were SDHIs from the first generation, used only against Basidiomycetes (i.e., benodanil, carboxin, furcarbanil, thifluzamide) and several multisite toxicants (i.e., anilazine, captan, dichlone, dithianon, folpet). Moreover, it was possible to identify four main profiles of multidrug resistance, in terms of biological effects:
-
A
Compounds with similar levels of activity against wild-type, MDR1 and MDR2 strains: multisite toxicants other than anilazine, SDHIs used only against Basidiomycetes (i.e., benodanil, carboxin, furcarbanil, thifluzamide), azoxystrobin and the inhibitor of oxidative phosphorylation fentin acetate.
-
B
Compounds for which resistance is observed only in MDR1 strains: dimoxystrobin and the two tested uncouplers fluazinam and malonoben.
-
C
Compounds for which resistance is observed only in MDR2 strains: the novel SDHI boscalid.
-
D
Compounds for which resistance is observed in both MDR1 and MDR2 strains: the multisite toxicant anilazine, the novel SDHIs of the pyrazole carboxamide group (i.e., isopyrazam, penthiopyrad), pyraclostrobin and trifloxystrobin.
Among the inhibitors of sterol biosynthesis, terbinafine, prochloraz,fenpropimorph, fenpropidin, fumecyclox and fenhexamid were highly effective in wild type strains (EC50 values below 0.1 mg l−1). On the opposite, the triazolinethione prothioconazole was weakly fungitoxic (EC50 values of 2.5 mg l−1) (Table 2). Towards multidrug resistant strains, high resistance factors (RFs ≥ 100) were recorded in both MDR1 and MDR2 phenotypes only for the thiolcarbamate tolnaftate. The allylamine terbinafine, which, like tolnaftate, inhibits squalene epoxidase, was equally effective in wild-type and MDR1 strains, whereas slight resistance was recorded in MDR2 strains. For fenhexamid and the DMIs tested (i.e., prochloraz, tebuconazole, prothioconazole), resistance was clearly observed in MDR2 strains. Finally, the MDR1 and MDR2 strains remained susceptible to the sterol Δ14 reductase inhibitors (i.e., fenpropidin, fenpropimorph, furmecyclox) (Table 2).
Within antimicrotubule fungicides, the benzimidazole derivatives carbendazim and thiabendazole were highly toxic towards strains exhibiting a wild-type β-tubulin (EC50 values below 0.1 mg l−1). Among multidrug resistant strains, resistance was only recorded for carbendazim in MDR1 ones (Table 2). As regards to the phenylcarbamate diethofencarb, its highest fungitoxicity occurred in strains with the β-tubulin amino-acid substitution E198A (Leroux et al. 1999). Moreover, MDR1 and MDR2 strains, exhibiting this altered β-tubulin, showed reduced sensitivity to diethofencarb (Table 2).
The anilinopyrimidines cyprodinil, mepanipyrim, pyrimethanil, as well as the phenylpyrrole fludioxonil were highly toxic to wild-type strains (EC50 values below 0.1 mg l−1) and resistance occurred in both MDR1 and MDR2 strains. The dicarboximides iprodione and procymidone, whose intrinsic fungitoxic activity was weak towards wild-type strains (EC50 values close to 1 mg l−1), exhibited also reduced sensitivity towards both MDR phenotypes. However the highest resistance levels were observed in MDR1 strains towards cyprodinil and fludioxonil, alone or in mixture (Table 2). Toxicity of the antibiotic cycloheximide and the modulators tested (i.e., amitryptiline, chlorpromazine, diethylstilbestrol, verapamil) was moderate towards wild-type strains (EC50 values above 3 mg l−1). Resistance was recorded only with cycloheximide in MDR2 strains (Table 2).
Overall, with the exception of tolnaftate, resistance factors did not exceed 15 in the MDR1 and MDR2 phenotypes. RF values greater than 10 were recorded for fludioxonil and cyprodinil in MDR1 strains and for penthiopyrad and prochloraz in MDR2 strains (Tables 1 and 2).
MDR3 field strains have the widest spectrum of cross-resistance
MDR3 field strains of B. cinerea were resistant towards more toxicants in comparison to MDR1 and MDR2 phenotypes (Tables 1 and 2). However, dichlone, dithianon, folpet, thiram (multisite toxicants), benodanil, furcarbanil (SDHIs), fentin acetate (inhibitor of oxidative phosphorylation), fenpropidin, fenpropimorph, furmecyclox (inhibitors of sterol Δ14-reductase), thiabendazole (benzimidazole) and the four putative modulators exhibited similar activity in MDR1, MDR2, MDR3 and wild-type strains (Tables 1 and 2).
