Abstract
The Rotterdam Study is a prospective cohort study ongoing since 1990 in the city of Rotterdam in The Netherlands. The study targets cardiovascular, endocrine, hepatic, neurological, ophthalmic, psychiatric, dermatological, oncological, and respiratory diseases. As of 2008, 14,926 subjects aged 45 years or over comprise the Rotterdam Study cohort. The findings of the Rotterdam Study have been presented in over a 1,000 research articles and reports (see www.erasmus-epidemiology.nl/rotterdamstudy). This article gives the rationale of the study and its design. It also presents a summary of the major findings and an update of the objectives and methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Rotterdam Study was designed in the mid-1980s as a response to the demographic changes that were leading to an increase of the proportion of elderly people in most populations [1]. It was clear that this would produce a strong rise in elderly people living with diseases, as most diseases cluster at the end of life, and that to discover the causes of diseases in the elderly one would have to study risk factors of those diseases [2]. A major approach to finding causes is the prospective follow-up study, which has proven quite effective in finding causes of heart disease and cancer.
The design of the Rotterdam Study
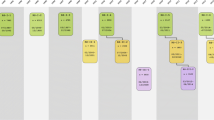
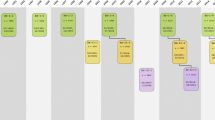
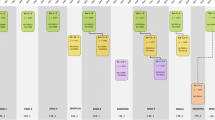
The design of the Rotterdam study is that of a prospective cohort study among, initially, 7,983 persons living in the well-defined Ommoord district in the city of Rotterdam in The Netherlands (78 % of 10,215 invitees). They were all 55 years of age or over and the oldest participant at the start was 106 years [3]. The study started with a pilot phase in the second half of 1989. From January 1990 onwards participants were recruited for the Rotterdam Study. Figure 1 gives a diagram of the various cycles in the study. In 2000, 3,011 participants (out of 4,472 invitees) who had become 55 years of age or moved into the study district since the start of the study were added to the cohort. In 2006 a further extension of the cohort was initiated in which 3,932 subjects were included, aged 45–54 years, out of 6,057 invited, living in the Ommoord district. By the end of 2008, the Rotterdam Study therefore comprised 14,926 subjects aged 45 years or over [4–6]. The overall response figure for all three cycles at baseline was 72.0 % (14,926 of 20,744).
Diagram of examination cycles of the Rotterdam Study (RS). RS-I-1 refers to the baseline examination of the original cohort (pilot phase 07/1989–12/1989; cohort recruitment 01/1990–09/1993). RS-I- 2, RS-I-3, RS-I-4, and RS-I-5 refer to re-examinations of the original cohort members. RS-II-1 refers to the extension of the cohort with persons in the study district that became 55 years since the start of the study or those of 55 years or over that migrated into the study district. RS-II-2 and RS-II-3 refer to re-examinations of the extension cohort. RS-III-1 refers to the baseline examination of all persons aged 45 years and over living in the study district that had not been examined already (i.e., mainly comprising those aged 45–60 years). RS-III-2 refers to the first re-examination of this third cohort which will be completed by the end of 2013. Examination RS-I-4 and RS-II-2 were conducted as one project and feature an identical research program. Similarly, examinations RS-I-5, RS-II-3, and RSIII-2 share the same program items
The participants were all examined in some detail at baseline. They were interviewed at home (2 h) and then had an extensive set of examinations (a total of 5 h) in a specially built research facility in the centre of their district. These examinations focussed on possible causes of invalidating diseases in the elderly in a clinically state-of-the-art manner, as far as the circumstances allowed. The emphasis was put on imaging (of heart, blood vessels, eyes, skeleton and later brain) and on collecting body fluids that enabled further in-depth molecular and genetic analyses. These examinations were repeated every 3–4 years in characteristics that could change over time. And so there were examination cycles from 1990 to 1993, from 1993 to 1995, from 1997 to 1999, from 2000 to 2001, from 2002 to 2004, from 2004 to 2005, from 2006 to 2008, from 2009 to 2011, from 2011 to 2012, and from 2012 onwards (Fig. 1). In December 2013 the second examination cycle for the third cohort (RS-III-2) will be finished.
The participants in the Rotterdam Study are followed for a variety of diseases that are frequent in the elderly (and many are also in the not so elderly): coronary heart disease, heart failure and stroke, Parkinson disease, Alzheimer disease and other dementias, depression and anxiety disorders, macular degeneration and glaucoma, respiratory diseases, liver diseases, diabetes mellitus, osteoporosis, dermatological diseases and cancer.
The Rotterdam Study has been approved by the institutional review board (Medical Ethics Committee) of the Erasmus Medical Center and by the review board of The Netherlands Ministry of Health, Welfare and Sports. The approval has been renewed every 5 years, as well as with the introduction of major new elements in the study (e.g., MRI investigations).
In the remainder of this article the objectives and major findings will be presented with an update of the research methods for cardiovascular diseases, dermatological diseases, endocrine diseases, liver diseases, neurological diseases, ophthalmic diseases, psychiatric diseases, respiratory diseases, as well as for genetic and biomarker studies and for pharmaco-epidemiologic studies. For relevant recent EJE references see [7–19].
Cardiovascular diseases
Objectives
Research on the epidemiology of cardiovascular disease focuses on the etiology, prognosis, and prediction of cardiovascular disorders (including coronary heart disease, stroke, heart failure) diabetes mellitus and metabolic syndrome. The main emphasis is on prevention and management of a first cardiovascular event but prevention of secondary events is also an area of interest. Putative risk factors include five groups: lifestyle factors, endocrine factors, factors involved in hemostasis, inflammation and endothelial function, metabolomic factors and genetic factors. We have five specific focused themes:
-
1.
Lifestyle: focused on evaluating the role of lifestyle factors (including nutrition, physical activity, sleep and smoking) in maintaining cardiovascular health as well as the interactions that lifestyle factors might have other factors (e.g. genes and medications).
-
2.
Biomarkers and genes: aimed to identify relevant biomarkers for the identification of novel mechanisms of disease. These incorporate both molecular and genetic factors together with their potential interactions. Genomics and metabolomics play a key role.
-
3.
Prediction and women’s cardiovascular health: aimed to improve the identification of individuals at increased risk of developing cardiovascular disease in order to point out windows of opportunities that could permit early preventive interventions and personalised care. A special focus is given to evaluating specific factors and formulating targeted strategies to prevent cardiovascular disease in women.
-
4.
High risk: focused on predictors and prognosis of chronic cardiovascular conditions, like heart failure, pulmonary hypertension, and atrial fibrillation.
-
5.
Imaging: this work theme aims to identify the contribution that new technologies can provide to the maximum benefit of early diagnosis and accurate prognostication. Major focus is on non-invasive assessment of atherosclerosis to improve the understanding of the atherosclerotic process and the prediction of cardiovascular disease, including measurement of coronary calcification with electron-beam and multi-detector CT (MDCT) and carotid plaque characterization by MRI.
Major findings
Recognized and unrecognized myocardial infarction
We found that a high proportion of incident myocardial infarctions remains clinically unrecognized. The incidence rate of recognized myocardial infarction in the Rotterdam Study was 5.0 per 1,000 person years. The incidence was higher in men (8.4) than in women (3.1). The incidence rate of unrecognized infarction was 3.8 per 1,000 person years. Men (4.2) and women (3.6) had approximately similar incidence. Hence, the proportion of unrecognized infarction is lower in men (33 %) than in women (54 %) [20]. We have further identified a two-fold increased risk in developing heart failure or atrial fibrillation among men with unrecognized myocardial infarction [21, 22].
Heart failure and atrial fibrillation
The Rotterdam Study enabled accurate assessment of the incidence and lifetime risk of heart failure and atrial fibrillation in an elderly population [23–25]. It was shown that inflammation and resting heart rate is associated with risk of heart failure [26, 27]. In addition we identified several new risk factors of atrial fibrillation. We found that markers of generalized atherosclerosis in persons without a history of myocardial infarction or angina were associated with a higher risk of atrial fibrillation [28]. Furthermore, high-normal thyroid function [29] and higher levels of dehydroepiandrosterone sulfate, a precursor in the biosynthetic pathway of androgenic and estrogenic sex hormones were associated with incidence of atrial fibrillation [30]. In collaboration with several community-based prospective studies we were able to develop a prediction model for atrial fibrillation, only using variables that are routinely collected in primary care settings [31]. In a large collaborative study as part of the CHARGE consortium, we investigated the genetic variation responsible for 6 traits related to cardiac structure and function. We found two replicated loci for left ventricular dimension and 5 replicated loci for aortic root size [32]. Another topic of interest was the search for genetic determinants of several rhythm and conduction disturbances on the ECG, notably RR-interval, QRS duration, and QT(c)-interval, PR-interval, as well as atrial fibrillation and sudden cardiac death. For example, we identified several new loci for PR interval [33], heart rate [34], and atrial fibrillation [35, 36] in meta-analyses from the CHARGE consortium.
Cardiovascular risk factors and prediction
Endocrine, inflammatory and hemostatic factors and risk of coronary heart disease were addressed in several studies. Subclinical hypothyroidism was an independent risk factor of atherosclerosis and myocardial infarction in older women [37]. In a recent study, we compared the change in the accuracy of risk predictions when newer risk markers, representative of various pathophysiologic pathways, were added to the established clinical risk predictors. Among the biomarkers, improvements in coronary heart disease risk prediction were most significant with the addition of amino-terminal pro-B-type natriuretic peptide (NT-proBNP)[38, 39]. Furthermore, plasma C-Reactive protein (CRP) and lipoprotein-associated phospholipase A2 (Lp-PLA2) activity were independent predictors of coronary heart disease [40, 41]. Earlier findings included the association of tissue plasminogen activator (TPA) with incident coronary heart disease [42]. Recently, we developed and validated a coronary heart disease prediction model tailored for the aging population based on competing risk methodology [43].
Non-invasive measures of atherosclerosis
Multiple studies focused on the predictive value of non-invasive measures of atherosclerosis for risk of coronary heart disease. Strong associations with risk of coronary heart disease were found for carotid intima-media thickness [44], pulse wave velocity [45], and coronary calcification as assessed by electron-beam CT [46]. The relatively crude measures directly assessing plaques in the carotid artery and abdominal aorta predict coronary heart disease equally well as the more precisely measured carotid intima-media thickness [47]. In persons at intermediate risk of cardiovascular disease, coronary artery calcium provided the best increment in coronary heart disease risk prediction and stratification (to reclassify persons into more appropriate coronary risk categories)[39, 48]. The burden of coronary calcification also provides incremental predictive information for heart failure, but not for cerebrovascular disease [49, 50].
Genetic studies
Genetic studies included candidate gene studies [51] and more recently genome-wide association studies of clinical disease and risk factor phenotypes. So far we have contributed to more than 100 Genome-wide association (GWA) studies in the field of cardiovascular disease. These GWA studies are primarily conducted in the framework of the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium [52, 53] however in many instances we include further studies. We identified 3 genetic loci associated with uric acid concentration and gout [54].
We also identified a significant association between the UMOD gene which encodes Tamm-Horsfall protein and chronic kidney disease [55]. We found four genes for systolic blood pressure, six for diastolic blood pressure and one for hypertension [56–58]. We found multiple loci that influenced erythrocyte phenotypes in the CHARGE Consortium [59]. In a meta-analysis in more than 80,000 individuals from 25 studies, we identified 18 loci for CRP levels. The study highlighted immune response and metabolic regulatory pathways involved in the regulation of chronic inflammation [60]. Novel associations of multiple genetic loci with plasma levels of factor VII, factor VIII, and von Willebrand factor were also detected [61]. We have also identified genetic loci associated with the measures of subclinical atherosclerosis burden. Our genome-wide association studies on the 3 measures of subclinical atherosclerosis identified several new genetic loci [62–64]. We have contributed to GWA studies on coronary artery disease [65, 66].
Nutrition and lifestyle
We found that subjects who had fish intake of more than 19 g/day had a significantly lower prevalence of mild/moderate coronary calcification [67]. Also, we found that an increase of 50 g in processed meat was associated with increased CRP levels [68]. In addition to this, the intake of processed meat was associated with a higher risk of type 2 diabetes which was independent of CRP levels [68]. Likewise, we studied whether dietary proteins, amino acids and acid load were associated with the risk of hypertension. It appeared that these factors were not a major determinant of the risk of hypertension in the Rotterdam Study [69–71].Besides main effects of nutrition, we studied gene-nutrient interactions. We found that dietary vitamin E intake may modulate the relation of SIRT1 genetic variants with body mass index [72]. Also, we studied the modification of magnesium, whole grain and a healthy diet score on fasting glucose and insulin by SNPs related to fasting glucose and insulin as part of the CHARGE consortium [73–75].
