Abstract
The interpretation of information used to defend an assessment that T. indica, the cause of Karnal bunt of wheat, has a high risk of establishing in Europe and of causing significant yield/quality losses is questioned. Karnal bunt has only established in locations that are arid or semi-arid with hot summers and cool/mild winters. There is very strong circumstantial evidence that substantial amounts of seed contaminated with teliospores of T. indica were sown in Europe in the past without the appearance of Karnal bunt. It is unlikely that sufficient numbers of teliospores would survive long enough on the soil surface under European conditions and then synchronise germination during the period at heading when wheat is vulnerable to infection to guarantee disease expression. Karnal bunt is regarded as a minor disease everywhere it occurs. Almost two thirds of European wheat cultivars inoculated by a severe boot injection method have been categorised as either resistant or highly resistant to T. indica. Yield/quality losses would be expected to be low even if the pathogen were capable of establishing in Europe. The status of T. indica as an important quarantine pest is based on the indirect economic consequences of the appearance of the pathogen and not on the direct damage it causes to wheat crops. Arguments in this and previous reviews advocating a more reasoned and comprehensive assessment of the threat to Europe, North America and other locations from T. indica need to be taken into consideration in any new pest risk analyses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Two different opinions have emerged as to the threat that Tilletia indica, the cause of Karnal bunt disease, poses to European wheat crops, A pest risk analysis (PRA) developed under the auspices of European Commission (EC)-funded programme QLK5-1999-01544 to define risks to the European Union (EU) from Karnal bunt contends that it is a serious disease and that the results of associated research support the status of T. indica as a 1/A1 harmful organism under EU legislation (Sansford et al. 2006). However, counter arguments have been advanced that T. indica is a minor pathogen and has a low risk of establishment in Europe (Jones 2007a, b). Its current status as an important worldwide quarantine pest based on direct damage caused to crops has also been questioned (Jones 2007b).
Recently, Sansford et al. (2008) defended the original conclusion about the high risk the pathogen poses to wheat crops in Europe contained in the EU-PRA by endeavouring to challenge key points made by Jones (2007a, b). The five main areas where differences of opinion exist are (1) past opportunities for entry of T. indica into Europe, (2) climatic requirements for disease, (3) inoculum threshold levels that result in disease expression, (4) the use of the Humid Thermal Index model in predicting potential disease distribution and (5) potential economic damage should the disease establish in Europe.
This new review is a response to the arguments raised by Sansford et al. (2008), whose conclusions are refuted. All five main areas of contention are discussed. The case for a low risk of establishment of T. indica in Europe is strengthened and the potential for the pathogen to establish outside its present range discussed with reference to the situation in the Americas. In addition, the importance of T. indica as a pathogen, even if it could establish on European wheat crops, is further questioned. The information and arguments provided in this review and also those of Jones (2007a, b) need to be considered in any new PRA on T. indica developed for Europe or elsewhere.
Karnal bunt
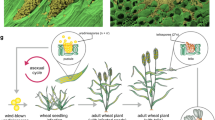
Karnal bunt is a seedborne disease affecting kernels of wheat grown in some arid or semi-arid areas of several countries with hot summers and mild/cool winters. It is known to occur in parts of India, Pakistan, Mexico, Nepal, Iran, Iraq, South Africa and the USA (Jones 2007a). Some other countries, which have been reported as sources of seed contaminated by teliospores of T. indica, have been implicated as locations where the disease may occur (Jones 2007a). In semi-arid regions, the infection of wheat by T. indica is associated with rains and periods of high humidity at heading when wheat is vulnerable to infection. In arid regions, irrigation is considered to play a role in the epidemiology of the pathogen.
Germination of teliospores of T. indica on the soil surface to produce sporidia and secondary sporidia that infect wheat spikes from emergence through anthesis and up to the soft dough stage (Goates and Jackson 2006) requires a soil water content >15% (g/g) and temperatures from 5 to 25°C with an optimum at about 20°C (Murray 2004; Smilanick et al. 1985). If freezing or drying interrupts the incubation of teliospores, the germination process resumes on the return of suitable conditions (Murray 2004; Smilanick et al. 1985; Zhang et al. 1984).
Secondary sporidia have traditionally been thought of as short-lived when desiccated (Smilanick et al. 1989). However, more recent work indicates that desiccated secondary sporidia can survive for extended periods (Carris et al. 2006; Goates 2005), which raises the question as to whether teliospore germination needs to occur precisely at heading to ensure infection. Sporidial inoculum generated by teliospores during rainy periods several weeks prior to heading could survive in the environment until wet and humid conditions during heading stimulate their revival, proliferation and dispersal to spikes.
Other information on the lifecycle of T. indica and factors relevant to its role as a pathogen of wheat has been reported by Bonde et al. (1997), Carris et al. (2006), Jones (2007a), Nagarajan et al. (1997), Murray (2004), Sansford et al. (2006), Singh (2005) and Warham (1986, 1992).
Opportunities for disease spread
Jones (2007a) provided strong evidence that over 1,700 consignments of wheat germplasm distributed to institutes in Europe and other locations by the Centro Internacional de Mejororamiento de Maiz y Trigo (CIMMYT), an international centre in Mexico dedicated to cereal improvement, from the early 1970s until the late 1980s were likely to have been contaminated with teliospores of T. indica. This germplasm, which was intended for yield trials, was sown in the field in Belgium, England, France, Greece, Italy, Finland, Hungary, Germany, Ireland, The Netherlands, Norway, Poland, Romania, Spain, Sweden, the former USSR, Wales and the former Yugoslavia (T. Payne, CIMMYT, El Batan, Texcoco, Mexico, pers. commun.). Data on yield in the different countries were returned to CIMMYT by cooperating institutes for compilation (T. Payne, CIMMYT, El Batan, Texcoco, Mexico, pers. commun.). CIMMYT germplasm was also sent to institutes in the USA and Canada during the same period. Reports of findings of teliospores of T. indica in CIMMYT/Mexican germplasm have appeared in the scientific literature (Diekmann 1987; Lambat et al. 1983; Mendes and Ferreira 1994; Nath et al. 1981; Zhang et al. 1984).
Sansford et al. (2008) have argued that there is no absolute proof that there have been numerous opportunities for T. indica to enter and spread in Europe with germplasm. This is correct, as there have been no published reports of European institutes finding teliospores in wheat germplasm. However, imported CIMMYT germplasm was probably not closely inspected by cooperating European agronomists. What is known is that much potentially contaminated germplasm was sown in Europe over many years and teliospores of T. indica were found in CIMMYT/Mexican germplasm during the same period. This is very strong circumstantial evidence.
