Abstract
Previous studies have linked maternal smoking during pregnancy with regular tobacco use in offspring, but findings are not consistent and confounding from genetic and environmental factors have not fully been taken into account. A comparison between siblings discordant for prenatal smoking exposure adjusts for confounding by shared familial (i.e., genetic and environmental) factors. We investigated the association between prenatal exposure to maternal smoking during pregnancy and the risk of regular smoking or snus (Swedish moist smokeless tobacco) use in young adult offspring, using a population based matched cohort study. The cohort consisted of 1,538 randomly sampled same-sex sibling pairs, discordant for maternal smoking during pregnancy, 19–27 years old, participating in a survey conducted in Sweden 2010–2011. Lifetime and current history of tobacco use was self-reported in the survey, and information about maternal smoking during pregnancy was retrieved from the Medical Birth Register. Conditional logistic regression and stratified Cox proportional hazards regression were used to calculate odds ratios, hazard ratios, and corresponding 95 % confidence intervals. Analyses of exposure-discordant siblings did not reveal significant associations between prenatal exposure to maternal smoking and lifetime or current daily tobacco use, intensity of use, or time to onset of daily tobacco use. These findings suggest that the previously reported higher risks of tobacco use in offspring of mothers who smoked during pregnancy, compared with offspring of non-smoking mothers, were likely due to confounding from genetic or environmental factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prenatal exposure to maternal smoking during pregnancy has been linked with later tobacco use both in epidemiological studies [1–16] and in animal studies [17, 18]. Nicotine from maternal smoking during pregnancy readily crosses the placental barrier and reaches the foetus [19]. Since nicotine may activate nicotine receptors in the foetal brain, it has been hypothesized that the foetal mesolimbic dopaminergic reward system might be altered, which would create a predisposition to tobacco use and dependence later in life [19].
Several prospective studies have investigated the association between maternal smoking and offspring tobacco use [1–3, 6–9, 13–16, 20–25]. However, findings are not consistent: for instance, prenatal exposure to maternal smoking has been linked with regular smoking in offspring in five studies [1–3, 8, 13], while no association was found in other studies [20, 24, 25]. Furthermore, intergenerational transmission of tobacco use may be influenced by both genetic and early-life environmental factors [26]. Therefore, in order to assess the independent effect of in utero exposure to nicotine, genetic and social influences must be accounted for.
Full siblings share around 50 % of their segregating genes and are usually brought up together. Therefore, comparing siblings automatically, albeit partially, adjusts for confounding from shared familial (i.e., genetic and environmental) factors [27]. One recent sibling study found no association between prenatal exposure to maternal smoking and initial use of cigarettes in exposure-discordant siblings [28]. However, initial episodes of smoking may not be an optimal outcome for studies of brain-priming effects of tobacco toxicants, since initiation with tobacco is more likely to be influenced by social circumstances [26]. Furthermore, the effect of prenatal exposure on tobacco use other than smoking (for instance, smokeless tobacco such as snus) has not been explored in depth, and smokeless tobacco is suggested to be as addictive as cigarettes [29, 30]. Finally, because previous findings indicate that women may be more sensitive to the influence of prenatal exposure to tobacco toxicants [14, 31], the modifying role of sex is worth to be elucidated in sibling studies.
In this study, we addressed the association between maternal smoking during pregnancy and the risk of regular smoking or snus use in adult offspring, based on a cohort study of full siblings. Moreover, we wanted to assess whether there was any difference between men and women regarding these potential relationships.
Materials and methods
Study population and data collection
The study is based on the Swedish Sibling Health Cohort—a cohort study of sibling pairs, discordant for maternal smoking during pregnancy. The study was initiated in 2009, with the explicit aim to study the influence of prenatal exposure to maternal smoking on health effects later in life. Using information from population-based Swedish registers (the Medical Birth Register [32], the Multi-Generation Register [33], and the Total Population Register [34]), we identified all same-sexed consecutive full sibling pairs discordant for maternal smoking during pregnancy. In order to ensure that participants were at least 18 years of age, and because exposure information was available from 1983 and onwards, we were restricted to pairs where both siblings were born 1983–1991. We identified 11,143 such pairs who were alive and living in Sweden in 2010. Of these, a random sample of 5,000 pairs were contacted and asked to participate in the study.
