Abstract
To examine the prevalence and incidence of diabetic eye disease (DED) among individuals with diabetes in Europe, a systematic review to identify all published European prevalence and incidence studies of DED in individuals with diabetes managed in primary health care was performed according to the MOOSE and PRISMA guidelines. The databases Medline, Embase and Web of Science were searched to 2 September 2017. Meta-analyses and meta-regressions were performed. The pooled prevalence estimates were applied to diabetes prevalence rates provided by the International Diabetes Foundation atlas and Eurostat population data, and extrapolated to the year 2050. Data of 35 prevalence and four incidence studies were meta-analyzed. Any diabetic retinopathy (DR) and diabetic macular edema (DME) were prevalent in 25.7% (95% CI 22.8–28.8%) and 3.7% (95% CI 2.2–6.2%), respectively. In meta-regression, the prevalence of DR in persons with type 1 diabetes was significantly higher compared to persons with type 2 diabetes (54.4% vs. 25.0%). The pooled mean annual incidence of any DR and DME in in persons with type 2 diabetes was 4.6% (95% CI 2.3–8.8%) and 0.4% (95% CI 0.5–1.4%), respectively. We estimated that persons with diabetes affected by any DED in Europe will increase from 6.4 million today to 8.6 million in 2050, of whom 30% require close monitoring and/or treatment. DED is estimated to be present in more than a quarter of persons with type 2 diabetes and half of persons with type 1 diabetes underlining the importance of regular monitoring. Future health services need to be planned accordingly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetic eye disease (DED), comprising diabetic retinopathy (DR) and diabetic macular edema (DME), is the leading cause of visual impairment (VI) and blindness in Europeans of working age [1, 2]. The World Health Organization (WHO) has classified diabetic retinopathy as a priority eye diseases due to an increasing global prevalence. The risk of DED has been shown to be related to glycemic control, presence of arterial hypertension, and disease duration as well as the type of diabetes with a higher risk in type 1 diabetes [3].
DED affects almost 80% of persons with type 2 diabetes after 10 years of disease [4]. Highly effective treatments have been developed to prevent visual loss due to DED, including laser photocoagulation, intravitreal injection of anti-vascular endothelial growth factor (VEGF) agents and corticosteroids. The effectiveness of these procedures, however, depends on early diagnosis of DED. Especially people with early stages remain asymptomatic, which is why in most European countries screening for DED in persons with diabetes is strongly recommended as outlined in current national and international guidelines [5].
The Meta-analysis for Eye Disease (META-EYE) study group has provided pooled global data on the prevalence and major risk factors of DR [3] and reported a prevalence of 34.6% for any DR in the global diabetic population. To day, we lack data on DME as well as regional data, which are needed as prevalence and incidence of diabetes and available diabetes care differ widely between continents and countries. Already today, diabetes and its complications are a major healthcare burden in Europe with diabetes healthcare expenditures second highest globally after North America [6]. In addition, Europe has the highest incidence of type 1 diabetes and European countries experience more pronounced population ageing compared to other parts of the world. Faced with continuously increasing diabetes prevalence, we need solid European data and future projections to plan health services according to need in Europe. Therefore, we performed a systematic literature review, meta-analysis and meta-regressions, as well as projections of the prevalence and incidence of DED in Europe.
Methods
Literature search and study selection
Following the international guideline for systematic literature searches and meta-analysis of observational studies in epidemiology (MOOSE) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [7, 8], we performed a systematic literature review, following a systematic review protocol, to identify all relevant publications on the prevalence and incidence of DED in Europe.
The databases Medline (through PubMed), Embase and Web of Science (WoS) were searched for European studies published in the last 20 years starting from January 1st 1996. The search query formatted for PubMed is presented in the Online Resource 1. The primary search was performed on August 1, 2016, and was repeated on September 2, 2017, to identify more recent publications. Reference lists of identified articles were hand searched for further relevant literature.
Inclusion and exclusion criteria
We included published population-based observational studies from Europe reporting prevalence or incidence of DR and/or DME. Cohort studies of diabetic persons and incident case–control studies were eligible for inclusion, if derived from primary healthcare. For incidence data, longitudinal studies reporting the incidence of any DED in a defined observation period of individuals with no evidence of DED at the initial screening were included. In case of multiple reports from the same cohort, we included data from the most recent publication. Studies based on retinal photographs as well as on clinical examination using dilated funduscopy were included. Case series, hospital-based studies, interventional studies, or studies relying on participant-reported diagnoses of DED were excluded. To minimize language bias, we did not exclude non-English publications. Data from non-English publications were extracted by native (Spanish, German) and advanced (French) speakers.
Diabetes diagnoses in the included studies were mainly provided by general practitioners (GP) or diabetes registries, and confirmed through blood glucose or glycated hemoglobin (HbA1c) measurements. To differentiate between type 1 and type 2 diabetes, when GP based diagnoses were absent, most studies used age (below or above 30 years of age) or a combination of age and insulin or oral antidiabetic therapy as defining criteria. All studies assessed DR according to standardized protocols following the Early Treatment Diabetic Retinopathy Study (ETDRS), the American Academy of Ophthalmology (AAO) International Clinical Diabetic Retinopathy Disease Severity Scale or the Retinopathy Working Party Protocol [9,10,11]. Data were re-categorized according to the international clinical diabetic retinopathy severity scale: mild, moderate or severe non-proliferative (NPDR) or proliferative DR (PDR). For diabetic macular edema, definitions were heterogenous and unclear in some studies. Thus, only clearly defined clinically significant DME as defined by the ETDRS was included in the analysis [10].
