Abstract
Fusarium head blight in small grain cereals has emerged as a major problem in the Nordic countries. However, the impact of this disease in oats has been less investigated than in other cereals. For this reason we have studied the infection process (the optimal time of infection and infection pathways) of Fusarium graminearum in oats and its subsequent effects on kernel infection, deoxynivalenol (DON) content and germination capacity. In a field experiment the oat cultivar Morton was spray-inoculated at different developmental stages, and the highest kernel infection and DON content and lowest germination percentage were observed when inoculation took place at anthesis. Field grown oats affected by a natural Fusarium head blight epidemic and spray-inoculated field and greenhouse oats were used to study the infection pathway. Results showed that the fungus entered primarily through the floret apex into the floret cavity, where it could infect via the internal surfaces of the palea, lemma and caryopsis. Both visual symptoms and fungal infections started at the apical portions of the florets and progressed to the basal portions. Hyphae of F. graminearum grew more profusely on the anthers than on other floret parts during initial stages of infection. Disease development within the oat panicle was slow and is primarily by physical contact between adjoining florets, indicating that the long pedicels give Type II resistance in oats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fusarium spp. are common pathogens of cereals and cause a wide range of diseases at all stages of plant development including seedling blight, root and foot rot, snow mould, leaf spot and Fusarium Head Blight (FHB) (McMullen et al. 1997; Parry et al. 1995). FHB results in shrivelled and chalky kernels with low germination rate (Gilbert and Tekauz 1995; Gilbert et al. 1997). Contamination of food and feed stuff with several mycotoxins due to FHB presents a serious health risk to humans and animals (Parry et al. 1995).
Fusarium graminearum, F. culmorum, F. avenaceum, F. poae, and Microdochium nivale are the most common FHB pathogens, although up to 17 causal organisms are associated with the disease (Parry et al. 1995). Fusarium graminearum dominates in North America (McMullen et al. 1997; Schroeder and Christensen 1963) while in cooler northern Europe environments, the most common Fusarium species are F. avenaceum, F. tricinctum, F. poae, F. culmorum and F. graminearum (Yli-Mattila 2010). From 416 samples of wheat, barley and oats collected from Norway during the years 1980–1983 F. culmorum (29%), F. avenaceum (25%), F. graminearum (8%) and F. nivale (7%) were isolated (Haave 1985). Fusarium poae, F. equiseti, F. oxysporium, F. tricinctum and F. sporotrichioides were also isolated, but at a lower frequency (Haave 1985). Recent studies in Norway have shown a shift in relative prevalence of FHB pathogens and increase in the importance of F. graminearum (Hofgaard et al. 2010). The Norwegian oat cv. Gere and the German cv. Bessin were withdrawn from the Norwegian seed market because of high deoxynivalenol (DON) contamination and poor germination rate (Bjørnstad and Skinnes 2008).
Infection by Fusarium spp. is influenced by factors such as moisture and temperature, cultivar susceptibility and cultivation practice (Shaner 2003). Infection and disease development are favoured by warm and humid conditions during flowering and early stages of kernel development (Parry et al. 1995). Fusarium spp. infect wheat and barley heads mainly around anthesis (Schroeder and Christensen 1963; Wagacha and Muthomi 2007), with decreasing severity at later stages of development (Andersen 1948). Mycotoxin content and effects on floret fertility, thousand grain weight and germination capacity vary according to time of infection (Del Ponte et al. 2007; Lacey et al. 1999; Yoshida et al. 2007). However, significant kernel infection and mycotoxin accumulation can occur in kernels inoculated as late as the hard dough stage (Del Ponte et al. 2007).
Barley and wheat florets are covered by a lemma and a palea having lignified thick-walled epidermal and hypodermal cells (Bushnell et al. 2003; Lewandowski et al. 2006). This makes direct penetration and infection of the external floret surfaces by head blight pathogens difficult and unlikely (Bushnell et al. 2003). However, the developing caryopses and internal surfaces of the palea and the lemma are thin-walled and can be penetrated more easily (Bushnell et al. 2003). Further, hyphae of F. graminearum are susceptible to desiccation (Skadsen and Hohn 2004). Therefore, spores germinating on external surfaces of florets that can gain access to internal surfaces avoid desiccation and increase the chances of successful infection.