For most tested compounds, resistance factors in MDR3 strains were greater than those in MDR1 and MDR2 strains (Tables 1 and 2). Generally, the observed RF [MDR3] values and the expected ones, calculated as RF [MDR1] x RF [MDR2] (Tables 1 and 2) were similar (ratio between 0.5 and 2), suggesting an additive effect of the Bc AtrB and Bc mfsM2 transporters. For penthiopyrad, diethofencarb and mepanipyrim, the observed RF [MDR3] values were lower than the expected ones (ratio below 0.5). Conversely, for several substances affecting respiration, including thifluzamide, azoxystrobin, trifloxystrobin and malonoben, the observed RF [MDR3] values were higher than the expected ones (ratio above 2.0) (Tables 1 and 2). Finally, tolnaftate was the only compound tested for which an RF greater than 30 was obtained with MDR3 strains (Table 2).
Interaction of putative MDR modulators with fungicides in various strains of Botrytis sp
As amitriptyline, chlorpromazine and diethylstilbestrol inhibited germ-tube elongation in B. cinerea, they were tested at concentrations below their respective EC50 values (Tables 2 and 3). When combined with fenhexamid, fludioxonil, iprodione, tolnaftate and pyrimethanil (this anilinopyrimidine was tested only with chlorpromazine), interactions were either additive or antagonistic in wild-type and MDR strains of B. cinerea. The most pronounced antagonism was recorded with diethylstilbestrol in B. pseudocinerea (Table 3).
Unlike amitriptyline, chlorpromazine and diethylstilbestrol, verapamil had only a mild effect on germ tube elongation in B. cinerea, allowing a 30 mg l−1 use in tests. Moreover, verapamil had no antagonistic effect with the strains and fungicides tested. In wild-type and MDR1 strains of B. cinerea, verapamil had additive effects with all the fungicides tested, except for prochloraz and tebuconazole. For these two DMIs, significant synergy was observed with verapamil. In MDR2 strains, verapamil also had synergic effects with tolnaftate, fenhexamid, fludioxonil and pyrimethanil (Table 4). The range of synergy observed in MDR3 strains was narrower than that for MDR2 strains, because verapamil synergistic effect was observed in MDR3 strains only with fenhexamid (Table 4). Furthermore, the EC50 values of tolnaftate alone were greater than 10 mg l−1 in all MDR phenotypes. Similar values were recorded in MDR1 and MDR3 strains when verapamil was added, but not in MDR2 strains for which the mean EC50 value was only 1.8 mg l−1 (data not shown). And last, in tests involving fenpropimorph, verapamil displayed synergy only in B. pseudocinerea (Table 4).
Discussion
Characteristics of transporters leading to MDR in B. cinerea
In B. cinerea, three ABC transporters (i.e., BcatrB, BcatrD, BcatrK) and two MFS transporters (i.e., Bcmfs1, BcmfsM2) have been shown to be determinants of MDR in laboratory or field mutants (Kretschmer et al. 2009; Stergiopoulos et al. 2002). In MDR1 and MDR2 field strains, resistance to botryticides is determined by the overproduction of BcatrB (levels between 50 to 150 times those of wild type strains) and of BcmfsM2 (levels about 600 times those of wild-type strains), respectively (Kretschmer et al. 2009). Based on the phenotypic characterization of these MDR1 and MDR2 strains (Tables 1 and 2);(Kretschmer et al. 2009; Leroux et al. 1999, 2010), the following antifungal compounds can be considered as putative substrates for:
-
mainly BcatrB: antimycin A, dimoxystrobin, fluazinam, malonoben, carbendazim, phenylpyrroles (i.e., fenpiclonil, fludioxonil)
-
mainly BcmfsM2: boscalid, naftifine, terbinafine, DMI fungicides, fenhexamid and cycloheximide.
-
both transporters: anilazine, isopyrazam, penthiopyrad, pyraclostrobin, trifloxystrobin, tolnaftate, diethofencarb, dicarboximides (i.e., iprodione, procymidone, vinclozolin) and anilinopyrimidines (i.e., cyprodinil, mepanipyrim, pyrimethanil).