Data extraction
After initial database search, results were merged using the reference management software Citavi (Version 5.3.1.0, Swiss Academic Software GmbH, Wädenswil, Switzerland) [12]. Abstracts were examined and, if eligible, full texts underwent further evaluation for eligibility by one reviewer (JQL). A random sample of 5% (n = 13) of all full-text articles was reassessed for eligibility by a second reviewer (CW). Any ambiguities or disagreements between reviewers were resolved through discussion with the senior researcher (RPF). Full-texts of potentially relevant publications were retrieved. Authors were contacted where needed.
Data of studies meeting the inclusion criteria were extracted into a data extraction form. Most studies reported non-weighted prevalence and incidence data. Where both weighted and non-weighted prevalence and incidence data were reported, we used the non-weighted data to ensure consistency and allow for comparison. Some studies reported the number of non-gradable images, but did not exclude these from the prevalence estimation. These data were adjusted by dividing the number of prevalent cases by the number of graded images.
Risk of bias and quality assessment
To assess risk of bias and quality of primary studies or systematic reviews identified from database searches, full-texts of eligible publications were examined using a previously described checklist [13]. The checklist was based on the principles of STROBE (Strengthening the Reporting of Observational Studies in Epidemiology for assessing observational studies) for primary observational studies and MOOSE [7, 14]. Risk of bias was categorized as low, medium, or high risk. One reviewer (JQL) scored the quality of included studies. Five percent (n = 13) of full-texts were screened by a second reviewer (CW) and discrepancies were discussed. Agreement between the two reviewers was high (Cohen’s kappa = 0.80). Studies with high risk of bias were excluded from further quantitative analysis after discussion with the senior reviewer (RPF).
Data analysis
Meta-analysis and meta-regression of prevalence and incidence data
For prevalence and incidence data, we performed a meta-analysis and meta-regression. All analyses were performed using the statistical software R (Version 3.4.0, R Foundation for Statistical Computing, Vienna, Austria) with the add-on package meta (Version 4.8-2) [15, 16]. After systematic review, prevalence estimates and mean annual incidence rates from studies meeting the inclusion criteria were extracted. Data for the multinational EUREYE study were extracted from the META-EYE study, as original data for DED were not published at the time of our literature search [3]. Three studies reported DED prevalence for a purely type 1 diabetes cohort. These were not included in the meta-analysis of overall DED prevalence, but included in the meta-regression analysis for diabetes type. Reported cumulative incidences were converted to mean annual incidences. Between-study heterogeneity was assessed with Higgins’ and Thompson’s I2 measure and tested by Cochran’s Q-test for heterogeneity [17, 18]. Random-effects models were calculated and summaries of meta-analysis results are presented in forest plots with exact binomial confidence intervals. Further possible small-study effects were analysed by funnel plots, with sample sized plotted on the y-axes, and Peter’s Test. Peter’s Test was considered appropriate for proportion studies because of less bias towards asymmetry and better performance against other regression based asymmetry tests. Univariate meta-regression was conducted to examine the influence of sample characteristics such as years of examination and country. Pooled subgroup estimates were compared for heterogeneity using Cochran’s Q-test. Meta-regression was performed for time-trends, region (northern, western, eastern, and southern Europe defined by the United Nations geoscheme for Europe [19]; northern compared to southern Europe determined by the orientation to the 45th parallel north), type of diabetes, reported mean HbA1c levels, and mean duration since diagnosis of diabetes, and the five most populous countries (Germany, France, the UK, Italy, and Spain).
The prevalence of DED in regard to the underlying diabetes type was examined. Some studies did not report results seperately for type 1 and type 2 diabetes. We defined studies which included more than 80% of persons with type 2 diabetes but did not report results separately for type 1 and type 2 diabetes as type 2 diabetes studies, similarly to other meta-analyses of diseases relating to diabetes [20, 21]. All studies including only persons with type 1 diabetes were located in northern Europe and excluded from the meta-analysis and meta-regression of regions and examination year. Comparable data for the incidence of DED could only be identified for persons with type 2 diabetes. Due to lack of data, it was not possible to perform a meta-regression of age.
EU estimates and future projection
Estimates of the number of persons currently affected by DED in the European Union (EU) were calculated by multiplying our age-stratified random-effects prevalence estimates with Eurostat population statistics and the diabetes prevalence rates sourced from the 8th edition of the International Diabetes Foundation (IDF) Diabetes Atlas [6, 22] (Supplementary Table 3). For our future projections, IDF national diabetes prevalence rate estimates for 2017 and 2045 were used and stratified in 10-year intervals, under the assumption that diabetes prevalence will increase in a linear manner. Random-effects pooled prevalence estimates were used to predict prevalence of DED in the EU and the five most populous countries. Assuming that incidence will remain stable and that age—as a proxy for disease duration—is the main factor affecting prevalence, projections until the year 2050 were calculated by multiplying the age-stratified pooled prevalence estimates with IDF diabetes prevalence rates and Eurostat European population projections data in the age-group over 40 years, which is in accordance with the mean lower age limit of included studies [6, 23, 24].