First symptoms of FHB appeared on non-extruded anthers of wheat (Pugh et al. 1933) and this led researchers to investigate the importance of anthers in floret colonization. Strange and Smith (1971) observed a prolific mycelial growth on anthers of wheat which were exposed to ascospores of F. graminearum. Later, Strange et al. (1974) isolated and characterized betaine and choline as the fungal growth stimulants resulting in prolific F. graminearum growth. These chemicals were found in greater concentrations in anthers than in other floret parts (Strange et al. 1974). Miller et al. (2004) showed likewise that F. graminearum had high affinity to anthers and pollen. Kang and Buchenauer (2000) also noted that retained anthers were colonized densely by F. culmorum hyphae, but that inoculated wheat heads were also invaded at other easily penetrable parts of the floret regardless of the presence or absence of anthers. In addition, wheat genotypes with high levels of anther extrusion tended to develop less FHB and had lower levels of DON contamination (Skinnes et al. 2010). However, there are reports that did not confirm this, and the role of anthers was regarded as equivocal (Engle et al. 2004).
The objectives of this study were: (a) to investigate the optimal time of F. graminearum infection in oats and its subsequent effects on kernel infection, DON contamination and germination capacity; (b) to determine the infection pathway of this pathogen into the oat floret cavity based on macro- and microscopic observations; and (c) to determine the importance of anthers at the initial stages of the infection process. Fusarium graminearum was chosen among other FHB causing pathogens due to the observed increase in prevalence and importance of the pathogen in Nordic countries (Hofgaard et al. 2010; Yli-Mattila 2010).
Materials and methods
A field inoculation experiment and supplemental greenhouse study on infection pathways were conducted in 2008 at University of Minnesota (UMN) Agricultural Research Station, St. Paul. The experiments were further supplemented by samples obtained from a natural epidemic in an oat field at Vollebekk Experimental Farm in 2007 and a greenhouse inoculation experiment conducted in 2009 at the Norwegian University of Life Sciences, Ås, Norway.
Optimal time of infection
The Minnesota field experiment was planted on May 01, 2008. Sixteen plots with four 1.8 m rows spaced 30 cm apart were seeded with the oat cv. Morton. This cultivar is late maturing, resistant to lodging and smut, and susceptible to crown rust and FHB. Plots were harvested at maturity on August 01, 2008.
The inoculum consisted of 41 F. graminearum isolates previously collected from commercial wheat and barley fields in Minnesota. Of the 41 isolates, 40 were collected from 2005 to 2007 while one isolate was from 1986. Isolates were collected, stored, and increased using the procedures described by Dill-Macky (2003) . The inoculum was adjusted to a spore concentration of 1 × 105 spores/ml and 8 ml of Tween20® per litre of inoculum was added. Inoculum was applied using a CO2-powered backpack sprayer fitted with a flat-fan spray tip (TeeJet SS8003; Spraying Systems Co., Wheaton, Illinois) pressurized at 276 KPa. Each row was sprayed for 6–8 s distributing ~32 ml of inoculum per metre of plot row.
Plots were inoculated at anthesis (when ~50% of the spikes had fully emerged) or 1 or 2 weeks after anthesis. Each plot was inoculated twice; at the indicated time and 3 days after initial inoculation. This was done to enhance disease development and to catch the later developing tillers. Uninoculated plots provided the control. Treatments were replicated four times in a completely randomized design.
Following each inoculation, the plants were mist irrigated for 30 min. Subsequently, plots were mist irrigated for 9 min at 1 h intervals during evenings (17:00 to 21:00 h) and mornings (04:00 to 06:00 h). Harvested grain was dried in a commercial seed drier set at 95°C for 7 days. Representative samples were taken from each treatment to determine kernel infection, DON content and germination capacity.
Data collection and analysis
Kernel infection was determined by the modified freezer blotter test (Brodal 1991; Limonard 1966) at the Kimen Seed Testing Laboratory, Ås, Norway. One hundred seeds from each sample were soaked in 1% NaOCl for 10 min for surface sterilization. Kernels were then placed on moist filter paper (300 g/m2, Munktell Filter AB, Falun, Sweden) in transparent, 24.5 × 24.5 × 2 cm polystyrene Nunc bio-assay incubation dishes (Nunc A/S, Roskilde, Denmark), and incubated at 20°C for 24 h. Subsequently, the dishes were transferred to a freezer at -20°C for 24 h to kill the embryo. Finally, dishes were kept under alternating 12 h of a combination of cool white light and UVA light and 12 h darkness at 20°C. After 2 weeks kernels were examined visually for the presence of Fusarium spp. colonies.
Germination tests also were done at the Kimen Seed Testing Laboratory. One hundred kernels were placed on moist paper towels in a room maintained at 10°C for 7 days and then moved to 20°C for 3 days. Seedlings and kernels were then classified as ‘normal’ seedlings, ‘abnormal’ seedlings, ‘healthy non-germinated’ kernels and ‘dead’ kernels according to the International Seed Testing Association (ISTA) standards. Normal seedlings and non-infected un-germinated kernels were grouped as ‘germinated’ while abnormal seedlings and dead kernels were considered as ‘un-germinated’.
