Abstract
Atrial fibrillation (AF) is the most common chronic arrhythmia in adults and its prevalence is increasing. Due to its serious cardiovascular complications there is a strong need to understand predisposing risk factors to develop effective prevention strategies. There are a few established risk factors but a number of further risk factors have been suggested including obesity, metabolic syndrome, sleep-disordered breathing, and inflammation. The aim of this study was to investigate established and emerging risk factors for AF in a cohort study of 4,267 adults in Busselton, Western Australia, without a history of AF at baseline in 1994/95 who were followed for 15 years for incident AF events. Baseline measurement included questionnaire, clinical assessment and blood sample. A total of 343 (8 %) experienced AF during follow-up. Cox regression analysis confirmed advancing age, male sex, taller height, being on hypertension treatment and higher body mass index (BMI) as the major common risk factors (all p < 0.001). However, further modelling showed the effect of being on hypertension treatment may be stronger in women (p = 0.001) and the effect of BMI stronger in men (p = 0.004). After adjustment for these factors, no other factors were strongly related (p < 0.001) although short PR interval, history of valvular heart disease, stroke, chronic obstructive pulmonary disease, lung function and adiponectin level were marginally related (p < 0.05). This cohort study of predictors for incident AF has confirmed the major established risk factors. However, recently suggested potential novel risk factors for AF (inflammation, sleep-disordered breathing, glucose/metabolic disorders) were not confirmed in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is a supraventricular tachyarrhythmia characterized by chaotic atrial activation leading to loss of atrial mechanical function and an irregular ventricular response. AF is the most common chronic arrhythmia in adults afflicting about 1–2 % of the general population, increasing to about 10 % of persons by 80 years of age [1, 2]. AF can lead to serious complications, including a fivefold increased risk of stroke, threefold increased risk of heart failure, and up to twofold increased risk of death compared to the general population [1–5]. AF is an escalating problem with the number of affected persons in the USA projected to more than double over the next four decades due to an aging population and longer survival with cardiovascular disease [6, 7]. The potential for a similar rise in the burden of AF in other countries underscores the need to develop effective prevention strategies for AF [8]. To achieve this we first need to understand the contemporary predisposing risk factors for AF.
Apart from advancing age and male gender, established risk factors for AF are cardiac conditions such as heart failure, valvular disease, and myocardial infarction, and cardiovascular risk factors such as hypertension and diabetes [1, 9, 10]. However, other factors potentially playing a role in the genesis of AF have gained attention, including obesity, metabolic syndrome, obstructive sleep apnoea, and inflammation [11–14]. The ‘epidemic’ of AF has occurred concurrently with obesity. Although earlier studies were discordant [9, 10], more recent population-based studies have indicated a 3–8 % higher incidence of AF with each unit increase in body mass index (BMI) independent of lipid levels, hypertension and diabetes [12, 13]. Obesity is also strongly associated with the metabolic syndrome, a pre-diabetic condition, in addition to an inflammatory state, and both conditions have recently been shown to carry an excess risk of AF [15, 16]. Three risk algorithms for incident AF have been published and all include age, hypertension treatment, systolic blood pressure (BP), and heart failure, two include BMI or weight, two include height, two include diabetes, and each includes particular coronary conditions or measures such as coronary heart disease, PR interval, cardiac murmur, and left ventricular hypertrophy [17–19].
The objective of this study was to investigate established and emerging risk factors for AF in a well characterised Australian cohort [20]. In particular, to establish if obesity, metabolic syndrome, sleep-disordered breathing, and inflammation are independent risk factors for incident AF.
Methods
Study design and participants
This is a prospective community-based cohort study. Invitations to participate were sent to adults listed on earlier Electoral Registers (registration to vote is compulsory in Australia) for the Busselton district and people were asked to complete a questionnaire and present at the survey centre 1994/95 for a range of tests and blood collection. The Busselton community is almost entirely of white Caucasian background. A total of 4,843 participated in the survey (response rate 57 %), after restricting to age 25–84 this became 4,465, after excluding 84 prevalent AF cases identified from a 15-year history of hospital admissions (see below) and from electrocardiogram (ECG) evidence (Minnesota code 8.3) at the survey, this became 4,381, and a further 114 were omitted due to missing body size and other data, leaving 4,267 for analyses. All participants gave informed consent and the 1994/95 survey was approved by the Human Research Ethics Committee of The University of Western Australia.