As well as the movement of CIMMYT germplasm, many US state and federal wheat breeding programmes took advantage of the warm winter weather in Mexico to multiply wheat lines and progeny out of season prior to 1983. This activity occurred in Karnal bunt-affected areas and was terminated in 1983 when Karnal bunt became a quarantine issue for the USA. It is possible that T. indica was contaminating multiplied wheat seed sent to the USA from Mexico between the early 1970s and 1983 (Babadoost 2000; B. J. Goates, USDA, Aberdeen, Idaho, USA, pers. commun.).
Sansford et al. (2008) do not refer to information provided by Jones (2007a) on the sowing in Europe of hundreds of metric tonnes of commercial wheat seed produced in areas of Mexico where crops were affected by Karnal bunt. Large seed consignments were sent to Greece (in 1973, 1976, 1979, 1980, 1981 and 1982), Portugal (in 1978) and Spain (in 1976, 1980, 1981) without phytosanitary precautions (G. Fuentes-Dávila, INIFAP-CIRNO, Valle del Yaqui, Sonora, Mexico, pers. commun.). Commercial seed consignments were also sent to the USA and other countries (Fuentes-Davila 1996; G. Fuentes-Davila, INIFAP-CIRNO, Valle del Yaqui, Sonora, Mexico, pers. commun.).
Much germplasm/seed contaminated with teliospores of T. indica must have been sown outdoors in Europe in the past. The chances of Karnal bunt appearing in trial plots and farmers’ fields in many European countries would have been high if the pathogen was capable of establishing. Similarly, one would have expected Karnal bunt to have established in the USA outside known affected arid/semi-arid areas if this were possible.
There is no doubt that thick-walled, long-lived teliospores of T. indica can travel long distances and survive as a contaminant of seed. There is also no doubt that teliospores of T. indica have been distributed widely with germplasm and commercial wheat seed in the past. The fact that T. indica, which was first described in India in 1930 (Mitra 1931), but may have been present in Pakistan as early as 1909 (Gill et al. 1993), has still not established outside the arid and semi-arid zones of the world with hot summers and mild/cool winters is a strong argument for the pathogen having very exacting environmental requirements. These requirements seem unlikely to exist in Europe, northern North America and many other locations.
Risk of establishment
Climatic factors influencing disease establishment
In an attempt to justify their assertion that Karnal bunt can establish in Europe, Sansford et al. (2008) argue that Europe has many areas with similar annual rainfall as India, a country where T. indica is found. Jones (2007a) reported that, although wheat is grown in all regions of India (Anon. n.d.a), Karnal bunt is most prevalent in northwest India with incidences in other wheat growing regions being either low, very low or zero (Gill et al. 1993; Joshi et al. 1983). As there have been many opportunities for T. indica to spread throughout India with wheat seed since its first detection (Gill et al. 1993; Singh 2005), there must be environmental factors, which have not been fully investigated, limiting its distribution and incidence. Similarly, Karnal bunt is not found on wheat in Mexico outside the dry, irrigated regions in the northwest, although spread of the pathogen with contaminated seed to other wheat-growing areas would have been inevitable (Jones 2007a). This information again suggests that the environmental requirements of the pathogen are quite exacting.
Rain in India is seasonal. Singh (2005) believes that irrigation provides moisture for teliospores to germinate in the absence of rains when wheat is vulnerable to infection and is the reason why incidence is higher in the northwest region of India than in the drier central and peninsular regions. Warham and Flores (1988) report that >70% of non-irrigated wheat farms in the arid Yaqui Valley in Mexico were free of Karnal bunt. Rains and high humidity are linked to the infection of wheat by T. indica and this indicates that moisture plays a key role in the epidemiology of the pathogen. However, as suggested by Jones (2007a) to explain the occurrence of the disease only in arid/semi-arid locations with hot summers and mild/cool winters, the presence of surface moisture at various times throughout the year, as in many parts of Europe, may result in teliospore germination when infection is not possible (termed ‘suicidal germination’) and be detrimental to survival. Conversely, long dry conditions at certain times of the year, which would not allow germination, may favour survival (Jones 2007a).
Sansford et al. (2008) have argued that, rather than just surviving in dry soils, teliospores also survive in wet soils. They refer to the irrigation of wheat in northwest India and its rotation with irrigated rice in the summer months. They point out that under these conditions, the pathogen still survives to infect wheat. Kaur et al. (2002) have also reported the survival of teliospores in paddy soil in the Punjab. There is other evidence for some teliospores remaining viable and ungerminated in moist soil (Inman et al. 2008).
It is possible that moisture may play a greater role in the erosion of viable inoculum on the soil surface than of inoculum buried in soil. Although it is acknowledged that moisture levels alone may not be the sole determinant for the survival and germination of teliospores, the current distribution of Karnal bunt suggests an association with disease expression and environments that are naturally dry for long periods during the year.
Sansford et al. (2008) raise the issue of Karnal bunt occurring in southern Brazil, which does not have an arid or semi-arid climate, to promote their contention that wheat crops grown in other climates, such as in Europe, are at risk from the disease. The location in question in Brazil has hot summers and temperate winters with rainfall throughout the year (W.C. da Luz, EMBRAPA, Passo Fundo, Rio Grande do Sul, Brazil, pers. commun.). Tilletia indica was reported in seed lots of three different wheat lines harvested in southern Brazil in 1989 (da Luz et al. 1993), but has not been recorded since. The occurrences in Brazil may have been the result of sowing imported seed contaminated with teliospores and favourable, but unusual, environmental conditions that allowed the survival of inoculum until wheat was vulnerable to infection. The erosion of surface inoculum reserves by steady precipitation throughout the year and/or a lack of favourable conditions at heading in subsequent years may explain its failure to establish (Jones 2007a). There is no evidence that T. indica has appeared in Brazil since the 1989 outbreak. The EU does not recognise Brazil as a country where Karnal bunt is found (Anon. 2002).
Teliospore factors influencing disease establishment
The physiology of teliospores of T. indica in relation to dormancy and germination is little understood. It is known that fresh teliospores can remain dormant for several months, which may allow them to survive monsoon rains or artificial wet periods without germinating. Dormant spores beneath the soil are most likely brought to the surface during tilling operations associated with seedbed preparation. It is only those teliospores on or very near the soil surface that can germinate normally to produce sporidia (Smilanick et al. 1985).
Carris et al. (2006) describe three types of dormancy. The first is the dormancy that prevents the majority of freshly harvested teliospores from germinating compared to teliospores from bunted grains that have been stored for several months. The second occurs in teliospores stored for one year or longer when germination rates rarely exceed 50%. Cold temperature-induced dormancy is the third type.
The work of Inman et al. (2008) contains evidence for a proportion of teliospores remaining viable and ungerminated for at least three years under the surface of European soils, which was most likely moist for considerable periods. This experimental result has been used to suggest that inoculum would be able to survive in European soils for extended periods and be available at heading to initiate disease. However, the link between survival of buried teliospores and potential distribution of Karnal bunt is unproven and tenuous. Similar longevity experiments have been undertaken in Arizona, Georgia, Kansas, Maryland and Montana and viable teliospores were recovered after burial for long periods (Babadoost et al. 2004; Bonde et al. 2004a, b). It is highly likely that teliospores of T. indica can survive for extended periods in soils at locations where the pathogen is never going to establish.