Results
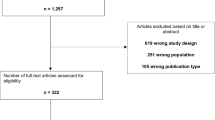
The systematic review process is presented in Fig. 1.
Prevalence of DED in Europe
35 studies providing prevalence data on any DED were included in the meta-analysis [3, 25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]. The total number of European individuals examined was 205,743. The majority of studies reported data from either diabetic cohorts or screening programs. Details on included studies are presented in Table 1.
Any DR was prevalent in 25.7% (95% CI 22.8–28.8%) of Europeans with diabetes type 1 and 2 (Table 2 and Supplementary Figure 1). 18.5% (95% CI 15.9–21.4%) of persons with diabetes were estimated to have mild or moderate NPDR. Ophthalmic treatment due to advanced DED was required in 2.2% and 3.7% of European persons with diabetes due to PDR and clinically significant DME, respectively (Supplementary Figures 2 to 4). Several treatment guidelines recommend severe NPDR to be treated as well [63, 64]. Including severe NPDR, the prevalence of DED stages requiring treatment increased to 7.9% (see Table 2). In seven (n = 14,158) out of 16 studies reporting prevalence of DME (n = 80,415), the definition of clinically significant DME was used.
No significant asymmetry was found in the funnel plots (see Supplementary Figures 7 to 10). However, the analysis was dominated by two studies with a much larger sample size (see Supplementary Figure 1). We analyzed time trends in the reported prevalence of any DR. Lowest pooled prevalence of any DR was found in recent studies initiated between 2002 and 2005, although the differences were not significant. Ten non-English publications were included: one in German, one in French and eight in Spanish. Mean age across studies ranged from 40 to 81 years. A vast majority of included studies were conducted in Spain and the UK. We corrected the estimates by performing meta-regression by region and country (Table 2). The majority of included studies was performed in northern and southern Europe (14 studies each), followed by western Europe (5 studies), and eastern Europe (1 study). The prevelance of any DR was significantly higher in southern (25.8%, 95% CI 10.9–34.2%) and northern Europe (29.6%, 95% CI 25.7–33.8%) compared to western (18.3%, 95% CI 22.3–29.7%) and eastern Europe (20.1%, 95% CI 10.9–34.2%; p < 0.01). A significant effect was also seen in mild or moderate DR (southern Europe: 27.1%, 95% CI 22.2–32.8%; western Europe: 17.3%, 95% CI 15.0–19.8%; p < 0.01). No significant effects were seen regarding severe NPDR, PDR and DME (Table 2). In the meta-regression of northern compared to southern Europe, no significant differences were found across all disease stages. Reported prevalence of any DR among persons with either type 1 or type 2 diabetes was significantly higher in Italy (34.1%, 95% CI 22.4–48.1%), the UK (29.8%, 95% CI 26.4–33.4%), and Spain (26.5%, 95% CI 23.8–29.3%) compared to France (14.6%, 95% CI 11.4–18.5%, p < 0.01).
The majority of studies included mainly or exclusively persons with type 2 diabetes. All three type 1 diabetes studies were performed in northern Europe: one in Estonia and two in Sweden [58,59,60]. Data were available for any DR, mild and moderate NPDR, severe NPDR and PDR. Across all diseases states, the estimated prevalences were higher in type 1 diabetes and odds-ratios (OR) ranged from 3.2 to 6.0 compared to type 2 diabetes, which was significant across all disease states except severe NPDR (Table 2). Two studies reported a prevalence of DED in undiagnosed DM: The Gutenberg Health Study (GHS) and the Diabetes Audit and Research in Tayside Scotland study (DARTS) [45, 65]. The reported prevalence of any DR ranged from 13% (95% CI 9.15–17.64%, sample size: 285) to 14.7% (95% CI 12.5–16.9%, sample size: 295), with mostly mild or moderate NPDR. These data were excluded from the meta-analysis.
No significant effects were found in the meta-analysis of mean HbA1c levels (p > 0.17) or mean duration since diagnosis (p > 0.14).
Incidence of DED in Europe
Data from a total of 71,307 persons with type 2 diabetes from two English and two Spanish studies were available for meta-analysis of the incidence of DED [66,67,68,69]. Of those, all studies reported incidence of any DR and two studies reported incidence of clinically significant DME [68, 69]. Three studies presented data on the incidence of sight threatening DR (STDR) or referable DR (RDR) [46, 66, 68, 70]. RDR or STDR were defined as pre-proliferative or proliferative retinopathy and/or maculopathy requiring referral and/or treatment. To allow for better consistency, we calculated the mean annual incidence from reported cumulative incidences. Details on included incidence studies are presented in Table 1. The analysis was dominated by the large Diabetic Retinopathy Screening Service for Wales study with 49,763 examined individuals [66]. Our random-effects pooled annual incidence of any DR, STDR and clinically significant DME was 4.6% (95% CI 2.3–8.8%), 0.5% (95% CI 0.2–0.8%), and 0.4% (95% CI 0.5–1.4%), respectively (Supplementary Figures 1 to 4).
EU estimates and future projection
In the EU, 6.4 million individuals over the age of 40 are currently affected by any DED. The highest numbers of affected persons are found in Germany and Italy with nearly one million individuals each (Fig. 2). France had the lowest number of affected individuals with only an estimated 360,000 affected by any DED, due to a low reported prevalence. The UK showed the second lowest number of affected individuals out of the five most populous countries, due to a low reported national prevalence of diabetes (5.9%) and a rather young population.