Measurements and follow-up
The conduct and measurements of the 1994/95 Busselton health survey have been described previously [20]. Survey participants were asked to complete a comprehensive health and lifestyle questionnaire and to undergo various measurements and tests including BP, anthropometry, 12-lead electrocardiogram (ECG; Minnesota coded, available for random three-quarters), spirometry, and fasting blood samples were collected. Sleep disordered breathing was based on the Lavie questionnaire [21] items relating to habitual snoring and the question “Do you fall asleep during the day, particularly if you are not busy?”. The physical activity measure was whether or not the participant reported doing any vigorous exercise in a usual week. Blood measures available for this analysis include serum total and HDL cholesterol, triglycerides, plasma glucose and insulin, creatinine, C-reactive protein (CRP), and adiponectin. The ECG-derived history variables were: left ventricular hypertrophy (LVH) (Minnesota codes 3.1, 3.3), left bundle branch block-(LBBB) (7.1), Long PR interval (6.3), and Short PR interval (6.5). As only three participants had multiple ECG abnormalities a combined ECG variable was created with categories LVH, LBBB, long PR interval, short PR interval, None of above and Unknown (ECGs were not available for a random 25 % of participants) where the one participant with both LVH and Long PR interval was included in the LVH category and the two participants with both LBBB and Long PR interval were included in the LBBB category. The MDRD formula for glomerular filtration rate was used as a measure of renal function [22]. The metabolic syndrome score was defined as the number of the five risk components (hypertension, hyperglycemia, hypertriglyceridemia, high density lipoprotein (HDL) cholesterol, waist circumference) meeting the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) 2005 criteria [23]. Insulin resistance was assessed by the homeostasis model assessment (HOMA) score calculated as insulin (uU/mL) × glucose (mmol/L)/22.5 [24].
The Human Research Ethics Committee of the Department of Health of Western Australia gave permission to access the hospital admission and death records from 1980 to 2010 for participants. History of various cardiac and other conditions at baseline were defined as having any hospital admission with a primary or secondary discharge diagnosis for that condition during the 15 years before the survey. The history variables based on hospital admissions were heart failure (ICD9 428), valvular heart disease (ICD9 394–397, 996.02, 996.71, V42.2, V43.3), myocardial infarction or coronary revascularisation (ICD9 410, 412 and procedure codes 5–363 and 5–361 in ICD9 and 36.01, 36.02, 36.05, 36.06, 36.07 and 36.1 in ICD9-CM), stroke, trans ischaemic attack (TIA) or systemic embolism (ICD9 431, 432, 433.x1, 434.x1, 435, 436 and 437.7), peripheral arterial disease (ICD9 440-448), chronic renal disease (ICD9 585, 586), chronic obstructive pulmonary disease (COPD) (ICD9 490–496). History of hypertension treatment was based on self-reported taking of anti-hypertensive medications at the survey or a history of hospital admissions (ICD9 401–405). Diabetes was based on self-reported doctor–diagnosed diabetes or on diabetes treatment (tablets/insulin) at the survey or a history of hospital admissions (ICD9 250).
Incident AF events during the 15-year follow-up period to the end of 2010 were defined as a hospital admission with a primary or other diagnosis of atrial fibrillation/flutter (ICD9-CM 427.31, 427.32, 427.3; ICD10 I48) and no mention of prosthetic heart valve (ICD9-CM 996.02, 996.71, V42.2, V43.3, 35.2; ICD10 T82, Z95, blocks 623, 628, 634, 637) or coronary artery bypass graft procedure (ICD9-CM 36.1; ICD10 blocks 672–679).
Statistical analysis
Variables with skewed distributions were log transformed for use in regression models and descriptive results for these variables are presented in both untransformed and transformed scales. Cox regression models for time from 1994/95 survey to first AF event were used to obtain various adjusted hazard ratios of incident AF (and their 95 % confidence intervals) for a range of established and potential new risk factors. Interactions of risk factors with sex and age were tested. Results from Cox regression models are presented either as estimated coefficients (with standard error and p value) or as estimated hazard ratios (with 95 % confidence interval and p value). The improvement in the prediction performance of models from adding additional risk factors was assessed using the area under the curve (also called C-statistic) [25] and the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) measures [26].