A postal questionnaire was mailed to these individuals in October 2010. Questions included current and lifetime history of tobacco use, lifetime symptoms of nicotine dependence, health status, alcohol use, family members’ tobacco use, and living circumstances during upbringing. All responders were given the choice to fill in the postal questionnaire or an identical web-based questionnaire. Telephone interviews were conducted in January and February 2011 with the purpose to increase the participation rate among sibling pairs, and therefore directed at a subsample of 1,700 individuals whose sibling had already answered the survey. Data collection was deemed complete when all 1,700 individuals in the sample either had participated, refused, or could not be reached after three call attempts. In-flow of postal questionnaires and web surveys was allowed until March 2011. Participants received a voucher for a movie ticket at the end of data collection. Information about reasons for non-participation and exclusions is reported in Fig. 1. In total, 4,368 individuals, including 1,608 complete sibling pairs, participated in the study, yielding a response rate of 44 % (32 % at the pair level). In 1,538 sibling pairs, both siblings consented to link their responses with registers to retrieve information about pregnancy and delivery of the respondent, and information about parental education.
Measures
Information about maternal smoking during pregnancy was retrieved from the Medical Birth Register, which contains information about pregnancies and deliveries for more than 98 % of all births in Sweden since 1973 [32]. Self-reported information about smoking at the first antenatal care visit (usually between the 8th and 12th gestational week) is available from 1983 and onwards, categorised as: no daily smoking; smoking 1–9 cigarettes per day; or smoking ≥10 cigarettes per day.
Information about probands’ lifetime and current tobacco use was self-reported in the questionnaire and assessed separately for smoking and snus. Individuals who had smoked at least 100 cigarettes and/or used snus at least 100 times in life were asked about their age at first cigarette/snus use; if they ever had smoked/used snus daily for at least 6 months; age at onset of daily use; and current smoking/snus use at the time of participation (including average number of cigarettes/snus dips per day if they were current users, or year and month when quitting if they were not current users).
Participants’ recall of parents’ and other family members’ smoking or snus use during their upbringing was also reported in the questionnaire. Information about both parents’ highest achieved educational level in 1991 was retrieved from the Swedish Register of Education [35]. Information about participants’ birth order, birth weight and maternal country of birth was obtained from the Medical Birth Register.
Exposure and covariates
Prenatal exposure to maternal smoking was categorised as any maternal daily smoking during pregnancy versus no maternal daily smoking. Parental postnatal tobacco use was categorised as any use if either parent smoked or used snus during the participant’s childhood or adolescence, versus no use if neither parent smoked or used snus. Maternal education was categorised as compulsory education, upper secondary education, or post-secondary education. Maternal country of birth was categorised as: Sweden, other Nordic country (i.e., Denmark, Finland, Iceland and Norway), or any other country. Birth order was categorised as first born versus later born, and sibling order as being the older or younger sibling in a pair. Calendar periods of birth were categorised based on the overall prevalence of smoking among pregnant women in Sweden: 1983–1986 (prevalence about 30 %) or 1987–1991 (declining prevalence between 28 and 24 %) [36].