It has been claimed that dormancy is only broken in those teliospores on or very near the soil surface (Sansford et al. 2008). Most teliospores are dormant immediately after they develop, but there is little evidence for a burial-induced dormancy except in soil in cold regions, which is most likely cold-induced (Inman et al. 2008). If a type of long-term dormancy is preventing germination under the soil surface, then it must be rapidly broken in at least a proportion of these teliospores after extraction from soil and incubation on water agar. Could trimethylamine, which is produced by teliospores of T. indica (Joshi et al. 1983) and is a known self-inhibitor (Trione 1977) or other inhibitors play a role in maintaining dormancy under the soil surface?
After recovery from the soil, the germination of teliospores on water agar is greater under light than in darkness (Smilanick et al. 1985; Zhang et al. 1984) and light has been reported to help break dormancy (Nagarajan et al. 1997). Other factors, such as oxygen availability (G.L. Petersen, USDA, Ft Detrick, Maryland, USA, pers. commun.), may also play a role. Transfer from soil to the soil surface could result in soil-associated constraints on physiological reactions that induce dormancy being lifted in a proportion of teliospores.
What is known is that usually less than half of teliospores buried in soil for extended periods are recoverable and less than half of those that are recoverable germinate when incubated on water agar (Babadoost et al. 2004; Bonde et al. 2004a, b; Inman et al. 2008). In addition, it is known that the recovery of teliospores incubated on soil surfaces in conditions of optimum moisture for germination usually rapidly diminishes and a large proportion of the remainder do not germinate when placed on water agar (Petersen et al. 2006). This indicates that the decline in viable inoculum may accelerate when teliospores reach the surface in a wet environment because of a breaking of dormancy in many followed by ‘suicidal germination’. Very few remain viable and ungerminated after a few months (Petersen et al. 2006). Cool climates with frequent rains and reduced evaporation, as in most of Europe for much of the year, would be expected to result in more opportunities for ‘suicidal germination’ with less chance of establishment.
Limitations on disease establishment in the USA
Teliospores of T. indica have been intercepted frequently at the USA border with Mexico on wheat seed and in railroad cars, personal baggage, etc. (Marshall et al. 2003). Viable teliospores have been found at 3,000 m altitude over burning stubble fields in Mexico (Bonde et al. 1987) indicating aerial dissemination over the border into the USA is also a possibility. Despite all the opportunities for movement into the USA, T. indica has only been seen in parts of California, Arizona and Texas (Rush et al. 2005).
Sansford et al. (2008) believe that a lack of reported disease outbreaks does not mean that T. indica is confined to California, Arizona and Texas in the USA. They argue that distribution may be far greater than has been recognised because (1) wheat grain from areas where the disease is not known to occur is being inspected for disease symptoms and not teliospores and (2) a long time interval may occur between arrival of teliospores in a new area and the observation of symptoms. This argument raises the possibility that T. indica may not be confined to hot arid and semi-arid regions of the world and may be able to establish in Europe. However, although teliospores of T. indica have undoubtedly spread throughout the USA in the past with seed and perhaps by wind, there is no evidence that this dissemination has resulted or will result in widespread disease. Contrary to the assertion by Sansford et al. (2008) that teliospores are not actively sought in US-produced grain, the extremely sensitive seed wash or sieve assay, which allows the detection of teliospores, has been undertaken in many states on a large scale, especially in the years immediately following the first outbreaks. Full details of all bunted kernal and sieve surveys for the disease and pathogen from 1996 until 2007 are given by Anon. (2008b). This unprecedented analysis of wheat grain in the USA for T. indica and Karnal bunt strongly indicates the disease has not spread in the USA over the past 12 years.
Karnal bunt has not been detected in Texas wheat fields since 2002, which has been attributed to strict quarantine regulations, unsuitable weather for infection in some years and the removal of wheat from rotation schedules (Workneh et al. 2008). However, wheat has been grown at least once in all of the fields where Karnal bunt has been found since 2002 and the disease has not reappeared (F. Workneh, Texas A&M University, Bushland, USA, pers. commun.). All fields in the central San Saba and some in the north-central Olney areas of Texas, where Karnal bunt has been reported, have now been deregulated because of a lack of disease (Anon. 2006, 2008a; T.W. Allen, University of Mississippi, Stoneville, USA, pers. commun.). This is further evidence for the two areas of Texas where Karnal bunt has occurred being marginal for establishment (Jones 2007a), as elimination is likely to be difficult to achieve in locations where environmental conditions are optimal for establishment. Areas in the USA affected by Karnal bunt are diminishing. In 2007, Maricopa County in Arizona was the only location surveyed in the USA where Karnal bunt had a status other than ‘not found’ (Anon. 2008b).
Approximately 35 years have elapsed since there have been possibilities for teliospores to spread aerially and on land transport from Mexico to the USA. Approximately 20–30 years have elapsed since teliospores were most likely introduced into many states with wheat germplasm and commercial seed lots from Mexico. Despite all the time that has elapsed, there is still no evidence that T. indica has been found in the USA other than in certain arid and semi-arid locations in southern California, Arizona and Texas. Again, this supports the contention that the pathogen has exacting environmental requirements that are unlikely to be found in most localities including Europe and northern North America.
Inoculum threshold level needed for disease establishment
Based on the reproductive strategy of T. indica, Jones (2007a) suggested that there may be a minimum inoculum threshold level for telisopores that allows establishment and concentrations below this level do not permit the pathogen to survive. This is a consequence of the Allee effect that is believed to reduce reproductive success for lower population densities of the pathogen. The Allee effect is thought to be important at the frontier of invasion and when the environment is non-conducive for the production of secondary sporidia (Garrett and Bowden 2002). Sansford et al. (2008) consider that the evidence does not support this theory and assert that disease can arise and be maintained by very few teliospores producing copious amounts of secondary sporidia (Murray and Sansford 2005). However, this is also unproven.
Many plant pathogens, whether aerial or soilborne, are believed to have inoculum threshold levels. Goates and Petersen (1999) have shown that quite high concentrations of teliospores of T. controversa in the soil in Montana and Utah were required to cause symptoms of dwarf bunt in a susceptible wheat cultivar. More inoculum was needed for disease to develop in a partially resistant cultivar. The authors concluded that, although only one teliospore was necessary to cause infection in theory, the chances of this occurring in nature were extremely remote. They believed that the results of their research clearly demonstrated the inter-relationship between teliospore concentration on the soil surface and the environment to cause different levels of disease and how more inoculum was required to produce disease under less-conducive conditions.