DON content was determined at the Mycotoxin Laboratory of UMN. Twelve grams of each sample were ground for 2 min with a Stein Laboratories Mill (Model M-2, Stein Laboratories Inc., Atchison, Kansas) and a 4 g sub-sample was used for extraction and derivation of DON using Gas Chromatography-Mass Spectroscopy as described by Mirocha et al. (1998).
One way analysis of variance on kernel infection, DON content and germination capacity was conducted using the MINITAB14 statistical software (Minitab Ltd., Coventry, UK). Values for each parameter are presented in the results section as mean ± standard error of the mean (SEM).
Infection pathways
St. Paul, Minnesota. Spikelets were collected for a laboratory study from the field experiment within 1 week after the first inoculation. These were examined for presence and location of lesions on apical or basal floret parts and glumes. Spikelets were dissected into glumes and florets after observation. The florets were further bisected into apical and basal portions. Cassettes containing parts from a single spikelet were soaked in 70% ethyl alcohol for 30 s followed by 10% bleach (Clorox®, 6% NaOCl) for 30 s and rinsed three times in sterile distilled water. These parts were placed on Komada’s medium agar- KMA (Komada 1975) Petri plates. The plates were incubated at 20°C for 7 days under alternating 12 h of a combination of UVA and cool white light and 12 h of darkness. A small hyphal plug from the edge of the resulting colonies was transferred to carnation leaf agar (CLA) Petri-plates. These plates were incubated under similar conditions as the KMA plates for 10–14 days. Colonies of F. graminearum were identified as such when they produced perithecia on the carnation leaf pieces by the end of the incubation period.
An oat cv. Winnona, a spring oat cultivar developed by UMN in 2005, was used for the greenhouse experiment. This is an early maturing, short cultivar with high resistance to loose smut and lodging and moderate resistance to crown rust. Kernels were seeded once a week for five consecutive weeks from June 6/2008 to July 4/2008. Six seeds were initially planted per pot (15 cm × 15 cm × 16 cm) and subsequently thinned to four seedlings. The soil mixture used was 50% field soil v/v and 50% growing medium (MetroMix® 200series, SunGro Horticulture Canada Ltd., Vancouver, British Columbia). At the 2–3 leaf stage, one teaspoonful of a slow release fertilizer Osmocote® (The Scotts Company, Marysville, Ohio) was applied per pot. Plants were treated with the fungicide Baylathon (Bayer Corporation Crop Protection Products, Kansas City, Missouri) and the systemic insecticide Marathon (Olympic Horticultural Products Inc. Mainland, Pennsylvania). Conserve® SC (Dow AgroSciences LLC, Indianapolis, Indiana) was specially applied to control thrips. Temperature in the greenhouse varied in the range 20–23°C.
Fully emerged panicles marked prior to inoculation were sprayed with F. graminearum inoculum (as used in the field experiment) until runoff using CO2-powered backpack sprayer. Plants were then placed in an adult plant dew chamber (100% relative humidity; 16 h fluorescent light) for 72 h before being returned to the greenhouse.
Spikelets were sampled randomly from the marked panicles 1 to 7 days after inoculation (DAI). Excised spikelets were placed in plastic bags and transported to the laboratory in a cooler containing ice. Florets for the anther colonization study were collected two, three, four and seven DAI.
Samples from the greenhouse were stained and fixed in a lactophenol blue (Fluka 61335 Lactophenol Blue Solution®) and alcohol solution. The solution consisted of one part lactophenol blue mixed with two parts 96% ethyl alcohol. This was heated to boiling point in a fume hood and poured into screw-capped glass tubes containing spikelets from each sampling time. These were left in the fume hood overnight and then kept in a refrigerator at 4°C for 7 days. Spikelets were washed twice with 50% alcohol for 15 min and rinsed twice with sterile distilled MilliQ water for 10 min. These were then transferred to a 25% glycerol solution for preservation until they were examined under the microscope (Lewandowski et al. 2006). This procedure resulted in fungal hyphae stained deep blue whilst most of the host plant tissue remained unstained.
Sixty florets from each sampling time were examined to study the pattern of floret colonization. For this observation, florets and floret parts were dissected and paleas and lemmas were mounted between two glass slides. Paleas and lemmas were examined under a compound microscope while the surface of the caryopsis was studied under a dissecting microscope. Anthers from fresh florets were mounted in a drop of water on a glass slide and examined under a compound microscope for the presence or absence of fungal hyphae.