Interestingly, the fungicides inhibiting the mitochondrial complexes II or III behave differently according to their structures (Table 1). Among SDHIs, also classified as carboxamides, carboxin and the oldest molecules (i.e., benodanil, furcarbanil, thifluzamide) are effective only against basidiomycetes, whereas the new ones (i.e., isopyrazam, penthiopyrad, boscalid) exhibit a wider spectrum of activity (Avenot and Michailides 2010). Our results indicate that in strains overproducing BcatrB or BcmfsM2, resistance is only recorded with the more recent carboxamides which harbour a lipophilic substituent at the ortho position. A similar behaviour has also been observed with carboxin analogues when such a substitution occurs at the meta position but not at the para position (Leroux et al. 2010). These observations suggest that structural features in addition to lipophilicity are involved in the interaction between carboxamides and the efflux pumps involved in MDR. As for strobilurins, the tested molecules belong to the four different chemical sub-groups: azoxystrobin (methoxy-acrylate), dimoxystrobin (oximino-acetamide), pyraclostrobin (methoxy-carbamate) and trifloxystrobin (oximino-acetate). In MDR strains overproducing either BcatrB or BcmfsM2, the highest resistance was obtained for the most lipophilic molecules (i.e., pyraclostrobin, trifloxystrobin) (Table 1). However, simultaneous resistance to azoxystrobin and trifloxystrobin has been recorded in laboratory mutants of Aspergillus nidulans and Mycosphaerella graminicola overproducing AnatrB and Mgmfs1 respectively (Andrade et al. 2000; Roohparvar et al. 2007a). These observations suggest that the interactions of strobilurins with fungal transporters differ between species. Fungicide accumulation assays have to be performed with B. cinerea germinated spores, in order to determine which SDHIs and strobilurins are transported by BcatrB and BcmfsM2.
In B. cinerea, the available data for efflux-mediated MDR concern phenylpyrrole, anilinopyrimidine and DMI fungicides. The kinetics of fludioxonil accumulation in various field strains and laboratory mutants suggest that BcatrB is the main transporter involved in the efflux of this phenylpyrrole. The other transporters such as BcatrK and BcmfsM2 are less important in this process (Kretschmer et al. 2009; Vermeulen et al. 2001). Moreover, the dicarboximide iprodione and the anilinopyrimidine cyprodinil were shown to inhibit fludioxonil transport by BcatrB (Vermeulen et al. 2001). A similar phenomenon was also recorded with plant defence compounds (e.g., camalexin, eugenol, resveratrol) and antibiotics produced by antagonistic microorganisms (e.g., phenazine-1-carboxamide) (Schoonbeek et al. 2002, 2003; Stefanato et al. 2009). On the other hand, the kinetics and levels of accumulation of pyrimethanil which are similar in wild-type, MDR1 and MDR2 strains, do not allow to prove that this anilinopyrimidine is transported by BcatrB and BcmfsM2 (Chapeland et al. 1999). At last, the available data obtained with the DMIs oxpoconazole, bitertanol, tebuconazole or triadimenol suggest that BcatrD and BcmfsM2 are the most important transporters for DMI efflux, with BcatrB and Bcmfs1 playing a secondary role (Chapeland et al. 1999; Hayashi et al. 2002; Kretschmer et al. 2009). However, BcatrD seems to target specifically DMIs, because laboratory mutants overproducing this ABC transporter are only resistant to this class of fungicides (Hayashi et al. 2002). Additional accumulation studies using rhodamine G, other fluorescent dyes (Reimann and Deising 2005) or radiolabeled DMIs in combination with various fungicides will allow to determine if several binding sites exist in BcmfsM2 as reported previously for CaCDR1 in C. albicans (Sharma et al. 2009).
The MDR modulator verapamil acts on the MFS transporter BcmfsM2
It has been shown previously that chlorpromazine potentiates the activity of DMIs in B. cinerea (Hayashi et al. 2003), and we confirmed this finding in wild-type strains, with tebuconazole (data not shown). Moreover, in laboratory mutants, this synergy appears to be positively correlated with the level of BcatrD expression, indicating that chlorpromazine is a modulator of this ABC transporter (Hayashi et al. 2003). By contrast, the lack of synergy with tolnaftate, pyrimethanil, iprodione, fludioxonil and fenhexamid in wild-type and MDR field strains suggests that chlorpromazine is not a modulator of BcatrB and BcmfsM2. A similar conclusion can be drawn for amitriptyline and diethylstilbestrol. In B. pseudocinerea, diethylstilbestrol displays strong antagonism towards certain fungicides, including iprodione and tolnaftate. It remains to determine if diethylstilbestrol prevents their penetration into B. pseudocinerea cells.
