Abstract
Meloidogyne ethiopica is a tropical root-knot nematode species which has recently been found in Europe. We examined its ability to survive in open fields located in regions with sub-Mediterranean and continental European climates. The outdoor microplot experiment consisted of two locations and lasted three growing and two winter seasons. It was demonstrated that M. ethiopica was able to survive at both locations and also that it retained its infection ability although temperatures below zero were recorded. The correct species was confirmed after each winter season by isozyme electrophoresis. Furthermore, the influence of temperature on the reproduction cycle of M. ethiopica was investigated. Meloidogyne ethiopica required 67, 48 and 36 days to complete the reproduction cycle at mean daily temperatures of 18.3, 22.7 and 26.3°C, respectively. At 13.9°C, M. ethiopica was not able to reproduce. The data obtained from these experiments were used to develop a correlation between temperature and the time needed for M. ethiopica to complete a reproduction cycle using a mathematical equation. Furthermore, eight vegetable crops that are important for agricultural production in Slovenia were tested for their suitability as hosts for M. ethiopica.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Root-knot nematodes (RKN, Meloidogyne spp.) are economically important plant parasites affecting a broad range of host plants including monocotyledons, dicotyledons, herbaceous and woody plants (Eisenback and Hirschmann 1991). They are obligate endoparasites causing substantial crop losses worldwide and attack plant roots by inducing characteristic galls (root-knots) where reproduction occurs. To date, more than 90 nominal species of the Meloidogyne genus have been described, of which 12 are considered to be emerging global plant pests: M. acronea, M. arenaria, M. chitwoodi, M. enterolobii, M. ethiopica, M. exigua, M. fallax, M. graminicola, M. hapla, M. incognita, M. javanica and M. paranaensis. These 12 species have received more attention than any other RKN because they are important agricultural pests that are distributed worldwide or have the potential to emerge as plant pests in the near future (Hunt and Handoo 2009).
Meloidogyne ethiopica was described by Whitehead in 1968 from the Mlalo region of Tanzania from a sample isolated from a single egg mass culture found on the type host tomato. It has been recorded in Africa in Tanzania, Kenya, Ethiopia, Mozambique, Zimbabwe and South Africa (Whitehead 1968, 1969); in Latin America in Brazil (Carneiro et al. 2003) and Chile (Carneiro et al. 2004); and in Europe in Slovenia (Širca et al. 2004).
In Slovenia, M. ethiopica was recorded for the first time in 2003 on tomato plants in a greenhouse situated in the village of Dornberk with no linkage to imported plant material (Širca et al. 2004). In Chile, M. ethiopica was found to be the major root knot nematode parasite of grapevines (Vitis vinifera) and other crops (Carneiro et al. 2007). This species is currently widely distributed throughout various locations in Chile and is associated with difficulties in eradication due to the lack of a rootstock that has resistance to this species (Aballay et al. 2009). In Brazil, M. ethiopica affects the root growth, yield and quality of grapevines and kiwi (Actinidia deliciosa), causing serious crop damage and economic losses (Carneiro et al. 2003).
Meloidogyne ethiopica is considered a tropical, polyphagous pest that is able to parasitise at least 80 different host plants, including many economically important dicotyledons and monocotyledons in the families Alliaceae, Amaranthaceae, Apiaceae, Asteraceae, Brassicaceae, Cucurbitaceae, Euphorbiaceae, Fabaceae, Poaceae, Polygonaceae, Rosaceae and Solanaceae (Whitehead 1968; O’Bannon 1975; Carneiro et al. 2003, 2004; Strajnar et al. 2009; Lima et al. 2009). However, few investigations have focused on host suitability to M. ethiopica. Host status is defined by the ability of the nematode species to multiply on the plant host (Liebanas and Castillo 2004), and it is measured as the final nematode population density compared to the initial population density (Pf/Pi), displaying the extent of nematode species multiplication that can occur on the certain host. The host status provides valuable information that can be used to build an efficient integrated pest management strategy. The cultivation of resistant non-host or poor hosts substantially reduces RKN populations, thus limiting the use of chemical nematicides. Therefore, our studies determined the host status of some crop species that are relevant to Slovenian and overall European agriculture.