Ås, Norway. During an extended period of mist and rain in July 2007 at Ås, a natural Fusarium epidemic developed in a commercial field of ‘Belinda’ oats. Panicles from plants at this site were sampled 8–10 days after anthesis. Symptomatic florets were fixed for microscopy in 2% paraformaldehyde and 1.25% glutaraldehyde in 0.05 M PIPES buffer with pH 7.2. Other bulk samples of panicles were kept frozen at −15°C. From the frozen samples, 100 florets with FHB-like symptoms were soaked in 1% NaOCl solution for 10 min and then dried in a chamber at 35°C. The florets were placed on potato dextrose agar (PDA) plates and kept under alternating 12 h of a combination of cool white light and UVA and 12 h darkness at 20°C for 10 days. At the end of the incubation period each culture was transferred to synthetic nutrient-poor agar (SNA) for subsequent identification of Fusarium species.
As a follow up to determine the role of anthers in floret infection, a limited greenhouse experiment was carried out in Norway in August—October 2009. The cv. Hurdal and four breeding lines: ‘38–8’, ‘3–11’, ‘1287’ and ‘1286’ from UMN were used. The first three are normal white-seeded oats displaying low anther extrusion while the latter two display strong anther extrusion likely because they carry the wild type allele for shattering. The plants were inoculated at anthesis with a macroconidial mixture of two F. graminearum isolates (Isolates 101177 and 101023, obtained from the Norwegian Veterinary Institute) with a concentration of 1 × 105 spores/ml. At 7 DAI, 25 florets were harvested from each genotype, stained and examined under the microscope as described in the infection pathway study carried out at UMN. The percentage of florets with hyphae (palea and/or lemma and/or caryopsis) was then calculated.
Results
Optimal time of infection
All three parameters: kernel infection, DON content, and germination capacity were significantly affected by the timing of F. graminearum inoculation (p value < 0.0001 for all parameters). Inoculation at anthesis resulted in a significantly higher percentage of infected kernels (89 ± 3.1%) compared to inoculation one (52 ± 3.9%) or two (30 ± 5.5%) weeks after anthesis. The uninoculated control had the lowest level of infected kernels (21.5 ± 2.63%), but it was not significantly different from inoculations 1 or 2 weeks after anthesis (Fig. 1).
Effect of time of inoculation with Fusarium graminearum on kernel infection, deoxynivalenol content, and germination capacity of oats (cv. Morton). Data is from a field inoculation experiment conducted in St. Paul, Minnesota in 2008. Kernel infection and germination capacity were measured in percentage while deoxynivalenol content was measured in ppm. One way analysis of variance was conducted and different letters belonging to each parameter represent significant difference (P < 0.0001). Bars represent standard error of means of four replicates
Similar patterns were found for DON content and germination. DON level was highest for inoculation at anthesis (19 ± 0.28 ppm) followed by inoculation 1 week later (3.5 ± 0.57 ppm). Inoculation 2 weeks after anthesis (1.1 ± 0.27 ppm) did not give significantly different DON levels from the uninoculated control (0.7 ± 0.21 ppm). Germination was highly suppressed (73 ± 1.75%) in grain inoculated at anthesis. However, the germination capacity of samples from the other inoculation times and the non-inoculated control were >90% and were not significantly different from each other (Fig. 1).
Infection pathways
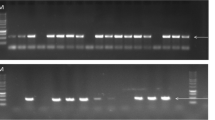
St Paul, Minnesota. First visual symptoms appeared at the floret tip and later spread to basal floret parts in samples from the field experiment that were collected within 1 week after the first inoculation date. Basal floret parts usually developed putative FHB symptoms only after the disease had spread to the entire floret. This was evident from the disparity in the percentage of symptomatic apical vs. basal floret parts (Table 1). Isolation of F. graminearum from the apical and the basal floret parts followed a similar pattern showing higher infection at the apical parts compared to the basal floret parts. The pathogen was observed to grow profusely from apical parts during the first stages of the infection process and spread to the basal portion of the floret (Table 1 and Fig. 2). However, both the apical and basal parts of the tertiary florets were usually found symptomatic and colonized in a few days after inoculation. The difference between symptomatic and colonized apical and basal floret parts of the tertiary floret was minimal compared to the primary and secondary florets (Table 1).
Higher rate of colonization of apical floret portions than basal floret portions. a Glumes and florets dissected into apical and basal parts were placed on Komada’s medium agar: a and b are basal parts while e and f are apical parts; c and d are glumes. b Additional Komada’s medium agar plates with apical and basal floral parts with a similar arrangement as described in a
Florets collected from the greenhouse experiment allowed for a more detailed examination of the infection process. First visual symptoms started to appear at 3 DAI. However, hyphal growth was observed on the different floret parts as early as 1 DAI (Table 2). The floret mouth is the major entry pathway into the floret cavity.