Methods update
Clinical follow-up
Information on clinical cardiovascular outcomes is collected through an automated follow-up system. The follow-up system involves linkage of the study base to digital medical records from general practitioners in the study area and subsequent collection of letters of medical specialists and discharge reports in case of hospitalisation. With respect to the vital status of participants, information is also obtained regularly from the municipal health authorities in Rotterdam. After notification, cause and circumstances of death are established by questionnaire from the treating physicians. Clinical cardiovascular outcomes are adjudicated according to established definitions based on international guidelines by study physicians and medical specialists in the field affiliated with the Rotterdam Study. Methods of follow-up data collection, adjudication of events, and definitions of cardiovascular end points have been described in detail previously in this journal [14]. Systematic follow-up data collection is done for the occurrence of cardiovascular mortality, coronary heart disease (including coronary death, myocardial infarction, and coronary revascularization procedures), heart failure, atrial fibrillation, and sudden cardiac death [14]. Diabetes mellitus is defined based on guidelines of the American Diabetes Association and the World Health Organization. We defined incident diabetes as fasting plasma glucose level ≥7.0 mmol/L, or the use of oral antidiabetic medication or insulin, or treatment by diet and registered by a general practitioner as having diabetes.
Cardiovascular risk factors
Besides traditional cardiovascular risk factors, five major groups of putative risk factors for cardiovascular conditions are examined. The first group are lifestyle factors, including dietary factors, physical activity, smoking, sleep and vitamin D (as described above). The second are endocrine factors, including diabetes, sex hormones, thyroid gland and adrenal gland hormones and natriuretic peptides (e.g. [29, 30, 37–39]. The third group comprises factors involved in hemostasis, inflammation and endothelial function (e.g. [40, 61, 76]). The fourth group covers genetic factors. In addition to the candidate gene approach, studies are more recently conducted through the genome-wide association approach (e.g. [32–36, 54–66, 73–75]). In genome-wide association studies, data from the Rotterdam Study are often combined with those from other studies in the context of the large collaborative CHARGE consortium [52, 53]. Within the fifth group we are applying both proton Nuclear Magnetic Resonance (1H NMR) and Mass Spectrometry (MS) for metabolic profiling in 2,000 participants of the Rotterdam Study including nearly 200 incident cases of coronary heart disease. Furthermore, in this context, special attention has been given to the contribution of different risk factors in relation to cardiovascular disease in women. Data has been collected to evaluate the impact of specific periods of potential vulnerability across a woman’s lifespan; menarche, pregnancy, and menopause.
Non-invasive measures of atherosclerosis
At baseline and follow-up examinations, ultrasonographic assessments of carotid intima-media thickness and carotid plaques were conducted in all participants [44]. At these examinations, also measurements of the ankle-brachial index and aortic calcification (on X-rays of the lumbar spine) were obtained [14]. Carotid–femoral pulse wave velocity, a measure of aortic stiffness, was measured in all *participants of RS-I-3, RS-II-1, and RS-III-1 with an automatic device [45]. Measurements of coronary calcification by electron-beam CT and more recently by MDCT were conducted from 1997 onwards in RS-I and RS-II [46, 48]. From 2003 to 2006, MDCT was used to also quantify calcification in the aortic arch and carotid arteries in RS-I and RS-II. Measurement of carotid plaque components using MRI was done from 2007 to 2012 in all participants from RS-I, RS-II and RS-III with carotid wall thickening on conventional carotid ultrasound. Repeated MRI measures over time were obtained in RS-I and RS-II.
Electrocardiographic, echocardiographic and other ultrasound measurements
At every exam, a 12-lead 10-second resting ECG is made and processed by the Modular ECG Analysis System (MEANS) to obtain a series of ECG measurements [77]. Abdominal aortic diameters were measured by ultrasound at RS-I-1, and from 2002 (RS-I-4) onwards in all three Rotterdam Study cohorts. Also from 2002 onwards (RS-I-4), repeated echocardiographic measurements are conducted of structural and functional left heart parameters [78]. From 2009 (RS-I-5) onwards, measurements of structure and function of the right heart are also collected, including estimates of pulmonary artery pressure. In the same round a 3-minute resting ECG was measured in all participants.
Nutrition and lifestyle
Nutritional data has been collected in RS-I-1, RS-I-5, RS-II-1, RS-II-3 and RS-III-1 by using semi quantitative food frequency questionnaires (FFQ). In RS-I-1 and RS- II-1, participants completed a checklist about foods and drinks they had consumed at least twice a month during the preceding year and a standardized interview using a validated 170-item semi-quantitative FFQ [79]. In RS-I-5, RS-II-3 and RS-III-I, a more comprehensive FFQ was used during the visits as described in detail previously [80–83]. Development and processing of nutrition data is being performed in close collaboration with the Department of Human Nutrition, Wageningen University, the Netherlands. In RS-I-III, RS-I-5, RS-II-I-3 and RS-III-I, physical activity data was assessed by means of an adapted version of the Zutphen Physical Activity Questionnaire and the LASA Physical Activity Questionnaire [84–86]. The questionnaire contained questions on walking, cycling, gardening, diverse sports, hobbies and on housekeeping. According to time spent in light, moderate and vigorous activity, metabolic equivalents of task were calculated.
For additional EJE references concerning cardiovascular disease see [12, 87–136].
Dermatological diseases
Objectives
Dermatoepidemiologic research in the Rotterdam Study focuses on the frequency of the most common skin conditions as well as on genetic and environmental factors associated with these skin diseases. The emphasis is on cutaneous malignancies such as basal and squamous cell carcinomas (BCC and SCC, respectively) and their precursor lesions (actinic keratosis), inflammatory dermatoses such as eczema and psoriasis, and varicose veins. Also, we examine the frequency and determinants including genetics and environmental exposures of skin aging (pigmentation, wrinkling and photodamage).
Methods
In 2010, dermatology studies were introduced in the Rotterdam Study. To the home interview several items have been added questioning ultraviolet light exposure, history of (personal and familial) psoriasis, history of skin cancer, the diagnostic criteria of British association of dermatology for atopic eczema, adjusted diagnostic criteria for psoriatic arthritis.
A full body skin examination by physicians trained in dermatology with a focus on the most common skin diseases is the core contribution of dermatology. The clinical presence and extent of specific skin diseases (i.e., actinic keratosis, malignancies, psoriasis, xerosis, hand and flexural eczema, alopecia, and signs of chronic venous insufficiency based on the ‘C’ of the CEAP classification) at time of examination is assessed in a standardized fashion. Other dermatological diseases will just be noted.
The extent of skin aging as a global score and broken down in different aspects such as wrinkling, pigmentary spots, and teleangiecatsia are scored using a validated photonumeric scales and computer algoritms. The Norwood-Hamilton classification and the Ludwig classification is used for male and female pattern hair loss, respectively. Fully standardized 3-dimensional photographs (Premier 3dMDface3-plus UHD, Atlanta, USA) of the face are taken to further assess skin characteristics. The colour of the facial skin and at the inner side of the upper arm are measured using a spectrophotometer (Konica Minolta Sensing, spectrophotometer CM-700d, Singapore).
As for other cancers, pathology data of the cutaneous malignancies is obtained from linkage to the national cancer registry and the Dutch pathology database (PALGA). In a further attempt to identify cohort members with psoriasis, medical files and dispenses at pharmacies have been investigated resulting in over 350 psoriasis cases.
Major findings
In the first follow-up study including the skin examinations of more than 2,000 cohort members, showed that actinic keratosis is very common in this elderly population (AK prevalence was 49 % for men and 28 % for women) [137]. After adjusting for other factors, baldness in men was associated with a strongly increased risk of actinic keratosis.
The first prevalence study of single and multiple BCC was done in the Rotterdam Study and showed that a total of 524 patients (4.8 % of included population) had developed this type of keratinocytic cancer and that 31.1 % had developed more than one tumor during observation [138]. A multi-failure survival model suggested that people with red hair and higher levels of education, and those who had their first BCC at younger age were significantly more likely to develop multiple malignancies. A recent update yielded more than 1,350 participants with a history of BCC and approximately 450 with a SCC. In collaboration with researchers from the Nurses Health Study, the association between common genetic variants and these skin cancers is currently being examined.
In a candidate gene study in almost 6,000 people, we confirmed known and identified new variants associated with digital skin colour extraction. Of the two new skin color genes, the genetic variants in UGT1A were significantly associated with hue and variants in BNC2 were significantly associated with saturation [139].
The psoriasis patients within the Rotterdam Study had predominantly mild disease. The distribution of subclinical artherosclerosis measures as well as the cardiovascular events were comparable between the 262 psoriasis patients and the reference population [140]. However, psoriasis patients were significantly more likely to have signs of nonalcoholic fatty liver disease based on ultrasonography than their controls after adjusting for potential confounders (adjusted OR 1.70, 95 % CI 1.13–2.58).
Endocrine diseases
Objectives
The main objective of the programme of endocrine epidemiology research is to study frequency and etiology of major disorders of the endocrine glands (pituitary, reproductive, thyroid, parathyroid, adrenal, and neuro-endocrine pancreas) and the musculoskeletal system. These include diabetes mellitus, osteoporosis, osteoarthritis, reproductive traits (fertility, age-at-menopause), and hypo- and hyper-thyroidism. The evaluation of risk factors for the above mentioned conditions includes serum measurements (such as classical hormones and other endocrine molecules) and genetic determinants of endocrine diseases and traits.
Major findings
In the process of obtaining digitized Xrays for all participants at all time-points of follow-up, we have evaluated the 3 major methods to score vertebral fractures: quantitative morphometry, semi-quantitative morphometry, and the qualitative ABQ method [141]. Prevalence of vertebral fractures differed substantially by the different methods, so standardization is crucial for patient care and for large scale epidemiological studies. Using the digitized Xray scores, we have for the first time determined population-based prevalence of Scheuermann’s disease in the Dutch population to be 4 % [142].
In the relationship of type 2 diabetes with bone health we observed that diabetic subjects with inadequately controlled glucose control had 1–5 % increased bone mineral density (BMD) and ~50 % increased fracture risk, compared to diabetics with adequately controlled glucose and to non-diabetics [143].
By studying bone health across different types of hip osteoarthritis (OA), we observed that subjects with atrophic OA (i.e., with joint space narrowing but without osteophytes) have ~50 % increased fracture risk as compared to controls without OA [144]. In addition, we found that hip geometry measures had modest ability to predict hip OA, in addition to clinical risk factors [145].
The research line endocrine diseases is also actively involved in collaborating with economists to study the biology of economic behaviour, in particular entrepreneurial behaviour (and related traits such as educational attainment). In one of the first collaborative analyses we found no evidence for a relationship of testosteron levels with entrepreneurial behaviour [146].
Much of the work of this research is made possible by large-scale collaboration in consortia, some of which focus on one particular disease or trait (e.g., GEFOS), while others are more broad spectrum strategic collaborations (e.g., CHARGE, ENGAGE). We are part of several such large consortia studying genetic and epidemiological risk factors for osteoporosis (GEFOS, GENOMOS, CHANCES, CHARGE), osteoarthritis (TREAT-OA, ArcoGen), diabetes (MAGIC), thyroid disease (CHARGE), and reproductive traits (CHARGE, REPROGEN, PCOSGEN).
Major GWAS findings
With >82,000 samples collected within the GEFOS consortium a landmark publication was achieved with the identification of 56 loci influencing bone mineral density in total explaining ~5 % of genetic variation in BMD, and of which 14 loci also were associated with fracture risk [147].
In a meta-analysis of 15,000 hip OA cases and 54,000 controls assembled in the arcOGen consortium, 5 novel loci influencing risk of hip OA were identified [148]. In an analysis of a so-called endo-phenotype of OA, i.e., joint space width narrowing (JSN) a GWAS among 10,000 subjects we identified a novel locus, called DOT1L, to influence cartilage thickness, as well as the risk for hip OA [149]. Interestingly, this gene product is a known drug target for treating leukemia with several drugs under development. Experiments are therefore now ongoing to evaluate the effect of these drugs on features of OA in animal and cell model systems. Finally, in the analysis of another endophenotype of OA, pain, our group led a large scale meta-analysis to chronic wide spread pain in 2700 cases and 14,000 controls and identified for the first time a genetic locus involved in pain, i.e., CCT5 [150].
In the largest meta-analysis of GWAS of age-at-menopause among 62,000 women and which our group led, we identified 13 loci associated with differences in age-at-menopause and explaining ~5 % of the genetic variation [151]. Interestingly, the majority of the loci most likely involves genes in the DNA repair pathway which points to the importance of this system in maintaining an error-free stemcell lineage to which the oocyte belongs.
In a meta-analysis of GWAS data on TSH levels and free T4 levels derived from up to 26,000 subjects, 26 loci were identified explaining 2–5 % of the genetic variation of TSH and fT4 respectively [152]. There was only limited overlap between the loci for TSH and fT4, and evidence was obtained for 5 loci to have sex-specific effects.