Outcome
Participants’ lifetime daily smoking/snus use was defined as daily use of cigarettes or snus (the Swedish moist smokeless tobacco) for a period of at least 6 months. Starting from these two variables, a cumulative indicator of any lifetime daily tobacco use (either smoking or snus) was derived. Variables for current smoking, snus use, and use of any tobacco (smoking and/or snus) were dichotomized as current versus not current daily use. Lifetime and current daily tobacco use in the older sibling was censored at the younger sibling’s attained age in 2011. Concerning intensity of use among current daily tobacco users, heavy smoking was categorised as >7 cigarettes per day (versus less), and heavy snus use as >10 snus dips per day (versus less), based on the median values of the overall distribution in the cohort. Time of progression from experimental to regular use was calculated as the difference in years between age at first cigarette/snus use and age of onset of daily smoking/snus use.
Statistical analyses
In matched sibling pairs, the association between prenatal exposure to maternal smoking during pregnancy and lifetime and current daily tobacco use, as well as intensity of use, was assessed as odds ratios (OR) and corresponding 95 % confidence intervals (CI) by means of conditional logistic regression. Progression from experimental to regular use was estimated using stratified Cox proportional hazards regression models. Hazard ratios (HR) and corresponding 95 % CIs were calculated among pairs where both siblings had smoked at least one cigarette (or used snus at least once for analysis on snus use). Participants who progressed during the same year of age were arbitrarily assigned a time to event of 0.5 years. The assumption of proportional hazards was investigated by studying graphs over the log cumulative hazards function and the Shoenfeld residuals, and verified by a global test of rho.
Parental postnatal tobacco use, birth order, sibling order, and calendar period of birth were considered possible confounders, and adjusted for in multivariate models. Sex, maternal country of birth, and parental education were investigated for effect modification in stratified analyses. Wald-test was used to formally test for statistical interaction. To study a possible dose–response relationship, all analyses were also stratified by level of exposure (maternal smoking of 1–9 cigarettes per day, or ≥10 cigarettes per day). Analysis of intensity of use (i.e. heavy smoking and heavy snus use) was based on 90 pairs where both siblings were current smokers at time of participation and 126 pairs of current snus users. Due to the low sample sizes, stratified analyses were not possible for heavy smoking or snus use.
Since it is likely that the efficiency of control of shared environmental factors decreases with increasing age difference between siblings, all analyses were replicated after excluding siblings born more than 5 years apart.
SAS version 9.2 for Windows (SAS Institute Inc., Cary, N.C., USA), and Stata Statistical Software: Release 11 (StataCorp. 2009. College Station, TX: StataCorp LP) were used for all analyses.
Results
Selected characteristics of the study population are shown in Table 1. A majority of participating pairs were female and were born within 3 years. In 67 % of all pairs the exposed sibling was older than the unexposed. In total, 1,121 participants (36 %) were the first born child, and 1,300 participants (42 %) were born in the period 1983–1986.
Table 2 gives the ORs for lifetime and current daily tobacco use according to prenatal exposure to maternal smoking. In 1,538 sibling pairs discordant for prenatal smoking exposure, there were 430 pairs who were also discordant for lifetime daily smoking, and thus informative in analysis. In 208 of these pairs, the exposed sibling was a daily smoker, whereas the unexposed sibling was a daily smoker in the remaining 222 pairs, thereby yielding a crude OR of 0.94 (95 % CI 0.78–1.13). Similarly, prenatal exposure to maternal smoking was not statistically associated with current daily smoking, lifetime or current daily snus use, or with lifetime or current use of any tobacco (Table 2). Furthermore, there was no dose–response relationship with number of cigarettes daily smoked by the mother during pregnancy and lifetime or current daily tobacco use in offspring (data not shown). Analyses stratified by whether the younger or older sibling was prenatally exposed (Table 2) resulted in a higher risk of any current tobacco use in pairs where the older sibling was exposed (OR 1.40, 95 % CI 1.12–1.75), but a lower risk in pairs where the younger sibling was exposed (OR 0.73, 95 % CI 0.53–0.99). Analyses on current use of snus and current smoking yielded the same pattern of associations. Adjusting for calendar period of birth, birth order or parental postnatal tobacco use did not materially change the estimates (data not shown), whereas the point estimate was close to unity after adjusting for sibling order (Table 2).