The observations from this study showed a similar pattern to the study of the samples collected from the field inoculation experiment. Apical parts were found symptomatic and colonized with F. graminearum hyphae more often than basal floret parts. From 420 paleas studied 1–7 DAI, 157 had hyphae growing on the apical halves while only 22 had hyphae growing on the basal halves. The same numbers of lemmas and caryopses were examined and 168 and 65 respectively showed hyphae growing on the apical halves while only 61 and 4 respectively had hyphae growing on the basal halves (Table 3).
Hyphae on external surfaces of the palea and lemma tend to accumulate on the apical halves, but a few random hyphae were also found on the basal halves (Fig. 3). Hyphae were seen to grow from the apical parts of the lemma into the floret cavity and colonize the caryopsis and the external surfaces of the upper parts of the palea wings. Fungal hyphae on internal surfaces were observed either at the very tip or at the edges of the distal half of the palea and lemma. Hyphae on basal internal surfaces were exclusively found on the wings of the palea and lemma where the two overlap, possibly extending hyphal growth from external surfaces (Figs. 3 and 4). Hyphae on the caryopses were typically first found on the trichomes; hyphal growth extending from colonized anthers. Basal portions (both dorsal and ventral surfaces) of the caryopses were rarely found colonized and only in completely damaged and dead florets (Fig. 3).
Number of oat florets colonized by Fusarium graminearum on apical and basal halves of the external and internal surfaces of the palea (a and b), and the lemma (c and d), and the dorsal and ventral surfaces of the caryopsis (e and f). Floret parts were studied under the microscope after staining with lactophenol blue solution
Hyphae of Fusarium graminearum on lemma and palea of oat florets stained with lactophenol blue solution. a Hyphae growing at the edge of apical part of the lemma, 4 days after inoculation (DAI). b, c Apical part of the palea with hyphal network of F. graminearum, 4 DAI. d Hyphae (indicated by the arrow) growing on the surface of the palea at the initial stage of infection, 1 DAI
Anthers appear to play an important role during the initial stages of the infection process (Fig. 5a and b). Hyphae of the fungus were observed to grow profusely out of anthers. Heavily colonized anthers appeared black to brownish in color (Fig. 5c and d). At 2 DAI and 3 DAI, more than 50% of the examined anthers were found colonized (Table 4) compared to lower numbers of the paleas, lemmas and caryopses (Table 2), suggesting that the anthers provide a more conducive environment for F. graminearum growth than any other floret part during the first few days after infection. The proportion of anthers colonized by F. graminearum at 4 DAI and 7 DAI was lower than that of 2 DAI and 3 DAI (Table 4). In addition, hyphae on anthers collected 4 DAI and 7 DAI appeared desiccated and thinner than those observed at 2 DAI and 3 DAI.
Oat anthers colonized by Fusarium spp. a Kernels of oat cv. Belinda with anthers colonized with Fusarium spp., b anthers colonized with Fusarium spp. on the surface of the lemma, and (c, d) anthers of the oat cv. Winnona colonized with hyphae of Fusarium graminearum, 3 days after inoculation. a and b are from the 2007 natural epidemic in Norway while c and d are from the greenhouse experiment at the University of Minnesota
The importance of anthers in FHB in oats and the possible influence of anther extrusion were demonstrated in the greenhouse experiment conducted in Ås. The shattering genotypes with strong anther extrusion (‘1286’ and ‘1287’) had 12–20% infected florets, whereas in the ‘normal’ oat genotypes (Hurdal, ‘3–11’, ‘38–8’), this varied from 40% to 52% (Fig. 6).
Percentage of infected florets of five oat genotypes inoculated with Fusarium graminearum at flowering and examined under the microscope after staining with lactophenol blue solution. Three genotypes (Hurdal, 3–11, 38–8) exhibit low anther extrusion and the remaining two (1286 and 1287) exhibit strong anther extrusion. Percentages are based on 25 florets of each genotype sampled at 7 days after inoculation
ÅS, Norway. The symptoms on florets collected from the 2007 natural epidemic in Norway were similar to symptoms observed from field-inoculated samples from Minnesota in 2008, with brownish water-soaked lesions first appearing at floret apices and spreading towards basal portions (Fig. 7a and b). Bleached areas surrounded by a brownish discolouration were often observed on glumes. Fungal hyphae were often observed to spread from primary floret to secondary and tertiary florets, suggesting disease spread in a single spikelet is due to physical contact between the florets rather than through the rachilla (Fig. 7c). Pathogens isolated from florets collected from the 2007 natural epidemic in Norway included F. avenaceum, F. poae, F. langsethiae and Microdochium nivale. As only FHB causing fungi were isolated, the symptoms described above were interpreted as resulting from FHB.