An interesting GWAS involved the analysis of Helicobacter pylori serologic status among members of the Rotterdam Study and the SHIP cohort [153]. Two novel loci were identified, TLR1 and FCGR2A, which can help explain inter-individual differences in risk for H pylori infection.
For many endocrine biomarkers GWAS have been performed to identify the genetic loci influencing their serum levels (e.g., homocysteine, testosteron, SHBG, thyroid hormone levels, etc.) and these are also involved in several mendelian randomization analyses in relation to major disease endpoints for which these biomarkers have been suggested to be predictive.
Methods update
For all participants DXA-based bone mineral density (BMD) measurements of the lumbar spine, dual hip and total body BMD, as well as determination of body composition parameters are assessed with a ProdigyTM total body fan-beam densitometer (GE Lunar Corp, Madison, WI, USA). Hip structural analysis (HSA) of DXA scans including hip strength indexes (using software by GE Lunar) is determined for all scans. Since 2009 we perform iDXA measurements (GE Lunar) which measures L1–L4 BMD, bilateral total hip and femoral neck BMD and total body BMD. From the total body scan, we measure lean mass and fat mass body composition, including total body, trunk, arm, legs, and android and gynoid regions of interest. X-ray examinations of vertebral bodies, hips, knees and hand/wrist are since 2009 obtained by a digitalized Fuji FCR system (FUJIFILM Medical Systems) for all participants. Analogue Xray photographs from previous time –points at all follow up measurements (~75,000 Xrays) have now all been digitized. All the Xrays have now been completely assessed for the presence of fractures and/or degenerative changes of the joints (e.g., Scheuermann’s Disease). Vertebral fractures and deformities are assessed using the classical quantitative McCloskey-Kanis method, the semi-quantitative Genant method, and a qualitative algorithm-based technique termed the ABQ method. Incident clinical fractures (of all bone sites) are obtained from computerized records of the general practitioners and hospital registries which are regularly checked by research physicians who review and code the fracture information.
Muscle strength is assessed in all participants with a hand grip dynamometer. Muscle mass estimates are derived from whole body iDXA measurements.
The incidence and progression of OA is assessed using Kellgren scores obtained from X-rays of hips, knees, hands, and spine. The complete set of X-rays (all participants, all follow-up time points) has now been evaluated for the Kellgren score at these 4 joints. Novel diagnostic assessments for OA are available using Magnetic Resonance Imaging (MRI) of the knees in a large subset of the population (n = ~ 1,000 RS-III). In addition, pain measurements were added in 2011 in this research line including a quantitative assessment of heat sensitivity on the arm using a standardized device, and indications of (wide-spread) pain in any part of the body using a puppet.
Several specific biomarker assessments in blood/serum/plasma and urine are done for the diagnosis and evaluation of risk factors of endocrine and metabolic diseases (e.g., glucose, TSH, freeT4, steroid hormones, calcium, CTXII, etc.). Fasting blood samples are collected along with challenged samples as part of a glucose tolerance test. Sputum is collected before and after a dexamethasone-suppression test. Finally, validated questionnaires evaluating nutrient intake (e.g., calcium and vitamins) and activities of daily living, allow to evaluate the role of environmental factors in endocrine conditions and locomotor diseases of the elderly.
For additional EJE references concerning endocrine diseases see [154–173].
Liver diseases
Objectives
Fibrogenesis of the liver is most probably not only the result of well known liver diseases, such as viral hepatitis, alcoholic liver disease or non-alcoholic fatty liver disease (NAFLD), but rather a complex interaction between a genetic predisposition and these liver disorders. Liver research in the Rotterdam Study will concern the association between these known causes of liver disease and the occurrence, magnitude, and progression of fibrosis in combination with genetic and environmental factors. Additional research focus will be on NAFLD. NAFLD is considered the hepatic manifestation of the metabolic syndrome and has become the most common chronic liver disease in Western countries in parallel with epidemics of obesity and type II diabetes mellitus. We aim to study the occurrence and risk factors of NAFLD in a general population and generate insight into the association with cardiovascular morbidity and mortality.
Methods
Abdominal ultrasound
From February 2009 trained technicians perform abdominal ultrasonography in Rotterdam Study participants. Liver, biliary tract, gall bladder, spleen, pancreas, and kidneys in combination with doppler examination of hepatic veins, hepatic artery and portal vein will be evaluated. All images are stored digitally and will be reevaluated by an ultrasound trained physician.
Assessment of steatosis
The diagnosis and grading of liver steatosis will be based on ultrasonographic liver brightness, hepatorenal echo contrast, deep attenuation and vessel blurring [174].
Non alcoholic fatty liver is diagnosed by presence of steatosis on ultrasound and exclusion of excessive alcohol consumption, presence of viral hepatitis, use of fatty liver inducing pharmacological agents, recent bariatric surgery and a history of inflammatory bowel disease.
Assessment of fibrosis
Ultrasonographic evaluation of the liver parenchyma and liver surface will be performed in order to assess severe fibrosis and/or cirrhosis. Additionally, sonographic signs of portal hypertension will be studied (splenomegaly, venous collaterals, portal vein diameter and flow, hepatic venous flow, and the presence of ascites).
To assess and quantify the grade of fibrosis trained technicians will perform elastography in all participants. This test measures non-invasively and quantitatively the liver stiffness using a probe which includes an ultrasonic transducer transmitting a vibration wave through the liver. The velocity of the ultrasonic wave correlates directly with tissue stiffness [175, 176].
Determinants of interest
The association between factors known to influence liver function and the occurrence of steatosis and fibrosis will be studied. Additionally the association of these conditions with age, gender, nutritional intake, concurrent alcohol intake, risk factors for viral hepatitis, BMI, waist-to-hip ratio, serum glucose, insulin, and diabetes mellitus, serum cholesterol and triglycerides will be studied. All clinical information will be obtained by interview (updated with liver specific questions) and clinical examination. For recent EJE references see [91, 177–180].
Neurological diseases
Objectives
Neuroepidemiologic research in the Rotterdam Study focuses on the frequency, etiology and early recognition of the most frequent neurologic diseases in the elderly. We study neurodegenerative diseases (dementia, including Alzheimer disease and Parkinson disease), cerebrovascular disease (both ischemic stroke and intracerebral hemorrhage), and recently migraine and polyneuropathy. In all of these disorders clinical symptoms typically become manifest late in the disease course, the occurrence of clinical disease does not reflect the underlying spectrum of disease-related pathology, and most of the clinical syndromes are etiologically heterogeneous. Therefore, an additional research focus is on the causes and consequences of pre-symptomatic brain pathology that can be assessed with non-invasive modalities, which include MR-imaging, cognitive testing, gait assessment, and recently electromyography (EMG).
Major findings
Neurodegenerative and cerebrovascular diseases are highly frequent in the elderly. The prevalence increases from age 55–65 years to age 90 years and above from less than 1 % to over 40 % for dementia [181], from less than 0.5 % to more than 4 % for Parkinson disease [182], and from approximately 1 % to nearly 10 % for stroke. The incidence figures follow this pattern of a strong increase with age over the entire age range, with the age-specific incidence of dementia being identical for men and women at least until the age of 85 [183] but with men having a higher age-specific incidence of both stroke and Parkinson disease than women throughout the age range [184, 185]. However, more recent numbers from 2000 onwards suggest that the relative incidence of dementia and stroke may be lower than in the 1990s [186–188]. Still, in absolute numbers these disease will dramatically increase in prevalence over the coming decades.
Vascular pathology and vascular risk factors are associated with worse cognitive performance [189], which also translates in people with vascular pathology or risk factors for vascular disease having an increased risk of dementia, including Alzheimer disease [190]. Moreover, several life style factors are associated with the risk of dementia and Alzheimer disease [191, 192], suggesting that onset of dementia may at least partly be delayed or prevented. However, many of these lifestyle factors have only a short-term effect, suggesting reverse causality to some extent [193]. Commonly used drugs may also have a role in development of dementia [194]. Similar risk factor profiles also underlie cognitive decline prior to the clinical diagnosis of dementia [195, 196].
The classical risk factors for stroke also associate with the risk of stroke in the Rotterdam Study [197, 198]. In contrast, some emerging putative risk factors are not associated with stroke [199], whilst others, such as inflammatory markers, may be etiologically relevant but thus far add little to the identification of people at risk [200]. Possibly underlying this is that a large amount of stroke goes clinically undetected [201]. Nearly 20 % of elderly people have at least one silent brain infarct, and thereby a nearly fourfold increased risk of clinical stroke, a more than doubled risk of dementia including Alzheimer disease, and an increased risk of depression [202].
With the advent of genome-wide association studies, the Rotterdam Study has contributed to large-scale collaborations and contributed to the identification of novel genes underlying the risk of Alzheimer disease and stroke [203–205]. In turn, we have also shown that although currently known genetic variants for dementia associate with cognition in non-demented elderly, these do not improve prediction [206].
Neuroimaging reveals that brain pathology is widespread [207] and can go clinically undetected for a long time. In addition to the silent infarcts, many apparently healthy elderly have ischemic changes in their cerebral white matter, i.e. white matter lesions, that are associated with an increased risk of dementia, stroke and depression. Also brain atrophy, especially of the hippocampus, is already present years before onset of even the earliest sign of cognitive impairment or subjective complaints. This emphasizes the need to shift the attention in etiologic research of neurodegenerative and cerebrovascular disease to the causes of pre-symptomatic and underlying brain changes. Technological advances in image acquisition, optimized imaging sequences and automated post-processing of multispectral MR data are major drivers of the rapid developments in this field. With our current imaging protocol we can now not only investigate established markers of brain pathology, such as infarcts, white matter lesions, and atrophy, but also extend towards novel markers, such as cerebral microbleeds and diffusion tensor imaging [208]. We have shown that risk factors profiles for subclinical disease overlap with those for clinical disease [209–213]. Also, subclinical white matter lesions and silent infarcts improve the clinical prediction of incident stroke [214]. The clinical relevance of subclinical pathology is demonstrated by strong associations of these markers with morbidity and mortality [215–218].
Finally, we have extensively studied the genetic basis of subclinical brain disease, both in genome-wide settings and in candidate-gene studies [219–225] (see further section on population imaging).
Most research on the preclinical phase of neurodegenerative diseases, particularly dementia, focuses on either cognitive performance or brain pathology on neuroimaging. Given that brain pathology is usually diffuse, it is conceivable that brain functions other than cognition will also be affected. In this light, we have shown that gait deteriorates significantly with age. Also, gait and cognition are linked following a specific pattern of certain cognitive domains only associated with specific aspects of gait, but not others [226] .
Methods update
Assessment of dementia and Alzheimer disease
In the baseline and follow-up examinations participants undergo an initial screen for dementia with the Mini Mental State Examination (MMSE) and the Geriatric Mental Schedule (GMS), followed by an examination and informant interview with the Cambridge Examination for Mental Disorders of the Elderly (CAMDEX) in screenpositives (MMSE < 26 or GMS > 0), and subsequent neurological, neuropsychological and neuroimaging examinations [181, 183]. Of subjects who cannot be reexamined in person, information is obtained from the GPs and the regional institute for outpatient mental health care. A consensus panel makes the final diagnoses in accordance with standard criteria (DSM-III-R criteria; NINCDS-ADRDA; NINDS-AIREN).
Assessment of Parkinsonism and Parkinson disease
Participants are screened in the baseline and follow-up examinations for cardinal signs of parkinsonism (resting tremor, rigidity, bradykinesia, or impaired postural reflexes). Persons with at least one sign present are examined with the Unified Parkinson’s Disease Rating Scale and a further neurologic exam. PD is diagnosed if two or more cardinal signs are present in a subject not taking antiparkinsonian drugs, or if at least one sign has improved through medication, and when all causes of secondary parkinsonism (dementia, use of neuroleptics, cerebrovascular disease, multiple system atrophy, or progressive supranuclear palsy) can be excluded [182, 185].
Assessment of stroke and stroke subtypes
History of stroke at baseline was assessed through interview and verified in medical records. Putative incident strokes get identified through the linkage of the study database with files from general practitioners, the municipality, and nursing home physicians’ files, after which additional information (including brain imaging) is collected from hospital records. A panel discusses all potential strokes and subclassifies strokes into ischemic, hemorrhagic or unspecified [184, 200]. We also systematically collect transient ischemic and neurological attacks [227].
Assessment of cognitive function
Global cognitive function is measured through the Mini Mental State Examination (MMSE) in all surveys. From the third survey (RS-I-3) onwards we added a 30 min test battery that was designed to assess executive function and memory function, and which includes a Stroop test, a Letter Digit Substitution Task, a Word Fluency Test, and a 15 words Word List Learning test. This test battery was expanded from the fourth survey onwards (RS-I-4) to include motor function assessment using the Purdue Pegboard Test. Moreover, from 2009 onwards we expanded further by including the Design Orientation Test (DOT) and a modified version of the International Cooperative Ataxia Rating Scale (ICARS), which assess visuo-spatial orientation and ataxia respectively [228, 229].