Based on the projected increase in prevalence of diabetes, an increase of persons with type 1 and type 2 diabetes with any DED of 34% (from 6.4 to 8.6 million people) was projected until 2050 (Supplementary Table 2). A total of 1.4 million will require close monitoring and treatment due to severe NPDR (around 690 000) or PDR (around 740 000). Cases of clinically significant DME are estimated at 1.2 million.
Discussion
We found any DR and clinically significant DME to be prevalent in 25.7% and 3.7% of Europeans with diabetes, with a mean annual incidence of 4.6% and 0.5%, respectively. The total numbers of EU inhabitants with DED will likely increase from 6.4 million persons today to 8.6 million in 2050.
The META-EYE study group reported 34.6% for any DR and 7% for PDR in a global analysis. In this pooled estimate, 35 studies were included [3]. In the United States (US), prevalences as high as 40.3% for any DED and 8.2% for sight-threatening diabetic retinopathy (STDR) were reported [71]. Recent studies reported a higher rate of required insulin-treatment in the US compared to Europe [72]. This may reflect a higher severity of diabetes in the US. In comparison, we found lower prevalence rates in European populations compared to both current global as well as US estimates. This further highlights the need for European data.
Most of the included studies were carried out in Spain or the UK. In the UK, a national screening programme has been established and succeeded in reaching a broad public, yielding a large amount of data. In Spain, patients with diabetes are referred to community eye clinics. These cover a large geographical area and “population-based” data can easily be obtained. The situation is more challenging e.g. in France, Germany and Italy, as the majority of individuals are screened by office-based ophthalmologists with no systematic screening programme in place. Prevalence estimates for France were much lower than for all other countries which might be due to participant selection in the published studies [26]. Only one eastern European study was included. Thus, our estimate for this European region should be interpreted with caution.
We found a significantly higher prevalence of DED in persons with type 1 diabetes, which is consistent with the literature. In the META-EYE study, prevalence of any DR, PDR and DME was higher in people with diabetes type 1 (RR: 2.7, 15.3 and 4.8). In another study from the US, the prevalence of DR in young people with type 1 and type 2 diabetes was found to be 20.1% and 7.2%, respectively [73]. Contrary to the META-EYE study, we did not find a significant decrease of the reported prevalence of DED. This is most probably due to the exclusion of studies published before 1996.
In most studies, DED was assessed clinically or by grading of colour fundus photographs (CFP). Additional diagnostic assessments commonly performed in clinical routine such as fluorescein angiography (FA) and optical coherence tomography (OCT) were used rarely or not at all in the included studies. FA plays a key role in the evaluation of the retinal vasculature, especially in the diagnosis of proliferative DED. However, due to the invasive nature of this imaging technique with intravenous injection of fluorescein dye, FA was only performed in a minority of participants in the included studies. Noninvasive OCT scans are commonly used and strongly recommended in clinical practice for the diagnosis and follow-up of DME patients [74]. Compared to clinical or photographic assessment, even slight changes in the central retina (subretinal or intraretinal fluid) may be detected by OCT. Being a fairly recently introduced and costly procedure, none of the included studies implemented OCT. Thus, the prevalence estimates for PDR and DME in our study may be an underestimate due to the absence of OCT and rare use of FA. Additionally, due to a high heterogeneity regarding the definition of DME between the studies, only the clearly defined clinically significant DME was included in our meta-analyses.
DED screening is essential in preventing vision loss associated with diabetes. Among the five most populous countries in the EU, a systematic national screening program has only been implemented in the UK. As showcased by the UK, implementing national DED screening translates to decreasing visual loss baused by DED but comes at a considerable cost [75]. Whether telemedical approaches or the involvement of non-ophthalmic medical personnel might be a solution is currently being assessed in a number of programmes across different countries. Already today, the majority of cases of blindness and severe visual impairment in Europe is caused by retinal diseases such as AMD and DED [1, 2]. All of these diseases need to be managed by specialized medical professionals and may require regular monitoring as well as treatment which requires considerable healthcare resources. With increasing prevalence of diabetes and thus its complications including DED novel approaches to healthcare service delivery are warranted as healthcare ressources are limited.
