Abstract
The importance of the spatial aspect of epidemics has been recognized from the outset of plant disease epidemiology. The objective of this study was to determine if the host spatial structure influenced the spatio-temporal development of take-all disease of wheat, depending on the inoculum spatial structure. Three sowing patterns of wheat (broadcast sowing, line sowing and sowing in hills) and three patterns of inoculum (uniform, aggregated and natural infestation) were tested in a field experiment, repeated over 2 years. Disease (severity, root disease incidence, plant disease incidence and, when applicable, line and hill incidences) was assessed seven times during the course of each season and the spatial pattern was characterized with incidence-incidence relationships. In the naturally infested plots, disease levels at all measurement scales were significantly higher in plots sown in hills, compared to plots sown in line, which were in turn significantly more diseased than plots with broadcast sowing. Disease aggregation within roots and plants was stronger in line and hill sowing than in broadcast sowing. Analysis of the disease gradient in the artificially infested plots showed that the disease intensified (local increase of disease level) more than it extensified (spatial spread of the disease), the effect of the introduced inoculum was reduced by 95% at a distance of 15 cm away from the point of infestation. Yield was not significantly affected by sowing pattern or artificial infestation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditionally, plant disease epidemiology has been concerned with disease dynamics, reducing space to the mathematical point (Zadoks 2001). This proved a useful simplification in many cases, as demonstrated by the practical usefulness of many non-spatial epidemiological models, such as the ones used in decision support systems (e.g. Audsley et al. 2005), and experimental studies considering mean disease levels (e.g. Schoeny and Lucas 1999). However the importance of the spatial aspect has been recognized from the outset of plant disease epidemiology (Van der Plank 1963) and the incorporation of the spatial dimension into epidemiological models has been an important research focus recently (Scherm et al. 2006). Indeed, the spatial structures of both the host and the disease are liable to influence disease spread and crop losses.
The host’s spatial structure has an effect on the temporal dynamics of epidemics when the disease spreads locally (i.e. when the probability of infection between an infected and a susceptible individual decreases with distance between these individuals). In phytopathology, this effect was experimentally demonstrated in the case of rice sheath blight caused by Rhizoctonia solani (Willocquet et al. 2000) and was explained theoretically, either in the case of vector-borne diseases (Caraco et al. 2001) or in a more general framework (Bolker 1999). Host aggregation creates spatial discontinuities, thus causing the speed of epidemics to fluctuate as host aggregates are depleted of susceptible individuals and new aggregates are being colonised. Compared to a random host distribution, an aggregated distribution speeds up the epidemics at early times (during the first host cluster’s colonisation) and slows it down later on in the epidemic development, because the rate of cross-infection between host aggregates is smaller than the rate of plant-to-plant infection in the case of a random distribution of plants (Gosme and Lucas 2009).
Disease aggregation also decreases the speed of epidemics in the case of locally transmitted diseases. Indeed, when the disease is aggregated, susceptible and infected individuals are somewhat segregated, which reduces the number of susceptible individuals in the vicinity of an infected one (Filipe et al. 2004; Bauch 2005). This effect was proven experimentally in the case of black rot of cabbage, caused by Xanthomonas campestris pv. campestris (Kocks et al. 1998), or the damping off of cress, caused by Pythium ultimum (Brassett and Gilligan 1988).
Take-all disease of wheat, caused by Gaeumannomyces graminis (Sacc.) Arx & Olivier var. tritici Walker (Walker 1972) (Ggt) should not depart from these general rules. Since the disease is transmitted by mycelium growth, root-to-root infection occurs over short distances, typically a few millimetres (Gilligan et al. 1994), which allows the appearance/maintenance of an aggregated spatial structure of the disease, and makes the host spatial structure at the centimetre scale relevant for the host-pathogen interaction. Thus a modification of the sowing pattern of the crop might be used as a lever to reduce disease spread. Several studies have showed an effect of host spatial structure on take-all dynamics. For example, sowing wheat in pairs of rows (increased host aggregation) reduced the development of take-all (Cook et al. 2000), but this was attributable to environmental modification caused by the canopy structure, and not the direct effect of the distance between plants (indeed, the distances between rows was 30 cm in the case of “normal” sowing and 18 or 42 cm in the case of paired rows, which is superior in all case to the dispersal distance of the pathogen as shown by Willocquet et al. (2008). Following observations that yield loss was reduced more by the lateral distance between the seed and the inoculum than by the horizontal distance between seed and inoculum (Kabbage and Bockus 2002), a model showed that when wheat is direct-drilled, planting the seeds exactly between rows of the previous year’s wheat crop could result in yield losses less than 40% of the yield losses predicted in the case where seeds are exactly on the row of the previous year (Garrett et al. 2004). As there is no efficacious fungicide available to control take-all, it is particularly important to consider all possibilities to reduce disease development, even by a small amount, and combine several partially efficient control methods in an integrated approach (Lucas 2006; Ennaïfar et al. 2007). Such methods include soil cultivation (ploughing can bury the infected residues (Colbach 1994)). But tillage also influences the disease spatial structure (Gosme et al. 2007), which might have an effect on the speed of the epidemics.