Assessment of gait patterns
Halfway through RS-III-1, we successfully implemented the assessment of gait in all participants using the GAITRite walkway (http://www.gaitrite.com/). Gait is assessed using a 5.79 meter long walkway (GAITRite Platinum; CIR systems, Sparta, NJ, USA: 4.88 meter active area; 120 Hertz sampling rate) with pressure sensors. Participants perform a standardized gait protocol consisting of three different walking conditions: normal walk, turning and tandem walk. In the normal walk, participants walk over the walkway at their own pace. This walk is repeated four times in both directions (yielding a total of 8 recordings). In turning, participants walk over the walkway at their own pace, turn halfway and return to the starting position (1 recording). In the tandem walk, participants walk tandem (heel-to-toe) over a line visible on the walkway (1 recording). A total of 30 spatiotemporal gait variables are calculated by the walkway software and downloaded offline for further analysis. Subsequently, principal components analysis on these thirty gait variables is performed to derive summarizing factors, referred to as gait domains. The following gait domains are used: Rhythm, Pace, Phases, Base of Support, Variability, Tandem, and Turn. Gait domains can be compared to cognitive domains, in which each domain reflects a different aspect of the overall concept [226].
Assessment of polyneuropathy
Starting in January 2013, we have successfully implemented a protocol to assess polyneuropathy. This includes a full work-up including questionnaire, neurological exam, and EMG in all participants. In coming years, we will publish on the prevalence, risk factors, and clinical correlates of polyneuropathy in the general population. The continuous measures of conductivity obtained through EMG can also serve as excellent endophenotype for genetic and biomarker studies.
Rotterdam Scan Study: brain imaging within the Rotterdam Study
In 1991, a random sample of 111 participants underwent axial T2-weighted magnetic resonance (MR) imaging to assess presence and severity of white matter lesions [207]. In 1995, a random sample of 563 non-demented participants underwent brain MR imaging in the context of the Rotterdam Scan Study. From August 2005 onwards (RS-II-2 and further), a dedicated 1.5 Tesla scanner is operational in the research center of the Rotterdam Study, and brain imaging is performed in all study participants without contra-indications [208].
Currently, the follow-up of this latter sample extends to up to 7 years. Therefore, in the coming years we will be able to investigate how cerebral microbleeds and DTI-markers relate to incident neurological diseases (see further section on population imaging).
Ophthalmic diseases
Objectives
The main objectives of the ophthalmic epidemiology studies are to investigate frequency, genetic and environmental risk factors, their interaction, (endo)phenotypic characteristics, and potential biomarkers or disease intermediates of frequently occurring complex eye disorders. We particularly focus on age-related macular degeneration, open angle glaucoma, and myopia; other areas of interest are retinal vessel diameters, and diabetic or vascular retinopathy.
Major findings
Age-related macular degeneration (AMD)
Although many major genes for AMD have already been identified these last years, missing heritability was still abundantly present. To identify more genes, we combined our efforts with international study groups which had a total study population consisting of 17,100 advanced AMD cases and > 60,000 control subjects [242]. Within this AMDGene consortium, 7 new genetic regions (COL8A1/FILIP1L; IER3/DDR1; SLC16A8; TGFBR1; RAD51B; ADAMTS9/MIR548A2; B3GALTL) for AMD were found. Taken together with the already known genes, a total of 19 AMD genes were identified in this consortium. Highest genetic associations were found for the ARMS2 and CFH genes. The pathogenetic pathways that can be deduced from these genes are the complement pathway, atherosclerosis signaling and lipid pathway, angiogenesis, and extra-cellular matrix remodeling. Further steps to diminish missing heritability are identification of genetic variants by NGSexome sequencing. This is the focus of attention of the newly formed AMDExome Chip consortium.
To perform more extensive analyses involving genetic as well as other risk factors, we also searched for connection with international studies. The 3 Continent AMD Consortium was established, a consortium of four population-based studies: Beaver Dam Eye Study (BDES) [243] and Los Angeles Latino Eye Study (LALES) [244] from the United States; Blue Mountain Eye Study (BMES) from Australia [245], and our own Rotterdam Study (RS) from the Netherlands. The total study population of this consortium is 25,000 subjects aged 45 + years, and many of these subjects have been followed for >15 years. A first effort of the consortium was harmonizing the outcome variable AMD, and a new severity scale was launched. Secondly, a detailed analysis of gene-diet interaction was performed, showing that the major AMD genes interact with the anti-oxidants lutein/zeaxanthin. Thirdly, we developed a prediction model for late AMD based on well-known genetic and environmental risk factors, age, sex, and early AMD phenotype. Incorporation of all these factors provided a predictive value of 0.88 (area under the curve; AUC) to predict late AMD in the discovery cohort the Rotterdam Study, and a predictive value of 0.85 (AUC) in the BDES and BMES at validation. The prediction model enabled distinction between those with virtually no risk of late AMD and those with up to 65 % cumulative risk. We think these findings will be useful for clinicians as well as researchers. They may facilitate recognition of high risk individuals who need life style advice to diminish their risk, as well as identification of a suitable study population for intervention trials.
Research aims for 2013 are evaluation of gene and gene-environment effects on (sub)clinical manifestations of disease as visible on various imaging devices (OCT; fundus autofluorescence; infrared); or as systemic biomarkers; and interaction with medication.
Open angle glaucoma (POAG)
After a first attempt was made to identify genes for optic disc parameters in 2011, we now investigated the genetic factors for intraocular pressure (IOP) in the Rotterdam Study. IOP is a highly heritable risk factor for primary open-angle glaucoma and is the only target for current glaucoma therapy. We performed a GWAS for IOP in 11,972 participants from the 3 Rotterdam Studies and ERF [246]. We then replicated our findings in 7,482 participants from 4 additional cohorts from the UK, Australia, Canada, and the Wellcome Trust Case–Control Consortium 2/Blue Mountains Eye Study. IOP was significantly associated with GAS7 and TMCO1, and risk variants also appeared to associate with glaucoma.
The genes are highly expressed in significant eye structures involved in glaucoma pathogenesis, and we think we have identified two clinically relevant genes involved in IOP regulation. Further action in this realm has been the formation of an international consortium for meta-analyses of GWAS data. Collaboration was found with 16 studies from 7 countries including 45,000 study participants, forming the International Glaucoma Genetics Consortium (IGGC). Genetic meta-analyses are currently being carried out.
Relations with environmental factors were studied with a focus on diet. We found that a low intake of retinol equivalents and vitamin B1 and a high intake of magnesium were associated with an increased risk of OAG [247]. These relations will be further explored.
Imaging of POAG hallmarks on OCT is a relatively new but competitive field. Quantification of the retinal nerve fiber and ganglion cell layer measured on multiple images taken over time would help to establish the early course of disease, and the use of continuous outcomes to estimate progression would facilitate statistical analyses. In a collaborative study with University of Iowa, we investigated test–retest variability of measurements on 3-D OCT data. We found that combined macular RNFL and RGCL thickness averaged over larger areas had the best test–retest variability, and may be the best outcome parameter to use in future analyses [248].
Future investigations will focus on in-depth elucidation of the genetic background of POAG, identification of metabolic factors, further development of techniques to improve measurements of retinal layers on OCT, risk modeling, and study of gene-environment interactions.
Myopia
After our successful identification of the first gene for common refractive errors and myopia in 2010 [249], we took the initiative to form a consortium with international GWA studies. CREAM (Consortium Refractive Error And Myopia) was formed consisting of 45,000 study participants, and a meta-analysis found 26 significant genomic loci for refractive error [250]. The new loci include candidate genes with functions in neurotransmission (GRIA4), ion transport (KCNQ5), retinoic acid metabolism (RDH5), extracellular matrix remodeling (LAMA2 and BMP2) and eye development (SIX6 and PRSS56). We also confirmed previously reported associations with GJD2 and RASGRF1. Risk score analysis using associated SNPs showed a tenfold increased risk of myopia for individuals carrying the highest genetic load. Our new research is aimed at further clarification of genetic mechanisms, the study of interaction with environmental factors such as education, and search for possible common pathways with glaucoma. Other actions which will be undertaken is development of a classification scheme for myopic retinal changes on various retinal images (photographs; OCT; autofluorescence), and the study of genotype-phenotype correlations using this classification.
Methods update
At baseline and follow-up examinations participants undergo ophthalmic measurements including best-corrected ETDRS visual acuity, refractive error, Goldmann applanation tonometry, keratometry, slit lamp examination of the anterior segment and visual field testing. In pharmacological mydriasis we make 35° color photographs of the macular area, and 20° simultaneous stereoscopic imaging of the optic disc and macular area. Since the fourth follow-up, 35° stereoscopic color photographs of the optical disc and the macular area were made (RS-I-5). Analog fundus photography was replaced by stereoscopic digital imaging of the macular area and optic disc since the third follow-up examination. Optic nerve head analysis with a Heidelberg Retina Tomograph, macular pigment density, and melanin optical density measurements were added during the third follow-up (RS-I-3). At fourth follow-up examination, fourier domain optical coherence tomography of the macular area and optical disc, axial length and width measurements of cornea, anterior chamber, lens, posterior chamber and retina measured with Lenstar; and fundus autofluorescence, infra-red and red-free measurements were added (RS-I-5).
Classification of AMD, POAG, and retinal vessel diameters remained unchanged; refractive error was evaluated as spherical value + half cylindrical value, following clinical standards.
Psychiatric diseases
Objectives
The aim of the psychiatric research in the Rotterdam Study is to investigate the determinants, correlates and consequences of common psychiatric problems in the elderly. The focus has been on depressive disorders but anxiety disorders, sleep disturbances, addiction to smoking, and complicated grief are also being studied.
Study design update
In the first years of the Rotterdam Study, psychiatric data collection was very limited. However, in the second visit most participants were screened for depressive symptoms and from the third examination onwards (RS-I-3), which began in 1997, depressive disorders have been ascertained in all participants. Assessments of anxiety disorders, sleeping disturbances, and complicated grief were added in the subsequent examination (RS-I-4) and have been performed in all follow-up visits of RS-I and II, and in the baseline of RS III. Recent additions to the protocol included a screening for psychotic symptoms and, starting in January 2012, ambulatory polysomnography.
Major determinants
Psychiatric research in the Rotterdam Study focuses on biological risk factors. The vascular depression hypothesis was tested with different measures of atherosclerosis, arterial stiffness and cerebral blood flow [252]. We also examined whether blood levels of vitamins and fatty acids, immune parameters, and markers of folate metabolism increased the likelihood of depression [253]. Diurnal patterns of cortisol secretion were related to psychiatric and other disorders such as subclinical atherosclerosis [254]. Only a few candidate gene studies were performed, currently GWAs data only is analysed in collaborative efforts focussing on depressive symptoms, sleep, anxiety and cortisol [255, 256]. Several studies of brain morphology are underway [257]. Current data collection includes a dexamethasone suppression test to measure hypothalamic–pituitary–adrenal axis activity in all participants, which is unique in a population-based study. Also, psychiatric problems and psychological traits such as happiness, sleep duration, and depression are increasingly studied as determinants of health and mortality [258].
Major outcomes
Information on depression is obtained from (a) psychiatric examinations, (b) self-reported histories of depression, (c) medical records, and (d) registration of antidepressant use [259]. The psychiatric examination during follow-up visits consists of a screening with the Center for Epidemiologic Studies Depression Scale (CES-D), and in the screen-positive participants a semi-structured interview performed by a trained clinician (Schedules for Clinical Assessment in Neuropsychiatry). To continuously monitor incidence of depression throughout follow-up, trained research-assistants scrutinize the medical records of the general practitioners and copy the information about possible depressive episodes.
The following anxiety disorders are assessed with a slightly adapted Munich version of the Composite International Diagnostic Interview: generalized anxiety disorder, specific and social phobia, agoraphobia without panic disorder, and panic disorder [260].
Sleep quality and disturbance is measured with the Pittsburgh Sleep Quality Index. In addition, sleep duration and fragmentation are assessed with actigraphy, a method that infers wakefulness and sleep from the presence or absence of limb movement [261]. In total, nearly 2000 persons participated in this actigraphy study: they wore an actigraph and kept a sleep diary for, on average, six consecutive nights.
Circadian rhythms: Sleep-wake activity patterns over a week are studied with actigraphy As a marker of circadian rhythms. In more than 1700 persons we calculated interdaily stability, i.e. the stability of the rhythm over days and the intra-daily variability, i.e. the fragmentation of the rhythm.
The Inventory of Complicated Grief is used to identify traumatic grief [262]. This is a condition distinct from normal grief and bereavement-related depression, characterized by symptoms like disbelief about the death and searching for the deceased.