Climatic conditions, particularly temperature, are important for the development and distribution of RKN, especially in relation to their ability to survive cooler environmental conditions. Meloidogyne ethiopica is a tropical species that requires certain temperatures for successful reproduction.
As an additional objective of our studies, we investigated the ability of M. ethiopica to survive the natural environmental conditions at two distinct locations in Slovenia over two consecutive winter seasons. In addition, the length of the M. ethiopica reproduction cycle at optimal, low and high temperatures were investigated in growth chamber experiments.
Materials and methods
A population of M. ethiopica isolated from infected tomato roots from the Dornberk site (Širca et al. 2004) was grown from egg masses and maintained on beans (Phaseolus vulgaris cv. Top crop) planted in sterile sand kept at 20–25°C in a glasshouse for 50 days. The inoculum was prepared from an extraction of eggs taken from the infected bean roots using 1% sodium hypochlorite that was applied for 4 min to dissolve the gelatinous matrix surrounding the root-knot nematode eggs (Hussey and Barker 1973). The suspension of eggs was washed through 850, 250 and 32 μm banked sieves, and the eggs that were extracted on the lower sieve were washed with tap water to remove the NaOCl (Ehwaeti et al. 1998). The extracted eggs were rinsed in 40 ml of water, placed into 50 ml polycarbonate centrifuge tubes and centrifuged at 1500 rpm for 5 min. The pellets were re-suspended in 40 ml of sucrose solution (454 g sucrose per 1 l of tap water) and centrifuged at 1000 rpm for 1 min (McClure et al. 1973). The supernatant containing the eggs was poured through a 32 μm banked sieve and washed with tap water. Finally, the eggs were removed from the sieve, rinsed and counted.
Host plant test
Dicotyledonous and monocotyledonous plants were tested for their suitability as hosts for M. ethiopica. Plant species included beet, Beta vulgaris var. conditiva cv. Bikor; cauliflower, Brassica oleracea var. botrytis cv. Snežna kepa; chard, Beta vulgaris var. cicla cv. Srebrnolistna; kale, Brassica oleracea var. subauda cv. Železna glava; kohlrabi, Brassica oleracea var. gongylodes cv. Dunajska bela; maize, Zea mays cv. Benicia; spinach, Spinacia oleracea cv. Norvak; and tomato, Solanum lycopersicum cv. San Marzano F1. Seeds were planted in 16 cm diameter pots filled with a mixture of fine sterilised sand (0.25–1.0 mm) and hydroculture granules (size 1–4 mm) in a 3:1 volume ratio. The experiment was set up outdoors under a roof to protect the plants against the rain and was conducted from May to July, 2009. During this period, the mean air temperature was 19.6°C, ranging from 12.9°C to 25.8°C in May, from 13.4°C to 24.3°C in June and from 15.4°C to 26.2°C in July. Temperature data were obtained from the Environmental Agency of Slovenia. The plants were watered and fertilized as needed. The nutrient concentration depended on the stage of plant development. Forty-five days old plants were inoculated with an aqueous suspension containing 5000 M. ethiopica eggs. The experiment was terminated 69 days after inoculation and the final population of M. ethiopica determined. Three to four test plants of each crop were evaluated for the nematode reproduction rate. Second stage juveniles were isolated from the substrate using a decanting method (Hržič 1973) followed by Bearman’s funnel extraction. The nematode eggs were isolated as previously described for the inoculum preparation. The reproductive factor R = Pf/Pi was used as a measure of host suitability and was calculated as the final nematode population (eggs + J2) divided by the initial nematode population (eggs only). Four host categories were defined based on the R values (Ferris et al. 1993): R > 10 = excellent host; 10 > R > 1 = good host; R close to 1 = maintenance host and R < 1 = poor host to non-host. Data were statistically analysed using an analysis of variance (ANOVA), and significant differences in the means of the reproduction factor values were ranked using Duncan’s multiple range test at P = 0.05 in the Statgraphics XV software package.