Discussion
Results from kernel infection, DON contamination and germination capacity indicate that anthesis is the most susceptible stage for F. graminearum infection in oats. Conversely, susceptibility to successful infection decreased at later stages of development. Inoculation 2 weeks after anthesis was statistically equivalent to the uninoculated control in terms of kernel infection, DON contamination and germination capacity. In addition, inoculation 1 week after anthesis did not affect germination capacity showing that the effect of F. graminearum infection on germination capacity is restricted to just a few days after anthesis. Fusarium graminearum infections occurring at anthesis may cause complete decay of the caryopses or result in infected kernels with severely depressed germination and elevated mycotoxin contamination.
The trend in barley and wheat is more or less similar showing infections at and a few days after anthesis are more severe and devastating in terms of production of shriveled and scabby kernels and mycotoxin contamination as compared to infections ocurring later in kernel development (Del Ponte et al. 2007; Lacey et al. 1999; McCallum and Tekauz 2002) . Barley is susceptible to F. graminearum infection up to 14 days after heading with the highest seed colonization occurring at 7 days after heading (McCallum and Tekauz 2002). In another study, effect of time of infection with F. graminearum was found to be dependent on type of flowering where cleistogamous barley cultivars were susceptible 10 days after anthesis when spent anthers were exposed, whereas chasmogamous barley cultivars were susceptible at anthesis (Yoshida et al. 2007). On the other hand, wheat (cv. Norm) was found to be susceptible to F. graminearum infection from flowering to the hard dough stage with highest incidence of scabby kernels and DON contamination recorded at early inoculations (Del Ponte et al. 2007). In another experiment in wheat, highest disease incidence and DON contamination occurred after inoculation at about mid-anthesis with decreasing effects of later inoculations (Lacey et al. 1999).
The field- and greenhouse-grown oats inoculated with F. graminearum, as well as the florets collected from the natural FHB epidemic in Norway, showed that the floret mouth is the principal pathway of the fungus into the floret cavity. In addition, the crevices between the palea wing and the lemma wing near the floret mouth served as an alternate avenue. Anthers also played an important role at the initial stages of the infection process. In barley (cv. Robust), Lewandowski et al. (2006) found the floret mouth and the crevices between the overlapping palea and lemma to be the principal entry pathways to F. graminearum. However, unlike our results, they found that anthers played a minimal role in floret colonization. Some of the differences in infection in oats and barley can be attributed to the difference between the floral structures of the two crops. Oat panicles have spikelets hanging down while barley has an erect spike type. Water from irrigation or rainfall accumulates at the tips of the individual oat florets while in barley it accumulates at the basal portion of florets between individual spikelets. This high moisture creates a more conducive environment for spore germination and infection than the relatively drier parts. This can be one reason why Lewandowski et al. (2006) found more lesions and mycelial colonies of F. graminearum colonizing external surfaces of basal portions of barley florets while we found on oats more on the apical portions.
The observations that anthers appear to play a significant role during the initial stages of infection in oats agrees with the observations in wheat by Pugh et al. (1933) and Strange and Smith (1971). Skinnes et al. (2010) found that a high degree of anther extrusion was strongly associated with reduced levels of FHB: unless they are extruded from the florets, anthers become an infection focal point, which has to be addressed by other mechanisms of active resistance. They suggested that Fusarium spp. have a high affinity to anthers because they are also successful saprophytes and anthers constitute dead tissue subsequent to anthesis (Skinnes et al. 2010).
Alternatively, as shown in cleistogamous barley, enclosed anthers escape infection until they are forced out of the floret by the developing kernel. This makes these type of barley cultivars more susceptible to Fusarium infection 7–10 days after anthesis (Yoshida et al. 2007). Similar to our observations, FHB symptoms in such barleys appeared first on the tips where the extended anthers were retained and exposed, and then progressed towards the basal portions. In the same experiment, chasmogamous barley cultivars showed severe FHB and high DON and nivalenol contamination when inoculated at anthesis (Yoshida et al. 2007). The results from our study show that similar factors may apply in the initial stages of oat infection by Fusarium spp. Oats become less susceptible as the floret tissues develop and the anthers are lost. Therefore oat genotypes extruding their anthers appear to be at a lower risk of FHB than genotypes that retain them, but this needs further investigation.