Although T. controversa has a different infection strategy to T. indica, a similar situation as regards a minimum threshold level of inoculum needed to cause disease being dependent on environmental and wheat cultivar resistance seems likely. Although one or two viable teliospores on the surface of a wheat field during the latter stages of crop development may be all that is theoretically required for disease, no results of experimental work undertaken to prove this point have ever been published. It is more likely that a minimum concentration of teliospores in a given area would be required to ensure disease under optimal conditions. A much higher concentration may be required under sub-optimal conditions or when cultivars with even just partial resistance are being grown.
In 2002, small numbers of T. indica teliospores were found in soil in fields in regulated areas of Texas that had records of Karnal bunt and also in neighbouring fields that had never been tested positive for bunted kernels (Stein et al. 2005). This was interpreted as evidence that a few teliospores are unlikely to cause disease. Further evidence for the existence of a minimum threshold level comes from research undertaken in a wheat field positive for T. indica in Arizona by Allen et al. (2006). Despite a 2-year history of Karnal bunt and the finding of between 25 and 704 teliospores in 25 g soil samples from experimental rows, no bunted kernels were recovered in wheat from the same rows in 2005 even though weather conditions at heading were conducive for infection (T.W. Allen, Mississippi State University, Stoneville, USA, pers. commun.). This result is hardly likely to have occurred had just a few viable teliospores been able to cause infection.
In years when Karnal bunt appeared, its incidence in regulated Texan wheat fields has been very low (Allen et al. 2008; Workneh et al. 2008). In 2002, teliospore numbers in selected regulated fields were found to range from 0 to 1,305/25 g of soil in spot locations (Allen et al. 2008). The research showed that there was a widespread distribution of teliospores with high concentrations in places. As no disease appeared in these fields after 2002, infection leading to disease development would seem not to be guaranteed even with relatively high teliospore levels. Disease would undoubtedly have occurred if it could be caused by just a few teliospores, as there were most likely many viable teliospores still remaining in the soil after 2002 and weather conditions in 2004 were conducive for infection (Workneh et al. 2008).
Concentrations of viable teliospores on the soil surface would most likely be influenced by the local climate throughout the year. It is possible that the inoculum threshold levels for particular environments have not been reached in many locations where teliospores of T. indica have been distributed in the past and this may be why disease has not spread far in the USA and has not been seen in Europe.
Use of the Humid Thermal Index model to predict disease distribution
Baker et al. (2005, 2006) used the Humid Thermal Index (HTI) disease prediction model of Jhorar et al. (1992) and wheat phenology models to determine if environmental conditions in Europe are suitable for infection by T. indica when wheat crops are vulnerable. Their analysis of the number of consecutive years during which the HTI was outside the critical range that allows infection by T. indica showed that significant areas of northern Europe (including England and Denmark) had gaps of at least four years and that 21% of all European arable areas had gaps of over four years. The risk of establishment was believed to be lower further north and further west in Europe, as northern regions may on occasion be too cold and western regions too moist at heading to favour infection and disease development. Areas calculated to be at greatest risk on the basis of regularity of conditions suitable for infection during periods of wheat vulnerability were in southern and eastern Europe though high temperatures in the extreme south were also unfavourable. The conclusion of the work was that on a year-by-year basis there are always years and locations in Europe favourable to infection and disease development. However, this prediction relies on the assumption that sufficient inoculum to cause disease would also be available on the soil surface when wheat was vulnerable to infection and this, as argued by Jones (2007a), is by no means certain. The HTI model when used to predict disease distribution does not take climatic conditions throughout the year, which are likely in many places to be deleterious to teliospore survival, into account (Jones 2007a). It is still by no means certain that threshold levels of germinating teliospores needed to permit disease establishment in Europe are sustainable or even achievable.
Sansford et al. (2008) state that the analysis of Jones (2007a) overlooks the critical work of Petersen et al. (2006) on the timing of teliospore germination in relation to European wheat crop phenology. They argue that the results of this research show that the HTI model is valid for predicting disease distribution because, even under high soil moisture conditions favourable for early germination of surface-borne teliospores, some remain available and capable of germination at the critical time for infection under a range of simulated European field temperature condition.
Petersen et al. (2006) have shown that only a very small proportion of original teliospores (estimated from histograms to be <1% for simulated English, Italian and Hungarian growing temperatures) deposited on wet soil surfaces survive until after the period of wheat vulnerability. However, these may not constitute a minimum threshold level even if their germination was synchronised. The teliospores may indeed be dormant. Petersen et al. (2006) maintain that the results suggest that some viable teliospores would most likely be present at the time of wheat heading in Europe, especially if they were produced at the end of the preceding growing season and retained some dormancy at the time of sowing and early crop development. However, they acknowledge that they were unable to predict if these teliospores would germinate at the optimum time to initiate infection in nature. Petersen et al. (2006) believe that further research on proliferation of secondary sporidia, the infection process and post-infection disease development under diverse European environments is required. They conclude that the general decline in viability in teliospores (under very wet conditions, but presumably also under fluctuating wet/dry conditions, which was believed by the authors to be closer to natural conditions and gave similar results) would lead to undetectable levels of disease unless conditions conducive for infection occurred at a frequency high enough to continue to regularly add viable teliospores to the soil. They also believe that, as there is no quantitative data that establishes a relationship between the number of teliospores on the soil surface and the levels of disease produced, it was not possible to predict the rate of disease decline in relation to infection frequency.
Petersen et al. (2006) admit that much uncertainty remains as to what can be deduced from their experimental results. Nevertheless, Sansford et al. (2006, 2008) have used the results to make a case for the survival of teliospores in sufficient numbers to allow disease establishment in many locations in Europe. Again, the failure of T. indica to establish in Europe despite many opportunities to do so in the past would suggest that unknown factors, which may be related to an unsustainable inoculum threshold level and an unsuitable environment, are playing a significant role in determining distribution.
Economic damage caused by Karnal bunt
Yield and quality loss
When first discovered in the USA, it was acknowledged that Karnal bunt was not a devastating disease, but had national and international implications for the US wheat export markets (Ykema et al. 1996). The incidence and severity of Karnal bunt has been very low in the USA with the vast majority of positive fields having an infection level of 0.02% or less (Rush et al. 2005). From a yield and quality perspective, the disease has not had any impact in the USA (Rush et al. 2005). Carris et al. (2006) state that Karnal bunt has historically caused minor overall yield and quality losses in countries where it occurs and that significant yield and quality losses are typically localised, occurring in highly susceptible cultivars grown in fields with high inoculum density during seasons with unusually favourable weather conditions. The American Phytopathology Society have concluded that Karnal bunt is a minor disease and what little risk does exist can be effectively managed without the use of quarantines (Beattie and Biggerstaff 1999). Karnal bunt has been described by Australian scientists as having a minimal impact on crop yield, but is considered a disease of political and quarantine importance (Stansbury et al. 2002).