Results
Table 1 shows the (baseline) characteristics of the cohort of 4,267 participants aged 25–84 years who had no history of AF at baseline. The anthropometric, lifestyle, health history and risk factor levels are typical of Australian population cohorts at that time. ECG abnormalities were rare with LVH, LBBB, Long PR interval and Short PR interval being present in about 1 % of the cohort. Similarly, history of heart failure, valvular heart disease, stroke/TIA, peripheral arterial diseases, and chronic renal disease were present in 1 % or less of the cohort. However, around 3 % had a history of MI, 3 % had a history of COPD, 6 % had diabetes, 3 % were taking lipid medications, and around 20 % had a history of hypertension. A total of 16 % met the NCEP ATP III metabolic syndrome criterion of 3 + disordered components.
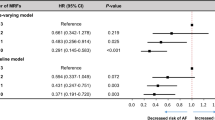
A total of 343 (8.0 %) experienced an AF event during follow-up and this was slightly higher in men (8.8 %) than women (7.4 %). Cox regression analysis confirmed the higher risk in men and showed an increasing risk with age but with a stronger age trend in women (sex × age interaction p value 0.0065). Given the basic constitutional nature of height and that height was strongly related (p = 0.0001) to risk of AF after adjustment for age and sex terms, all remaining potential risk factors were assessed after adjustment for age, sex and height. Table 2 (Model 1) shows the fitted model involving age, sex and height. The estimated coefficients in this model translate to the risk of AF increasing by a factor of 3.25 (95 % CI 2.81–3.77) for each additional 10 years of age in women and by a factor of 2.49 (95 % CI 2.18–2.85) for each additional 10 years of age in men. As a consequence, although the relative risk in men vs women is 2.66 at 25 years of age this gap decreases with age and the risks for men and women are similar for people aged over 60 years. The risk of AF increases by 35 % (adjusted hazard ratio 1.35; 95 % CI 1.16–1.57) for each additional 9 cms (one SD) of height.
Table 3 (left side) shows the estimated hazard ratio for each potential risk factor after adjustment for age, sex and height. Hypertension treatment (p < 0.0001) and BMI (p < 0.0001) were the strongest risk factors with waist circumference, history of heart failure, history of valvular disease, history of stroke, COPD, fasting insulin and HOMA, metabolic syndrome, and FEV1 % (protective) also moderately or strongly related (p < 0.01) to risk of AF. Further modelling indicates the effect of hypertension treatment may be greater in women (hypertension × sex interaction p = 0.0014) and the effect of BMI may be greater in men (BMI × sex interaction p = 0.0035). Table 2 (Model 2) shows the fitted model involving age, sex, height, history of hypertension treatment and BMI. This model shows that, after adjusting for age, sex and height, hypertension treatment increases the risk of AF by a factor of 2.14 (95 % CI 1.56–2.94) in women and by a factor of 0.98 (95 % CI 0.69–1.40) in men, and that a gain of 4.2 (one SD) in BMI increases risk of AF by 16 % (adjusted hazard ratio 1.16; 95 % CI 1.02–1.32) in women and by 64 % (adjusted hazard ratio 1.64; 95 % CI 1.35–1.99) in men.
Table 3 (right side) shows the estimated hazard ratio (and p value) for each remaining potential risk factor after adjustment for age, sex, height, hypertension treatment and BMI. No further risk factors were strongly related (p < 0.001) to risk of AF, although several remain moderately or weakly associated including short PR interval (HR = 6.21; p = 0.011), history of valvular heart disease (HR = 2.44, p = 0.034), stroke/TIA (HR = 2.02, p = 0.019), COPD (HR = 1.73, p = 0.012), adiponection (p = 0.007), and FEV1 %Pred (p = 0.003). Note that the metabolic syndrome score adds no further risk assessment value after hypertension treatment and BMI have been considered.
The C-statistics for model prediction performance were 0.845 for the model with just age and sex, this increased marginally to 0.848 when height was added, then further to 0.856 when hypertension treatment was added, and then further increased to 0.861 when BMI was added. The NRI measure of improved risk classification was significantly improved when height was added (p = 0.008) and when hypertension treatment was added (p = 0.002) but not when BMI was added (p = 0.129) whereas the IDI measure improved significantly when hypertension treatment (p = 0.002) and BMI were added (p = 0.001) and not when height was added (p = 0.087).
Discussion
This prospective community cohort analysis of 4,267 people without a history of AF and with 343 incident AF occurrences over a 15-year follow-up period has confirmed a number of established risk factors for AF. Age is a major risk factor, albeit slightly stronger in women, and our results of a more than doubling of risk with every additional decade is consistent with previous studies [10, 27]. Whilst we found that men have higher risk than women overall as in most previous studies we also found that the higher relative risk for men decreased with age and older (>60 years) men and women had similar risk. This finding is at odds with the observed increased risk of AF in men in the Cardiovascular Health Study of older people [27], but an age × sex interaction was also found in the Framingham Study [17]. Curiously, gender was not included as a risk factor in the AF clinical risk score developed from the ARIC Study or from three combined cohorts [18, 19].