Stratified analyses by sex resulted in higher ORs of snus use among exposed women than among exposed men, although no estimate was significantly different from the expected value of one (Table 3). Only small differences were detected in analyses stratified by maternal country of birth (e.g. p values for lifetime daily use: 0.89 for smoking, 0.17 for snus use, and 0.67 for any tobacco use) or by parental education (e.g. p values for lifetime daily use: 0.37 for smoking, 0.83 for snus use, and 0.98 for any tobacco use). Restricting analyses to siblings born 5 years apart or less did not result in different estimates than analyses based on the entire sample (data not shown).
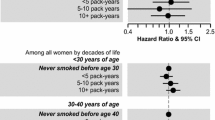
Prenatal exposure to maternal smoking did not influence intensity of use in offspring [OR for heavy smoking and heavy snus use were 0.91 (95 % CI 0.50–1.67), and 1.38 (95 % CI 0.79–2.42), respectively]. Likewise, we could not detect any associations with progression to daily use (Table 4). Stratified analyses by sex did not reveal any difference between men and women for intensity of use or for progression to daily use (data not shown).
Discussion
In this study of exposure-discordant siblings, we found no evidence of an association between prenatal exposure to maternal smoking and several measures of tobacco use in early adulthood. These results are in line with those presented by the only previous study addressing maternal smoking as risk factor for offspring’s tobacco use in a sibling design [28]. These findings do not support the notion of a causal effect of prenatal exposure on tobacco habits in offspring. The results rather indicate that the higher risks of regular or heavy tobacco use found in earlier prospective studies [1–3, 8, 13–16], were in fact confounded by unmeasured genetic or environmental factors.
However, participants’ age may be an important cue for the interpretation of discrepancies between studies. In fact, previous prospective studies that found no association between prenatal exposure to smoking and regular [20, 24, 25] or heavy tobacco use in offspring [21, 23] included participants in the same age range of the siblings in this study, whereas most of the studies reporting a positive association were based on adolescent samples. Therefore, one cannot exclude the possibility that genetic or prenatal influences may manifest through early escalation to heavy tobacco use and dependence rather than with its persistence into adulthood.
A secondary aim of this study was to clarify if sex modified the association between prenatal exposure to maternal smoking and adult offspring’s tobacco use. It has previously been hypothesized that women are more influenced by prenatal exposure to maternal smoking [31], perhaps due to hormonal modulation of the brain reward system, as seen in experimental animal studies [37]. However, the overall picture emerging from previous prospective studies doesn’t clearly speak in favour of such sex difference. In fact, four studies found a stronger association among women [7, 8, 14, 25], one study found a stronger association among males [24], whereas the majority of previous studies did not find any difference between men and women [1, 3, 6, 16, 20, 21, 23]. Accordingly, results from this sibling study do not support the hypothesis of women being more influenced by prenatal exposure to maternal smoking.
Associations in this study seemed to diverge depending on whether the exposed sibling was the older (OR of current tobacco use above unity) or the younger (OR of current tobacco use below unity) in the pair. The estimates were symmetrical around the null, indicating a possible effect of calendar period. It should be noted that the prevalence of tobacco use in Sweden has profoundly changed over time. While the prevalence of cigarette smoking has decreased, the prevalence of snus use has continuously increased [38]. The prevalence of snus use has historically been low among women, but it has become more common during the last decade (especially among teenagers) [38]. Changes in the overall prevalence of tobacco use is likely to mirror societal norms regarding tobacco use, which likely influences smoking, both in general and during pregnancy. Adjusting for calendar period did not materially change the estimates. However, it is possible that this adjustment could not account for the effect of calendar period in this data. This possibility is supported by a secondary analysis, showing that the different association patterns according to whether the older or the younger sibling was exposed, were entirely attributable to pairs where the siblings were born in different calendar periods (1983–1986 vs 1987–1991), thus many years apart (average 4 years, to be compared with 2 years among siblings born in the same calendar period).