To our knowledge, this is the first study to provide pooled European prevalence and incidence estimates based on published data, as well as a projection into the future. Our study was conducted according to current gold standards, applied stringent quality criteria and included meta-analysis and meta-regression where possible. However, based on the available published studies there are several limitations. Reported prevalence rates of advanced diseases, such as severe NPDR, PDR and clinically significant DME, as well as incidence rates were few and mostly based on rather small numbers of cases. Due to a lack of published data, only limited information was available on DED in type 1 diabetes and a more detailed analysis on rare diabetes types was not possible. We could not assess risk factors such as insulin requirement in persons with type 2 diabetes. Only a limited number of studies reported mean HbA1c levels or mean duration since diagnosis of diabetes. In our meta-regression, no significant effects were identified which might be due to lack of data, inconsistencies in obtaining these data across studies and recall bias amongst other factors. As we defined studies with mainly type 2 diabetes persons as type 2 diabetes studies, prevalences of all stages of DED for type 2 diabetes may be slightly inflated as persons with type 1 diabetes are more likely to develop DED. Heterogeneity among studies was high mostly due to varying diagnostic procedures and grading protocols being used. We addressed this by re-categorising different stages using established and commonly used DED classifications and assessing risk of bias, excluding studies with a high risk of bias. In addition we used a random-effects model which provides more reliable estimates in such instances [76]. Studies applied heterogenous diagnostic criteria for diabetes. In most studies, the diagnosis was based on information provided by the patient’s GP or existing diabetes registries, and/or blood glucose and HbA1c measurements. A few studies relied on self-reported diagnoses, but biochemical blood glucose and/or HbA1c measurements were performed to confirm the reported diagnosis [29]. Only one Italian study relied on self-reported diagnoses of diabetes only [30]. This may have influenced the reported prevalence of DED. However, studies have shown that persons diagnosed with diabetes are commonly aware of their diagnosis and self-reported diabetes diagnoses were highly accurate [77, 78]. Regarding incidence data, the observation period varied between 4 and 8 years, which we addressed by calculating the mean annual incidence which might underestimate actual incidence as cummulative incidences tend to rise with a longer time of observation. Our estimates should be applied carefully to European countries for which no data were available as the actual situation may differ from countries which contributed data. We applied our pooled prevalence estimate to a population aged 40 years and older, which is in accordance with the meta-analyzed studies. However, the very elderly are not well represented in our data. As the risk of DED increases with duration of the diabetes, we might have underestimated the age-related increase as life expectancies increase and thus life-time risk of both diabetes and its complications increase. In the estimation of persons with DED requiring therapy, an overlap of individuals with severe NPDR and clinically significant DME, or PDR and clinically significant DME is most likely. Nonetheless, according to current treatment guidelines, treatment modalities differ for severe NPDR and PDR (improvement of metabolic control, panretinal lasercoagulation) and clinically significant DME (intravitreal injection of anti-VEGF or corticosteroids; central laser treatment in certain cases) [74].
In conclusion, DED is present in a quarter of persons with type 2 diabetes and half of persons with type 1 diabetes, with a considerable proportion requiring very frequent monitoring and/or treatment for their DED. Population ageing and increasing diabetes prevalence are likely to lead to a 30% increase in affected Europeans under the modelled assumptions stated in the methods. New models of delivering screening and treatment for DED need to be assessed to cope with this. Future healthcare planning will need to take this into account.
References
Finger RP, Fimmers R, Holz FG, Scholl HPN. Incidence of blindness and severe visual impairment in Germany: projections for 2030. Investig Ophthalmol Vis Sci. 2011;52:4381–9.
Bourne RRA, Jonas JB, Bron AM, Cicinelli MV, Das A, Flaxman SR, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: magnitude, temporal trends and projections. Br J Ophthalmol. 2018;102:575–85. https://doi.org/10.1136/bjophthalmol-2017-31125.
Yau JWY, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. https://doi.org/10.2337/dc11-1909.
Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015;2:17.
Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, van Lansingh C, et al. Guidelines on diabetic eye care: the international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125:1608–22.
International Diabetes Federation. IDF diabetes atlas. 8th ed. Brussels: International Diabetes Federation; 2017.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–12.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9.
Retinopathy Working Party. A protocol for screening for diabetic retinopathy in Europe. Diabet Med. 1991;8:263–7.
Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified airlie house classification. Ophthalmology. 1991;98:786–806. https://doi.org/10.1016/s0161-6420(13)38012-9.
Wilkinson CP, Ferris FL, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. https://doi.org/10.1016/s0161-6420(03)00475-5.
Meurer P, Schluchter M. Wissenschaftliches Arbeiten mit Citavi 5. Hinweise zum Schreiben wissenschaftlicher Arbeiten mit der Software» Citavi–Literaturverwaltung und Wissensorganisation « 2015: Zugriff am.
Li JQ, Terheyden JH, Welchowski T, Schmid M, Letow J, Wolpers C, et al. Prevalence of retinal vein occlusion in Europe: a systematic review and meta-analysis. Ophthalmologica. 2018;49:1–7.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology [STROBE] statement: guidelines for reporting observational studies. Gac Sanit. 2008;22:144–50.
R Core Team. R. A language and environment for statistical computing. 2015. https://www.R-project.org. Accessed 2 Sept 2017
Schwarzer G. Meta: an R package for meta-analysis. R News. 2007;7:40–5.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. https://doi.org/10.1002/sim.1186.
Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37:256–66.
United Nations Statistics Division. Standard Country and area codes for statistical use (M49). https://unstats.un.org/unsd/methodology/m49/. Accessed 3 May 2019.
Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. New York: Springer; 2007.
Ben Q, Xu M, Ning X, Liu J, Hong S, Huang W, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer. 2011;47:1928–37. https://doi.org/10.1016/j.ejca.2011.03.003.
Eurostat. Population on 1 January. 2016. http://ec.europa.eu/eurostat/tgm/table.do?tab=table&init=1&plugin=1&language=en&pcode=tps00001. Accessed 11 May 2017.
Eurostat. People in the EU – population projections. http://ec.europa.eu/eurostat/statistics-explained/index.php/People_in_the_EU_%E2%80%93_population_projections (2015). Accessed 14 Jul 2017.
Eurostat. Population projections. http://ec.europa.eu/eurostat/tgm/table.do?tab=table&init=1&plugin=1&language=de&pcode=tps00002 (2016). Accessed 11 May 2017.