The general consensus is that yield/quality losses caused by T. indica have never been great and economic repercussions have never been serious (Beattie and Biggerstaff 1999; Bonde et al. 1997; Cardwell et al. 2003; Carris et al. 2006; Jones 2007b; Rush et al. 2005; Singh 2005). However, to justify in part their claim that Karnal bunt could seriously affect European wheat, Sansford et al. (2008) state that “grain infection frequently exceeds 3%” in India. Published evidence does not support this statement. Incidence may have been occasionally high in some fields in the past (Gill et al. 1993), but even during the years of worst incidence, losses were only 0.2 to 0.5% of total production (Joshi et al. 1980). Data on disease incidence reported by Gill et al. (1993) and used by Sansford et al. (2008) to justify high losses would seem in many instances to refer to percentages of grain samples affected at markets, which is not an indication of field incidence. Gill et al. (1993) correlate years of low disease incidence to the cultivation of less susceptible wheat cultivars. For other information on disease incidence in India, the reader is referred to Kaur et al. (2002) and Sharma et al. (2004). In Mexico, average yield loss as been estimated at 0.12%/year (Brennan et al. 1992). Infection levels of 3% in individual farmers’ fields have been reported as rare even in years when disease levels were at their highest (Warham 1992).
Sansford et al. (2008) assert that there is a “lack of resistance in current European wheat cultivars” and “most were susceptible and some highly susceptible” in inoculation experiments undertaken by Riccioni et al. (2008). They suggest that this lack of resistance would mean that yield/quality losses would be higher in Europe than where T. indica is endemic. However, this is unproven and most likely incorrect.
Because high levels of inoculum are used in inoculation experiments, the amount of disease generated is usually far greater than would be expected under field conditions. Results have to be treated cautiously and in comparison with the reactions of host cultivars with known resistance/susceptibility. Riccioni et al. (2008) used existing disease rating scales developed to interpret interactions between wheat and T. indica to assign each cultivar tested by a severe boot injection method a ‘susceptibility category’. Forty of the 41 European bread and durum wheat cultivars tested were categorised as being less susceptible than the highly susceptible Indian cultivar ‘WL 711’. Six were categorised as highly resistant, 20 as resistant, 11 as moderately susceptible, three as susceptible and only one as highly susceptible. Therefore, information provided by Riccioni et al. (2008) suggests that almost two thirds of European cultivars challenged by boot injection have some physiological resistance to T. indica. Consequently, the publication that Sansford et al. (2008) cite to support their assertion that European wheat cultivars are mostly susceptible or highly susceptible actually reports the opposite.
The boot injection and spray inoculation methods used by Riccioni et al. (2008) do not show that average levels of Karnal bunt on European wheat would be any higher than average levels on Indian wheat today. Even if T. indica could establish in Europe, any highly susceptible cultivars found under field conditions would most likely be phased out and replaced by existing resistant lines. A breeding programme may not be needed. No resistant cultivars have been released in the USA as a response to Karnal bunt (B. J. Goates, USDA, Aberdeen, Idaho, USA, pers. commun.). Karnal bunt is being effectively managed in the USA with existing cultivars and very little damage is being caused (Rush et al. 2005).
Sansford et al. (2008) assert that under European quality assurance schemes, grain contaminated with T. indica is likely to be rejected or downgraded to animal feed. However, this may not necessarily be the case even if T. indica could establish in Europe. In the UK, grain is cleaned if an analysis of a sample by the grain merchant determines that this is necessary. If grain needs cleaning, this is done before it goes to the millers. Millers will only accept grain that meets their standards. If T. indica could establish in Europe, it is highly likely that bunted gain would be handled just like other bunts and smuts of wheat, which are currently found in Europe. Tilletia. indica poses no serious human or animal health issues as does grain affected by Fusarium head blight, which is the disease currently of great concern in Europe and elsewhere because of harmful mycotoxins that can appear in flour and animal feed (Bottalica and Perrone 2002; Schollenberger et al. 2002).
Costs associated with disease introduction
Sansford et al. (2008) state that costs associated with an outbreak of Karnal bunt were fully considered in the EU-PRA (Sansford et al. 2006). These figures were used by Jones (2007b) to show that the effects of Karnal bunt on yield and quality amounted to <0.5% of the total outbreak costs. Just over 99.5% of costs were attributed to other considerations, such as restrictions on trade, surveillance, seed and grain testing, disinfestations of grain silos and trucks and other quarantine measures to prevent local and international spread. This analysis emphasised the relative low cost of actual damage to the crop compared to the high costs arising as a consequence of marketing implications. It showed that the economic impact was almost entirely a result of the unjustified status of T. indica as an important quarantine pest.
The use of a PRA to determine quarantine pest status
A PRA is a tool for determining whether a candidate organism is harmful and suitable for quarantine pest status. The Sanitary and Phytosanitary Agreement of the World Trade Organisation requires that quarantine restrictions, which may impede trade, be justified by PRAs (Anon. n.d.b). Pest risk analysts have to consider the probability of entry, establishment and spread of an exotic pathogen plus the potential to cause economic and/or environmental damage. If the organism has the potential to establish and cause serious damage, management strategies aimed to prevent introduction are then devised. However, these strategies should not be any more trade restrictive than is absolutely necessary.
Factors associated with climate, teliospore physiology and a relatively high teliospore threshold level required to cause disease in environmentally sub-optimal locations have been proposed as possible explanations for the limited distribution of T. indica (Jones 2007a). Although not conclusive, this information provides a valid and strong argument for T. indica not being able to establish in Europe and many other locations. Uncertainties and arguments in favour of a low risk of establishment need to be included in any new PRA if the analysis is to accurately reflect all possibilities and opinions.
Jones (2007b) has argued that losses to wheat in Europe, even if T. indica could establish, would be slight, as in the USA. Indeed, the results of Riccioni et al. (2008) suggest that the majority of European wheat cultivars seem likely to have resistance. Jones (2007b) has called for world plant health authorities to reconsider the quarantine significance of the pathogen given that most costs should it enter a country and establish are almost entirely based on its effects on trade, such as control, surveillance and hygiene measures that would need to be taken to satisfy wheat importing nations. Indirect impacts caused by the appearance of the disease seem to be the major reason for its important quarantine pest status and not its direct effect on the crop.
Quarantine measures restricting the importation of grain for processing into flour would seem in need of rationalisation on both sides of the Atlantic. Despite the declaration by Sansford et al. (2008) that grain poses a significant risk, there is no evidence that Karnal bunt has been introduced to any country with grain. Contaminated seed is a much more important pathway of introduction because seed is planted and this places any accompanying inoculum in a situation where it can infect crops. Wheat grain imports could be transferred from docks to mills in closed containers with little chance of any pathogens escaping into the rural environment even if establishment was possible. A lowering of the perceived risk from grain would be a first step into placing Karnal bunt disease in its proper perspective (Jones 2007b).