In our experimental conditions, verapamil enhances the activity of the DMIs tested (i.e., prochloraz, tebuconazole) in wild-type and MDR field strains of B. cinerea. No such synergy was detected in crossed-paper assays (Hayashi et al. 2003), suggesting that this bioassay is less sensitive than tests in which verapamil is mixed with fungicides (see Material and Methods). As BcatrD seems to be the major transporter involved in the efflux of DMIs in B. cinerea wild-type strains (Hayashi et al. 2002), our results strongly suggest that verapamil inhibits this ABC transporter. Moreover, synergy with tolnaftate observed only in MDR2 strains suggests that verapamil also blocks BcmfsM2, but not BcatrB. Verapamil also enhances the activity of fludioxonil, fenhexamid and pyrimethanil in MDR2 strains. No such effect is observed with diethofencarb and iprodione. According to a study conducted with CaCDR1 expressed in S. cerevisiae and the modulator curcumin, synergy appears to be specific to competitive drugs (i.e., rhodamine 6G, “extended” triazoles) and is not observed with not competitive drugs (e.g., “compact” triazoles, cycloheximide) (Sharma et al. 2009). Similarly, we can consider tolnaftate, fludioxonil, pyrimethanil, fenhexamid and possibly DMI fungicides to bind to BcmfsM2 at a site overlapping that for verapamil and different from that for iprodione and diethofencarb. In B. cinerea, additional experiments, including fungicide uptake assays and elaborate binding studies, are required to characterise the interaction between verapamil and the MFS transporter BcmfsM2. Finally, the specific synergy observed between verapamil and fenpropimorph in B. pseudocinerea, which is naturally more susceptible to this fungicide than B. cinerea (Leroux et al. 1999), suggests the involvement of a particular efflux pump.
Practical implications
The chemical control of grey mould has been hindered by the selection of B. cinerea strains resistant to a particular class of fungicides or to many active ingredients with different modes of action. In the first case, the specific resistance generally results from qualitative changes at the target site and is moderate to strong, regardless of the stage of the fungus (Leroux 2004). In the second case, corresponding to MDR, which is correlated with the overproduction of efflux pumps, resistance is generally weak and mostly concerns spore germination and germ-tube elongation (Leroux et al. 1999). This observation suggests that the activity of BcatrB and BcmfsM2 varies with fungal stage. However, the resistance risk seems to be low in practice, when MDR strains are well implanted, because the efficacy of botryticide programmes in Champagne vineyards has never been significantly affected (Petit et al. 2010).
Coumpounds that inhibit fungal transporters involved in fungicide activity or virulence represent a novel tool for controlling plant diseases (de Waard et al. 2006). Till now, most available data concern medical drugs whose use is, of course, precluded in agriculture (Hayashi et al. 2003). However, it should be feasible to develop inhibitors of specific efflux pumps involved in the transport of synthetic fungicides or natural plant defence molecules (Stergiopoulos et al. 2002). In B. cinerea, there is a need for polyvalent modulators fitted to prevent the transport of most botryticides by at least BcatrB, BcatrD and BcmfsM2. Moreover, the selective activity between target and non-target organisms is a prerequisite for such discovery (de Waard et al. 2006). Another possible approach would consist of using respiratory inhibitors which indirectly affect the functioning of membrane transporters. Indeed, in B. cinerea, the uncouplers CCCP and fluazinam have been shown to prevent the cellular efflux of fludioxonil or DMIs from MDR1, MDR2 and wild-type strains (Chapeland et al. 1999; Kretschmer et al. 2009). The combination of fluazinam with fenhexamid, fludioxonil or an anilinopyrimidine fungicide may be of practical value for treating grey mould. The available mixture of fludioxonil + cyprodinil (Forster and Staub 1996) does not display any synergy in wild-type and MDR strains (as determined from the effects of these fungicides alone, and as a mixture, on germ-tube elongation; see Table 2). The combination of botryticides must be integrated into global anti-resistance strategies, which, in French vineyards, involves a maximum of one application per year for each class of fungicides. Moreover, as two treatments (at flowering, and then at bunch closure or veraison) are currently recommended, the reasoned use of botryticides would help to restrict the risk of specific resistance and MDR in vineyards.