The high rate of colonization of anthers can explain the typical symptomatic apical floret parts. The lag in time of infection in other floret parts compared to the anthers suggests that early infections on the anthers lead to later infections of the other floret parts during the first few days after inoculation. The thinner hyphae observed on anthers collected at 4 DAI and 7 DAI might simply be due to desiccation of hyphae after the plants were transferred from the dew chamber to the greenhouse after 3 DAI. In addition, as disease progresses, the fungus finds other nutrient sources as it grows into the floret cavity colonizing the internal surfaces of the palea, lemma and the caryopses.
Spread of infection within a single spikelet, mainly due to physical contact between florets but never between different spikelets, confirmed that oats have high type II resistance to FHB (Langevin et al. 2004). This observation also explained the relatively small difference between the percentage of symptomatic apical and basal parts of the tertiary florets in the field inoculated samples from the UMN trial. As the tertiary florets are located between the apical parts of the primary and secondary florets, spread from infected apical parts of primary and secondary florets is relatively easy. In addition, the tertiary floret is much smaller than the other florets, likely making it possible for the whole floret to be invaded in a relatively short time, reducing any difference between symptomatic apical and basal parts. An established infection in the primary and secondary florets may make the later flowering florets more vulnerable to infection from earlier flowering florets. A more detailed microscopic study of the infection biology of F. graminearum investigating the spread of infection from spikelet to spikelet as done in wheat by Brown et al. (2010) would give thorough results and complement our findings.
Abbreviations
- FHB:
-
Fusarium head blight
- DON:
-
Deoxynivalenol
- DAI:
-
Days after inoculation
References
Andersen, A. L. (1948). The development of Gibberella zeae head blight of wheat. Phytopathology, 38, 595–611.
Bjørnstad, Å., & Skinnes, H. (2008). Resistance to Fusarium infection in oats (Avena sativa L.). Cereal Research Communications, 36, 57–62.
Brodal, G. (1991). Occurrence, pathogenicity and transmission of seed-borne fungi on grasses in Norway. Dissertation: Agricultural University of Norway.
Brown, N. A., Urban, M., Van De Meene, A. M. L., & Hammond-Kosack, K. E. (2010). The infection biology of Fusarium graminearum: defining the pathways of spikelet to spikelet colonisation in wheat ears. Fungal Biology, 114, 555–571.
Bushnell, W. R., Hazen, B. E., & Pritsch, C. (2003). Histology and physiology of Fusarium head blight. In K. J. Leonard & W. R. Bushnell (Eds.), Fusarium head blight of wheat and barley (pp. 44–83). Saint Paul: The American Phytopathological Society.
Del Ponte, E. M., Fernandes, J. M. C., & Bergstrom, G. C. (2007). Influence of growth stage on Fusarium head blight and deoxynivalenol production in wheat. Journal of Phytopathology, 155, 577–581.
Dill-Macky, R. (2003). Inoculation methods and evaluation of Fusarium head blight resistance in wheat. In K. J. Leonard & W. R. Bushnell (Eds.), Fusarium head blight of wheat and barley (pp. 184–210). Saint Paul: The American Phytopathological Society.
Engle, J. S., Lipps, P. E., Graham, T. L., & Boehm, M. J. (2004). Effects of choline, betaine, and wheat floral extracts on growth of Fusarium graminearum. Plant Disease, 88, 175–180.
Gilbert, J., & Tekauz, A. (1995). Effects of fusarium head blight and seed treatment on germination, emergence, and seedling vigour of spring wheat. Canadian Journal of Plant Pathology, 17, 252–259.
Gilbert, J., Tekauz, A., & Woods, S. M. (1997). Effect of storage on viability of fusarium head blight-affected spring wheat seed. Plant Disease, 81, 159–162.
Haave, R. (1985). Forekomst og patogenitet av Fusarium-arter på korn i Norge. Dissertation, Agricultural University of Norway.
Hofgaard, I. S., Aamot, H. U., Klemsdal, S. S., Elen, O., Jestoy, M., & Brodal, G. (2010). Occurrence of Fusarium spp. and mycotoxins in Norwegian wheat and oats. In I. S. Hofgaard and E. Fløistad (Eds.), Nordic Baltic fusarium seminar (p. 37). Bioforsk, Ski.
Kang, Z. S., & Buchenauer, H. (2000). Cytology and ultrastructure of the infection of wheat spikes by Fusarium culmorum. Mycological Research, 104, 1083–1093.
Komada, H. (1975). Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soil. Review of Plant Protection Research, 8, 114–125.
Lacey, J., Bateman, G. L., & Mirocha, C. J. (1999). Effects of infection time and moisture on development of ear blight and deoxynivalenol production by Fusarium spp. in wheat. Annals of Applied Biology, 134, 277–283.