Lenoble P, Kheliouen M, Bourderont D, Klinger V, Nasica X, Benseddik Y, Holl P. Screening diabetic retinopathy using a telediagnosis system. Results of the upper Rhine survey. J Fr Ophtalmol. 2009;32:91–7. https://doi.org/10.1016/j.jfo.2009.01.006.
Schulze-Dobold C, Erginay A, Robert N, Chabouis A, Massin P. Ophdiat((R)): five-year experience of a telemedical screening programme for diabetic retinopathy in Paris and the surrounding area. Diabetes Metab. 2012;38:450–7. https://doi.org/10.1016/j.diabet.2012.05.003.
Soulie-Strougar M, Charles A, Metral P, Quercia P, Souchier M, Chirpaz L, et al. Screening diabetic retinopathy in Burgundy with an itinerant nonmydriatic camera. J Fr Ophtalmol. 2007;30:121–6.
Raum P, Lamparter J, Ponto KA, Peto T, Hoehn R, Schulz A, et al. Prevalence and cardiovascular associations of diabetic retinopathy and maculopathy: results from the Gutenberg Health Study. PLoS ONE. 2015;10:e0127188. https://doi.org/10.1371/journal.pone.0127188.
Zietz B, Kasparbauer A, Ottmann S, Spiegel D, Palitzsch KD. Diabetic retinopathy and associated risk factors in type-1 and type-2 diabetics in the Upper Palatinate. Dtsch Med Wochenschr. 2000;125:783–8. https://doi.org/10.1055/s-2007-1024518.
Giuffre G, Lodato G, Dardanoni G. Prevalence and risk factors of diabetic retinopathy in adult and elderly subjects: the Casteldaccia Eye Study. Graefes Arch Clin Exp Ophthalmol. 2004;242:535–40. https://doi.org/10.1007/s00417-004-0880-4.
Goldaracena MB, Escudero JM, Arrondo A, Villarrubia A, Aramendia B, Iturralde R. Prevalencia de retinopatía diabética en una población diabética registrada en atención primaria. Prevalence of diabetic retinopathy. A population based study in primary health care. 1998.
Hernández Mira G, Macarro Merino A, Fernández Perianes J, Fernández Vigo J. Prevalencia de retinopatía diabética en Extremadura. Av Diabetol. 1996;12:165–71.
Gil Hernández MA, Abreu Reyes P, Pareja Rios A, Martinez Piñero A. Estudio de la retinopatía diabética en la población de la isla de Hierro. Arch Soc Canar Oftal. 2000;11:23–6.
Lopez IM, Diez A, Velilla S, Rueda A, Alvarez A, Pastor CJ. Prevalence of diabetic retinopathy and eye care in a rural area of Spain. Ophthalmic Epidemiol. 2002;9:205–14.
Martinez RM, Moya MM, Bellot BA, Belmonte MJ. Diabetic retinopathy screening and teleophthalmology. Arch Soc Esp Oftalmol. 2012;87:392–5. https://doi.org/10.1016/j.oftal.2012.04.004.
Romero-Aroca P, Sagarra-Alamo R, Baget-Bernaldiz M, Fernández-Ballart J, Méndez-Marin I. Prevalence and relationship between diabetic retinopathy and nephropathy, and its risk factors in the North-East of Spain, a population-based study. Ophthalmic Epidemiol. 2010;17:251–65.
Romero-Aroca P, Fernandez-Alart J, Baget-Bernaldiz M, Mendez-Marin I, Salvat-Serra M. Diabetic retinopathy epidemiology in type II diabetic patients. Effect of the changes in the diagnostic criteria and stricter control of the diabetes between 1993 and 2005 on the incidence of diabetic retinopathy. Arch Soc Esp Oftalmol. 2007;82:209–18.
Bueso E, Fernandez-Vigo J, Perez C, Merino A, Perianes J. Prevalence of diabetic retinopathy in the Regional Community of Extremadura. 1997–2001 (Extremadura Project to Prevent Blindness). Arch Soc Esp Oftalmol. 2005;80:187–94.
Santos Bueso E, Macarro Merino A, Garrote Florencio T, Fernández-Vigo López J. Prevalencia de retinopatía diabética en el centro de salud de Torrejoncillo (Cáceres). Av Diabetol. 2002;18:203–7.
Santos-Bueso E, Fernandez-Perez C, Macarro A, Fernandez-Vigo J. Prevalence of diabetic retinopathy in the city of Badajoz 2002 (Extremadura project to prevent blindness). Arch Soc Esp Oftalmol. 2007;82:153–8.
Teruel MC, Fernandez-Real JM, Ricart W, Valent FR, Valles PM. Prevalence of diabetic retinopathy in the region of Girona. Study of related factors. Arch Soc Esp Oftalmol. 2005;80:85–91.
Vleming EN, Castro M, Lopez-Molina MI, Teus MA. Use of non-mydriatic retinography to determine the prevalence of diabetic retinopathy in diabetic patients. Arch Soc Esp Oftalmol. 2009;84:231–6.
Broadbent DM, Scott JA, Vora JP, Harding SP. Prevalence of diabetic eye disease in an inner city population: the Liverpool Diabetic Eye Study. Eye (London). 1999;13(Pt 2):160–5. https://doi.org/10.1038/eye.1999.43.