Major findings
Depression: The incidence and recurrence of depression in the elderly was estimated by continuously monitoring depression during a follow-up period of, on average, 8 years [259]. In total, 566 depressive syndromes and 1,073 episodes of clinically relevant depressive symptoms occurred. For depressive syndromes, the incidence rate was 7.0 (95 % CI 6.0–8.3) per 1,000 person-years and the recurrence rate was 27.5 (95 % CI 23.7–32.1) per 1,000 person-years. The recurrence rate of depressive syndromes was equal for women and men.
In a series of initial studies we found some evidence for the vascular depression hypothesis. More severe coronary and extra-coronary atherosclerosis were associated with a higher prevalence of depression, as were cerebral haemodynamic changes [252]. However, our data did not support a specific symptom profile of vascular depression as previously defined [263]. Most importantly, we found no longitudinal relation between peripheral atherosclerosis and incident depression [264]. Recently we prospectively studied cerebral vascular risk factors such as white matter lesions, silent infarcts or blood flow in relation to depression [265]. We found evidence that small vessel disease predicted the onset of depression. This suggests that atherosclerotic processes in the brain are a specific risk factor for depression, but provides little support for a vascular depression phenotype as a distinct entity.
Sleep: We investigated the relationships of sleep duration with both cardiovascular risk factors and psychiatric disorders. We found a marked U-shaped association of actigraphically measured total sleep time with BMI and obesity [266]. We also investigated and aimed to explain sex differences in subjective and actigraphic sleep parameters [267]. If assessed by diary or interview, elderly women consistently reported shorter and poorer sleep than elderly men. In contrast, actigraphic sleep measures showed shorter and poorer sleep in men. These discrepancies were partly explained by sleep medication use and alcohol consumption.
Anxiety: We found that prevalent anxiety disorders fulfilling DSM-IV criteria may be much less co-morbid with depressive disorders than previously thought if the disorders are assessed with different diagnostic instruments. On the other hand, a history of depression is very common in persons with prevalent anxiety disorder [268].
Smoking: Typically, determinants of smoking cessation are studied by comparing former with current smokers [269]. We also used a prospective approach of studying smoking cessation in 1,200 smokers (mean years of smoking: 40 years, minimum: 10 years). Smoking status was repeatedly assessed during follow-up every 3- to 4-years. Thus, an individual could contribute any number of person-years to the analyses. In other words, people were classified as smokers or quitters. This approach enabled us to detect a genetic effect on the incidence of smoking cessation [270].
Complicated grief: In our population-based study of 5741 elderly persons, current grief was reported by 1,089 participants, of these 277 (25 % or 4.8 % of total) were diagnosed with complicated grief, the vast majority of which had no clinical symptoms of anxiety or depression. Persons with complicated grief were older, had a lower level of education, and more often had lost a child [271]. Ongoing work suggests that complicated grief occurs together with structural brain atrophy more often than expected by chance.
Genetics of common psychiatric disorders: In the past year, we have performed a series of genome-wide association studies of the above psychiatric and psychological phenotypes, mostly as part of the CHARGE consortium. Several analyses have yielded no convincing genome wide significant results—clearly the initial studies were underpowered, psychiatric phenotypes do not present very homogenous entities and are highly polygenetic. Disappointingly, the genome wide analyses of intermediate phenotype in the field of psychiatry such as cortisol or executive function have hardly been more successful [256, 272]. To study the genetics of cortisol, we have established a dedicated consortium of population-based studies: CORNET.
Finally, ongoing psychiatric research projects examine whether and how psychological well-being or psychiatric problems contribute to survival. Most importantly, we are interested in whether the effects are specific to certain behaviour or emotions, are independent of confounding by physical disease, or can be explained by lifestyle, immunological or hormonal regulation [273].
Respiratory diseases
Objectives
The objectives are to investigate the incidence of respiratory diseases, to study genetic and environmental risk factors for these diseases, and the effect of respiratory diseases on quality of life, hospitalizations and mortality. The respiratory diseases of interest in the RS encompass chronic obstructive pulmonary disease (COPD), asthma, asthma and COPD overlap syndrome, respiratory tract infections, pneumonia and lung cancer. COPD is defined as a chronic airway disease characterized by airflow limitation that is not fully reversible and usually progressive [292]. COPD is a worldwide leading cause of chronic morbidity, disability and mortality; by 2020, COPD will become the third most common cause of death worldwide [293]. In patients with COPD, comorbidities and exacerbations of COPD contribute to the severity and the prognosis of the disease. Asthma is characterized by intermittent attacks of breathlessness, wheezing and cough, often nocturnal or elicited by exposure to specific allergens (such as house dust mites, animal danders and pollens) or to non-specific triggers (such as exercise and cigarette smoke). Although the onset of asthma is most common during childhood, asthma can also occur in adulthood [294]. Asthma in the elderly is underdiagnosed and undertreated, and there is a paucity of knowledge on the clinical course and outcomes of asthma in this population [295].
Major findings
In the first cohort of the Rotterdam Study (RS-I) of 7,983 participants, 648 cases have been identified with incident COPD after a median follow-up time of 11 years, resulting in an overall incidence rate of 9.2/1,000 person-years (PY) (95 % CI 8.5–10.0) [296]. The incidence rate of COPD was higher in smokers than in never-smokers (12.8/1,000 PY; 95 % CI 11.7–13.9 vs. 3.9/1,000 PY; 95 % CI 3.2–4.7) and higher in men than in women (14.4/1,000 PY; 95 % CI 13.0–16.0 vs. 6.2/1,000 PY; 95 % CI 5.5–7.0). In the second and third cohort of the Rotterdam Study (RS-II and RS-III) of 3,011 and 3,932 participants, respectively, a diagnosis of COPD has been validated in 782 subjects based on spirometry, files from the general practitioners and hospital discharge letters.
COPD and comorbidities
Since COPD does not only affect the lungs, but is also associated with extrathoracic manifestations and comorbidities, an important line of research focuses on the association of COPD with other diseases (such as cardiovascular, cerebrovascular, endocrine, oncological and neurological diseases). COPD has been shown to be an independent risk factor for ischemic stroke and the risk increases by severity of airflow limitation. We investigated the prevalence of carotid artery wall thickening by ultrasonography and characterized the carotid artery plaque composition by high-resolution magnetic resonance imaging in subjects with COPD compared with non-smoking and smoking subjects without airflow limitation. COPD subjects had a twofold increased risk of carotid wall thickening compared with control subjects with a normal lung function (odds ratio, 2.0; 95 % CI 1.44–2.85), and the risk increased significantly with severity of airflow limitation [297]. Importantly, vulnerable lipid core plaques were more frequent on magnetic resonance imaging in COPD cases than in control subjects (odds ratio, 2.1; 95 % CI 1.25–3.69). Since vulnerable carotid artery plaques place persons at risk for ischemic stroke through disruption of the plaque surface and thromboembolism, these observations offer new insights into the link between COPD and ischemic stroke.
COPD and systemic inflammation
We investigate the role of systemic inflammation in the pathogenesis of COPD and its comorbidities. Using a Mendelian randomization approach, we investigated the role of C-reactive protein (CRP) and interleukin-6 (IL-6) in the pathogenesis of COPD. We demonstrated that increased serum levels of high sensitivity (hs) CRP (>3 mg/l), a marker of systemic inflammation, are significantly associated with an increased incidence of COPD (hazard ratio (HR), 1.7; 95 % CI 1.16–2.49) compared with subjects with low CRP levels (<1 mg/l), but that variations (single nucleotide polymorphisms and haplotypes) in the CRP gene are not associated with COPD [298]. Since IL-6 induces the expression of acute phase proteins such as CRP in hepatocytes, we performed a Mendelian randomization study of IL-6 in the Rotterdam Study. Increased plasma levels of IL-6 at baseline were significantly associated with an increased risk to develop COPD [299]. However, genetic variation in the IL-6 gene did not alter the risk for COPD. These findings suggest that IL-6 and hsCRP are markers of systemic inflammation, but are not driving the pathogenesis of the pulmonary dysfunction in COPD (makers of disease).
Genetic determinants of lung function and COPD
COPD is an obstructive airway disease, characterized by a reduced ratio of forced expiratory volume in one second (FEV1) to forced vital capacity (FVC) (i.e. FEV1/FVC). The first genome-wide association study (GWAS) of pulmonary function measures, performed in the Framingham Heart Study, identified single nucleotide polymorphisms (SNPs) near the HHIP (hedgehog-interacting protein) gene on chromosome 4q31 which were associated with the FEV1/FVC ratio. We meta-analyzed GWAS for FEV1/FVC in 20,890 participants of European ancestry from four CHARGE Consortium studies: Atherosclerosis Risk in Communities, Cardiovascular Health Study, Framingham Heart Study and Rotterdam Study. We confirmed the HHIP locus and identified seven novel loci associated with FEV1/FVC at genome-wide significance [P < 5 × 10(-8)], including GPR126 (G-protein-coupled receptor 126), ADAM19 (A disintegrin and metalloproteinase 19), AGER (advanced glycation endproducts receptor), FAM13A, PID1, HTR4 (serotonin receptor 4) and PTCH1 (patched 1, receptor for HHIP) [300]. Thanks to collaboration between the CHARGE and Spirometa Consortium, 16 additional genetic loci have been shown to be associated with lung function, including MMP15, TGFB2, HDAC4 and RARB (retinoic acid receptor B) [301]. Since several identified genes (e.g. RARB, HHIP and PTCH1) play crucial roles in lung development by regulating branching morphogenesis during foetal life, the results of these GWAS suggest that genetic variants associated with lung development and growth might be important genetic determinants of lung function in childhood and adulthood, both in healthy subjects and in patients with airway disease (asthma and COPD) [302].
Since only approximately 20 % of smokers develop COPD, genetic risk factors are suspected to be involved in the pathogenesis of the disease. In the Rotterdam Study, we confirmed the association between the SNPs near the HHIP gene and COPD, and demonstrated a significant interaction with the number of pack-years of smoking [303]. In a collaborative effort of fifteen cohorts, we performed meta-analyses of GWAS for airflow obstruction, a key pathophysiologic characteristic of COPD, defined as FEV1 and FEV1/FVC both less than their respective lower limits of normal. We confirmed the association of airflow obstruction to the chromosome 15 CHRNA5/CHRNA3 (nicotinic acetylcholine receptor, subunits alpha 5 and alpha 3) gene cluster, even in never smokers, and implicated the HTR4 gene in the pathogenesis of COPD [304]. In gene expression studies we confirmed the presence of CHRNA5/3 in lung, bronchial epithelial cells and airway smooth muscle cells, suggesting that the CHRNA5/3 region might act as a genetic risk factor for airflow obstruction independent of smoking. Lastly, in genome-wide joint meta-analyses of SNP and SNP-by-smoking associations on FEV1 and FEV1/FVC across 19 studies, we identified three novel loci not previously associated with pulmonary function. SNPs in or near DNER, KCNJ2 and SOX9, and the HLA region (HLA-DQB1 and HLA-DQA2), were associated with FEV1/FVC or FEV1 [305]. These findings demonstrate that joint testing of SNP and SNP-by-environment interaction is able to identify novel loci associated with complex traits that are missed when considering only the genetic main effects.
Methods update
Lung function measurements
Spirometry is performed by trained paramedical personnel using an electronic spirometer with pneumotachograph (Jaeger Master Screen PFT, Cardinal Health, Hoechberg, Germany), according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines. Forced expiratory volume in one second (FEV1), forced vital capacity (FVC) and FEV1/FVC ratio are measured. The spirogram (volume-time curve) and the maximal expiratory flow-volume curve are recorded. The spirometries are interpreted by two research physicians according to the ATS/ERS guidelines; a senior respiratory physician decides in case of discordance between both physicians.
Diffusing capacity of the lungs (DLCO) is measured by single-breath determination of carbon monoxide (CO) uptake in the lung using the Jaeger Master Screen PFT Pro Diffusion apparatus according to the ATS/ERS task force on standardization of lung function testing [306]. The test gases used to calculate DLCO include the tracer gas methane, to measure alveolar volume (VA), and carbon monoxide (CO 0.3 %). The DLCO and the diffusing capacity for CO per unit of alveolar volume, also known as transfer coefficient of the lung (i.e. DLCO/VA or KCO), are corrected for blood hemoglobin levels, measured at the same visit of the participants to the Rotterdam Study center.
