Abstract
Apple chlorotic leaf spot virus (ACLSV) isolates from sand pear (Pyrus pyrifolia) were characterized by analyzing the sequences of their coat protein (CP) genes and serological reactivity of recombinant coat proteins (rCPs). The sequences of CP genes from 22 sand pear isolates showed a high divergence, with 87.3–100% identities at the nucleotide (nt) level and 92.7–100% identities at the amino acid (aa) level. Phylogenetic analysis on the aa sequence of CP showed that the analyzed ACLSV isolates fell into different clusters and all isolates from sand pear were grouped into a large cluster (I) which was then divided into two sub-clusters (A and B). Sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE), western blot and enzyme-linked immunosorbent assay (ELISA) analyses demonstrated that rCPs of eight ACLSV isolates (PP13, PP15-2, PP24, PP43, PE, PP54, PP56 and ACLSV-C) from two sub-clusters had different mobility rates and serological reactivity. The rCPs of five isolates grouped into the sub-cluster A showed stronger reactivity with antibodies against rCPs of a sand pear isolate ACLSV-BD and virions of a Japanese apple isolate P-205 than that with the antibody against a Chinese apple isolate ACLSV-C. Three isolates grouped into the sub-cluster B showed stronger reactivity with the antibody against ACLSV-C. The antigenic determinants of CPs from these eight isolates and isolates ACLSV-BD and P-205 were predicted. These results contribute to a further understanding of molecular diversity of the virus and its implication in serological detection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apple chlorotic leaf spot virus (ACLSV) occurs world-wide and is known to infect most pome and stone fruit tree species of the family Rosaceae, including apple, pear, peach, almond, plum, apricot and cherry (Németh 1986). The infection of commercially cultivated apple and pear trees caused by the ACLSV is usually symptomless. However, the multiple-infection of the virus together with Apple stem grooving virus (ASGV) and/or Apple stem pitting virus (ASPV) can cause top-working disease of apple and pear in sensitive rootstocks (Yanase et al. 1979; Desvignes and Boyé 1989). In stone-fruit trees the virus leads to severe symptoms, including dark green sunken mottle in peach, rosette formation and graft incompatibility in apricot, pseudopox and bark split in plum, and bark split in cherry (Dunez et al. 1972; Desvignes and Boyé 1989; Cieślińska et al. 1995; Jelkmann and Kunze 1995).
ACLSV is the type species of the genus Trichovirus, the family Flexiviridae (Martelli et al. 1994; Adams et al. 2004). It is flexuous filamentous, approximately 640–760 nm in length, consisting of a single-stranded positive-sense RNA and multiple copies of a 22 kDa coat protein (CP) (Yoshikawa and Takahashi 1988; Martelli et al. 2007). The complete sequence of ACLSV has been determined for eight isolates from plum (P863 and PBM1) (German et al. 1990; Jelkmann 1996), apple (P-205, A4, B6 and MO-5) (Sato et al. 1993; Yaegashi et al. 2007), cherry (Bal1) (German-Retana et al. 1997) and peach (TaTao5) (Marini et al. 2008), respectively. The genome consists of 7,474–7,555 nt excluding the poly (A) tail at its 3′-end, which contains three overlapping open reading frames (ORFs 1, 2 and 3) encoding a 216 kDa protein involved in replication, a 50 kDa movement protein (MP) and a 22 kDa CP, respectively.
Early studies showed differentiations in serological and biological characteristics among ACLSV isolates from different sources. Different ACLSV serotypes were detected from naturally-infected apple and plum trees (Barba and Clark 1986). It was observed that pathogenic variations of some ACLSV isolates from apple and plum were related to their differences in physical and serological characteristics (Paunović 1989). Furthermore, Cieślińska et al. (1995) found that the ACLSV CP from a plum isolate SX/2 showed a faster migration rate than that from other tested isolates in sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and the serological reactivity of the isolate was also different from apple and cherry isolates. Sequence analysis revealed that the CP from SX/2 had 93% and 91% homologies at amino acid (aa) level with isolate P863 from plum and P-205 from apple (Malinowski et al. 1998).