Effect of temperature on Meloidogyne ethiopica development
The time needed for one reproduction cycle was determined in a growing chamber experiment at four separate experiments at mean daily temperatures: 13.9, 18.3, 22.7 and 26.3°C. Bean plants, Phaseolus vulgaris cv. Meraviglia di venezia nano; chicory, Cichorium intybus var. foliosum cv. Castelfranco; cucumber, Cucumis sativus cv. Dolge zelene and tomato, Solanum lycopersicum cv. Volovsko srce were used to determine the time. The plants were planted in 9 x 9 cm pots filled with 560 g fine sterilised sand (particle site 0.25–1.0 mm). The appropriate amount of water and nutrients for hydroponic growth were added according to the manufacturer’s instructions (Flora series, General hydroponics Europe) to achieve 15% moisture based on the dry sand weight. The growth conditions included 13 hours of illumination and 60% relative humidity at average daily temperatures of 13.9°C (15°C day/12.7°C night), 18.3°C (21°C day/15°C night), 22.7°C (25°C day/20°C night) and 26.3°C (30°C day/22°C night). Chicory was used as host plant for the experiment at 13.9°C, whereas bean, cucumber and tomato were used at 18.3, 22.7 and 26.3°C average temperature. The tomato, cucumber, chicory, bean plants were at 43, 43, 37 and 15 days after seeding, respectively inoculated with an aqueous suspension of 3000 M. ethiopica eggs per plant. The aqueous solution of eggs was prepared according to previously described methods. To evaluate the duration of the M. ethiopica reproduction cycle, the nematode egg development was examined in two pots of each host plant every fourth day starting at 20 days post inoculation (DPI) in the experiment at 26.3°C, 24 DPI at 22.7°C, 51 DPI at 18.3°C and 60 DPI at 13.9°C.
In addition, the temperature-dependent development equation was calculated by regressing the development times against temperature using TableCurve 2D (Systat Software, USA). The equation was based on best fit, algebraic simplicity and a logical description of the nature of the data.
Microplot experiments
The experiments to determine survival of M. ethiopica were conducted over three growing and two winter seasons in two locations in Slovenia. The first site, representing a continental climate was located in Ljubljana, the second site was located in a sub-Mediterranean climate in Bilje. For both locations two 50 l vessels were buried and filled with soil. In May 2007, two tomato plants (cv. Volovsko srce) were planted in the vessel and inoculated with eggs of M. ethiopica. In 2008 and 2009 new tomato seedling were re-planted in the vessels. Every year in the autumn, the tomato stems were cut, leaving the infected roots in the soil. The survival of the nematodes was examined after the winter seasons 2007/2008 and 2008/2009. A re-identification of the nematode species was done by isozyme phenotyping of young egg-laying females (Esbenshade and Triantaphyllou 1985) using PhastSystem (GE Healthcare Life Sciences) gel electrophoresis.
Results
Host plant test
Both, dicotyledonous and monocotyledonous plants were suitable hosts for M. ethiopica. Reproductive factors showed the highest reproduction rates on tomato (R = 91.20), intermediate rates on spinach, beet and chard, and the lowest on cauliflower, kale, kohlrabi and maize (Table 1). In terms of host status tomato, spinach, chard and beet were considered as excellent hosts. Among the excellent hosts, tomato had reproductive factors 1.8, 5.0 and 7.6 times higher than spinach, chard and beet, respectively (Table 1). Maize and kohlrabi were identified as good hosts, but they had significantly lower reproductive factors of 2.87 and 4.47, respectively. Kale turned out to be a maintenance host with an R value above 1.0, while cauliflower was shown to be a poor host with an R value below 1.0 (Table 1).
Effect of temperature on Meloidogyne ethiopica development
No reproduction of M. ethiopica was observed at 13.9°C. For up to 120 DPI, chicory plants showed no root galling or signs of reproduction. At the average daily temperatures of 18.3, 22.7 and 26.3°C M. ethiopica was able to complete reproduction cycle. Eggs were observed after 51, 24 and 20 DPI at the average daily temperatures of 18.3, 22.7 and 26.3°C, respectively (Fig. 1).
Number of Meloidogyne ethiopica eggs after inoculation with 3000 eggs from tomato, cucumber and bean plants in three experiments with mean daily temperatures of 18.3, 22.7 and 26.3°C. The graphs show the number of days needed for development of maximum number of eggs indicating completed reproduction cycle at certain temperature
In 18.3°C temperature experiment nematode eggs were observed at 51 DPI on tomato and bean plants. Eggs were detected on cucumber plants eight days later. Maximum of M. ethiopica eggs were obtained at 67 DPI on bean, cucumber and tomato plants (Fig. 1).