If Karnal bunt could establish in Europe, Sansford et al. (2008) would be justified under the present political circumstances in believing that the very costly indirect effects arising as a result of current plant health policy on Karnal bunt are sufficient justification for defining T. indica as an important quarantine pest. However, this is not based on its biological potential as a pathogen. Jones (2007b) calls for international cooperation that will result in a more realistic evaluation of the threat to global wheat crops posed by T. indica.
Summary
Jones (2007a, b) contends that T. indica has a low risk of establishment in Europe and in North America north of its present range, and is a minor pathogen of wheat based on direct crop losses. Sansford et al. (2008) have attempted to refute the arguments of Jones (2007a, b) that support this contention. Interpretations of research work and other information used by Sansford et al. (2008) to come to their conclusions are challenged in this review.
Karnal bunt has had many opportunities to spread since it was first recognised in India in 1930 (Mitra 1931). Records from CIMMYT and other sources show that significant amounts of potentially contaminated wheat seed originating in Mexico were sown in many locations around the world, including Europe and northern North America, about 20 to 36 years ago (Jones 2007a). However, T. indica is still only confirmed in arid and semi-arid regions with hot summers and mild/cool winters. This strongly suggests that the pathogen may not be able to establish in different environments. There would appear to be a low risk of establishment in Europe and northern North America.
Sansford et al. (2008) maintain that there may be a long period between introduction of T. indica to a new wheat-growing area and the establishment of a detectable disease. This argument is used to suggest that Karnal bunt may have already established in areas of the USA outside the arid and semi-arid zones in the southwest, but has not yet been recognised. Some time undoubtedly elapses between pathogen introduction and symptom discovery, but extensive surveys of grain for bunted kernels and teliospores undertaken in the USA from 1996 onwards (Anon. 2008b) make undiscovered outbreaks unlikely. In addition, the disease seems to be in retreat in the USA (Anon. 2006, 2008a, b).
Sansford et al. (2008) speculate that few teliospores are needed for disease establishment. Arguments are proposed that a minimum threshold level of teliospores seems likely for disease development and that this level may vary according to the local environment and resistance of local wheat cultivars. It is possible that minimum threshold levels needed for establishment in many environments may be quite high and have not been exceeded or cannot be maintained.
The HTI model for predicting incidence of Karnal bunt (Jhorar et al. 1992) relies on sufficient viable teliospores to initiate disease being present on the soil surface at heading (Sharma and Nanda 2003). However, problems arise when the HTI model alone is used to make predictions about the potential distribution of T. indica (Baker et al. 2005, 2006), as it does not take into account factors that relate to teliospore survival throughout the year. Although teliospore longevity studies show that some teliospores may remain viable buried in European soils for long periods (Inman et al. 2008), this does not prove that conditions in Europe are suitable for the establishment of Karnal bunt. Research indicates that teliospores rapidly germinate or lose viability on wet or alternating wet/dry soil surfaces under most simulated European wheat-growing temperatures with only a few surviving until heading (Petersen et al. 2006). There is no proof that sufficient numbers of surviving teliospores would synchronise their germination at the time of heading to ensure disease expression.
Inoculation studies indicate that almost two thirds of a selection of European bread and durum wheat cultivars inoculated by boot injection has some resistance to T. indica (Riccioni et al. 2008). Therefore, if T. indica could establish in Europe, it is hard to justify the claim by Sansford et al. (2008) that the incidence of Karnal bunt and damage caused by the disease would be high because of the susceptibility of European wheat cultivars.
The main conclusions of Sansford et al. (2006, 2008), as regards the risk that T. indica poses to European wheat crops, remain unsubstantiated. Many of the arguments of Sansford et al. (2006, 2008) rely on assumptions that require verification. Some appear to be as a result of misinterpretations of the data. More research has to be undertaken in order for teliospore physiology and the epidemiology of the pathogen to be fully understood. Only with this information may the suitability of different environments for the establishment of T. indica be found and risks refined. However, given current knowledge, it seems highly probable that the pathogen is unable to establish in environments other than those similar to where it now occurs.
Uncertainties and viable alternative theories on risk need to be documented and more fully discussed in future PRAs for T. indica. World plant health authorities need to place T. indica in a proper perspective that may allow current regulations governing wheat trade to be relaxed.
References
Allen, T. W., Boratyniski, T. N., & Rush, C. M. (2006). Tilletia indica teliospore distribution in a naturally infested Arizona wheat field. Phytopathology, 96 (Supplement 6), S5.
Allen, T. W., Maples, H. W., Workneh, F., & Rush, C. M. (2008). Distribution and recovery of Tilletia indica teliospores from regulated wheat fields in Texas. Plant Disease, 92, 344–350.
Anon. (2002). Unofficial Consolidation of the European Community Plant Health Directive 2000/29/EC as last amended by Commission Directive 2002/28/EC (of 19 March 2002). Brussels, Belgium: Commission of the European Communities, Directorate-General for Agriculture.
Anon. (2006). Removal of regulated areas in Texas. Federal Register November 22, 2006. USDA-APHIS, Washington, USA. Retrieved May 10, 2008 from http://a257.g.akamaitech.net/7/257/2422/01jan20061800/edocket.access.gpo.gov/2006/E6-19769.htm
Anon. (2008a). Removal of regulated areas in Texas. Federal Register April 7, 2008. Retrieved May 10, 2008, from http://edocket.access.gpo.gov/2008/E8-7194.htm
Anon. (2008b). Reported status of Karnal bunt—Tilletia (Neovossia) indica. National Agricultural Pest Information System (NAPIS) Pest Tracker. NAPIS Map Option. Retrieved May 20, 2008 from http://pest.ceris.purdue.edu/searchpest.php?txtSearch=karnal+bunt&selectName=FLBBNBF
Anon. (n.d.a). Agro-climatic zones and wheat growing zones of India. Retrieved May 10, 2008 from http://krishisewa.com/krishi/Azone.html
Anon. (n.d.b). The WTO agreement on the application of sanitary and phytosanitary measures (SPS Agreement). World Trade Organization, Geneva, Switzerland Retrieved May 10, 2008 from http://www.wto.org/English/tratop_e/sps_e/spsagr_e.htm
Babadoost, M. (2000). Comments on the zero-tolerance quarantine of Karnal bunt of wheat. Plant Disease, 84, 711–712.
Babadoost, M., Mathre, D. E., Johnston, R. H., & Bonde, M. R. (2004). Survival of telisopores of Tilletia indica in soil. Plant Disease, 88, 56–62.