Comparison of the genomes showed that there was a high molecular diversity among ACLSV isolates from different hosts (German-Retana et al. 1997; Yaegashi et al. 2007). The variations were mostly presented in three main regions: the hyper-variable segment downstream of the methyl-transferase domain in the ORF1, the C-terminal part of the ORF2, and the N-terminal part of the ORF3 (German-Retana et al. 1997). Although the CP was relatively conserved, some studies indicated that there was sequence diversity in CP genes from different ACLSV isolates or strains (Al Rwahnih et al. 2004; Yaegashi et al. 2007). However, little information was known about the effect of sequence variation of CP on their serological reactivity. Also, previous studies on the virus were mostly focused on apple and stone fruit isolates, only a little of CP sequence information was obtained from European pear (Pyrus communis) and Xinjiang pear (Pyrus sinkiangensis) (Cai et al. 2005). Sand pear (Pyrus pyrifolia) is widely grown in China. Previous surveys indicated that over 80% of pear trees in China were infected by ACLSV (Wang et al. 1994). In the present study, the molecular characteristics of ACLSV isolates from sand pear grown in China were compared with other isolates from different fruit trees. Furthermore, the potential antigenic determinants were deduced based on the analyses of their CP sequences and serological reactivity revealed by western blot and enzyme-linked immunosorbent assay (ELISA). The results will contribute to further understanding of molecular population structure of the virus from different hosts.
Materials and methods
Virus sources
A total of 22 Apple chlorotic leaf spot virus (ACLSV) isolates from sand pear including 3 isolates (PP57, PP58 and PP61) from western Hubei province (Enshi), 2 (PP63 and PP64) from Yunnan province and 17 from the National Germplasm Conservation Center of Pyrus pyrifolia (Wuhan, Hubei) were used in this study. Two plum isolates (PL1 and PL2) from Hubei province, a peach isolate (PE) from Shanxi province, and an apple isolate ACLSV-C from China reported previously by our group (Hong and Wang 1999; Zheng et al. 2007) were employed as references.
RNA extraction and reverse transcription-polymerase chain reaction
Total RNA extracted from 0.1 g leaves with cetyltriethylammonium bromide (CTAB) method (Li et al. 2008) was used as template for the amplification of the CP gene of ACLSV by nested-RT-PCR. The outer primer sequences for amplification of the region flanking the CP gene were ACUNI6745(+): 5′-GAGAATTTCAGTTTGCTCGA-3′ and ACUNI3′(-): 5′-AGTCTACAGGCTATTTATTATAAGT-3′ (Yaegashi et al. 2007). The inter primer sequences for amplification of the CP cistron were CP(+)E: 5′-CCGAATTCATGGCAGCAGTTCTGAATC-3′ and CP(-)SC: 5′-GAGAGCTCCTAGATGCAAAGATCAG-3′ with the EcoR I and Sac I restriction sites underlined (Zheng et al. 2007). The expected size of nested-RT-PCR product is 598 bp.
First-strand cDNA synthesis was performed using 0.5 mM of the reverse primer ACUNI3′(-) and M-MLV reverse transcriptase (Promega, Madison, USA) at 42°C for 1 h. PCR reactions were taken in a 25 μl volume with reaction mixtures containing 2.5 μl of 10 × PCR buffer, 0.5 mM of each dNTP, 0.5 mM of each primer, one unit of Taq DNA polymerase (TaKaRa, Dalian, China), and corresponding templates. The template for the first PCR was 3 μl of originally synthesized cDNA and 1 μl of the first PCR product diluted 1:20 was used for the second PCR. The PCR reaction condition was follows: 94°C for 5 min, followed by 35 cycles of denaturation for 30 s at 94°C, annealing for 1 min at 56°C for the first PCR and 55°C for the second PCR, extension for 1 min at 72°C, and final extension for 10 min at 72°C. The two PCR reactions were conducted in a 96-well PCR Thermal Cycler (Model PTC-200, MJ Research, USA). The PCR products were separated by electrophoresis in 1% agarose gel, stained with ethidium bromide and visualized under UV light.
Cloning, sequencing and sequence analysis of the CP gene of ACLSV
The PCR products of the complete CP gene were ligated into pMD18-T vector (TaKaRa, Dalian, China). The plasmids with target fragment were verified by enzyme digestion with EcoR I and Sac I (TaKaRa, Dalian, China). Three positive clones of each ACLSV isolate were sequenced by Shanghai Sangon Biological Engineering & Technology and Service Co. Ltd, Shanghai, China.