The objective of this study was to determine if host and/or inoculum spatial structure influenced the spatio-temporal development of the disease. In order to do so, the effect of three sowing patterns of wheat (broadcast sowing, line sowing and sowing in hills) on disease dynamics and spatial structure was investigated under three different inoculum patterns (natural infestation with low level of inoculum expected, artificial infestation with aggregated inoculum, artificial infestation with uniform inoculum).
Material and methods
Experimental design
Take-all epidemics were triggered with artificial infestation in a first wheat crop (preceding crops: faba bean, following maize), the statistical design was a split-split-plot replicated three times, with main plots receiving the sowing pattern, second main plot corresponding to sampling date and subplots receiving the inoculum pattern. The experiment was repeated in two successive years (2004–2005 and 2005–2006) on two different fields. For simplicity, seasons are named after the year of harvest: 2005 and 2006. Each year, the 40 m × 70 m field was divided into three blocks and each block was split into three sowing patterns (SP): LS: line sowing (3 m wide, 7 rows per metre), BS: broadcast sowing (4 m wide) and HS: hill sowing (4.2 m wide, 19 cm between hills across sowing rows, 50 cm between hills within rows). Soil cultivation was the same among all treatments: ploughing followed by rotavator in the first year, harrowing in the second year. Wheat seeds (variety Caphorn, coated with Celest® against bunt, head blight and septoria leaf blotch) were sown on the same day in all treatments (18/10/2004 and 17/10/2005) at the same density of 300 seeds/m2. After seedling emergence, 21 (24 in 2006) subplots measuring 1 × 1 m were delineated in each combination block*SP, three for each of seven sampling dates (plus three for the harvest assessment in 2006), making 189 or 216 subplots every year. Subplots were separated by 2 m in order to avoid cross-contamination. For each sampling date and in each combination block*SP, three inoculum patterns (IP) were randomly attributed: AI, aggregated inoculum; UI, uniform inoculum; and NI, natural infestation expected to be nil or very low in these experimental conditions (1st wheat).
Inoculum preparation
Ggt (isolate IV-26, belonging to the G2 group (Lebreton et al. 2004) and isolated in 2000 in a fourth wheat crop) explants were taken from the growing edge of a colony on PDA amended with antibiotics (Penicillin and streptomycin, 0.075 and 0.15 g/l respectively). Fifteen 5 mm explants were introduced in each of 4 plastic bags containing 250 g of oat grains and 250 ml of water, previously autoclaved twice at 120°C for 1 h with a 24 h interval. Plastic bags were placed in the dark at 20°C for incubation and shaken vigorously every week for 4 weeks.
Field infestation
One month after sowing (17/11/04 and 24/11/05), the inoculum was introduced in the field following two spatial patterns: AI: 9 infestation points at the vertices of a 17 cm grid, centred on the middle of the subplot, UI: 25 infestation points (including 9 within the subplot) at the vertices of a 34 cm grid. The infestation points placed outside the subplot were supposed to avoid edge effect and simulate a truly uniform distribution of inoculum. Infestation point consisted of 4 (+/−1) infested oat kernels buried 5 cm below the soil surface.
Sampling
In order to assess the kinetics of the disease, seven sampling dates were taken between early spring and harvest (GS26 to 85, Zadoks et al. 1974). The first sampling was done at one end of the field and later samplings were taken from progressively further down the field, in order to avoid trampling of future sampling areas. In each 1 × 1 m subplot, plants were sampled following two sampling plans, depending on the sowing pattern. For hill sowing, three plants were sampled for each of the 12 hills, one in the middle and one at each extremity of the hill. The second sampling plan was used for broadcast sowing and for line sowing: five plants (20 cm apart) were sampled in each of the seven rows, which in broadcast sowing corresponded to 35 plants at the vertices of a 20 × 14 cm grid. Each plant was individually labelled so that the distance between each plant and the infestation points was known.