Clinical assessment of COPD
For the validation of the COPD cases, we have access to spirometry reports, files from the general practitioners, hospital discharge letters and pharmacy dispensing data for subjects participating in the Rotterdam Study. The diagnosis of COPD is based on an obstructive spirometry examination according to the modified Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (FEV1/FVC < 70 %) and classified into mild, moderate, or severe airflow limitation by FEV1% predicted of ≥80 %, 50–80 % or <50 % respectively. Clinical outcomes are collected during our continuous follow-up and encompass respiratory and non-respiratory death, hospitalizations due to exacerbations of COPD (defined as severe exacerbations) and exacerbations of COPD treated with antibiotics and/or systemic corticosteroids (i.e. moderate exacerbations). In addition, according to the GOLD 2011 update, the influence of respiratory symptoms is evaluated. Dyspnoea score is based on five dyspnoea-related questions and scored from 0 (never dyspnoeic) to 5 (even dyspnoeic at rest). In addition to the spirometric classification of COPD severity according to GOLD 2007 (GOLD grade I, II and III based on mild, moderate and severe airflow limitation), we also assess COPD subjects according to the updated classification of GOLD 2011 [292]. COPD cases are classified as GOLD A, B, C and D according to the combined assessment of airflow limitation, symptoms and exacerbation history. COPD subjects A have few symptoms and low risk (spirometric classification: GOLD grade 1–2 and ≤1 exacerbations per year); COPD subjects B have more symptoms but low risk; COPD cases C have few symptoms but high risk (GOLD grade 3–4 as spirometric classification or ≥2 exacerbations per year) and lastly COPD cases D report more symptoms and have high risk. Chronic bronchitis is defined as the self-reported presence of cough and sputum for at least 3 months in each of two consecutive years.
Genetic and biomarker studies
Objectives
The team in this research line focusses on bio-banking activities of the participants of the Rotterdam Study and investigates biological determinants of disease (i.e., DNA, RNA, proteins, metabolites). Bio-banking involves collecting, storing and managing the biological tissues of participants of the Rotterdam Study at all follow-up measurements. This concerns mainly blood and urine but increasingly also other tissues and bio-materials, such as sputum, hair and faeces. The research of this group concerns assessment of biological determinants of disease (biomarkers) in these bio-materials. The analysis of markers using genomic technologies (using SNP arrays and next generation sequencing) has substantially increased in volume and importance over the past years.
Major findings
Rotterdam Study investigators are playing leading roles in the emerging large global consortia focussed on assessing the contribution of complex disease gene variants by prospective meta-analysis across many epidemiological cohorts, such as in CHARGE and ENGAGE, and in many disease/phenotype focused efforts such as GEFOS, REPROGEN, TREATOA, DIAGRAM, etc. Since 2005 the genome wide association study (GWAs) has changed the field of complex genetics, and identified a still growing list of common variants contributing to disease risk and explaining genetic variance of traits. While this large scale global collaboration has originated from the GWAS era, we now see similar consortia being built around the newer genomics datasets with RNA expression profiles, DNA methylation profiles, and the NGS datasets on DNA, RNA and microbiomes.
The Rotterdam Study has GWAS data for almost the complete dataset summing to over 12,000 DNA samples, and is involved as a major collaborative centre for meta-analysis studies of GWAS data, including national programs (RIDE, NGI-NCHA), EU-funded projects (GEFOS, TREATOA, ENGAGE), and voluntary collaborations (GIANT, MAGIC, CHARGE). Especially, from the CHARGE consortium (the Rotterdam Study together with the Framingham Study, AGES, CHS, and ARIC) many important publications have emerged on a wide variety of phenotypes and diseases from all major research lines in the Rotterdam Study [325]. You can find them discussed under the subheadings of each individual research line.
Data collection, storage and management
At each examination at the research centre, blood, serum, plasma (citrate, heparine, and EDTA based), and sputum are collected. Fasting blood samples are collected along with challenged samples as part of a glucose tolerance test. Sputum is collected before and after a dexamethasone-suppression test. Sputum is frozen at −196 °C before and after the challenge and stored at −80 °C. To obtain serum and plasma, tubes are centrifuged according to a protocol standardising time and conditions from the drawing of blood to centrifugation. All samples are snap frozen at −196 °C using liquid nitrogen and stored at −80 °C. Overnight urine samples are collected at home, frozen at −196 °C at the research centre and stored at −80 °C.
DNA is isolated from whole blood at one laboratory at Erasmus MC by a manual salting-out protocol and is subsequently stored in Eppendorff tubes at −20 °C. A copy of the complete DNA collection of ~13,000 samples has been transferred to Matrix 2D-barcode tubes in 96 well format at another location. This copy has been subjected to normalization of DNA concentrations and made suitable for handling in 96- and 384-well micro-titer plates for subsequent downstream genomic analysis.
Starting with the RS-III round of data collection, blood drawing has also been taken place with PAXgene tubes, from which whole RNA is isolated and stored at −80 °C. This is now ongoing for the whole study population following the cycles of visits to the research centre.
Similarly, with the RS-III round, collection of faeces material has been initiated for the intestinal microbiome analysis. A collection pot is distributed at the research centre visit which is to be used at home and then by postal mailed returned to Erasmus MC where DNA is isolated and stored at −80 °C. This is now ongoing for the whole study population following the cycles of visits to the research centre.
For data management, an in-house customized sample-management system has been developed. All genomic data of the Rotterdam Study (e.g., SNP array, RNA expression, Next Generation Sequencing (NGS) DNA and RNA, microbiome NGS) are generated in one laboratory which keeps all raw data, while QC-ed and extracted data are stored and managed in the central data repository of the Rotterdam Study.
Blood serum/plasma assessments
For all participants, serum cholesterol, HDL, LDL, triglycerides, glucose and glucose levels are assessed. In urine, micro albumin and creatinine are determined in all participants. Recently, a new “baseline” serum biomarker dataset has been generated at the Clinical Chemistry Laboratory and the Endocrine Laboratory consisting of RS-I-3, RS-II-2, and RS-III-1 samples. These measurements include a steroid profile by mass-spectrometry (e.g., estrogens, androgens, vitamin D, cortisol), thyroid hormones (TSH, free T4), interleukins, CRP, IGF1, insulin, iron-parameters (iron, ferritin and transferrin saturation), fibrinogen, homocysteine, folic acid, riboflavine, pyridoxine, SAM/SAH ratio, cobalamine, Lp-PLA2, Fas/Fas-L, and abeta42/40.
Human genomics facilities
The Rotterdam Study uses one Erasmus MC laboratory for all its genomic studies on DNA, RNA, methylation, microbiome, etc. The facilities use high-end automated machinery including 2 Caliper pipetting robots (including a TwisterII, and integrated plate reader (OD 260/280), 2 Tecan EVO 150 Freedom pipetting robots, a Deerac Equator NS808 nanoliter liquid dispenser, PCR machines, an ABI7900HT Taqman machine (running 1 ng gDNA in 2 microliter reactions), 2 Illumina iScan micro-array readers, one Roche 450 GS Junior sequencer, and two Illumina HiSeq2000 sequencing machines. DNA sample handling is centred on 384-well plates. Single SNP genotyping studies are done mostly using Taqman and Sequenom genotyping with throughputs at 30,000 genotypes per day. We work with reduced amounts of input genomic DNA of 1 nanogram per genotype. This facility has been generating all GWAS data for the Rotterdam Study as well as its RNA expression profiles, methylation profiles, and all NGS data including whole exome sequences, RNA sequencing, and microbiome sequencing.
Genome-wide association studies (GWAS) datasets
Genome-Wide Association Studies (GWAS) are based on genotyping epidemiological cohorts with high density SNP arrays with 500,000—5 million SNPs. The method has been shown to successfully identify common genetic factors for hundreds of traits and diseases (see www.genome.gov/ GWAstudies). Through a large grant from the Dutch research organisation NWO one of the world’s largest GWAs datasets has been facilitated involving over 12,000 DNA samples from the Rotterdam Study cohorts. This GWAS dataset consists of (a) a small dataset of ~450 women with 500 K Affymetrix arrays (Nsp250 + Sty250), and (b) a large dataset of ~12,000 samples covering almost all RS-I, RS-II, RS-III DNA samples consisting of 550 K (RS-I, II; single + duo array format) and 610 K (RS-III; quattro array format) Illumina array genotypes.
The Illumina GWAS dataset of the Rotterdam Study (with approximately 500,000 SNPs having been genotyped) also forms the basis to generate so-called “imputed” datasets derived thereof. In this process the genotypes of SNPs which have been genotyped in reference datasets (such as HapMap with ~8 million SNPs genotyped), are being estimated for all Rotterdam Study samples using the basis Illumina 500 K SNP dataset configurations in each subject. With the advent of large reference datasets becoming available based on whole genome/exome NGS, imputation activities using the Rotterdam GWAS dataset will remain an active area of development. So far, the Rotterdam Study GWAS dataset has been imputed to HapMap version 2 and 3 (with ~7.5 million resulting SNP genotypes in the Rotterdam Study dataset), and the 1000 genome dataset version 4 (with ~18 million resulting SNP genotypes in the Rotterdam Study daaset). Currently, imputations are being generated for the Genome of the Netherlands (GoNL) whole genome NGS dataset [326], and the 10KUK whole genome sequencing dataset.
The Rotterdam Study GWAS dataset is actively being used by all research lines within the Rotterdam Study as can be read under the subheadings of each research line in this review of the Rotterdam Study. In addition, it also serves as a control GWAS dataset for other research centers in- and outside The Netherlands for both SNP frequencies as well as copy number variations (CNVs), in which capacity it has been used in ~100 publications up to date. Most importantly, it has formed the start of a very successful collaboration in the CHARGE consortium (combining GWAS datasets of major epidemiological cohort studies across the world) which has >70 phenotype working groups in which almost all research lines of the Rotterdam Study are active.
Candidate gene SNP studies
In the past, we have genotyped over 300 individual polymorphisms as part of candidate gene studies across the complete Rotterdam Study cohort using Taqman and Sequenom genotyping techniques. These mostly concern individual potentially functional single nucleotide polymorphisms (SNPs) per gene, but sometimes also haplotype tagging SNPs (e.g., ESR1, ESR2, HSD11B1, fibrinogen), and also high density SNP screening (e.g., the vitamin D receptor gene). Currently, for candidate gene/SNP studies we perform look-ups in the GWAS datasets and/or perform individual SNP genotyping (e.g., SNPs not on the array, badly imputed SNPs, functional SNPs).
RNA expression datasets
With the availability of good RNA from Rotterdam Study participants, starting with the RS-III subjects, studies have been initiated analysing the expression pattern of a single gene across samples or of the complete RNA collection in a sample (expression profiling). An expression profiling dataset has now been generated of the first ~1,000 samples of the RS-III dataset, using the Illumina Human HT-12 v4 array containing ~48,000 probes. Subsequently, these same RNA samples are currently being subjected to NGS (see below) so that a very rich expression dataset will become available. While RNA expression is known to differ between tissues, so far we only have RNA isolated from whole blood as a tissue.
Methylation datasets
In ~1,000 samples of the RS-III dataset we have generated DNA methylation profiles of ~480,000 CpG sites across the human genome using the Illumina Infinium HumanMethylation450 array. As this same set of RS-III subjects was also used for the RNA expression profiling, deep genomic studies can now take place in combination with GWAS data and NGS data in these ~1,000 subjects.
New developments: Next Generation Sequencing datasets
A major development in genomics studies has been the introduction in the past few years of high-throughput parallel sequencing methods (also known as Next Generation Sequencing or NGS) which allow DNA (and RNA) sequencing at unprecedented high speed and low costs. This development has brought sequencing of whole genomes within reach of individual laboratories, rather than the large global effort that was needed to sequence the human genome at first pass. This development has led to a revival in Mendelian Genetics by solving many “cold cases” (because causal mutations could directly be found in a few samples, rather than by linkage analysis in many family samples and laborious sequencing analyses of dozens of candidate gene exons in the area). In addition, it has stimulated the cohort studies that had generated GWAS datasets in the past 5 years, to generate NGS data in part or all of their samples to find local/regional variants of interest and variants that are very rare. While whole genome sequencing is often seen as the ultimate goal in these efforts, currently almost all labs turn to Whole Exome Sequencing (WES) because it is much more cost-effective. In WES the parts that code for amino acids as part of the encoded proteins (i.e., the exons) are first captured from the whole genome by hybridization techniques upon which the selected parts (the exons) are then subjected to NGS. WES costs 5-10 times less euro’s than whole genome sequencing (so, more samples can be done with the same amount of money) and generates interpretable findings. (note that for the vast majority of areas in the human genome we have no good idea what they signify as opposed to exons that encode functionally important parts, i.e., the parts that make up the proteins). A large grant from the NGI-sponsored Netherlands Consortium for Healthy Ageing (NCHA), allowed to generate whole exome NGS data for ~3,000 samples by the Erasmus MC genomics facility on the Illumina HiSeq2000 sequencing machines. The samples for this experiment were selected to constitutes a random sample from the RS-I dataset. These NGS data have been generated, and the samples are now in the process of QC and calling of variants. This process is complicated and no golden standard exists, so these steps take place together with colleagues at Baylor College, the Broad Institute, and NGS facilities in Germany and Netherlands. Through a collaborative grant from the NIH Alzheimer initiative we will also obtain an additional ~2,000 samples with whole exome NGS data from RS-I. Also here we collaborate with colleagues in CHARGE since NGS data have been generated in large parts of the ARIC study, the Framingham Study, and the CHS study. Within the several working groups these data are currently being analysed in relation to specific phenotypes.