Height is another immutable risk factor and in our study each additional 9 cms of height was associated with a 35 % higher AF risk. A case–control study of middle-aged individuals found height to be strongly related to lone AF and this was independent of atrial size which is positively correlated with height [28]. Height was also found to be an independent predictor of incident AF in the ARIC Study [18] and a study of middle-aged Swedish men [29]. Further, a large study of women found that the association between birth weight and AF was substantially attenuated after adjustment for height [30]. Whether birth weight or adult height is the true determinant will be difficult to resolve.
A recent meta-analysis involving 5 population-based cohort studies conclusively demonstrated the increased risk of AF in obese individuals and risk increased with increasing BMI [31]. Our findings corroborate BMI is an important risk factor but our finding of a stronger association with BMI in men has not been previously reported. The afore-mentioned meta-analysis reported sex-specific results but, opposite to our finding, showed larger relative risks in women for overweight and obese groups in comparison to the normal weight group. We found a significant protective effect of physical activity (doing some vigorous exercise each week) but this attenuated and was not significant after adjustment for BMI. The same was observed in a more detailed study of physical activity in relation to AF in women [32].
Our finding of a greater risk of AF in people on treatment for hypertension is not surprising as this is well established and published AF risk scores all include anti-hypertensive treatment [17–19]. However, our finding of a stronger effect of hypertension treatment on AF risk in women has not previously been reported and appears to be at odds with the Framingham Study that also investigated the sex × hypertension interaction [17]. Further, in contrast to previous studies, we did not find high BP to be a significant additional risk factor after adjustment for being on anti-hypertensive treatment [2, 17–19, 33].
After accounting for age, sex, height, history of hypertension and BMI we found no further risk factors to be strongly related (p < 0.001) to AF risk but did find some to remain moderately or weakly associated (p < 0.05). Some of these additional risk factors have already been established in other studies. Cardiac conditions have been shown to be associated with increased risk of AF [10]. The Framingham AF risk score includes PR interval, significant cardiac murmur and history of heart failure [17] whereas the ARIC AF risk score includes precordial murmur, left atrial enlargement and LVH [18]. In our Busselton Study we found short PR interval, history of valvular heart disease and history of stroke all had relative risk estimates exceeding 2 and with marginal statistical significance (0.01 < p < 0.05). This is likely to be due to lack of statistical power due to the low prevalence of these conditions in this cohort.
Other risk factors associated (p < 0.05) with AF in our Busselton Study include history of COPD, FEV1 %Pred and serum adiponectin. Whilst there are reports of an association between COPD/FEV1 and AF from both cross-sectional [34] and prospective studies [35], the evidence is limited. Few prospective studies have investigated adiponection in relation to AF and none found it to be an independent risk factor [36].
A number of other metabolic and inflammatory related factors, none of which were significant in our Busselton Study, have been suggested as potential new risk factors for AF. Diabetes but not fasting glucose or circulating insulin levels has been shown to be associated with AF [37, 38]. C-Reactive protein, a measure of inflammation, has been associated with AF in a number of studies and despite our negative results the evidence base is accumulating [2, 39–41]. Despite the report of Gami et al. [42] and the conclusion in a recent review that obstructive sleep apnoea (OSA) is established as a risk factor for AF [43], our Busselton finding for sleep disordered breathing was not significant, perhaps due to the limitations of self-report measures or that only those with OSA and not those with only sleep disordered breathing are at increased risk.
Strengths of this study are that it was community-based, included a wide variety of established and potential new risk factors, and with 343 incident AF cases, had over 80 % power to detect hazard ratios of 1.2 or more for a one SD change in a quantitative risk factor and hazard ratios of 1.5 or more for a binary risk factor with 10 % or higher prevalence. Limitations include the low prevalence of some cardiac conditions partly due to a potential healthy response bias arising from recruiting participants from the Electoral Register and for some conditions partly due to use of hospital admissions history only to identify conditions. Some of these low prevalence conditions therefore could not be conclusively confirmed as risk factors. As baseline ECG data were (randomly) missing for about one quarter of the cohort (n = 1,127) we only had their 15-year hospital admissions history to identify prevalent AF. We estimated that an additional 8 prevalent AF cases were therefore missed and hence not excluded from the cohort. As a sensitivity check we re-fitted all models using only the cohort with baseline ECG data (n = 3,140) and found the estimates to be essentially the same albeit with slightly wider confidence intervals due to smaller sample size. Finally, the use of linked hospital admission data to detect incident AF cases means we may have over-estimated the true time to AF onset for the identified incident AF cases and were not able to detect new cases of AF that were diagnosed in primary practice and over the 15 year follow-up period did not have any hospital admission where AF was recognised. Any bias from this is likely to be towards the null hypothesis and thus our hazard ratios may be conservative [44].