Disease assessment
Sampled plants were kept in a cold room at 4°C before being washed and scored (within 1 week after sampling). For each infected plant, the total number of roots, the number of infected roots and disease severity (on a 0, 1, 5, 10, 20…80, 90, 95, 99, 100 scale) were scored. The number of healthy plants as well as the number of roots of ten randomly selected healthy plants per subplot was also noted. These observations allowed the computing of the following variables: disease severity, disease incidence at the root scale (proportion of infected roots), disease incidence at the plant scale (proportion of infected plants) and, when applicable, disease incidence at the line scale (proportion of infected sowing lines) and disease incidence at the hill scale (proportion of infected sowing hills).
Yield assessment
At the end of the 2006 season, yield was estimated by manually collecting ears in 27 1 × 1 m subplots (three sowing patterns, three inoculum patterns and three blocks). They were then threshed, oven-dried for 48 h at 70°C and weighted in order to estimate dry weight yield.
Infestation efficiency
The efficiency of the artificial infestations was checked with a chi-squared test, using R statistical software (R Development Core Team 2009). The test was used to determine if the plants directly in contact with the infestation points had a higher probability of being diseased compared with the other plants.
Temporal dynamics of mean disease level
A model taking into account primary and secondary infections (Brassett and Gilligan 1988) was fitted to the data as a function of degree-days since sowing (base 0°C). The model is:
where Y is disease incidence, t is the sum of degree-days since sowing and α, β and Z are parameters of the model (α is linked to primary infections, β is linked to secondary infections, and Z is the maximum incidence).
The model was fitted to the data for each year, sowing pattern, inoculum pattern and variable separately. The model was fitted by maximum likelihood with a MCMC method (Gibbs sampler) with flat priors, under the assumption of a binomial distribution of the data (number of diseased roots, plants, lines, hills). For disease severity, the mean severity for each year, sowing pattern and inoculum pattern was multiplied by 100 and rounded to the nearest integer. The first 25.104 iterations were discarded and the chain was then continued for 5.106 steps, recorded every 50 steps in order to avoid autocorrelation. Convergence was assessed visually by plotting the evolution of each parameter in two independent chains.
Effect of the treatments on disease level
The effect of the treatments on disease level was tested with a mixed model. The data from the artificially and naturally infested subplots were analysed separately.
In the case of the naturally infested subplots, the fixed effect was sowing pattern (SP). The random effects were year and date nested within year. The comparison between the model with SP effect vs. the model with only random effects was used in order to test the effect of sowing pattern.
In the case of artificially infested subplots, the fixed effects were sowing pattern (SP), inoculum pattern (IP) and their interaction (SP:IP) (except for line disease incidence and hill disease incidence which of course exist only in the case of line or hill sowing patterns). The random effect was date of observation. The comparison between nested models obtained by sequentially removing fixed effects was used in order to test the significance of these effects. If the interaction between SP and IP was significant, the analysis was done for each inoculum pattern separately.
Five variables were considered: disease severity, root disease incidence, plant disease incidence and, when appropriate, line disease incidence and hill disease incidence. Disease severity was arcsine-root transformed before being submitted to a linear mixed model, fitted by maximum likelihood using function lmer (from package lme4 of R statistical software, Bates and Maechler 2009). For all other variables, a generalized mixed model was used, using function lmer with the binomial link function (i.e. under the assumption that the number of diseased roots, plants, lines and hills, followed a binomial distribution between 0 and the total number of observed roots, plants, lines and hills, respectively). In case of a significant effect, a multiple comparison test with Tukey contrasts (using function glht from multcomp package of R statistical software, Hothorn et al. 2008) was used to obtain adjusted p values for the multiple comparisons between the different levels of the effect.
Relationship between disease variables at two successive levels in the hierarchy
The relationships between severity and root disease incidence, root disease incidence and plant disease incidence, plant disease incidence and line disease incidence, and finally plant disease incidence and hill disease incidence were fitted to the data by least squares using function nls of R statistical software. The following equation was used and one fit was done for each combination of year, SP, IP):
where I high is the incidence at the higher level and I inf is the incidence at the lower level (or severity) and c is the parameter being fitted.