The nucleotide (nt) and deduced aa sequences were compared using DNAMAN package (Version 5.2.9, Lynnon BioSoft, Quebec, Canada). Multiple sequence alignment was performed using the Clustal X program (http://www.clustal.org) (Chenna et al. 2003). The phylogenetic tree was derived by importing the aligned sequences (produced with Clustal X) into the MEGA 3.1 and constructed using the neighbour-joining method with 1,000 bootstrap replicates (Kumar et al. 2004). The genetic distances between intra- and inter-clusters were computed using the MEGA 3.1 with 1,000 bootstrap replicates. The antigenic determinants of CP were predicted using the Kolaskar and Tongaonkar method (1990) (http://imed.med.ucm.es/Tools/antigenic.pl). The sequence sources and GenBank accession numbers of the ACLSV CP genes for sequences analysis were listed in Table 1.
Expression of ACLSV CP gene in Escherichia coli
Primarily, the CP gene of isolate ACLSV-BD from sand pear was inserted into prokaryotic expression vector pET-28a(+) (Novagen, Madison, WI, USA) for 229 aa recombinant protein production and the antiserum against its recombinant coat protein (rCP) was prepared. The recombinant plasmid was denoted as pET-ACLSV-BD.
Based on the divergence deduced from phylogenetic analysis, the CP gene of six ACLSV sand pear isolates (PP13, PP15-2, PP24, PP43, PP54 and PP56) were selected for producing recombinant proteins that were used for further analyses of electrophoretic mobility and serological reactivity. The isolates PE from peach and ACLSV-C from apple were used as references. As there was an internal EcoR I restriction site in the CP genes of isolates PP54, PP56 and ACLSV-C, the primer sets CLCP-F/CLCP-R (5′-CCGAGCTCATGGCAGCAGTTCTGAATC-3′/5′-GAGTCGACCTAGATGCAAAGATCA-3′) with Sac I and Sal I restriction sites were designed for the CP gene amplification of these eight isolates. The recombinant plasmids were denoted as pET-PP13, pET-PP15-2, pET-PP24, pET-PP43, pET-PP54, pET-PP56, pET-PE and pET-ACLSV-C. The expected sizes of expressed products were 231 aa.
Escherichia coli (E. coli) strain BL21 (DE3) was transformed with all expression constructs. Protein production was induced by adding 1 mM isopropyl-β-D-thiogalactoside (IPTG) into 2-h preincubated bacterial culture at 37°C for 6 h in Luria-Bertani (LB) medium containing 50 mg l−1 kanamycin and then evaluated by 12% SDS-PAGE. Gels were stained with 0.25% coomassie blue G250 solution. GeneTools analysis software (SYNGENE, USA) was used to quantify the expressed proteins on SDS-PAGE gels.
Preparation of antiserum against rCP of ACLSV
For immunization, the expressed rCP of isolate ACLSV-BD was recovered as described (Vaira et al. 1996). Briefly, the band corresponding to the rCP visualized with ice-cold 0.5 M potassium chloride (KCl) was excised from gel after SDS-PAGE. The gel containing the rCP was homogenized in a small volume of chilled 0.01 M phosphate-buffered saline (PBS, pH 8.0). Rabbits were immunized according to Xu et al. (2006) with slight modifications. Approximately 1 ml suspension containing 1 mg of the rCP was emulsified with Freund’s complete adjuvant (Sigma) for the first injection, 0.5 mg of the rCP in Freund’s incomplete adjuvant (Sigma) for the rest three injections. Blood was collected two weeks after the last injection.
The raised antiserum was purified according to the methods described previously (Burke et al. 1982) with some modifications. Total proteins from induced cells were separated on 12% SDS-PAGE and then electrotransferred onto a polyvinylidene difluoride membrane (PVDF) (HybondTM-P, Amersham Biosciences). To improve the separation efficiency of rCP from total E. coli proteins, the electrophoresis period was properly prolonged. After staining with ponceau S solution, membrane areas containing the rCP were collected, blocked, and then incubated with antiserum. Antibody eluted with 0.2 M Glycine was preserved in a freezer at −20°C or used immediately for further experiments.
Western blot and protein A sandwich enzyme-linked immunosorbent assay
Western blot and protein A sandwich enzyme-linked immunosorbent assay (PAS-ELISA) were used to detect proteins expressed in E. coli cells with three antibodies PAb-BD, PAb-C and PAb-P-205, raised against the rCPs from isolate ACLSV-BD, virions of an apple isolate ACLSV-C from China (Hong et al. 1999) and an apple isolate P-205 from Japan, respectively. The antibody against P-205 was kindly supplied by Yanase (Fruit Tree Research Station, Yatabe, Ibaraki, Japan).