This prospective community cohort study of predictors for incident AF has confirmed age, sex, height, hypertension and BMI as the major common risk factors but also suggests the effects of age and hypertension may be stronger in women and the effect of BMI stronger in men. Although not statistically confirmed in this Busselton Study, the less common risk factor of having a cardiac condition remains important. Furthermore, recently suggested potential novel risk factors for AF (inflammation, sleep-disordered breathing, obstructive lung disease, glucose/metabolic disorders) were not confirmed in this study. Thus prevention efforts should continue to focus on BP and weight reduction.
References
Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82(7):2N–9N.
Greenlee RT, Vidaillet H. Recent progress in the epidemiology of atrial fibrillation. Curr Opin Cardiol. 2005;20(1):7–14.
Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–52.
Chugh SS, Blackshear JL, Shen W-K, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37(2):371–8.
Stewart S, Hart CL, Hole DJ, McMurray JJV. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley Study. Am J Med. 2002;113:359–64.
Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–5. doi:10.1001/jama.285.18.2370.
Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–25. doi:10.1161/circulationaha.105.595140.
Benjamin EJ, Chen P-S, Bild DE, Mascette AM, Albert CM, Alonso A, et al. Prevention of atrial fibrillation: report from a National Heart, Lung, and Blood Institute workshop. Circulation. 2009;119(4):606–18. doi:10.1161/circulationaha.108.825380.
Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96(7):2455–61.
Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. JAMA. 1994;271(11):840–4. doi:10.1001/jama.1994.03510350050036.
Schoonderwoerd BA, Smit MD, Pen L, Van Gelder IC. New risk factors for atrial fibrillation: causes of ‘not-so-lone’ atrial fibrillation. Europace. 2008;10(6):668–73. doi:10.1093/europace/eun124.
Wang TJ, Parise H, Levy D, D’Agostino RB, Wolf PA, Vasan RS, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA, J Am Med Assoc. 2004;292(20):2471–7. doi:10.1001/jama.292.20.2471.
Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish diet, cancer, and health study. Am J Med. 2005;118(5):489.
Dublin S, French B, Glazer NL, Wiggins KL, Lumley T, Psaty BM, et al. Risk of new-onset atrial fibrillation in relation to body mass index. Arch Intern Med. 2006;166(21):2322–8. doi:10.1001/archinte.166.21.2322.
Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, et al. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. 2008;117(10):1255–60. doi:10.1161/circulationaha.107.744466.
Marott SCW, Nordestgaard BG, Zacho J, Friberg J, Jensen GB, Tybjaerg-Hansen A, et al. Does elevated C-reactive protein increase atrial fibrillation risk? A mendelian randomization of 47,000 individuals from the general population. J Am Coll Cardiol. 2010;56(10):789–95. doi:10.1016/j.jacc.2010.02.066.
Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D’Agostino RB Sr, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. The Lancet. 2009;373(9665):739.
Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, et al. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk In Communities [ARIC] Study). Am J Cardiol. 2011;107(1):85–91.
Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2(2):e000102. doi:10.1161/JAHA.112.000102.
Knuiman MW, Hung J, Divitini ML, Davis TM, Beilby JP. Utility of the metabolic syndrome and its components in the prediction of incident cardiovascular disease: a prospective cohort study. Eur J Cardiovasc Prevent Rehabil. 2009;16(2):235–41. doi:10.1097/HJR.0b013e32832955fc.
Knuiman MW, James AL, Divitini ML, Bartholomew HC. Correlates of habitual snoring and witnessed apnoeas in Busselton, Western Australia. Aust N Z J Public Health. 2005;29(5):412–5.