We also adjusted for sibling order as siblings have been shown to influence each other’s smoking [39]. However, this adjustment did not substantially modify the estimates, which is not that surprising considering that role-modelling is more likely to be exerted by the older on the younger sibling than vice versa.
This sibling study is the first to investigate the role of prenatal exposure to maternal smoking on regular and established tobacco use in adults, including both cigarette and snus use. The strength of this study foremost rests on the design, a matched population-based cohort, planned specifically to test hypotheses about effects of prenatal exposure to maternal smoking on offspring’s health outcomes. The cohort of same-sex siblings was based on registers with high quality and coverage, where data has been continuously collected for more than 30 years. Furthermore, our source population constituted a nationally representative sample, randomly selected from all exposure-discordant siblings born 1983–1991. By comparing exposure-discordant siblings with each other, confounding was considerable reduced, both regarding genetic and environmental influences shared between siblings [27].
Some concerns should be taken into account in sibling-comparison studies. Analysis based on exposure-discordant pairs implies a selection of pairs that are also likely to differ regarding non-shared causes of the exposure, including random measurement error [40]. Therefore, studies on exposure-discordant sibling pairs are more likely to suffer from misclassification of exposure than studies based on the general population, which could result in a dilution of true associations [40]. Since maternal smoking during pregnancy is likely underreported [41], this could be a potential problem especially in pairs where the mother reported smoking when pregnant with the younger sibling, but not with the older. These pairs could be falsely classified as discordant. Only exposure to daily smoking during pregnancy was considered, which might have diluted possible effects of low levels of exposure. Moreover, in sibling studies confounding from unshared factors linked to sibling order or calendar period, could induce bias [40]. In our study, in 46.5 % of all pairs the siblings were born within 2 years, and excluding all siblings born more than 5 years apart did not affect the estimates. Also, it has been shown that the social environment plays a more important role for initial tobacco use, whereas genetic factors may have a higher impact on progression of use [26]. Lastly, smoking among adults tends to be underreported [41], which could have resulted in attenuated estimates. However, the prevalence of tobacco use among participants in this study was comparable with the prevalence in the general population in 2011 [42].
In summary, we found no evidence of associations between prenatal exposure to maternal smoking and tobacco use when exposure-discordant siblings were compared. These findings suggest that the higher risks of tobacco use previously found in offspring of mothers who smoked during pregnancy were at least partly confounded by genetic or environmental factors.
References
Al Mamun A, O’Callaghan FV, Alati R, et al. Does maternal smoking during pregnancy predict the smoking patterns of young adult offspring? A birth cohort study. Tob Control. 2006;15(6):452–7.
Lawlor DA, O’Callaghan MJ, Mamun AA, Williams GM, Bor W, Najman JM. Early life predictors of adolescent smoking: findings from the Mater-University study of pregnancy and its outcomes. Paediatr Perinat Epidemiol. 2005;19(5):377–87.
O’Callaghan FV, O’Callaghan M, Najman JM, Williams GM, Bor W, Alati R. Prediction of adolescent smoking from family and social risk factors at 5 years, and maternal smoking in pregnancy and at 5 and 14 years. Addiction. 2006;101(2):282–90.
O’Callaghan FV, Al Mamun A, O’Callaghan M, et al. Maternal smoking during pregnancy predicts nicotine disorder (dependence or withdrawal) in young adults—a birth cohort study. Aust N Z J Public Health. 2009;33(4):371–7.
Agrawal A, Knopik VS, Pergadia ML, et al. Correlates of cigarette smoking during pregnancy and its genetic and environmental overlap with nicotine dependence. Nicotine Tob Res. 2008;10(4):567–78.
Cornelius MD, Leech SL, Goldschmidt L, Day NL. Prenatal tobacco exposure: is it a risk factor for early tobacco experimentation? Nicotine Tob Res. 2000;2(1):45–52.