For western blot, total proteins from induced cells were separated on 12% SDS-PAGE and electro-transferred onto a PVDF membrane. The membranes blocked with 5% (w/v) non-fat milk in PBST (0.01 M PBS, 0.05% Tween-20, pH 7.4) were incubated with primary antibodies at dilutions of 1:100 for PAb-BD, 1:500 for PAb-C or 1:30 for PAb-P-205, respectively. Alkaline phosphatase-conjugated goat anti-rabbit IgG diluted at 1:5,000 (Sigma, Germany) were used as the secondary antibody. Antigen-antibody reactions were visualized by incubation in the substrate solution containing 0.35 mg ml−1 nitroblue tetrazolium (NBT) and 0.18 mg ml−1 5-bromo-4-chloro-3-indolyl phosphate (BCIP) (Amresco). GeneTools analysis software (SYNGENE, USA) was used to quantify hybridization signals on the blots.
The rCPs were purified using Ni-NTA Superflow (QIAGEN) according to the manufacturer’s manual and estimated through Bradford’s method (Bradford 1976). PAS-ELISA was done as described by Hong et al. (1999), with primary and secondary antibodies diluted at 1:100 and 1:50 for PAb-BD, 1:500 and 1:250 for PAb-C, and 1:30 and 1:15 for PAb-P-205. Polystyrene microtitre 96-well plates were coated with the purified rCPs, each at 0.2 μg per well. The values were analyzed by Statistical Product and Service Solutions (SPSS) software for significant difference at the 5% level.
Results
Molecular variability of CP genes of ACLSV isolates from sand pear
The CP genes of 22 ACLSV isolates from sand pear, 2 isolates from plum and 1 isolate from peach were sequenced and annotated in GenBank (accession numbers EJ608984, GU327981-GU328004). The nt and deduced aa sequences of these 25 ACLSV isolates along with three other previously reported Chinese ACLSV isolates (Table 1) were compared using DNAMAN package (Version 5.2.9). The results showed that all sand pear isolates shared similarities, ranging 87.3–100% and 92.7–100% at the nt and the aa levels, respectively.
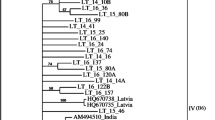
The phylogenetic tree was generated from the aa sequence alignment of CPs of 25 isolates from this study and 45 isolates available in GenBank (Table 1). Results suggested that these ACLSV isolates could be divided into different clusters (Fig. 1). All ACLSV isolates from sand pear were clustered into a large cluster (I), which consisted of two sub-clusters (A and B). Nineteen out of 22 ACLSV sand pear isolates showed high sequence identity with five isolates from stone fruit tree (PE, PL1, PL2, HBP and SX/2), ranging between 94.8% and 100% at the aa level, were grouped into the sub-cluster A, whereas other three sand pear isolates (PP39, PP54 and PP56) together with a pear isolate (Pear) from India, an apple isolate (ACLSV-C) from China and other isolates from Japan and India, ranging between 96.4% and 100%, were grouped into the sub-cluster B. The genetic distances based on the aa sequences were 0.022 ± 0.005 and 0.016 ± 0.005 for sub-cluster A and sub-cluster B, respectively. Whereas, the genetic distance between sub-clusters A and B was 0.049 ± 0.013.
Phylogenetic analysis of Apple chlorotic leaf spot virus (ACLSV) coat proteins. CP sequences were derived from 25 isolates obtained in this study (Accession numbers EJ608984, GU327981-GU328004) and 45 previously reported isolates (Accession numbers listed in Table 1). The tree was constructed with MEGA 3.1 using the Neighbor-joining method (Kumar et al. 2004). The designations of 45 isolates available in GenBank were followed by their accession numbers of CP sequences. The numbers at the nodes indicate the percentage of 1,000 bootstraps occurred in this group. Values below 60% were suppressed. The bar represents 0.05 substitution per site
It was notable that a previously sequenced ACLSV isolate (Kuerle) from kuerle pear (Pyrus sinkiangensis Yü) specifically grown in Xinjiang province, north-western China (Cai et al. 2005) showed low identities with 22 sand pear isolates, ranging from 82.5 to 84.5% (nt) and 89.1 to 91.7% (aa). The kuerle pear isolate was grouped into a cluster (II) together with some apple and stone fruit isolates from Japan and India. The genetic distance between cluster I and II was 0.088 ± 0.018. Four reported isolates PBM1, P863, Bal1 and TaTao5 showed greater distance and were divided into different clades.