If c = 1, aggregation is maximum. The fact that c = n (where n is the number of sampled elements in each element of the higher scale) denotes a random distribution of disease at the considered scale (McRoberts et al. 2003). The expected values of c under the hypothesis of a random distribution are: 31 for the relationship between root disease incidence and plant disease incidence (because there were 31 roots per plant on average), 3 for the relationship between plant disease incidence and hill disease incidence (because three plants were sampled in each hill) and 5 for the relationship between plant disease incidence and line disease incidence (because five plants were sampled in each line).
The estimated c parameters were then subjected to an analysis of variance (function aov of R statistical software), separately on the naturally infested subplots (effects year and sowing pattern were tested) and artificially infested subplots (effects sowing pattern and inoculum pattern were tested), in order to test the effects on the aggregation of diseased roots within plants and diseased root length (i.e. severity) within roots. When an effect was significant, multiple comparisons of means were carried out using Tukey’s honest significant difference test (function TukeyHSD of R statistical software).
Disease gradients
For each of the seven sampling dates, a disease gradient was fitted to the relationship between root disease incidence of each plant and the distance to the nearest infestation point (in the artificially infested subplots). This relationship was modelled with the following equation, which takes into account two sources of inoculum: a natural background inoculum (independent of the coordinates of the plant) and the introduced inoculum, whose effect decreases with distance following a sigmoid curve (Otten et al. 2001):
where y is the disease incidence, d is the distance between a plant and the nearest infestation point, y nat is the probability of infection due to the natural inoculum, y art-∞ is the asymptotic level of incidence (in the theoretical case where d = −∞) corresponding to the artificial inoculum, d 0 is the abscissa of the inflection point and r is proportional to the steepness of the sigmoid curve.
The model was fitted to the data for all sowing patterns and inoculum patterns together (in order to have enough plants for each distance), by pooling the plants in 4-cm distance-classes. The model was fitted by maximum likelihood with a MCMC method (Gibbs sampler) with flat priors, under the assumption of a binomial distribution of the number of diseased roots, using the same burn-in, thinning and iteration numbers as in the model for temporal dynamics of the disease. In order to determine if the sowing pattern or the inoculum pattern had an effect on the gradient, a mixed model (with fixed effects sowing pattern and inoculum pattern, and random effect observation date) was performed on the residuals of the fit.
The parameters of this model have a mathematical interpretation but no direct biological meaning (except y nat, which corresponds to the incidence in the control subplots). The estimated parameters were then used to compute two biologically meaningful variables:
The effect of artificial inoculum at the point of infestation
The effective distance of dispersal (distance at which the probability of infection due to the artificial inoculum is decreased by 95% compared to its value at the point of inoculum):
Effects of the treatments and the disease on yield
The effect of the treatments on yield was tested with an analysis of variance, testing the effect of SP, IP and SP:IP interaction. The correlation between disease variables (separately for each variable and each sampling date) was also investigated using Spearman’s correlation coefficient.
Results
Infestation success
Seedling emergence was homogeneous and the plants developed without problems during both years, the different sowing patterns lead to different spatial structure of plants. Temperatures were normal throughout both seasons, but the spring in 2006 was characterized by its lack of rain. Unfortunately, artificial infestations failed in 2005: the chi-squared test did not show any significant difference between the proportion of infected plants among the plants directly in contact with the inoculum and the other plants (χ 2 = 2.46, p = 0.116). Furthermore, a germination test in Petri dishes that was done at the end of the experiment on saved inoculum showed that the growth capability on PDA of the 2005 mycelium was reduced by 50% compared to a healthy reference inoculum, showing that the failure of the artificial infestations in 2005 was probably due to the poor quality of the inoculum batch used in that year. For this reason, all subplots are considered as naturally infested in 2005. On the other hand, infestations were successful in 2006 (χ 2 = 613.19, p < 0.0001).
Disease curves
Disease curves at different scales are presented in Fig. 1. In naturally infested subplots, the disease was more important in 2005 than 2006. The parameters estimated from Eq. 1 seem to indicate that this difference might be due to the maximum level of disease that can be achieved (Z): both primary and secondary infections are lower in 2005 than in 2006 (whatever the variable and whatever the sowing pattern) but the maximum attainable level being higher in 2005, the disease level is also higher. However this interpretation must be taken with caution as the parameter estimates for α, β and Z are highly correlated, and the parameter values seem to indicate that artificial infestation leads to a reduction in primary infections.