In the experiment at 22.7°C, the eggs were collected on bean plants at 24 DPI and four days later on cucumber and tomato plants. The maximum of nematode eggs were detected at 48 DPI (Fig. 1). Additionally, the highest nematode multiplication rate was determined in this experiment where the final number of eggs increased by 113 times on bean plants.
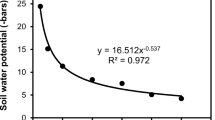
At 26.3°C, the eggs were first observed on tomato plants at 20 DPI followed by cucumber plants four days later. The number of eggs reached maximum at 36 and 40 DPI on tomato and cucumber plants, respectively (Fig. 1). Beans were excluded from the experiment at 26.3°C since growth conditions were unsuitable for bean plants (plants wilted and declined). The results demonstrated that approximately 67, 48 and 36 days are required for the reproductive cycle to be completed at 18.3, 22.7 and 26.3°C, respectively (Fig. 1). These results were used for the estimation of a correlation between temperature and the duration of the M. ethiopica reproduction cycle. The reproduction curve was defined with the following equation:
The adjusted regression coefficient and the standard error for the calculated equation were 0.9999 and 0.05, respectively. The reproduction curve covers temperatures ranging between 18.3°C and 26.3°C (Fig. 2).
Microplot experiments
The microplot experiments were conducted from May 2007 to June 2009. The monthly soil temperatures at a depth of 5 cm in Ljubljana were approximately 2°C lower compared to the Bilje site. In the first season (May–October 2007), the average soil temperatures in Bilje ranged from 13°C to 27°C and from 11.6°C to 24.3°C in Ljubljana (Fig. 3). The temperatures during the winter were the key factors affecting the survival of M. ethiopica. In the winter of 2007/2008, the soil temperatures in Ljubljana were below zero for 20 days, while at the Bilje location, the soil temperatures were always above zero. After the first winter season, re-infection of tomato plants was found at both locations. Re-identification confirmed that plants were indeed infected by M. ethiopica. In the 2008 growing season, the average temperatures ranged from 12.6°C to 23.7°C in Ljubljana and from 14.8°C to 26.4°C in Bilje (Fig. 3). The temperatures in the second winter 2008/2009 were below zero for 20 days in Ljubljana and for 10 days in Bilje. In the 2009 growing season, a re-identification of the nematodes confirmed that the M. ethiopica population had survived the low winter temperatures and retained infection ability in the open field at both locations.
Discussion
In these studies, the effects of temperature and host plants on survival and reproduction of M. ethiopica were investigated. Meloidogyne ethiopica was first observed in Tanzania, Africa. Published reports from South America have described plant damage caused by M. ethiopica, and the species has recently been classified into the group of pest species that require special attention (Hunt and Handoo 2009). Meloidogyne ethiopica infestations in Chilean vineyards are a permanent problem (Carneiro et al. 2007; Aballay et al. 2009) and also represent a serious risk to grapevine production in Brazil, where there are 67,800 ha in cultivation (Carneiro et al. 2003; Lima et al. 2009). In Europe, M. ethiopica has only been recorded in Slovenia (Širca et al. 2004).
The ability of M. ethiopica to survive under continental climate conditions that are characterised as mild, with hot summers and cold winters, as well as in a sub-Mediterranean climate characterised by hot summers and mild winters defines the potential risks of spread and establishment for this tropical root-knot nematode species. Meloidogyne ethiopica survived under field conditions despite the fact that the winter temperatures fell below zero degrees on several occasions. The survival of the nematode population was monitored for two winter and three growing seasons by root galling which is an indicator of nematode infection. In the second growing season increased root infestation was observed at both field locations. The resultant observations were contrary to our expectation that low temperatures in the winter season would reduce the nematode population. Instead, our results suggest that M. ethiopica can survive through the winter period in Europe and, therefore, represent a serious risk for agriculture production, especially in southern Europe.