Ding J, Strachan MWJ, Reynolds RM, Frier BM, Deary IJ, Fowkes FGR, et al. Diabetic retinopathy and cognitive decline in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes. 2010;59:2883–9. https://doi.org/10.2337/db10-0752.
Ellis JD, Zvandasara T, Leese G, McAlpine R, Macewen CJ, Baines PS, et al. Clues to duration of undiagnosed disease from retinopathy and maculopathy at diagnosis in type 2 diabetes: a cross-sectional study. Br J Ophthalmol. 2011;95:1229–33. https://doi.org/10.1136/bjo.2010.196204.
Jones CD, Greenwood RH, Misra A, Bachmann MO. Incidence and progression of diabetic retinopathy during 17 years of a population-based screening program in England. Diabetes Care. 2012;35:592–6. https://doi.org/10.2337/dc11-0943.
Kohner EM, Aldington SJ, Stratton IM, Manley SE, Holman RR, Matthews DR, Turner RC. United Kingdom Prospective Diabetes Study, 30: diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch Ophthalmol. 1998;116:297–303.
Leese GP, Morris AD, Swaminathan K, Petrie JR, Sinharay R, Ellingford A, et al. Implementation of national diabetes retinal screening programme is associated with a lower proportion of patients referred to ophthalmology. Diabet Med. 2005;22:1112–5. https://doi.org/10.1111/j.1464-5491.2005.01603.x.
Ling R, Ramsewak V, Taylor D, Jacob J. Longitudinal study of a cohort of people with diabetes screened by the Exeter Diabetic Retinopathy Screening Programme. Eye (London). 2002;16:140–5. https://doi.org/10.1038/sj/EYE/6700081.
Scanlon PH, Carter SC, Foy C, Husband RF, Abbas J, Bachmann MO. Diabetic retinopathy and socioeconomic deprivation in Gloucestershire. J Med Screen. 2008;15:118–21. https://doi.org/10.1258/jms.2008.008013.
Thomas RL, Dunstan FD, Luzio SD, Chowdhury SR, North RV, Hale SL, et al. Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. Br J Ophthalmol. 2015;99:64–8. https://doi.org/10.1136/bjophthalmol-2013-304017.
Younis N, Broadbent DM, Harding SP, Vora JR. Prevalence of diabetic eye disease in patients entering a systematic primary care-based eye screening programme. Diabet Med. 2002;19:1014–21.
Gunnlaugsdottir E, Halldorsdottir S, Klein R, Eiriksdottir G, Klein BE, Benediktsson R, et al. Retinopathy in old persons with and without diabetes mellitus: the Age, Gene/Environment Susceptibility-Reykjavik Study (AGES-R). Diabetologia. 2012;55:671–80. https://doi.org/10.1007/s00125-011-2395-y.
Hapnes R, Bergrem H. Diabetic eye complications in a medium sized municipality in southwest Norway. Acta Ophthalmol Scand. 1996;74:497–500.
Bertelsen G, Peto T, Lindekleiv H, Schirmer H, Solbu MD, Toft I, et al. Tromsø eye study: prevalence and risk factors of diabetic retinopathy. Acta Ophthalmol. 2013;91:716–21.
Kilstad HN, Sjølie AK, Gøransson L, Hapnes R, Henschien HJ, Alsbirk KE, et al. Prevalence of diabetic retinopathy in Norway: report from a screening study. Acta Ophthalmol. 2012;90:609–12.
Medeiros MD, Mesquita E, Papoila AL, Genro V, Raposo JF. First diabetic retinopathy prevalence study in Portugal: RETINODIAB Study—evaluation of the screening programme for Lisbon and Tagus Valley region. Br J Ophthalmol. 2015;99:1328–33. https://doi.org/10.1136/bjophthalmol-2015-306727.
Kuiv R, Tein P, Algvere PV, Bäcklund LB, Holm O. Photographic detection of retinopathy in insulin-treated diabetes. Acta Ophthalmol. 1997;75:447–56.
Larsson L, Alm A, Bergenheim T, Lithner F, Bergström R. Retinopathy in diabetic patients aged 15–50 years in the county of Umeå, Sweden. Acta Ophthalmol Scand. 1999;77:430–6.
Kernell A, Dedorsson I, Johansson B, Wickström CP, Ludvigsson J, Tuvemo T, et al. Prevalence of diabetic retinopathy in children and adolescents with IDDM A population-based multicentre study. Diabetologia. 1997;40:307–10.
Tóth G, Szabó D, Sándor GL, Szalai I, Lukács R, Pék A, et al. Diabetes and diabetic retinopathy in people aged 50 years and older in Hungary. Br J Ophthalmol. 2017;101:965–9. https://doi.org/10.1136/bjophthalmol-2016-309016.
Olafsdottir E, Andersson DKG, Dedorsson I, Stefansson E. The prevalence of retinopathy in subjects with and without type 2 diabetes mellitus. Acta Ophthalmol. 2014;92:133–7. https://doi.org/10.1111/aos.12095.
The Royal College of Ophthalmologists. Diabetic Retinopathy Guidelines December 2012; 2012.
Chakrabarti R, Harper CA, Keeffe JE. Diabetic retinopathy management guidelines. Expert Rev Ophthalmol. 2012;7:417–39.
Ponto KA, Koenig J, Peto T, Lamparter J, Raum P, Wild PS, et al. Prevalence of diabetic retinopathy in screening-detected diabetes mellitus: results from the Gutenberg Health Study (GHS). Diabetologia. 2016;59:1913–9.