From the first NGS efforts in reference datasets using whole exome sequencing, many thousands of novel DNA sequence variants were discovered in the exons, mostly rare to very rare and not well-covered on the existing GWAS arrays. Illumina and Affymetrix therefore decided to generate an array devoted to analyse these new exome variants called the Infinium HumanExome array. This array has been used to genotype many DNA samples across the globe and several collaborative analyses are currently using data generated with this array. In the Rotterdam Study we generated exome array genotypes of ~3,000 samples of RS-I (with version 1 of this array), and this dataset is part of several collaborative efforts, including the Dutch BBMRI exome array effort (with ~35,000 collective samples), an effort in GIANT on anthropometric traits, and the CHARGE exome array effort across many different phenotype working groups (with ~60,000 collective samples).
The RNA we have isolated from ~1,000 RS-III whole blood samples is currently being subjected to NGS analysis. This effort is part of the Dutch BBMRI bio-banking initiative together with other Dutch biobanks, such as LifeLines and the Netherlands Twins Register.
We have isolated DNA from faeces of ~1,000 RS-III subjects and this DNA is currently being analysed by 16S RNA sequencing using the Roche 454 Junior NGS machine. We are now setting up the protocols to start running whole DNA sequencing from these samples (called meta-genomics).
For additional EJE references see [9, 121, 277, 285, 327–349].
Pharmaco-epidemiologic studies
Objective
A major objective of the pharmaco-epidemiologic studies is to investigate the role of drugs as determinants of disease in the Rotterdam Study. This includes studying efficacy and effectiveness of drugs, as well as adverse reactions to drugs. As the drugs used in the Rotterdam Study are licensed and often on the market since several years, research focuses on effect modifiers on the association between drugs and disease. A topic of particular interest is pharmacogenetics and genomics as genes may may account for most of the variation in drug response.
Major findings
Important findings have been published on pharmaco-epidemiological topics concerning the main outcomes in the Rotterdam Study. Subsequently, we will summarize pharmacogenetic- and other findings.
Pharmacogenetic findings
Pharmacogenetic activities in the Rotterdam Study can be distinguished into hypothesis-generating ‘genome-wide association’ (GWA) studies, and in hypothesis-testing candidate gene studies. As the performance of GWA studies on gene-drug interactions require a very large sample size, the Rotterdam Study actively participates in the CHARGE consortium [350]. This is currently ‘work under progress’ and up till now most comes from GWA studies within the Rotterdam Study on specific topics. A very attractive topic consists of coumarin anticoagulants as the measurement of anticoagulation through the International Normalized Ratio (INR) is not only a very well standardized secondary outcome but also in indirect measure of drug adherence. Interesting were the results of a GWA in users of the anticoagulant phenprocoumon which confirmed the important genetic variant for VKORC1, CYP2C9 and 4F2 in acenocoumarol users [351], and later also in phenprocoumon dosage [352]. Although 4F2 was a relatively new finding, further studies on 4F2 revealed that its contribution was very modest [353]. Another genetic determinant, i.e. GATA-4, did not have a clinically relevant effect on the maintenance dose of acenocoumarol or phenprocoumon [353]. A recently developed ‘European Pharmacogenetics of Anticoagulant Therapy (EU-PACT) acenocoumarol dose algorithm performed as well in the Rotterdam Study as in the original trial [354]. Two analyses in the Rotterdam Stuy investigated the potential interaction between coumarin anticoagulants and other drugs, i.e. antidepressants and proton pump inhibitors. The risk for overanticoagulation during acenocoumarol maintenance treatment was more than doubled in combination with fluvoxamine and venlafaxine. There was no increase in risk for the other SSRIs, but numbers of exposed cases were low for all SSRIs except paroxetine [355]. As for the potential interaction between proton pump inhibitors and coumarins, the risk for overanticoagulation was most pronounced for esomeprazole and lansoprazole [356].
A promising subject for pharmacogenetic research consists of the effect of metformine in diabetics. Previously, we demonstrated that there were important genetic variations in OCT1 and MATE1 transporters with consequences for metformine response in diabetics [357–359]. Recently, we participated in a consortium meta-analyis demonstrating that a gene variant near ATM is significantly associated with metformin treatment response in type 2 diabetic patients from the Netherlands and the UK [360]. Other interesting study results comprised our finding that several statins are substrates for the multidrug resistance-associated protein 2 transporter, encoded by the ABCC2 gene [361], and that the cytochrome P450 CYP1A2 encoding gene variant rs2472299G >A, female sex, and nonsmoking were significantly inversely related to coffee intake [362].
Other findings
Electrolyte disorders such as hyponatremia and hypo- and hyperkalemia are common causes of drug-induced hospital admissions in the aged. Already since decades and in most western countries, antihypertensive diuretics are the most frequent iatrogenic cause of electrolyte disorders [363].
In a cross-sectional analysis in the Rotterdam Study, a total of 776 subjects (15.0 %) had at least 1 electrolyte disorder, with hyponatremia (7.7 %) and hypernatremia (3.4 %) being most common. Diuretics were independently associated with several electrolyte disorders: thiazide diuretics (hyponatremia, hypokalemia, hypomagnesemia), loop diuretics (hypernatremia, hypokalemia), and potassium-sparing diuretics (hyponatremia) [364]. The risk of hyponatremia was increased eightfold in users of thiazides, independent of sex and glomerular filtration rate, but not of age and BMI [365]. Apart from modifying sodium- and potassium levels, thiazides are known to retain calcium and may even be protective against hip fracture [366]. In women, short-term use of loop diuretics was associated with an increased level of FDP, reflecting increased bone resorption by osteoclasts [367].
A productive collaboration between pharmaco-epidemiology and outcome groups in the Rotterdam Study led to a study on the association between corticosteroids and open-angle glaucoma [368]. In the past, the suspicion that corticosteroids may induce glaucoma. Adjusted for age, sex, high myopia and family history of glaucoma, none of the classes of steroids were associated with the incidence of open-angle glaucoma. In this collaboration, two other drug groups were studied for their association with eye disorders. First, an association has been suggested in the past that antithrombotics may also induce open-angle glaucoma. Although there was a significant intra-ocular pressure (IOP)-lowering effect of anticoagulants of -0.31 mm Hg, the IOP-lowering effect of anticoagulants disappeared after additional adjustment for the use of systemic beta-blockers [369]. Second, Long-term use of statins appears to be associated with a reduced risk of OAG, independent of the IOP. These findings are in line with the idea that statins have neuroprotective properties [370]. Apart from its cardiovascular indications, more and more epidemiological data suggest other beneficial effects. Besides biomechanical mechanisms, the pathogenesis of osteoarthritis may involve inflammation, vascular alterations and dysregulation of lipid metabolism. In the Rotterdam Study, statin use was associated with more than a 50 % reduction in overall progression of osteoarthritis of the knee, but not of the hip [371]. Underlining the suggestion that the protective effect of statins on mortality is partly explained by an anti-inflammatory effect, was that long-term statin use (>2 years) was associated with a 39 % decreased risk of death in COPD patients. Stratified according to the level of systemic inflammation, long-term statin use was associated with a 78 % reduced mortality if hsCRP level >3 mg/L, versus a non significantly 21 % reduced mortality if hsCRP level ≤3 mg/L [372].
New developments
Drugs are attractive determinants in clinical epidemiologic research because they are probably the most important therapeutic intervention in health care, and the majority of all marketed drugs have proven biological activity. As a nice consequence of the availability of complete medication histories in Dutch health care, the role of drug exposure can be assessed in a detailed way. In the Rotterdam Study, there is an almost complete coverage of the population as of January 1, 1991, thanks to the fact that all pharmacies which serve the Ommoord district are on one computer network. This facilitates the use of detailed analyses with the drug as a time-varying determinant [373]. Apart from the performance of collaborative GWA studies on gene-drug interactions, as alluded to above, there are two interesting methodological developments in the pharmaco-epidemiological studies in the Rotterdam Study. First, based on earlier publications on marginal structural modeling [374], we are currently analyzing the association between statins and cardiovascular mortality with such models in which we try to use more precise exposure definitions than use/non-use. Second, in an effort to translate pharmacokinetic data from clinical trials to population-based research, PK/PD-modelling was performed on the association between sotalol and the length of the QTc-interval on ECGs [375].
Imaging studies
Objective
Biomedical imaging allows for non- or minimally-invasive assessment of structural and functional changes that may reflect specific pathology. Recent developments in image data acquisition and analysis enable to use these techniques on a large scale. The Population Imaging Unit within the Rotterdam Study aims to assess imaging biomarkers of disease in a pre-symptomatic phase at the population level. Advantages of imaging measures include that they mark early disease, can be assessed reliably and reproducibly, and are quantitative rather than qualitative which makes them more powerful than most conventional outcome measures such as clinical phenotypes.
The main imaging modalities that are currently being applied in the Population Imaging Unit are multidetector computed tomography (MDCT) and magnetic resonance imaging (MRI).
Imaging infrastructure and storage
Mdct
CT imaging is currently performed with hospital-based 16-slice or 64-slice MDCT scanners (SOMATOM Sensation 16 or 64, Siemens, Forcheim, Germany), located at Erasmus University Medical Center. Scanners are operated by clinical technicians. CT images are acquired without contrast-enhancement and according to standardized protocols. Imaging data are transferred from the CT scanner to a securely backed-up research picture archiving system.
Mri
From August 2005 onwards, a dedicated 1.5 Tesla MRI scanner (GE Healthcare, Milwaukee, Wisconsin, USA) is operational in the Rotterdam Study research center. This scanner is operated by trained research technicians and all imaging data are collected according to standardized imaging protocols. Changes or updates in hardware or software configuration are avoided and regular quality checks are performed to secure validity of cross-subject and cross-scan comparisons. Imaging is performed without administration of contrast agents. All imaging data are directly transferred from the scanner facility to the Erasmus University Medical Center. Data are stored on a securely backed-up research picture archiving system, using programmed scripts to check for completeness of the data received.
Data management and processing
Assessment of incidental findings
All imaging data are visually evaluated within days after acquisition by trained physicians for the presence of clinically relevant incidental findings [202]. Expert radiologists are consulted for all abnormal findings and the management of clinically relevant findings is based on protocols defined by expert panels. These protocols are updated on a regular basis incorporating the current best available knowledge regarding treatment and prognosis of the various abnormalities discovered.
Automated processing of MRI data
Though some measurements are still performed manually or scored visually, the majority of imaging data is now processed using semi- and fully-automated computer algorithms. The Population Imaging Unit collaborates with the Biomedical Imaging Group Rotterdam (BIGR) of Erasmus University Medical Center in the application and development of automated processing pipelines for high-throughput of large data quantities. These pipelines comprise on the one end image quality checks and procedures for non-uniformity correction, normalization and image registration and on the other end advanced algorithms to extract image features to use for analyses.
Grid architectures and networked processing pipelines are used to process the large quantities or imaging data that are acquired in the Rotterdam Study.
Major findings
The Rotterdam Study research lines currently applying imaging within the Population Imaging Unit are those on neurological diseases and cardiovascular diseases.
Brain imaging (MRI)
Neurodegenerative and cerebrovascular disease are common disorders in the elderly that exert a large influence on brain functioning. Identifying underlying pathology in a preclinical state may help to recognize persons at risk, assess determinants of disease and develop preventive measures. Main objective for the Population Imaging Unit with respect to brain imaging is therefore to identify and quantify brain imaging biomarkers that mark the development of neurodegenerative and cerebrovascular disease.
From August 2005 onwards (RS-II-2 and onwards), brain imaging in the Population Imaging Unit is performed in all study participants without contra-indications in the context of the Rotterdam Scan Study. The current structural scanning protocol includes 4 high-resolution axial sequences (3D T1-weighted; 2D PD-weighted; 2D FLAIR; and 3D T2* GRE), 2D phase-contrast imaging, and diffusion tensor imaging (DTI). Total scanning time for these sequences amounts to approximately 30 min. Currently, over 5,700 unique brain MRI scans and over 4,800 follow up scans have been acquired with this protocol. Starting 2012, functional imaging is incoporated in the MRI protocol in the form of a resting-state fMRI sequence (8 min), which is added to the existing structural scans. To date, rs-fMRI scans have been acquired in approximately 1,000 individuals and this will continue into 2014.
Fully automated methods are applied to quantify atrophy of brain tissues and structures and the severity of white matter lesions [218, 391, 392]. Automated hippocampal segmentation has been successfully applied on multi-scanner acquired MR images (on scans acquired in the Rotterdam Scan Study in 1995 [211] and follow up examinations in 2006), showing that a decline in hippocampal volume over a 10-year follow up period predicted onset of clinical dementia [393]. Apart from focusing only on supratentorial brain structures, we have more recently also incorporated segmentation of the cerebellar volume into our automated processing pipelines, enabling us to study the cerebellum in aging. In 3,962 persons, we found that smaller cerebellar volumes with increasing age were mainly driven by loss of white matter. Furtermore, diabetes, higher serum glucose and lower cholesterol levels were most important risk factors for cerebellar atrophy in aging.