Observed incidences (symbols) and curves fitted to the model of Brassett and Gilligan (Brassett and Gilligan 1988). A to C: 2005 naturally infested subplots; D to F: 2006 naturally infested subplots; G to I: 2006 aggregated inoculum; J to L: 2006 uniform inoculum; A, D, G and J: hill sowing; B, E, H and K: line sowing; C, F, I and L: broadcast sowing. Squares and thin line: disease severity; crosses and dashed line: root disease incidence; circles and thick line: plant disease incidence; triangle and dotted line: hill disease incidence (in hill sowing) or line disease incidence (in line sowing)
Effect of the treatments on disease level
In the naturally infested subplots, there was a significant effect of sowing pattern on transformed severity, as well as root disease incidence and plant disease incidence, with significantly more disease when the plants were sown in hills compared to line, and more in lines compared to broadcast sowing (Table 1).
In the artificially infested subplots (Table 2), inoculum pattern had a significant effect on hill disease incidence (χ 2(1) = 4.85, p = 0.028), with more infected hills when the inoculum was uniform. IP had no significant effect on line disease incidence (χ 2(1) = 1.27, p = 0.259). Sowing pattern had no effect on plant disease incidence (χ 2(2) = 0.35, p = 0.838), but inoculum pattern had an effect (χ 2(1) = 4.59, p = 0.032), with more infected plants when the inoculum was uniform compared to aggregated inoculum. For root disease incidence and severity, there was a significant interaction between sowing pattern and inoculum pattern (χ 2(2) = 134.31, p = <0.001 and χ 2(2) = 8.78, p = 0.012 respectively). The effect of sowing pattern was then tested for each inoculum pattern separately. For aggregated inoculum, there was no significant difference between line sowing and broadcast sowing for both root disease incidence and severity, but the disease in plots sown in hills was significantly lower. For uniform inoculum, root disease incidence was significantly higher in hill sowing than in line sowing, which in turn was significantly higher than broadcast sowing, but there was no significant effect of sowing pattern on severity. In conclusion, the results showed a trend for a higher disease level (plant disease incidence, root disease incidence and severity) when plants were sown in lines, compared to broadcast sowing (although not always significant). The ranking of hill sowing was different depending on inoculum pattern: in natural inoculum, plants sown in hills showed the highest disease level for all disease variables, while in aggregated inoculum, plants sown in hills had a significantly lower root disease incidence and severity than plants sown in line or broadcast sowing. Finally for uniform inoculum, plants sown in hills had a higher root disease incidence and severity. The effect of inoculum pattern varied depending on sowing pattern: for line and broadcast sowing, there was a trend for more disease with aggregated inoculum while for hill sowing, the highest disease was obtained with uniform inoculum.
Relationships between variables at two successive scales
The chosen relationship between disease variables measured at two successive scales (Eq. 2) provided a good fit to the data. In all cases, parameter c (Table 3) indicates that the disease was significantly aggregated (estimated c < expected c under the hypothesis of a random distribution), except for the relationship between disease incidence at the plant scale and at the line scale in the case of naturally infested subplots sown in lines (there is no theoretical relationship for the severity-incidence relationship, but the c parameter was close to 1, i.e. maximum aggregation).
In the naturally infested subplots, there was a significant effect of sowing pattern on both the severity-root disease incidence and root disease incidence-plant disease incidence relationships (F2,2 = 68.45, p = 0.0144; and F2,2 = 31.108, p = 0.0312, respectively), with a significantly higher c parameter for broadcast sowing than either line or hill sowing (which are not significantly different). The “year” effect was only significant for the root disease incidence-plant disease incidence relationship (F1,2 = 66.542, p = 0.01470).
In the artificially infested subplots, there was a significant sowing pattern effect on the severity-root disease incidence relationship (F2,2 = 47.707, p = 0.01972), with a significantly higher c for hill sowing than either line or broadcast sowing (which are not significantly different). There was also a significant effect of inoculum pattern (F1,2 = 73.676, p = 0.0133), with a higher c in the uniformly inoculated subplots. Neither sowing pattern nor inoculum pattern had significant effects on the root disease incidence-plant disease incidence relationships.
Disease gradient
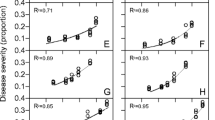
Disease gradient (root disease incidence as a function of the distance between the plant and the nearest infestation point), observed in 2006 with all treatments pooled, is presented in Fig. 2. The corresponding parameters are given in Table 4.