The multiple alignment of ACLSV CPs obtained from this study and GenBank (Table 1) revealed uneven distribution of the variances along the CP (Fig. 2). The high variability appeared in the N-terminal part of CP which partially overlapped with MP. The C-terminal part of CP was relatively conserved. The five amino acids combination at positions 40, 59, 75, 130 and 184 (S40-L59-Y75-T130-L184) identified by Yaegashi et al. (2007) was presented in 25 ACLSV isolates analyzed in this study, suggesting that these isolates were belonged to B6 type. An aa residue E at position 70 was conserved in three sand pear isolates (PP39, PP54 and PP56) and isolate ACLSV-C grouped into the sub-cluster B.
Multiple sequence alignment of the amino acid sequences of coat proteins from different Apple chlorotic leaf spot virus (ACLSV) isolates. Arrows indicate the five amino acids that show co-variation conserved in B6 type ACLSV isolates (Yaegashi et al. 2007). Dots indicate identical amino acid residues among all isolates. Consensus sequence was shown in bottom row. Single representative for identical sequences was chosen for the analysis
Effectiveness of raised antiserum against rCP of ACLSV
Western blot analysis showed that the raised antiserum could react strongly with homologous protein expressed in E. coli. However, it also obviously reacted with other native products from E. coli cells (Fig. 3a and b). In order to reduce the nonspecific reaction, the antibody was purified and its specificity was greatly improved (Fig. 3c).
SDS-PAGE and western blot analyses of recombinant coat protein (rCP) of ACLSV-BD isolate. The rCP of ACLSV-BD isolate was expressed in E. coli cells and analyzed by SDS-PAGE (a) and western blotting with unpurified- (b) and purified- (c) antibodies against its rCP. Lane M: molecular weight marker; Lane 1: proteins extracted from E. coli cells with pET-ACLSV-BD vector under induction of IPTG for 6 h; Lane 2: proteins extracted from E. coli cells with pET-ACLSV-BD vector without induction; Lane 3: proteins extracted from E. coli cells with empty pET-28a(+) vector
SDS-PAGE analysis of rCPs from different ACLSV isolates
SDS-PAGE analysis revealed that the expressed rCPs showed different migration rates (Fig. 4a). All these rCPs consisted of 231 aa, including an N-terminal His·Tag/thrombin/T7·Tag and the CP. The predicted CP molecular weights of isolates PP13, PP15-2, PP24, PP43, PE, PP54, PP56 and ACLSV-C were 21.6, 21.6, 21.6, 21.7, 21.7, 21.5, 21.5 and 21.5 kDa, respectively. Interestingly, the sizes of CPs from first five isolates PP13, PP15-2, PP24, PP43 and PE grouped into the sub-cluster A were larger than those of the other three isolates PP54, PP56 and ACLSV-C. However, these five larger rCPs migrated faster in the SDS-PAGE gel, possibly due to their differences in aa composition. Similar patterns were observed in a previous study (Al Rwahnih et al. 2004).
Migration pattern and serological reaction type of coat proteins of Apple chlorotic leaf spot virus (ACLSV) isolates. Coat proteins of eight ACLSV isolates were analyzed by SDS-PAGE (a) and western blotting with three antibodies PAb-BD (b), PAb-C (c) and PAb-P-205 (d), raised against isolates ACLSV-BD, ACLSV-C, and P-205, respectively. Lane M: molecular weight marker; Lane 1–9: proteins extracted from E. coli cells with pET-28a(+), pET-PP13, pET-ACLSV-C, pET-PP43, pET-PP54, pET-PP24, pET-PP56, pET-PP15-2, and pET-PE vectors under induction of IPTG for 6 h, respectively. Equal loading of total expressed proteins was illustrated in Fig. 4a. Hybridization signals on western blots were quantified by scanning with GeneTools software (right column in Fig. 4b–d)
Differences in serological reactivity among rCPs of ACLSV isolates
The rCPs of eight ACLSV isolates (PP13, PP15-2, PP24, PP43, PP54, PP56, PE and ACLSV-C) were analyzed by western blot (Fig. 4b–d) and PAS-ELISA (Table 2) with three antibodies PAb-BD, PAb-C and PAb-P-205, respectively. Hybridization signals on the blots were quantified using GeneTools software to compare the serological reactivity between rCPs from eight isolates. Results showed that the rCPs of eight ACLSV isolates could react with these three antibodies, but produced different intensities of reaction signals. The rCPs of five isolates PP13, PP15-2, PP24, PP43 and PE grouped into the sub-cluster A (Fig. 1) showed stronger serological reactivity with PAb-BD (Fig. 4b) and PAb-P-205 (Fig. 4d), respectively, than that with PAb-C (Fig. 4c). In contrast, three isolates PP54, PP56 and ACLSV-C grouped into the sub-cluster B (Fig. 1) showed stronger reaction signals with PAb-C (Fig. 4c). To confirm the differential serological reactivity between two sub-clusters CPs and three antibodies, we further performed PAS-ELISA. The PAS-ELISA results were in consistency with western blot studies (Table 2). Both western blot and PAS-ELISA studies demonstrated that PAb-BD and PAb-C could differentiate CPs from tested isolates of two sub-clusters in their reaction activity, while PAb-P-205 exhibited similar serological patterns as PAb-BD.