Thomas RL, Dunstan F, Luzio SD, Roy CS, Hale SL, North RV, et al. Incidence of diabetic retinopathy in people with type 2 diabetes mellitus attending the Diabetic Retinopathy Screening Service for Wales: retrospective analysis. BMJ. 2012;344:e874. https://doi.org/10.1136/bmj.e874.
Younis N, Broadbent DM, Vora JP, Harding SP. Incidence of sight-threatening retinopathy in patients with type 2 diabetes in the Liverpool Diabetic Eye Study: a cohort study. Lancet. 2003;361:195–200. https://doi.org/10.1016/S0140-6736(03)12267-2.
Romero-Aroca P, de La Riva-Fernandez S, Valls-Mateu A, Sagarra-Alamo R, Moreno-Ribas A, Soler N. Changes observed in diabetic retinopathy: eight-year follow-up of a Spanish population. Br J Ophthalmol. 2016. https://doi.org/10.1136/bjophthalmol-2015-307689.
Salinero-Fort MA, San FJ, de Burgos-Lunar C, Arrieta-Blanco FJ, Gomez-Campelo P. Four-year incidence of diabetic retinopathy in a Spanish cohort: the MADIABETES study. PLoS ONE. 2013;8:e76417. https://doi.org/10.1371/journal.pone.0076417.
Younis N, Broadbent DM, Harding SP, Vora JP. Incidence of sight-threatening retinopathy in Type 1 diabetes in a systematic screening programme. Diabet Med. 2003;20:758–65.
Kempen JH, O’Colmain BJ, Leske MC, Haffner SM, Klein R, Moss SE, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–63. https://doi.org/10.1001/archopht.122.4.552.
Centers for Disease Control and Prevention. Age-adjusted percentage of adults with diabetes using diabetes medication, by type of medication, United States, 1997–2011; 2015.
Ting DSW, Cheung GCM, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44:260–77.
Schmidt-Erfurth U, Chong V, Loewenstein A, Larsen M, Souied E, Schlingemann R, et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol. 2014;98:1144–67.
Pasquel FJ, Hendrick AM, Ryan M, Cason E, Ali MK, Narayan KV. Cost-effectiveness of different diabetic retinopathy screening modalities. J Diabetes Sci Technol. 2016;10:301–7.
Higgins JP, Deeks JJ, Higgins J, Green S. 9.5. 4 Incorporating heterogeneity into random-effects models. Cochrane handbook for systematic reviews of interventions, Version. 2011;5.
Hocking A, Laurence C, Lorimer M. Patients’ knowledge of their chronic disease: the influence of socio-demographic characteristics. Aust Fam Physician. 2013;42:411.
Pastorino S, Richards M, Hardy R, Abington J, Wills A, Kuh D, Pierce M. Validation of self-reported diagnosis of diabetes in the 1946 British birth cohort. Prim Care Diabetes. 2015;9:397–400.
Diabetic Retinopathy Study Research Group. Report No. 7: a modification of the Airlie House classification of diabetic retinopathy. Investig Ophthalmol Vis Sci. 1981;21:210–26.
Massin P, Angioi-Duprez K, Bacin F, Cathelineau B, Cathelineau G, Chaine G, et al. Detection, monitoring and treatment of diabetic retinopathy. Recommendations of ALFEDIAM. Committee of above-mentioned experts and validated by the board of directors and scientific board of ALFEDIAM. Diabetes Metab. 1996;22:203–9.
Harding S, Greenwood R, Aldington S, Gibson J, Owens D, Taylor R, et al. Grading and disease management in national screening for diabetic retinopathy in England and Wales. Diabet Med. 2003;20:965–71.
Facey K, Cummins E, Macpherson K, Morris A, Reay L, Slattery J. Health technology assessment report 1: organisations of services for diabetic retinopathy screening. Glasgow: Health Technology Board for Scotland; 2002.
Klein R, Klein BEK, Magli YL, Brothers RJ, Meuer SM, Moss SE, Davis MD. An alternative method of grading diabetic retinopathy. Ophthalmology. 1986;93:1183–7.
Funding
European Society of Retina Specialists (EURETINA) project grant, the Jackstaedt Stiftung, and the Else Kroehner Fresenius Stiftung. None of the funders were involved in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the article for publication. All researchers confirm their independence from funders. All authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Contributions
RPF conceived and designed the study. JQL, JLe, CW, and IPC undertook the literature search, extracted data and performed quality assessment of the included studies. JQL, TW, and MS performed the data analysis. JQL, TW, MS, FGH, and RPF interpreted data. JQL and RPF wrote the first draft of the report. RPF as guarantor accepts full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish. All authors provided critical comments and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors had any financial relationships with any of the organisations that might have an interest in the submitted work (ADA Policy Statement on Duality of Interest). No other relationships or activities that could appear to have influenced the submitted work.
Ethical approval
Ethics committee approval or written informed consent was not required, as our review relied entirely on publicly available published data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, J.Q., Welchowski, T., Schmid, M. et al. Prevalence, incidence and future projection of diabetic eye disease in Europe: a systematic review and meta-analysis. Eur J Epidemiol 35, 11–23 (2020). https://doi.org/10.1007/s10654-019-00560-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-019-00560-z