Observed root disease incidence (dots) as a function of distance to the nearest infestation point (cm) and time (degree-days since sowing), in 2006 with all treatments pooled together (each dot is the mean over all plants in the considered distance-class (between 3 and 123 plants per class). The surface was obtained by fitting the equation \( y(d) = 1 - \left( {1 - {y_{\rm{nat}}}} \right) \times \left( {{{{1 - {y_{{{\rm{art}} - \alpha }}}}} \left/ {{\left( {1 + \exp \left( {\left( {{\hbox{d}} - {{\hbox{d}}_0}} \right) \times {\hbox{r}}} \right)} \right)}} \right.}} \right) \) to the incidence as a function of distance to the inoculum for each date separately
The disease gradient showed that the effective distance of dispersion (deff) of Ggt was 8.4 cm at the first assessment date and that it increased progressively and stabilised around 15 cm between assessment dates 5 and 7. The natural inoculum (y nat) and the artificial inoculum at the point of infestation (y art0) followed a logistic curve when plotted against sum of degree-days, with the asymptote being 0.07 and 0.8 for the natural and artificial inoculum respectively, the point of inflection was at 1518 and 1397 degree-days respectively and the slope of the curve at the point of inflexion was 0.0015 and 0.0016 respectively. The mixed model on the residuals of the fit did not show any significant effect of sowing pattern or inoculum pattern.
Yield
Yield in dry grains estimated from 1 m2 subplots ranged between 5.73 t ha−1 and 9.77 t ha−1 (mean 7.81 t ha−1). The results from the analysis of variance showed that none of the tested effects (sowing pattern, inoculum pattern, SP:IP interaction) had a significant effect on yield. Yield was almost always negatively correlated with disease incidence (at all dates and measurement scales), but the only significant correlation was observed at sampling date 6, i.e. milk stage of the crop growth, with severity, (ρ =−0.40; p = 0.044) and line disease incidence (ρ =−0.77 ; p = 0.025).
Discussion
Sowing pattern generally had an effect on disease level, but this effect depended on inoculum pattern (and/or level in the case of naturally infested subplots). In the naturally infested subplots (all subplots from 2005 and control subplots of 2006), the significant effect of sowing pattern on disease severity and incidences at all disease measurement scales (Table 1) indicates that host aggregation increased epidemic development. This result is in accordance with the observations made on other pathosystems (Willocquet et al. 2000) and indicates that the natural inoculum was spread out rather uniformly. Indeed, if the inoculum had been aggregated, one would have expected a lower proportion of diseased plants in the hills than in broadcast sowing, because of the lower hill-to-hill infection rate (Gosme and Lucas 2009). The significantly lower level of aggregation of both diseased root length within roots and diseased roots within plants (Table 3) that was observed in the broadcast sowing pattern, compared to line and hill sowing pattern, might be due to a lower intensification of disease within roots and plants (since it is accompanied by a lower disease incidence at all scales). This might have been caused by different root architecture and/or soil micro-climate and it would be worth examining this effect in more detail, as it could be one cause (along with the greater plant-to-plant distance) of the lower disease level observed in broadcast sowing.
In the artificially infested subplots (2006 only), there was a trend for more disease in subplots sown in lines, compared with broadcast sowing (Table 2). The fact that the ranking of hill sowing changed depending on inoculum pattern might be due to the interaction between the effect of sowing pattern on disease development on the one hand and the particular geometry between inoculum pattern and sowing pattern on the other hand: although the disease might increase more quickly in infected hills, the number of hills close to inoculation points was smaller when the inoculum was aggregated (in the case of uniform inoculum, the non-inoculated row of hills was only 16 cm away from the source of inoculum while in the case of the aggregated inoculum pattern, it was 33 cm away). One might have expected the disease to develop more slowly in the case of aggregated inoculum compared to uniform inoculum, because inoculum aggregation should lead to a local saturation of the disease (Bauch 2005), but no such effect could be observed in this study. This can be explained by the fact that the scale at which the inoculum was considered “aggregated” or “uniform” (17 or 34 cm between infestation points, in a 1 × 1 m subplot) was not the relevant scale for disease dispersion (see below).