Griesler P, Kandel D, Davies M. Maternal smoking in pregnancy, child behaviour problems, and adolescent smoking. J Res Adolesc. 1998;8(2):159–85.
Kandel DB, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. Am J Public Health. 1994;84(9):1407–13.
Kandel DB, Griesler PC, Schaffran C. Educational attainment and smoking among women: risk factors and consequences for offspring. Drug Alcohol Depend. 2009;104:S24–33.
Kardia SL, Pomerleau CS, Rozek LS, Marks JL. Association of parental smoking history with nicotine dependence, smoking rate, and psychological cofactors in adult smokers. Addict Behav. 2003;28(8):1447–52.
Lieb R, Schreier A, Pfister H, Wittchen HU. Maternal smoking and smoking in adolescents: a prospective community study of adolescents and their mothers. Eur Addict Res. 2003;9(3):120–30.
Oncken C, McKee S, Krishnan-Sarin S, O’Malley S, Mazure C. Gender effects of reported in utero tobacco exposure on smoking initiation, progression and nicotine dependence in adult offspring. Nicotine Tob Res. 2004;6(5):829–33.
Tehranifar P, Liao Y, Ferris JS, Terry MB. Life course socioeconomic conditions, passive tobacco exposures and cigarette smoking in a multiethnic birth cohort of U.S. women. Cancer Causes Control. 2009;20(6):867–76.
Rydell M, Cnattingius S, Granath F, Magnusson C, Galanti MR. Prenatal exposure to tobacco and future nicotine dependence: population-based cohort study. Br J Psychiatry. 2012;200(3):202–9.
Cornelius MD, Goldschmidt L, Day NL. Prenatal cigarette smoking: long-term effects on young adult behaviour problems and smoking behaviour. Neurotoxicol Teratol. 2012;34(6):554–9.
Rydell M, Magnusson C, Cnattingius S, Granath F, Svensson A, Galanti MR. Exposure to maternal smoking during pregnancy as a risk factor for tobacco use in adult offspring. Am J Epidemiol. 2014. doi:10.1093/aje/kwu074.
Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Prenatal nicotine exposure alters the responses to subsequent nicotine administration and withdrawal in adolescence: serotonin receptors and cell signalling. Neuropsychopharmacology. 2006;31(11):2462–75.
Levin ED, Lawrence S, Petro A, Horton K, Seidler FJ, Slotkin TA. Increased nicotine self-administration following prenatal exposure in female rats. Pharmacol Biochem Behav. 2006;85(3):669–74.
Hellstrom-Lindahl E, Nordberg A. Smoking during pregnancy: a way to transfer the addiction to the next generation? Respiration. 2002;69(4):289–93.
Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study. A J Psychiatry. 2003;160(11):1978–84.
Cornelius MD, Leech SL, Goldschmidt L, Day NL. Is prenatal tobacco exposure a risk factor for early adolescent smoking? A follow-up study. Neurotoxicol Teratol. 2005;27(4):667–76.
Isohanni M, Moilanen I, Rantakallio P. Determinants of teenage smoking, with special reference to non-standard family background. Br J Addict. 1991;86(4):391–8.
Munafo MR, Wileyto EP, Murphy MFG, Collins BN. Maternal smoking during late pregnancy and offspring smoking behaviour. Addict Behav. 2006;31(9):1670–82.
Porath AJ, Fried PA. Effects of prenatal cigarette and marijuana exposure on drug use among offspring. Neurotoxicol Teratol. 2005;27(2):267–77.
Roberts KH, Munafo MR, Rodriguez D, et al. Longitudinal analysis of the effect of prenatal nicotine exposure on subsequent smoking behaviour of offspring. Nicotine Tob Res. 2005;7(5):801–8.
Kendler KS, Chen XN, Dick D, et al. Recent advances in the genetic epidemiology and molecular genetics of substance use disorders. Nat Neurosci. 2012;15(2):181–9.