Beilby J, Divitini ML, Knuiman MW, Rossi E, Hung J. Comparison of cystatin C and creatinine as predictors of cardiovascular events in a community-based elderly population. Clin Chem. 2010;56(5):799–804. doi:10.1373/clinchem.2009.135962.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome—an American Heart Association/National Heart, Lung, and Blood Institute scientific statement—executive summary. Circulation. 2005;112(17):E285–90.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment—insulin resistance and beta-cell function from fasting plasma-glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Chambless LE, Diao GQ. Estimation of time-dependent area under the ROC curve for long-term risk prediction. Stat Med. 2006;25(20):3474–86. doi:10.1002/Sim.2299.
Pencina MJ, D’Agostino RB, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–72. doi:10.1002/Sim.2929.
Marcus GM, Alonso A, Peralta CA, Lettre G, Vittinghoff E, Lubitz SA, et al. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 2010;122(20):2009–15.
Mont L, Tamborero D, Elosua R, Molina I, Coll-Vinent B, Sitges M, et al. Physical activity, height, and left atrial size are independent risk factors for lone atrial fibrillation in middle-aged healthy individuals (vol 10, pg 15, 2008). Europace. 2008;10(3):386.
Rosengren A, Hauptman PJ, Lappas G, Olsson L, Wilhelmsen L, Swedberg K. Big men and atrial fibrillation: effects of body size and weight gain on risk of atrial fibrillation in men. Eur Heart J. 2009;30(9):1113–20.
Conen D, Tedrow UB, Cook NR, Buring JE, Albert CM. Birth weight is a significant risk factor for incident atrial fibrillation. Circulation. 2010;122(8):764–70.
Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity—results of a meta-analysis. Am Heart J. 2008;155(2):310–5.
Everett BM, Conen D, Buring JE, Moorthy MV, Lee IM, Albert CM. Physical activity and the risk of incident atrial fibrillation in women. Circ-Cardiovasc Qual. 2011;4(3):321–7.
Healey JS, Connolly SJ. Atrial fibrillation: hypertension as a causative agent, risk factor for complications, and potential therapeutic target. Am J Cardiol. 2003;91(10):9G–14G.
Shibata Y, Watanabe T, Osaka D, Abe S, Inoue S, Tokairin Y, et al. Impairment of pulmonary function is an independent risk factor for atrial fibrillation: the Takahata Study. Int J Med Sci. 2011;8(7):514–22.
Buch P, Friberg J, Scharling H, Lange P, Prescott E. Reduced lung function and risk of atrial fibrillation in The Copenhagen City Heart Study. Eur Respir J. 2003;21(6):1012–6.
Rienstra M, Sun JX, Lubitz SA, Frankel DS, Vasan RS, Levy D, et al. Plasma resistin, adiponectin, and risk of incident atrial fibrillation: the Framingham Offspring Study. Am Heart J. 2012;163(1):119–24.
Fontes JD, Lyass A, Massaro JM, Rienstra M, Dallmeier D, Schnabel RB, et al. Insulin resistance and atrial fibrillation (from the Framingham Heart Study). Am J Cardiol. 2012;109(1):87–90.
Huxley RR, Alonso A, Lopez FL, Filion KB, Agarwal SK, Loehr LR, et al. Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: the Atherosclerosis Risk in Communities study. Heart. 2012;98(2):133–8.
Smith JG, Newton-Cheh C, Almgren P, Struck J, Morgenthaler NG, Bergmann A, et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;56(21):1713–9.
Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB, et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121(2):200–7.
Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108(24):3006–10.
Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–7.
Needleman M, Calkins H. The role of obesity and sleep apnea in atrial fibrillation. Curr Opin Cardiol. 2011;26(1):40–5.
Selmer C, Olesen JB, Hansen ML, Lindhardsen J, Olsen AMS, Madsen JC, et al. The spectrum of thyroid disease and risk of new onset atrial fibrillation: a large population cohort study. Brit Med J. 2012;345.
Acknowledgments
The 1994/95 Busselton health survey was supported by a grant from the Health Promotion Foundation of Western Australia. This analysis was supported by a grant from the National Health and Medical Research Council of Australia (Project number 1020373). We would like to acknowledge the Data Linkage Branch (Department of Health WA) for extraction and provision of the linked hospital admission and death data, the Busselton Population Medical Research Institute for permission to access the survey data, and the residents of Busselton for their long-standing support of the Busselton health surveys.
Conflict of interest
The authors declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Knuiman, M., Briffa, T., Divitini, M. et al. A cohort study examination of established and emerging risk factors for atrial fibrillation: the Busselton Health Study. Eur J Epidemiol 29, 181–190 (2014). https://doi.org/10.1007/s10654-013-9875-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-013-9875-y