Baker, R., Gioli, B., Porter, J. R., & Sansford, C. (2006). Maps of Europe and key durum and bread wheat production locations showing the area at risk from T. indica establishment based on existing disease models (DL1·2) and revised maps using new pathogen data obtained in work packages 2, 3 and 4 (DL1·3). EC Fifth Framework Project QLK5-1999-01554: Risks associated with Tilletia indica, the newly-listed EU quarantine pathogen, the cause of Karnal bunt of wheat, Deliverable Reports 1·2 and 1·3. Retrieved May 10, 2008 from http://karnalpublic.pestrisk.net/deliverables
Baker, R. H. A., Sansford, C. E., Gioli, B., Migletta, F., Porter, J. R., & Ewert, F. (2005). Combining a disease model with a crop phenology model to assess and map pest risk: Karnal bunt disease (Tilletia indica) of wheat in Europe. In: Plant Protection and Plant Health in Europe: Introduction and Spread of Invasive Species, Symposium Proceedings No 81 (pp. 89–94) British Crop Protection Council, Alton, Hampshire, UK.
Beattie, B. R., & Biggerstaff, D. R. (1999). Karnal bunt, a wimp of a disease—but an irresistible political opportunity. Choices, Second Quarter, 1999, 4–8.
Bonde, M. R., Berner, D. K., Nester, S. E., Peterson, G. L., Olsen, M. W., Cunfer, B. M., et al. (2004a). Survival of Tilletia indica teliospores in different soils. Plant Disease, 88, 316–324.
Bonde, M. R., Nester, S. E., Olsen, M. W., & Berner, D. K. (2004b). Survival of teliospores of Tilletia indica in Arizona field soils. Plant Disease, 88, 804–810.
Bonde, M. R., Petersen, G. L., Schaad, N. W., & Smilanick, J. L. (1997). Karnal bunt of wheat. Plant Disease, 81, 1370–1377.
Bonde, M. R., Prescott, J. M., Matsumato, T. T., & Petersen, G. L. (1987). Possible dissemination of teliospores of Tilletia indica by practice of stubble burning. Phytopathology, 77, 639.
Bottalica, A., & Perrone, G. (2002). Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. European Journal of Plant Pathology, 108, 611–624.
Brennan, J. P., Wareham, E. J., & Byerlee, D. (1992). Evaluating the economic impact of quality-reducing, seed-borne diseases. Lessons from Karnal bunt of wheat. Agricultural Economics, 6, 345–352.
Cardwell, K. F., Dowell, F., Mitchel, J., Riemenschneider, R., Spaide, R., & Volke, G. (2003). 5.12 Phytosanitary versus regulatory risk of losses due to wheat Karnal bunt in the USA. Abstracts of Offered Papers at the 8th International Congress of Plant Pathology, Christchurch, New Zealand, 2–7 February 2003, 2, 59.
Carris, L. M., Castlebury, L. A., & Goates, B. J. (2006). Non-systemic bunt fungi—Tilletia indica and T. horrida: a review of history, systematics and biology. Annual Review of Phytopathology, 44, 113–133.
da Luz, W. C., Mendes, M. A. S., Ferreira, M. A. S. V., & Urben, A. F. (1993). Tilletia indica on wheat in the south of the state of Rio Grande do Sul and measures for its eradication. Fitopatologica Brasileira, 18 (Supplement), 329.
Diekmann, M. (1987). Monitoring the presence of Karnal bunt (Tilletia indica) in germplasm exchange at ICARDA. Phytopathologia Mediterranea, 26, 59–60.
Fuentes-Davila, G. (1996). Karnal bunt in Mexico. Transcripts of the American Phytopathological Society Karnal Bunt Symposium June 14–August 16, 1996. Retrieved May 10, 2008 from http://www.apsnet.org/online/proceedings/karnal/kbspaper/artmex.htm
Garrett, K. A., & Bowden, R. L. (2002). An Allee effect reduces the invasive potential of Tilletia indica. Phytopathology, 92, 1152–1159.
Gill, K. S., Sharma, I., & Aujila, S. S. (1993). Karnal bunt and wheat production. Punjab Agricultural University, Ludhiana, India: PAU.
Goates, B. (2005). Durability of secondary sporidia of floret infecting Tilletia Species; Implications for epidemiology. Phytopathology, 95, 961.
Goates, B. J., & Jackson, E. W. (2006). Susceptibility of wheat to Tilletia indica during stages of wheat development. Phytopathology, 96, 962–966.
Goates, B. J., & Petersen, G. L. (1999). Relationship between soilborne and seedborne inoculum density and the incidence of dwarf bunt of wheat. Plant Disease, 83, 819–824.
Inman, A., Magnus, H. A., Riccioni, L., Hughes, K., Coates, M., Barnes, A., et al. (2008). Survival of Tilletia indica teliospores under European soil conditions. Plant Pathology, 57, 290–300.
Jhorar, O. P., Mavi, H. S., Sharma, I., Mahi, G. S., Mathauda, S. S., & Singh, G. (1992). A biometeorological model for forecasting Karnal bunt disease of wheat. Plant Disease Research, 7, 204–209.
Jones, D. R. (2007a). Arguments for a low risk of establishment of Karnal bunt in Europe. European Journal of Plant Pathology, 118, 93–104.
Jones, D. R. (2007b). A reappraisal of the current status of Tilletia indica as an important quarantine pest for Europe. European Journal of Plant Pathology, 118, 105–13.
Joshi, L. M., Singh, D. V., & Srivastava, K. D. (1980). Wheat Disease Survey—I. Karnal bunt 1975–1980. Wheat Pathology Series No. 7., Division of Mycology and Plant Pathology, Indian Agricultural Research Institute, New Delhi, India.
Joshi, L. M., Singh, D. V., Srivastava, K. D., & Wilcoxson, R. D. (1983). Karnal bunt: a minor disease that is now a threat to wheat. The Botanical Review, 49, 309–329.
Kaur, S., Singh, M., & Singh, K. (2002). Factors affecting telial and sporidial inoculum of Neovossia indica. Indian Phytopathology, 55, 14–18.
Lambat, A. K., Nath, R., Mukewar, P. M., Majumdar, A., Rani, I., Kaur, P., et al. (1983). International spread of Karnal bunt of wheat. Phytopathologica Mediterranea, 22, 213–214.
Marshall, D., Work, T. T., & Cavey, J. F. (2003). Invasion pathways of Karnal bunt of wheat into the United States. Plant Disease, 87, 999–1003.
Mendes, M. A. S., & Ferriera, M. A. S. V. (1994). Pathogenic fungi detected in vegetable germplasm introduced into Brazil from 1990 to 1992. Fitopatologica Brasileira, 19, 449–454.
Mitra, M. (1931). A new bunt disease of wheat in India. Annals of Applied Biology, 18, 178–9.
Murray, G. M. (2004). Evaluation of published data for Tilletia indica to compare existing disease models in relation to data obtained in Workpackage 2, 3 and 4. EC Fifth Framework Project QLK5-1999-01554: Risks associated with Tilletia indica, the newly-listed EU quarantine pathogen, the cause of Karnal bunt of wheat, Deliverable Report 1·4. Retrieved May 10, 2008 from http://karnalpublic.pestrisk.net/deliverables
Murray, G. M., & Sansford, C. E. (2005). How Tilletia indica overcomes the Allee effect. In: Proceedings of the 15th Biennial Australasian Plant Pathology Society Conference, 2005, APPS, 256.