The observed differences in serological reactivity might be related to the CP sequences of tested ACLSV isolates. The antigenic determinants in the CPs of these eight ACLSV isolates and isolates P-205 and ACLSV-BD revealed by the Kolaskar and Tongaonkar method (1990) predicted nine epitope regions in their CPs (Fig. 5). The predicated epitope region VI around aa 101–109 was highly conserved among all analyzed isolates. The epitope region VIII around aa 135–142 was presented in these nine Chinese isolates, and absent in isolate P-205. Other predicted epitopes showed some variances among different isolates. It was notable that the aa V20 in epitope region I, M37/I37 in epitope region II, I80/T80 in epitope region V, and S188 in epitope region IX presented in isolates PP13, PP15-2, PP24, PP43, PE and ACLSV-BD were replaced with A20, T37, V80 and T188, respectively, in isolates PP54, PP56, ACLSV-C and P-205. It was also observed that isolates PP54, PP56 and ACLSV-C had two substitutions of V60 in epitope region III and E70 in epitope region IV, which were replaced with aa M60 and K70, respectively, in isolates PP13, PP15-2, PP24, PP43, PE and ACLSV-BD. It is likely that these aa variations at the epitope sites differentiate the serological reactivity between two sub-clusters of isolates.
Prediction of antigenic determinants of the coat proteins from Apple chlorotic leaf spot virus (ACLSV) isolates. Sequences of PP13, PP15-2, PP24, PP43, PE, ACLSV-BD, PP54, PP56, ACLSV-C and P-205 isolates used for antibody preparation, western blot and ELISA analyses were selected. Nine epitope regions predicted by the Kolaskar and Tongaonkar method (1990) were underlined. Dots indicated the identical aa residues
Discussion
In this study, we investigated the molecular diversity and serological reactivity of field isolates of ACLSV in sand pear cultivars. A phylogenetic analysis of full-length CP sequences of the ACLSV revealed different clusters from 22 sand pear and 3 stone fruit isolates from China, and 45 from GenBank. This study confirmed that the ACLSV population was very diverse as indicated by Al Rwahnih et al. (2004). All ACLSV isolates from sand pear were grouped into the same cluster (I) that was further diversified into two sub-clusters (A and B) (Fig. 1). Although the bootstrap value to differentiate two sub-clusters was weak, the cluster relations were always the same no matter how many sequences were used as references. Most sand pear isolates (19 out of 22) together with some isolates from stone fruit were grouped into the same sub-cluster (A). Our results suggested a possible relationship between ACLSV clustering and their host sources (Al Rwahnih et al. 2004). A previously reported ACLSV isolate “Kuerle” (Cai et al. 2005) from a Xingjiang (north-western China)-originated pear “kuerle pear” was grouped into another cluster (II) with other apple and stone fruit isolates from various countries. The CP of the Kuerle isolate has low identities (ranging 82.5–84.5% and 89.1–91.7% at the nt and aa levels, respectively) to that of all pear isolates analyzed in this study. Such a variation associated with kuerle pear might be due to different geographical isolation.
Comparison of the aa sequences of CP revealed that all 25 Chinese ACLSV isolates grouped into the same cluster (I) had the five amino acids combination (S40-L59-Y75-T130-L184) (Fig. 2), the signature sites of “B6 type” ACLSV proposed by Yaegashi et al. (2007). Among all Chinese isolates, only Kuerle isolate showed variation among these amino acids combination (S40-M59-Y75-A130-L184). This study provided further evidence that the B6 type ACLSV could be distinguished into two different migration patterns, corresponding to two phylogenetic sub-clusters. Western blot and PAS-ELISA analyses using three antibodies also revealed different serological reactivity between two sub-clusters. Multiple alignment of the ACLSV CP aa sequences showed that four Chinese isolates PP39, PP54, PP56 and ACLSV-C had a special aa E70 substitution of K70 that was present in most other isolates. The rCPs of five tested ACLSV isolates (PP13, PP15-2, PP24, PP43 and PE) with K70 showed stronger serological reactivity with antibodies PAb-BD and PAb-P-205 against isolates ACLSV-BD and P-205 which also containing K70 than that with the PAb-C against an apple isolate ACLSV-C containing E70. It is also noticed that the isolate SX/2 from plum, which showed weak serological reactivity with antibodies against apple and cherry isolates, has a special aa Q substitution at position 70 (Cieślińska et al. 1995; Malinowski et al. 1998). Based on these results, the serological reactivity of tested ACLSV isolates might depend on the aa substitution at position 70 within the postulated antigenic determinant IV, supporting a general view that specific aa sequences of CP are correlated with antigenic properties of the virus (Poul and Dunez 1990; Malinowski et al. 1998). However, further mutational and serological analyses will be necessary for confirming the co-variation relationships between individual aa substitutions and serological reaction types.