Lahey BB, D’Onofrio BM. All in the family: comparing siblings to test causal hypotheses regarding environmental influences on behaviour. Curr Dir Psychol Sci. 2010;19(5):319–23.
D’Onofrio BM, Rickert ME, Langstrom N, et al. Familial confounding of the association between maternal smoking during pregnancy and offspring substance use and problems. Arch Gen Psychiatry. 2012;69(11):1140–50.
Benowitz NL. Nicotine and smokeless tobacco. CA Cancer J Clin. 1988;38(4):244–7.
Benowitz NL, Porchet H, Sheiner L, Jacob P 3rd. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther. 1988;44(1):23–8.
Sullivan KM, Bottorff J, Reid C. Does mother’s smoking influence girls’ smoking more than boys’ smoking? A 20-year review of the literature using a sex- and gender-based analysis. Subst Use Misuse. 2011;46(5):656–68.
The National Board of Health and Welfare. The Swedish medical birth register: a summary of content and quality. Stockholm, Sweden: The National Board of Health and Welfare; 2003.
Ekbom A. The Swedish multi-generation register. Methods Mol Biol. 2011;675:215–20.
Statistics Sweden. Tables on the population in Sweden 2009. Örebro: Statistics Sweden; 2010.
Statistics Sweden. Registret över befolkningens utbildning [The Swedish Register of Education]. Statistics Sweden. http://www.scb.se/sv_/Vara-tjanster/SCBs-data-for-forskning/SCBs-datalager/Registret-over-befolkningens-utbildning. Accessed Febr 13 2014.
The National Board of Health and Welfare. Graviditeter, förlossningar och nyfödda barn: Medicinska födelseregistret 1973–2011, Assisterad befruktning 1991–2010 [Pregnancies, Deliveries and Newborn Infants: The Swedish Medical Birth Register 1973–2012, Assisted Reproduction, treatment 1991–2011]: The National Board of Health and Welfare, 2013.
Dluzen DE, Anderson LI. Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neurosci Lett. 1997;230(2):140–2.
The National Board of Health and Welfare. Folkhälsorapport 2009 [Public Health Report 2009]. Stockholm: The National Board of Health and Welfare; 2009.
Slomkowski C, Rende R, Novak S, Lloyd-Richardson E, Niaura R. Sibling effects on smoking in adolescence: evidence for social influence from a genetically informative design. Addiction. 2005;100(4):430–8.
Frisell T, Öberg S, Kuja-Halkola R, Sjölander A. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;23(5):713–20.
Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11(1):12–24.
Swedish National Institute of Public Health. Tobacco habits—results 2012. Swedish National Institute of Public Health, Östersund, Sweden. 2013. http://www.fhi.se/Statistik-uppfoljning/Nationella-folkhalsoenkaten/Levnadsvanor/Tobaksvanor/. Accessed 29 July 2013.
Acknowledgments
We thank Simon Lind and Dr Hanna Hultin for assistance with statistical analysis. This study was funded with grant 2008-0876 from the Swedish Council for Working Life and Social Research.
Conflict of interest
MR had financial support from the Swedish Council for Working Life and Social Research, and MRG from the National Board of Health and Welfare and from Stockholm County Council; MRG has received grants from Swedish National Institute for Public Health.
Ethical standard
The study was approved by the Regional Ethical Review Board in Stockholm, Sweden (DNR 2009/160-31, DNR 2009/1529-39/5, DNR 2009/1993-32 and DNR 2010/1982-32). Responders gave their written informed consent to participate in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rydell, M., Granath, F., Cnattingius, S. et al. In-utero exposure to maternal smoking is not linked to tobacco use in adulthood after controlling for genetic and family influences: a Swedish sibling study. Eur J Epidemiol 29, 499–506 (2014). https://doi.org/10.1007/s10654-014-9912-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-014-9912-5