Nagarajan, S., Aujla, S. S., Nanda, G. S., Sharma, I., Goel, L. B., Kumar, J., & Singh, D. V. (1997). Karnal bunt (Tilletia indica) of wheat—a review. Review of Plant Pathology, 76, 1207–1214.
Nath, R., Lambat, A. K., Mukewar, P. M., & Rani, I. (1981). Interceptions of pathogenic fungi on imported seed and planting material. Indian Phytopathology, 34, 282-286.
Petersen, G. L., Leth, V., Thinggard, K., & Sansford, C. (2006). Report on teliospore dormancy and germination under a range of abiotic conditions, interpreting the results in relation to European conditions and predicting the likely timing of teliospore germination in Europe. EC Fifth Framework Project QLK5-1999-01554: Risks associated with Tilletia indica, the newly-listed EU quarantine pathogen, the cause of Karnal bunt of wheat, Deliverable Report 4·1. Retrieved May 10, 2008 from http://karnalpublic.pestrisk.net/deliverables
Riccioni, L., Inman, A., Magnus, H. A., Valvasson, M., Porta-Puglia, A., Conca, G., et al. (2008). Susceptibility of European bread and durum wheat cultivars to Tilletia indica. Plant Pathology, 57, 612–622.
Rush, C. M., Riemenschneider, R., Stein, J. M., Boratyniski, T., Bowden, R. L., & Royer, M. H. (2005). Status of Karnal bunt of wheat in the United States 1996 to 2004. Plant Disease, 89, 212–223.
Sansford, C., Baker, R., Brennan, J., Ewert, F., Gioli, B., Inman, A., et al. (2006). Report on the risk of entry, establishment and socio-economic loss for Tilletia indica in the European Union (DL6·1) and determination and report on the most appropriate risk management scheme for Tilletia indica in the EU in relation to the assessed level of risk (DL6·5) EC Fifth Framework Project QLK5-1999-01554: Risks associated with Tilletia indica, the newly-listed EU quarantine pathogen, the cause of Karnal bunt of wheat, Deliverable Reports 6·1 and 6·5. Retrieved May 10, 2008 from http://karnalpublic.pestrisk.net/deliverables
Sansford, C. E., Baker, R. H. A., Brennan, J. P., Ewert, F., Gioli, B., Inman, A., et al. (2008). The new pest risk analysis for Tilletia indica, the cause of Karnal bunt of wheat, continues to support the quarantine status of the pathogen in Europe. Plant Pathology, 57, 603–611.
Schollenberger, M., Jara, H. T., Suchy, S., Drochner, W., & Müller, H.-M. (2002). Fusarium toxins in wheat flour collected in an area of southwest Germany. International Journal of Food Microbiology, 72, 85–89.
Sharma, I., & Nanda, G. S. (2003). Effect of prolonged period of fog/rain from sowing of wheat to pre-boot stage on Karnal bunt development. Annals of Plant Protection Services, 11, 93–95.
Sharma, I., Nanda, G. S., Singh, H., & Sharma, R. C. (2004). Status of Karnal bunt disease of wheat in Punjab (1994–2004). Indian Phytopathology, 57, 435–439.
Singh, D. V. (2005). Karnal bunt of wheat: A global perspective. Indian Phytopathology, 58, 1–9.
Smilanick, J. L., Hoffmann, J. A., & Royer, M. H. (1985). Effect of temperature, pH, light and desiccation on teliospore germination of Tilletia indica. Phytopathology, 75, 1428–1431.
Smilanick, J. L., Prescott, J. M., Hoffmann, J. A., Secrest, L. R., & Weise, K. (1989). Environmental effects on survival and growth of secondary sporidia and teliospores of Tilletia indica. Crop Protection, 8, 86–90.
Stansbury, C. D., McKirdy, S. J., Diggle, A. J., & Riley, I. T. (2002). Modeling the risk of entry, establishment, spread, containment, and economic impact of Tilletia indica, the cause of Karnal bunt of wheat, using an Australian context. Phytopathology, 92, 321–331.
Stein, J. M., Maples, H. W., & Rush, C. M. (2005). Epidemiology of Tilletia indica in regulated wheat fields in Texas. Plant Disease, 89, 828–833.
Trione, E. J. (1977). Endogenous germination inhibitors in teliospores of the wheat bunt fungus. Phytopathology, 67, 1245–1249.
Warham, E. J. (1986). Karnal bunt disease of wheat; a literature review. Tropical Pest Management, 32, 229–242.
Warham, E. J. (1992). Karnal bunt of wheat. In U. S. Singh, A. N. Mukhopadhyay, J. Kumar, & H. S. Chaube (Eds.), Plant diseases of international importance (Vol. 1, pp. 1–24). New Jersey, USA: Prentice Hall.
Warham, E. J., & Flores, D. (1988). Farmer surveys on the relationship of agronomic practices to Karnal bunt disease in the Yaqui Valley, Mexico. Tropical Pest Management, 34, 373–381.
Workneh, F., Allen, T. W., Nash, G. H., Narasinhan, B., Srinivasan, R., & Rush, C. M. (2008). Rainfall and temperature distinguish between Karnal bunt positive and negative years in wheat fields in Texas. Phytopathology, 98, 95–1000.
Ykema, R. E., Floyd, J. P., Palm, M. E., & Petersen, G. L. (1996). First report of Karnal bunt of wheat in the United States. Plant Disease, 80, 1207.
Zhang, Z., Lange, L., & Mathur, S. B. (1984). Teliospores survival and plant quarantine significance of Tilletia indica (causal agent of Karnal bunt) particularly in relation to China. EPPO Bulletin, 14, 119–128.
Acknowledgements
Blair Goates, Plant Pathologist, ARS-USDA, Aberdeen, Idaho is thanked for providing information on the current Karnal bunt situation in the USA, and for discussions relating to the physiology/epidemiology of T. indica. The advice of Gary Petersen, ARS-USDA, Ft. Detrick, Maryland, Fekede Workneh, Texas Agricultural Experimental Station, Bushland, Texas and Tom Allen, Delta Research and Extension Center, Stoneville, Mississippi is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
David Jones is a retired employee of the Central Science Laboratory (CSL). Statements and opinions expressed in this paper are those of the author and are not the views of CSL.
Rights and permissions
About this article
Cite this article
Jones, D.R. Towards a more reasoned assessment of the threat to wheat crops from Tilletia indica, the cause of Karnal bunt disease. Eur J Plant Pathol 123, 247–259 (2009). https://doi.org/10.1007/s10658-008-9364-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-008-9364-4