References
Adams, M. J., Antoniw, J. F., Bar-Joseph, M., Brunt, A. A., Candresse, T., Foster, G. D., et al. (2004). The new plant virus family Flexiviridae and assessmentof molecular criteria for species demarcation. Archives of Virology, 149, 1045–1060.
Al Rwahnih, M., Turturo, C., Minafra, A., Saldarelli, P., Myrta, A., Pallás, V., et al. (2004). Molecular variability of Apple chlorotic leaf spot virus in different hosts and geographical regions. Journal of Plant Pathology, 86, 117–122.
Barba, M., & Clark, M. F. (1986). Detection of strains of Apple chlorotic leafspot virus by F(ab)2-based indirect ELISA. Acta Horticulturae, 193, 297–304.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Burke, B., Griffiths, G., Reggio, H., Louvard, D., & Warren, G. (1982). A monoclonal antibody against a 135-K Golgi membrane protein. The EMBO Journal, 1, 1621–1628.
Cai, Y., Xiang, B. C., Xi, D. H., Liu, S. X., & Du, Y. J. (2005). Cloning and prokaryotic expression of CP gene of Apple chlorotic leaf spot virus Kuerle isolate and preparation of its specific antiserum. Journal of Agricultural Biotechnology, 13, 533–537.
Chenna, R., Sugawara, H., Koike, T., Lopez, R., Gibson, T. J., Higgins, D. G., et al. (2003). Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Research, 31, 3497–3500.
Cieślińska, M., Malinowski, T., & Zawadzka, B. J. (1995). Studies on several strains of Apple chlorotic leaf spot virus (ACLSV) isolated from different fruit tree species. Acta Horticulturae, 386, 63–71.
Desvignes, J. C., & Boyé, R. (1989). Different diseases caused by the Chlorotic leaf spot virus on the fruit trees. Acta Horticulturae, 235, 31–38.
Dunez, J., Marenaud, G., Delbos, R. P., & Lansac, M. (1972). Variability of symptoms induced by the Apple chlorotic leaf spot (CLSV). A type of CLSV probably responsible for bark split disease of prune trees. Plant Disease Reporter, 56, 293–295.
German, S., Candresse, T., Lanneau, M., Huet, J. C., Pernollet, J. C., & Dunez, J. (1990). Nucleotide sequence and genomic organization of Apple chlorotic leaf spot closterovirus. Virology, 179, 104–112.
German-Retana, S., Bergey, B., Delbos, R. P., Candresse, T., & Dunez, J. (1997). Complete nucleotide sequence of the genome of a severe cherry isolate of Apple chlorotic leaf spot trichovirus (ACLSV). Archives of Virology, 142, 833–841.
Hong, N., & Wang, G. P. (1999). The biological and biochemical characterization of Apple chlorotic leaf spot virus. Acta Phytopathologica Sinica, 29, 77–81.
Hong, N., Wang, G. P., Boscia, D., & Martelli, G. P. (1999). The purification of Apple chlorotic leaf spot virus and the production of antiserum against the virus. China Fruits, 1, 15–18.
Jelkmann, W. (1996). The nucleotide sequence of a strain of Apple chlorotic leafspot virus (ACLSV) responsible for plum pseudopox and its relation to an apple and plum bark split strain. Phytopathology, 86, 101–101.
Jelkmann, W., & Kunze, L. (1995). Plum pseudopox in German prune after infection with an isolate of Apple chlorotic leafspot virus causing plum line pattern. Acta Horticulturae, 386, 122–125.
Kolaskar, A. S., & Tongaonkar, P. C. (1990). A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Letters, 276, 172–174.