For this reason, it is better to analyse the data in the artificially infested subplot in relation to the nearest infestation point (Fig. 2 and Table 4). The disease gradient confirms that the chosen scales for inoculum aggregation might not have been relevant to the pathogen: it showed a weak dispersal capability of the disease, e.g. the effect of the introduced inoculum was reduced by 95% only 15 cm away from the point of infestation. The effective distance estimated in this study is comparable to the dispersal distances found in the literature for take-all: a maximum dispersal of 19 cm (10 cm in average) was observed with inoculum made of soil taken from an infested field (Prew 1980). Other results showed a dispersal at distances up to 20 cm (within a row) or 25 cm (with broadcast sowing) with an inoculum source made of 150 infested oat kernels (Willocquet et al. 2008). The fact that the abscissa of the inflection point of the disease gradient was constant and the slope became steeper and steeper indicates that the epidemic spreads more by local intensification than extensification. This result seems in accordance with the almost constant size of disease foci observed in the field over one growing season (Gosme et al. 2007). Contrary to what might have been expected, no effect of the sowing pattern on the disease gradient could be detected. This might be due to the fact that the method used here (analysis of the residues of the fit) could only detect a systematic over- or under-estimation of the proportion of diseased roots for a given sowing pattern: if, for example, one sowing pattern leads to an over-estimation for short distances and an under-estimation for longer distances, it is possible that the positive and negative residues would cancel each other.
No effect of infestation was observed on yield, which is not surprising considering the small amount of inoculum that was used (9 infestation points in 1 m2). Another study carried out under similar conditions but with 140 infestation points per square meter showed a decrease in yield of about 80% (Willocquet and Dunoyer 2006). The fact that the sowing patterns did not influence yield showed that the sowing patterns tested were not harmful to the crop; if changing the host spatial structure reduces the epidemic severity, it will then be one more element of the crop management plan that can be optimized to achieve integrated pest management and improved yield. Other studies have also shown how planting strategies can affect take-all epidemic dynamics, either by modifying the environment (Cook et al. 2000) or directly by spatial effects of the host relative to the available inoculum (Kabbage and Bockus 2002; Garrett et al. 2004). Our study corroborates these latter observations by showing that sowing pattern has an effect not only through the distance between the seedlings and the inoculum but also through the subsequent development of the disease during the secondary phase of the epidemics. Consequently, there is potential for designing innovative plant patterns, as long as inoculum distribution is known or suspected, as they do not appear to affect crop yield while they can reduce disease propagation.
References
Audsley, E., Milne, A., & Paveley, N. (2005). A foliar disease model for use in wheat disease management decision support systems. The Annals of Applied Biology, 147, 161–172.
Bates, D., & Maechler, M. (2009). lme4: Linear mixed-effects models using S4 classes.
Bauch, C. T. (2005). The spread of infectious diseases in spatially structured populations: an invasory pair approximation. Mathematical Biosciences, 198, 217–237.
Bolker, B. M. (1999). Analytic models for the patchy spread of disease. Bulletin of Mathematical Biology, 61, 849–874.
Brassett, P. R., & Gilligan, C. A. (1988). A model for primary and secondary infection in botanical epidemics. Zeitschrift für Pflanzenkrankenheiten und Pflanzenschutz, 95, 352–360.
Caraco, T., Duryea, M. C., Glavanakov, S., Maniatty, W., & Szymanski, B. K. (2001). Host spatial heterogeneity and the spread of vector-borne infection. Theoretical Population Biology, 59, 185–206.
Colbach, N. (1994). Influence of crop succession and soil tillage on wheat take-all (Gaeumannomyces graminis var. tritici). (Paper presented at the Third congress of the European Society for Agronomy, 18–22 September 1994, Padova University, Abano-Padova, Italy).
Cook, R. J., Ownley, B. H., Zhang, H., & Vakoch, D. (2000). Influence of paired-row spacing and fertilizer placement on yield and root diseases of direct-seeded wheat. Crop Science, 40, 1079–1087.
Ennaïfar, S., Makowski, D., Meynard, J.-M., & Lucas, P. (2007). Evaluation of models to predict take-all incidence in winter wheat as a function of cropping practices, soil, and climate. European Journal of Plant Pathology, 118, 127–143.
Filipe, J. A. N., Maule, M. M., & Gilligan, C. A. (2004). On “analytical models for the patchy spread of plant disease”. Bulletin of Mathematical Biology, 66, 1027–1037.
Garrett, K. A., Kabbage, M., & Bockus, W. W. (2004). Managing for fine-scale differences in inoculum load: seeding patterns to minimize wheat yield loss to take-all. Precision Agriculture, 5, 291–301.
Gilligan, C. A., Brassett, P. R., & Campbell, A. (1994). Modelling of early infection of cereal roots by the Take-all fungus: a detailed mechanistic simulator. The New Phytologist, 128, 515–537.