References
Andrade, A. C., Gd, S., Nistelrooy, J. G. M., & Waard, M. A. (2000). The ABC transporter AtrB from Aspergillus nidulans mediates resistance to all major classes of fungicides and some natural toxic compounds. Microbiology, 146(8), 1987–1997.
Avenot, H. F., & Michailides, T. J. (2010). Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi. Crop Protection, 29(7), 643–651.
Chapeland, F., Fritz, R., Lanen, C., Gredt, M., & Leroux, P. (1999). Inheritance and mechanisms of resistance to anilinopyrimidine fungicides in Botrytis cinerea (Botryotinia fuckeliana). Pesticide Biochemistry and Physiology, 64(2), 85–100.
Colby, S. (1967). Calculating synergistic and antagonistic responses of herbicide combinations. Weeds, 15, 20–22.
de Waard, M., Andrade, A. C., Hayashi, K., Schoonbeek, H., Stergiopoulos, I., & Zwiers, L. (2006). Impact of fungal drug transporters on fungicide sensitivity, multidrug resistance and virulence. Pest Management Science, 62(3), 195–207.
Elmer, P. A. G., & Michailides, T. J. (2004). Epidemiology of Botrytis cinerea in orchard and vine crops. In ‘Botrytis: biology, pathology and control’ (pp. 243–272). Dordrecht: Kluwer Academic Publishers.
Fillinger, S., Leroux, P., Auclair, C., Barreau, C., Al Hajj, C., & Debieu, D. (2008). Genetic analysis of fenhexamid-resistant field isolates of the phytopathogenic fungus Botrytis cinerea. Antimicrobial Agents and Chemotherapy, 52(11), 3933–3940.
Forster, B., & Staub, T. (1996). Basis for use strategies of anilinopyrimidine and phenylpyrrole fungicides against Botrytis cinerea. Crop Protection, 15(6), 529–537.
Gottesman, M. M. (2002). Mechanisms of cancer drug resistance. Annual Review of Medicine, 53, 615–627.
Hayashi, K., Schoonbeek, H. J., & De Waard, M. A. (2002). Expression of the ABC transporter BcatrD from Botrytis cinerea reduces sensitivity to sterol demethylation inhibitor fungicides. Pesticide Biochemistry and Physiology, 73(2), 110–121.
Hayashi, K., Schoonbeek, H. J., & De Waard, M. A. (2003). Modulators of membrane drug transporters potentiate the activity of the DMI fungicide oxpoconazole against Botrytis cinerea. Pest Management Science, 59(3), 294–302.
Kretschmer, M., Leroch, M., Mosbach, A., Walker, A.-S., Fillinger, S., Mernke, D., et al. (2009). Fungicide-driven evolution and molecular basis of multidrug resistance in field populations of the grey mould fungus Botrytis cinerea. PLoS Pathogens, 5(12), e1000696.
Leroux, P. (2004). Chemical control of Botrytis and its resistance to chemical fungicides. In ‘Botrytis: biology, pathology and control’ (pp. 195–222). Dordrecht: Kluwer Academic Publishers.
Leroux, P., Chapeland, F., Desbrosses, D., & Gredt, M. (1999). Patterns of cross-resistance to fungicides in Botryotinia fuckeliana (Botrytis cinerea) isolates from French vineyards. Crop Protection, 18(10), 687–697.
Leroux, P., Fritz, R., Debieu, D., Albertini, C., Lanen, C., Bach, J., et al. (2002). Mechanisms of resistance to fungicides in field strains of Botrytis cinerea. Pest Management Science, 58(9), 876–888.
Leroux, P., Gredt, M., Leroch, M., & Walker, A.-S. (2010). Exploring mechanisms of resistance to respiratory inhibitors in field strains of Botrytis cinerea, the causal agent of gray mold. Applied and Environmental Microbiology, 76(19), 6615–6630.
Morschhäuser, J. (2010). Regulation of multidrug resistance in pathogenic fungi. Fungal Genetics and Biology, 47(2), 94–106.