Kumar, S., Tamura, K., & Nei, M. (2004). MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Briefings in Bioinformatics, 5, 150–163.
Li, R., Mock, R., Huang, Q., Abad, J., Hartung, J., & Kinard, G. (2008). A reliable and inexpensive method of nucleic acid extraction for the PCR-based detection of diverse plant pathogens. Journal of Virological Methods, 154, 48–55.
Malinowski, T., Komorowska, B., Cieślińska, M., Zawadzka, B., & Candresse, T. (1998). Characterization of SX/2, an Apple chlorotic leaf spot virus isolate showing unusual coat protein properties. Acta Horticulturae, 472, 43–50.
Marini, D. B., Gibson, P. G., & Scott, S. W. (2008). The complete nucleotide sequence of an isolate of Apple chlorotic leaf spot virus from peach (Prunus persica (L.) Batch). Archives of Virology, 153, 1003–1005.
Martelli, G. P., Candresse, T., & Namba, S. (1994). Trichovirus, a new genus of plant viruses. Archives of Virology, 134, 451–455.
Martelli, G. P., Adams, M. J., Kreuze, J. F., & Dolja, V. V. (2007). Family Flexiviridae: a case study in virion and genome plasticity. Annual Review of Phytopathology, 45, 73–100.
Németh, M. (1986). Virus, mycoplasma and rickettsia diseases of fruit trees. Budapest: Akademiai Kiado.
Paunović, S. (1989). Properties of two Apple chlorotic leaf spot virus isolates. Acta Horticulturae, 235, 39–48.
Poul, F., & Dunez, J. (1990). Use of monoclonal antibodies for the identification of different antigenic domains in Apple chlorotic leaf spot virus. Archives of Virology, 114, 191–202.
Sato, K., Yoshikawa, N., & Takahashi, T. (1993). Complete nucleotide sequence of the genome of an apple isolate of Apple chlorotic leaf spot virus. Journal of General Virology, 74, 1927–1931.
Vaira, A. M., Vecchiati, M., Masenga, V., & Accotto, G. P. (1996). A polyclonal antiserum against a recombinant viral protein combines specificity with versatility. Journal of Virological Methods, 56, 209–219.
Wang, G. P., Hong, N., Zhang, Z. P., Hu, S. C., & Dong, Y. F. (1994). Identification of virus species in pears cultivated in northern China. China Fruits, 2, 1–4.
Xu, Z. Y., Hong, N., Xiang, B., & Wang, G. P. (2006). Partial molecular characterization of a Chinese isolate of Grapevine leafroll-associated virus 2 and production of antisera to recombinant viral proteins. Journal of Plant Pathology, 88, 87–92.
Yaegashi, H., Isogai, M., Tajima, H., Sano, T., & Yoshikawa, N. (2007). Combinations of two amino acids (Ala40 and Phe75 or Ser40 and Tyr75) in the coat protein of Apple chlorotic leaf spot virus are crucial for infectivity. Journal of General Virology, 88, 2611–2618.
Yanase, H., Yamaguchi, A., Mink, G. I., & Sawamura, K. (1979). Back transmission of Apple chlorotic leafspot virus (type strain) to apple and production of apple topworking disease symptoms in Maruba Kaido (Malus prunifolia Borkh. var. ringo Asami). Annals of the Phytopathological Society of Japan, 45, 369–374.
Yoshikawa, N., & Takahashi, T. (1988). Properties of RNAs and proteins of Apple stem grooving and Apple chlorotic leaf spot viruses. Journal of General Virology, 69, 241–245.
Zheng, Y. Y., Wang, G. P., Hong, N., Song, Y. S., & You, H. (2007). Partial molecular characterization of Apple chlorotic leaf spot virus from peach and apple trees and prokaryotic expression for cp gene. Acta Phytopathologica Sinica, 37, 356–361.
Acknowledgements
This study was financially supported by Chinese Ministry of Agriculture, Industry Technology Research Project (grant no: 200903004) and the earmarked fund for Pear Modern Agro-industry Technology Research System (nycytx-29-08). The authors would like to thank Professor Yangdou Wei, University of Saskatchewan, Canada, for critical revision for the manuscript, and Professor Yanxiu Liu, Huazhong Agricultural University, China, for help with the English of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, Y., Hong, N., Wang, L. et al. Molecular and serological diversity in Apple chlorotic leaf spot virus from sand pear (Pyrus pyrifolia) in China. Eur J Plant Pathol 130, 183–196 (2011). https://doi.org/10.1007/s10658-011-9744-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-011-9744-z