Gosme, M., & Lucas, P. (2009). Disease spread across multiple scales in a spatial hierarchy: effect of host spatial structure, and of inoculum quantity and repartition. Phytopathology, 99, 833–839.
Gosme, M., Willocquet, L., & Lucas, P. (2007). Size, shape and intensity of aggregation of take-all disease during natural epidemics in second wheat crops. Plant Pathology, 56, 87–96.
Hothorn, T., Bretz, F., & Westfall, P. (2008). Simultaneous inference in general parametric models. Biometrical Journal, 50, 346–363.
Kabbage, M., & Bockus, W. W. (2002). Effect of placement of inoculum of Gaeumannomyces graminis var tritici on severity of take-all in winter wheat. Plant Disease, 86, 298–303.
Kocks, C. G., Zadoks, J.-C., & Ruissen, T. A. (1998). Response of black rot in cabbage to spatial distribution of inoculum. European Journal of Plant Pathology, 104, 713–723.
Lebreton, L., Lucas, P., Dugas, F., Guillerm-Erckelboudt, A.-Y., Schoeny, A., & Sarniguet, A. (2004). Changes in population structure of the soilborne fungus Gaeumannomyces graminis var. tritici during continuous wheat cropping. Environmental Microbiology, 6, 1174–1185.
Lucas, P. (2006). Diseases caused by soil-borne pathogens. In B. M. Cooke, D. G. Jones, & B. Kaye (Eds.), The epidemiology of plant diseases (2nd ed., pp. 373–386). Dordrecht: Kluwer Academic.
McRoberts, N., Hughes, G., & Madden, L. V. (2003). The theoretical and practical application of relationships between different disease intensity measurements in plants. The Annals of Applied Biology, 142, 191–211.
Otten, W., Hall, D., Harris, K., Ritz, K., Young, I. M., & Gilligan, C. A. (2001). Soil physics, fungal epidemiology and the spread of Rhizoctonia solani. The New Phytologist, 151, 459–468.
Prew, R. D. (1980). Studies on the spread of Gaeumannomyces graminis var. tritici in wheat. I. Autonomous spread. The Annals of Applied Biology, 94, 391–396.
R Development Core Team. (2009). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Scherm, H., Ngugi, H. K., & Ojiambo, P. S. (2006). Trends in theoretical plant epidemiology. European Journal of Plant Pathology, 115, 61–73.
Schoeny, A., & Lucas, P. (1999). Modelling of take-all epidemics to evaluate the efficacy of a new seed-treatment fungicide on wheat. Phytopathology, 89, 954–961.
Van der Plank, J. E. (1963). Plant diseases: Epidemics and control. New York: Academic.
Walker, J. (1972). Type studies on Gaeumannomyces graminis and related fungi. Transactions of the British Mycological Society, 58, 427–457.
Willocquet, L., & Dunoyer, A. (2006). Damage caused by wheat take-all at varying spatial patterns of injury. Phytopathology, 96, S123.
Willocquet, L., Fernandez, L., & Savary, S. (2000). Effect of various crop establishment methods practised by Asian farmers on epidemics of rice sheath blight caused by Rhizoctonia solani. Plant Pathology, 49, 346–354.
Willocquet, L., Lebreton, L., Sarniguet, A., & Lucas, P. (2008). Quantification of within-season focal spread of wheat take-all in relation to pathogen genotype and host spatial distribution. Plant Pathology, 57, 906–915.
Zadoks, J. C. (2001). Plant disease epidemiology in the twentieth century—A picture by means of selected controversies. Plant Disease, 85, 808–816.
Zadoks, J. C., Chang, T. T., & Konzak, C. F. (1974). A decimal code for the growth stages of cereals. Weed Research, 14, 415–421.
Acknowledgements
This research was partly supported by the Institut National de la Recherche Agronomique, Agrocampus Rennes and Région Bretagne. We are grateful to Serge Carrillo for technical assistance and to Alex Cook for kindly providing the code for the Gibbs sampling. We also thank the anonymous reviewers for helpful comments and suggestions on the statistical analysis and presenting of the results.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gosme, M., Lucas, P. Effect of host and inoculum patterns on take-all disease of wheat incidence, severity and disease gradient. Eur J Plant Pathol 129, 119–131 (2011). https://doi.org/10.1007/s10658-010-9700-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-010-9700-3