Petit, A.-N., Vaillant-Gaveau, N., Walker, A.-S., Leroux, P., Baillieul, F., Panon, M.-L., et al. (2010). Determinants of fenhexamid effectiveness against grey mould on grapevine: Respective role of spray timing, fungicide resistance and plant defences. Crop Protection, 29(10), 1162–1167.
Reimann, S., & Deising, H. B. (2005). Inhibition of efflux transporter-mediated fungicide resistance in Pyrenophora tritici-repentis by a derivative of 4’-hydroxyflavone and enhancement of fungicide activity. Applied and Environmental Microbiology, 71(6), 3269–3275.
Robert, J., & Jarry, C. (2003). Multidrug resistance reversal agents. Journal of Medicinal Chemistry, 46(23), 4805–4817.
Roohparvar, R., De Waard, M. A., Kema, G. H. J., & Zwiers, L. H. (2007). Mgmfs1, a major facilitator superfamily transporter from the fungal wheat pathogen Mycosphaerella graminicola, is a strong protectant against natural toxic compounds and fungicides. Fungal Genetics and Biology, 44(5), 378–388.
Roohparvar, R., Huser, A., Zwiers, L. H., & De Waard, M. A. (2007). Control of Mycosphaerella graminicola on wheat seedlings by medical drugs known to modulate the activity of ATP-binding cassette transporters. Applied and Environmental Microbiology, 73(15), 5011–5019.
Schoonbeek, H., Raaijmakers, J., & De Waard, M. (2002). Fungal ABC transporters and microbial interactions in natural environments. Molecular Plant-Microbe Interactions, 15, 1165–1172.
Schoonbeek, H. J., van Nistelrooy, J. G. M., & de Waard, M. A. (2003). Functional analysis of ABC transporter genes from Botrytis cinerea identifies BcatrB as a transporter of eugenol. European Journal of Plant Pathology, 109(9), 1003–1011.
Schuetzer-Muehlbauer, M., Willinger, B., Egner, R., Ecker, G., & Kuchler, K. (2003). Reversal of antifungal resistance mediated by ABC efflux pumps from Candida albicans functionally expressed in yeast. International Journal of Antimicrobial Agents, 22(3), 291–300.
Sharma, M., Manoharlal, R., Shukla, S., Puri, N., Prasad, T., Ambudkar, S. V., et al. (2009). Curcumin modulates efflux mediated by yeast ABC multidrug transporters and is synergistic with antifungals. Antimicrobial Agents and Chemotherapy, 53(8), 3256–3265.
Stefanato, F. L., Abou-Mansour, E., Buchala, A., Kretschmer, M., Mosbach, A., Hahn, M., et al. (2009). The ABC transporter BcatrB from Botrytis cinerea exports camalexin and is a virulence factor on Arabidopsis thaliana. The Plant Journal, 58(3), 499–510.
Stergiopoulos, I., & De Waard, M. A. (2002). Activity of azole fungicides and ABC transporter modulators on Mycosphaerella graminicola. Journal of Phytopathology, 150(6), 313–320.
Stergiopoulos, I., Zwiers, L. H., & De Waard, M. A. (2002). Secretion of natural and synthetic toxic compounds from filamentous fungi by membrane transporters of the ATP-binding cassette and major facilitator superfamily. European Journal of Plant Pathology, 108(7), 719–734.
Vermeulen, T., Schoonbeek, H., & DeWaard, M. A. (2001). The ABC transporter BcatrB from Botrytis cinerea is a determinant of the activity of the phenylpyrrole fungicide fludioxonil. Pest Management Science, 57(5), 393–402.
Walker, A.-S., Gautier, A., Confais, J., Martinho, D., Viaud, M., Le Pecheur, P., et al. (2011). Botrytis pseudocinerea, a new cryptic species causing gray mold in French vineyards in sympatry with Botrytis cinerea. Phytopathology, 101(12), 1433–1445.
Acknowledgment
The authors would like to thank the companies Syngenta and Dupont de Nemours for providing samples of technical SDHI fungicides and for their interest in this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leroux, P., Walker, AS. Activity of fungicides and modulators of membrane drug transporters in field strains of Botrytis cinerea displaying multidrug resistance. Eur J Plant Pathol 135, 683–693 (2013). https://doi.org/10.1007/s10658-012-0105-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-012-0105-3