Langevin, F., Eudes, F., & Comeau, A. (2004). Effect of trichothecenes produced by Fusarium graminearum during Fusarium head blight development in six cereal species. European Journal of Plant Pathology, 110, 735–746.
Lewandowski, S. M., Bushnell, W. R., & Evans, C. K. (2006). Distribution of mycelial colonies and lesions in field-grown barley inoculated with Fusarium graminearum. Phytopathology, 96, 567–581.
Limonard, T. (1966). A modified blotter test for seed health. Netherlands Journal of Plant Pathology, 72, 319–321.
McCallum, B. D., & Tekauz, A. (2002). Influence of inoculation method and growth stage on fusarium head blight in barley. Canadian Journal of Plant Pathology, 24, 77–80.
McMullen, M., Jones, R., & Gallenberg, D. (1997). Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Disease, 81, 1340–1348.
Miller, S. S., Chabot, D. M. P., Ouellet, T., Harris, L. J., & Fedak, G. (2004). Use of a Fusarium graminearum strain transformed with green fluorescent protein to study infection in wheat (Triticum aestivum). Canadian Journal of Plant Pathology, 26, 453–463.
Mirocha, C. J., Kolaczkowski, E., Xie, W. P., Yu, H., & Jelen, H. (1998). Analysis of deoxynivalenol and its derivatives (batch and single kernel) using gas chromatography mass spectrometry. Journal of Agricultural and Food Chemistry, 46, 1414–1418.
Parry, D. W., Jenkinson, P., & McLeod, L. (1995). Fusarium ear blight (scab) in small-grain cereals—a review. Plant Pathology, 44, 207–238.
Pugh, G. W., Johann, H., & Dickson, J. G. (1933). Factors affecting infection of wheat heads by Gibberella saubinetti. Journal of Agriculatural Research, 46, 771–797.
Schroeder, H. W., & Christensen, J. J. (1963). Factors affecting resistance of wheat to scab caused by Gibberella zeae. Phytopathology, 53, 831–838.
Shaner, G. E. (2003). Epidemiology of Fusarium head blight of small grain cereals in North America. In K. J. Leonard & W. R. Bushnell (Eds.), Fusarium head blight of wheat and barley (pp. 84–119). Saint Paul: The American Phytopathological Society.
Skadsen, R. W., & Hohn, T. A. (2004). Use of Fusarium graminearum transformed with gfp to follow infection patterns in barley and Arabidopsis. Physiological and Molecular Plant Pathology, 64, 45–53.
Skinnes, H., Semagn, K., Tarkegne, Y., Maroy, A. G., & Bjornstad, A. (2010). The inheritance of anther extrusion in hexaploid wheat and its relationship to Fusarium head blight resistance and deoxynivalenol content. Plant Breeding, 129, 149–155.
Strange, R. N., & Smith, H. (1971). A fungal growth stimulant in anthers which predisposes wheat to attack by Fusarium graminearum. Physiological Plant Pathology, 1, 141–150.
Strange, R. N., Majer, J. R., & Smith, H. (1974). The isolation and identification of choline and betaine as the two major components in anthers and wheat germ that stimulate Fusarium graminearum in vitro. Physiological Plant Pathology, 4, 277–290.
Wagacha, J. M., & Muthomi, J. W. (2007). Fusarium culmorum: infection process, mechanisms of mycotoxin production and their role in pathogenesis in wheat. Crop Protection, 26, 877–885.
Yli-Mattila, T. (2010). Ecology and evolution of toxigenic Fusarium species in cereals in northern Europe and Asia. Journal of Plant Pathology, 92, 7–18.
Yoshida, M., Kawada, N., & Nakajima, T. (2007). Effect of infection timing on Fusarium head blight and mycotoxin accumulation in open- and closed-flowering barley. Phytopathology, 97, 1054–1062.
Acknowledgement
The first author is indebted to the collaborative program between the University of Minnesota and the Norwegian University of Life Sciences for a travel grant, and to Prof. Ruth Dill-Macky for the supervision of her M.Sc. experiments in Saint Paul during the summer in 2008. The authors also acknowledge the financial support of the Norwegian Research Council and the breeding company Graminor to the project Safe Grains: Mycotoxin prevention through resistant wheat and oats (Project number 178273/I10). The authors would also like to thank the anonymous reviewers for their valuable comments and suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tekle, S., Dill-Macky, R., Skinnes, H. et al. Infection process of Fusarium graminearum in oats (Avena sativa L.). Eur J Plant Pathol 132, 431–442 (2012). https://doi.org/10.1007/s10658-011-9888-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-011-9888-x