Approximately eighty plant species have been reported as hosts for M. ethiopica (Whitehead 1968; O’Bannon 1975; Carneiro et al. 2003, 2004; Strajnar et al. 2009; Lima et al. 2009 ), including several crops grown in moderate climatic zones. Strajnar et al. (2009) tested several vegetable crops that are relevant to Slovenian agricultural production, and all of the tested plants proved to be suitable hosts for M. ethiopica. This wide host range is similar to other major RKN species such as M. incognita and M. javanica which also reproduce by mitotic parthenogenesis. Mitotic parthenogenesis is believed to drive and maintain a wide host range because it slows co-evolution and genetic drift (Trudgill 1997). In this study, we determined the host suitability of eight crops that are important to Slovenian agricultural production. A high reproduction rate of M. ethiopica was observed on plants from the family Chenopodiaceae and Solanaceae. Bean plants proved to be excellent hosts, enabling the nematode population to increase 113 times in a single reproduction cycle. Lima et al. (2009) also determined that plants from the Fabaceae family are susceptible to M. ethiopica infection. Cauliflower and kale were poor or maintenance hosts for M. ethiopica; they are members of the Brassicaceae family. One of the potential explanations for the reduction in the population of these plants could be the biocidal activity of Brassicaceae plant cells that possess a glucosinolate-myrosinase system and therefore are capable of producing a number of biologically active compounds including isothiocyanates, nitriles, epithionitriles and thiocyanates (Fahey et al. 2001). Mojtahedi et al. (1993) established a reduction in a M. chitwoodi population with glucosinolate degradation products. Therefore, Brassicaceae plants could represent an efficient approach for M. ethiopica management.
Our study determined the generation times at low, optimal and high temperatures, and the reproduction curve between 18.3°C and 26.3°C was calculated. The data also indicated that plant species did not influence the duration of the nematode reproduction cycle. The highest nematode population was obtained at a daily temperature of 22.7°C, which is considered to be the optimal temperature for M. ethiopica reproduction. The correlation between temperature and RKN development time has been studied for M. javanica by Madulu and Trudgill (1994). In these experiments, the time from J2 inoculation to the development of the first J2 of the next generation was defined as a complete reproduction cycle. These experiments were carried out at temperatures ranging from 18°C to 30°C, and their results showed a linear relationship between developmental time and temperature. The first-hatched J2 were collected after 44, 32, 25 and 24 days at temperatures of 21.1, 23.8, 26.9 and 29.9°C, respectively. Similar results were observed in our study when the first eggs were collected after 51, 24 and 20 days at temperatures of 18.3, 22.7 and 26.3°C, respectively, suggesting that M. ethiopica has a developmental time similar to M. javanica.
The reproduction curve determined from our results represents a useful tool for further applied research on this pest species. The reproduction curve defined the duration of M. ethiopica reproduction cycles at certain temperatures ranging from 18.3 to 26.3°C. These results can be used in further tests of host status determinations, tests of host resistance to M. ethiopica, or any tests where the maximum number of the final pest population is important. The reproductive curve can also be used to predict the number of M. ethiopica generations that might occur on crops in a growing season.
These results confirm the importance of this pest species, not only for tropical areas but also for Europe. The facts that M. ethiopica can survive and maintain its infection ability under climatic conditions present in Slovenia together with its polyphagous nature underpin the importance of this RKN species. The data obtained in this study represent groundwork for more intensive studies needed for a thorough pest risk analyses (PRA) to determine the threat this pest poses to agricultural production in Europe.
References
Aballay, E., Persson, P., & Mårtensson, A. (2009). Plant-parasitic nematodes in chilean vineyards. Nematropica, 39, 85–97.
Carneiro, R. M. D. G., Gomes, C. B., Almeida, M. R. A., Gomes, A. C. M. M., & Martins, I. (2003). First record of Meloidogyne ethiopica Whitehead, 1968 on kiwi in Brazil and reaction on different plant species. Nematologia Brasileira, 27, 151–158.
Carneiro, R. M. D. G., Randing, O., Almeida, M. R. A., & Gomes, A. C. M. M. (2004). Additional information on Meloidogyne ethiopica Whitehead, 1968 (Tylenchida: Meloidogynidae), a root-knot nematode parasitising kiwi fruit and grape-vine from Brazil and Chile. Nematology, 6, 109–123.
Carneiro, R. M. D. G., Almeida, M. R. A., Cofcewicz, E. T., Magunacelaya, J. C., & Aballay, E. (2007). Meloidogyne ethiopica, a major root-knot nematode parasitising Vitis vinifera and other crops in Chile. Nematology, 9, 635–641.
Ehwaeti, M. E., Phillips, M. S., & Trudgill, D. L. (1998). The viability of Meloidogyne incognita eggs released from egg masses of different ages using different concentrations of sodium hypochlorite. Nematologica, 44, 207–217.
Eisenback, J. D., & Hirschmann, H. T. (1991). Root-Knot nematodes: Meloidogyne species and races. In W. R. Nickle (Ed.), Manual of agricultural nematology (pp. 191–274). New York: Marcel Dekker, Inc.
Esbenshade, P. R., & Triantaphyllou, A. C. (1985). Use of enzyme phenotypes for identification of Meloidogyne species. Journal of Nematology, 17, 6–20.
Fahey, J., Zalcmann, A., & Talalay, P. (2001). The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry, 56, 5–51.
Ferris, H., Carlson, H. L., Viglierchio, D. R., Westerdahl, B. B., Wu, F. W., Anderson, C. E., et al. (1993). Host status of selected crops to Meloidogyne chitwoodi. Supplement to Journal of Nematology, 25, 849–857.
Hržič, A. (1973). Extraction of nematodes from soil with whirling motion. Zaštita bilja, 122, 53–60.
Hunt, D. J., & Handoo, Z. A. (2009). Taxonomy, identification and principal species. In R. N. Perry, M. Moens, & J. L. Starr (Eds.), Root-knot nematodes (pp. 55–88). London: CABI.
Hussey, R. S., & Barker, K. R. (1973). A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Disease Reporter, 57, 1025–1028.
Liebanas, G., & Castillo, P. (2004). Host suitability of some crucifers for root-knot nematodes in southern Spain. Nematology, 6, 125–128.
Lima, E. A., Mattos, J. K., Moita, A. W., Carneiro, R. G., & Carneiro, R. M. D. G. (2009). Host status of different crops for Meloidogyne ethiopica control. Tropical Plant Pathology, 34, 152–157.
Madulu, J. D., & Trudgill, D. L. (1994). Influence of temperature on the development and survival of Meloidogyne javanica. Nematologica, 40, 230–243.
McClure, M. A., Kruk, T. H., & Misaghi, I. (1973). A method for obtaining quantities of clean Meloidogyne eggs. Journal of Nematology, 5, 230.
Mojtahedi, H., Santo, G. S., Wilson, J. H., & Hang, A. N. (1993). Managing Meloidogyne chitwoodi on potato with rapeseed as green manure. Plant disease, 77, 42–46.
O’Bannon, J. H. (1975). Nematode survey in Ethiopia. Institute of Agricultural Research, Addis Ababa, Ethiopia and FAO, Rome, [unpubl.].
Strajnar, P., Širca, S., Geric Stare, B., & Urek, G. (2009). Characterization of the root-knot nematode, Meloidogyne ethiopica Whitehead, 1968, from Slovenia. Russian Journal of Nematology, 17, 135–142.
Širca, S., Urek, G., & Karssen, G. (2004). First report of the root-knot nematode Meloidogyne ethiopica on tomato in Slovenia. Plant disease, 88, 680.
Trudgill, D. L. (1997). Parthenogenetic root-knot nematodes (Meloidogyne spp.); how can these biotrophic endoparasites have such an enormous host range? Plant Pathology, 46, 26–32.
Whitehead, A. G. (1968). Taxonomy of Meloidogyne (Nematoda: Heteroderidae) with description of four new species. Transactions of the Zoological Society of London, 31, 263–401.
Whitehead, A. G. (1969). The distribution of root-knot nematodes (Meloidogyne spp.) in tropical Africa. Nematologica, 15, 315–333.
Acknowledgements
This work is the result of research which was financially supported by the grant L4-1021 of the Slovenian Research Agency and the Ministry of Agriculture, Forestry and Food of the Republic of Slovenia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strajnar, P., Širca, S., Knapič, M. et al. Effect of Slovenian climatic conditions on the development and survival of the root-knot nematode Meloidogyne ethiopica . Eur J Plant Pathol 129, 81–88 (2011). https://doi.org/10.1007/s10658-010-9694-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-010-9694-x