Phase-contrast imaging allows for assessment of blood flow in the carotids and basilar artery. This yields measures of total brain perfusion [394], which when lower was found to be relate to worse cognition, an association that is mediated by brain atrophy [395].
The 3D T2* GRE sequence that we use was specifically developed to increase the conspicuity of cerebral microbleeds [396]. With this optimized sequence, we found that microbleed prevalence gradually increases with age, from 6.5 % in persons aged 45 to 50 years to 35.7 % in participants of 80 years and older; and that overall, 15.3 % of all subjects over the age of 45 years has at least 1 microbleed; a much higher prevalence than was reported before [370, 371]. We found supportive evidence that deep or infratentorial microbleeds reflect arteriolosclerotic angiopathy, whereas strictly lobar microbleeds are caused by cerebral amyloid angiopathy [397, 398]. We furthermore demonstrated that incidence of microbleeds over a 3-year time interval is high and that risk factors for new microbleeds again differ according to microbleed location, in line with our findings regarding prevalent microbleeds [399]. These findings impact research into the causes of cerebral amyloid angiopathy, as well as fuel the ongoing discussion about the safety of antithrombotic therapy in persons with microbleeds [400, 401]. Our current studies focus on the clinical relevance of cerebral microbleeds, in relation to clinical outcomes such as stroke, dementia and mortality, over a mean follow-up of 5 years.
Diffusion tensor imaging (DTI) allows the assessment of the microstructural integrity of white matter. White matter microstructure looses its integrity with increasing age, but this can largely be explained by presence of white matter atrophy and white matter lesions [402].
Nevertheless, the microstructural integrity in the normal appearing white matter and in white matter lesions relates to cognitive function regardless of concurrent macrostructural changes, emphasizing the importance of the microstructural integrity of white matter [403]. Also, we found that white matter changes in normal appearing white matter are present and can be quantified on diffusion tensor imaging before white matter lesions actually develop. This suggests that white matter lesions develop gradually, and that visually appreciable lesions are only the tip of the iceberg of white matter pathology. We recently developed within the Rotterdam Study a method for fully automated tractography of over 20 major white matter tracts, yielding tract-specific measures of white matter integrity, enabling a more in-depth exploration of structural integrity in relation to functional processes in aging [404].
Imaging of atherosclerotic calcifications (MDCT)
Main objectives with respect to imaging of vascular calcifications are to investigate distribution of and risk factors for atherosclerotic calcifications in the general elderly population and to study prognosis associated with calcifications in different vessel beds.
From September 2003 until February 2006, all participants from RS-I-4 and RS-II-2 who completed a center visit were invited to a MDCT scan of the coronary arteries and a second scan of the aortic arch and carotid arteries. A total of 2,521 participants (response rate 79 %) were scanned. The cardiac scan reached from the apex of the heart to the tracheal bifurcation. The second scan reached from the aortic arch to the intracranial circulation. Images were analyzed by trained reviewers and calcification in the different vessel beds (coronaries, aortic arch, extracranial and intracranial carotids) were quantified using the Agatston score [405].
As expected, we found that calcification load was higher overall in men compared to women, though aortic arch calcification was more prevalent among women [406]. Age and current smoking were found to be the strongest independent risk factors for arterial calcification [407]. Furthermore, strong and graded associations of prevalent stroke were found with carotid artery, aortic arch and coronary artery calcification, independent of cardiovascular risk factors [408]. When vessel calcification was studied in relation to vascular brain disease (non-invasively imaged with MRI), we found that larger intracranial carotid calcification load related to larger white matter lesion load, and that larger extracranial carotid calcification load related to the presence of cerebral infarcts, independently of ultrasound carotid plaque score. This suggests that calcification of atherosclerotic plaque yields other information in addition to merely the presence of plaques. This importance of vessel calcification is further corroborated by our recent finding that larger calcification volumes are associated with worse cognitive performance in the general population.
Despite extensive research on the identification of lifestyle- and environmental cardiovascular risk factors, a large part of the variability in the total burden of atherosclerosis remains yet unexplained. This inevitably indicates that other, unknown factors also considerably contribute to the development of atherosclerosis. During the last years, it has become clear that genetic factors may play an important role in the development of atherosclerosis. We determined whether previously identified genetic loci for coronary calcification were also associated with calcification in other locations than the coronary arteries Indeed, we found that the genetic basis for aortic arch and carotid artery calcification largely overlaps with that of coronary artery calcification. However, we found that the genetic variants contributed differentially to the amount of atherosclerotic calcification in these vessel beds. This suggests that also on the genetic level, differences in the etiology of atherosclerosis across vessel beds exist. Additionally, we also investigated the genetic basis of atherosclerosis using the genetics of strong risk factors of atherosclerosis, i.e. serum cholesterol levels and blood pressure and demonstrated that both serum lipids and blood pressure share a genetic predisposition with the formation of atherosclerosis in multiple vessel beds. Worldwide, intracranial atherosclerosis is one of the leading causes of stroke, yet it is understudied in population of white descent. We therefore focused specifically on the prevalence and risk factors of intracranial internal carotid artery calcification in our population, and found that the overall prevalence of intracranial atherosclerosis was as high as 82.2 %. Conventional cardiovascular risk factors are associated with intracranial atherosclerosis, but risk factor profiles differed between men and women, with excessive alcohol intake and smoking were strong risk factors in men, whereas diabetes and hypertension were in women. Our current studies focus on long-term risk of disease associated with calcifications in various vessel beds, again with a particular focus on intracranial atherosclerosis.
Carotid plaque imaging (MRI)
Carotid wall thickening and atherosclerosis are highly prevalent at older age and are considered a major cause of cerebrovascular events [197]. Carotid atherosclerotic plaque constituents such as lipid core and hemorrhage, so-called “vulnerable” components, are considered important factors in development of clinical neurological events [409]. With MRI, it is possible to separately identify these plaque components [410]. Main objectives with respect to carotid imaging in the Rotterdam Study are to investigate distribution of and risk factors for carotid plaque components in the general elderly population and to study prognosis associated with specific carotid plaque composition.
From October 2007 onwards, all participants with carotid wall thickening of 2.5 mm or larger on ultrasound (approximately 25 % of the Rotterdam Study population) are invited for carotid MRI. Imaging is performed with a bilateral phased-array surface coil (Machnet, Eelde, The Netherlands), stabilizing subjects in a custom-designed head holder to reduce motion. The imaging protocol consists of a series of high-resolution MRI sequences to image the carotid bifurcations on both sides: a PDw Fast Spin Echo (FSE) Black-blood (BB) sequence; a PDw-FSE-BB with an increased in-plane resolution; a PDw- Echo Planar Imaging (EPI) sequence and a T2w-EPI sequence; and 2 three-dimensional (3D) sequences: a 3D-T1w-Gradient Echo (GRE) sequence; and a 3D phased-contrast MR-Angiography. Total scanning time amounts to approximately 30 min.
Plaques are reviewed by trained raters for the presence of three different plaque components (calcification, hemorrhage and lipid core). Furthermore, carotid plaque size is quantified by obtaining maximum carotid wall thickness and degree of luminal stenosis using the NASCET criteria [411] on the PDw-FSE images. Postprocessing techniques aimed at automated quantification of plaque volume and identification of different plaque components are currently being developed.
So far, over 1,300 participants underwent a complete carotid MRI scan and data are currently being analyzed. There is a complete overlap between carotid and brain MRI participants, allowing for the investigation of carotid plaque constituents in relation to brain imaging markers. In the first 1,006 scans, we found that intraplaque haemorrhage and lipid core were present in almost 25 % of plaques, respectively, and occurred simultaneously in 9 % of plaques. Different risk factors are associated with these plaque components: hypertension (and in particular high pulse pressure) and current smoking were risk factors for the presence of intraplaque haemorrhage, and hypercholesterolaemia was the only risk factor for lipid core presence.
New developments
As also mentioned above, focus has shifted from purely structural imaging to also including functional imaging data, by incorporating resting-state functional MRI into the brain imaging protocol. Changes in the intrinsic activity of resting-state networks are presumed to represent alterations in functional brain connectivity and may mark neurodegeneration in an early, presymptomatic stage. Our initial studies will focus on the relation between functional brain connectivity and established structural markers in age-related brain diseases such as hippocampal volume, white matter lesions and microbleeds. In a later stage, we will investigate whether functional connectivity can be used an early imaging marker for dementia, by itself or in combination with other imaging markers and risk factors.
Regarding structural imaging markers, an emerging potential marker is Virchow–Robin (VR) spaces, spaces filled with interstitial fluid that surround the blood vessels in the brain and which can be dilated. Despite increasing literature on these dilated VR spaces, a major limitation of current research is the lack of a robust and generalizable rating method on MRI. We recently developed a novel rating method for VR spaces, which we successfully applied in 2 population-based studies, encompassing 3 different scanning protocols. We will use this rating method to explore determinants and consequences of dilated VR spaces in our general aging population.
Besides ever-increasing advances in imaging hardware, software and sequence design, major advances in the short and long run are to be expected from (fully) automated image analysis. Computer processing of images will enable to make fully use of all information contained within the image, introducing new imaging biomarkers. Besides, the vast amount of imaging data that are acquired in population-based studies like the Rotterdam Study renders visual assessment or manual measurements virtually impossible, strengthening the need for (fully) automated methods of data extraction and analysis.
Otolaryngological diseases
Objectives
Otolaryngological research in the Rotterdam study focuses on the frequency, etiology and consequences of auditory and vestibular disorders. We are mainly interested in dysfunctions located in the labyrinth of the inner ear, expressed by cochlear hearing loss and a deviant vestibulo-ocular reflex (VOR) for fast head movements. Etiology of both peripheral disorders will be studied, possibly revealing one or more common risk factors as both systems are connected and have similar sensory mechanisms. Additionally, we will investigate possible associations between reduced sensory input and general neuroepidemiological measures.
Methods
From 2011 we started measuring hearing and vestibular function within the Rotterdam Study population. Hearing loss is assessed at both ears by performing pure-tone audiometry in a sound proof room. Hearing thresholds are determined with headphones at frequencies 0.25, 0.5, 1, 2, 4 and 8 kHz. To distinguish between cochlear and middle-ear pathology, also bone-conduction thresholds are measured at frequencies 0,5 and 4 kHz. Additionally, speech perception in noise is tested at the better ear, using a validated triplet digit test [416] with speech-shaped noise at a fixed presentation level of 65 dB SPL. The ability to understand speech in noise is a functional measure that includes both sensory and central aspects of the auditory system.
Peripheral vestibular function is assessed by The Head Impulse Test (HIT), which measures the vestibule-ocular reflex (VOR) for a number of sudden head movements initiated by the tester [417]. Gain and delay are the main parameters that will be used to quantify vestibular function. An advanced infrared video-oculography system is used to track the eye movements simultaneously with the position of the head, enabling VOR recordings, even for fast head movements. Additionally, central vestibular processing is assessed by measuring the VOR during repetitive slow head movements at frequencies 0.5, 1 and 2 Hz while fixating the eyes at a projected visual target. The oculomotor function is tested by eye tracking of a moving target at frequencies 0.5, 1 and 2 Hz, while the head is fixated.
The general interview contains ten questions related to hearing and balance problems. In case of hearing-aid use, the participant has to answer five additional questions of the International Outcome Inventory of Hearing Aids (IOI-HA) [418]. In case of frequent tinnitus, ten additional questions of the Short Tinnitus Handicap Inventory (THI-S) are added [419].
Determinants of interest
One of our main goals is to find medical and genetic factors that are associated to age-related hearing loss. Age-related hearing loss is a common disorder that deprives older people of key sensory input. It leads to social withdrawal and is even been found to be independently associated with poorer cognitive functioning and incident dementia. Still, little is known about the mechanisms that are responsible for developing hearing loss and the way it affects general cognitive functions within the elderly population. Determinants of interest are genetic factors, cardiovascular disease, use of medication, endocrine diseases and neuroepidemiological factors.
Even less is known about age-related vestibular dysfunction. Problems related to balance and dizziness are frequently reported in elderly people, but not much is known about the prevalence and type of vestibular dysfunction that may cause these problems. This will be the first large cohort study in which vestibular function will be measured. The same determinants as mentioned for hearing will be studied for the vestibular system. Additionally, the relation between hearing and peripheral vestibular function will be extensively investigated.
Management
The Rotterdam Study is directed by a Management Team comprising the scientific principal investigators Sarwa Darwish Murad (PI Hepatic diseases), Cornelia van Duijn (PI Genetic epidemiologic studies), Oscar Franco (PI Cardiovascular diseases and ErasmusAGE), André Goedegebure (PI Otolaryngological diseases), Albert Hofman (chairman, PI Rotterdam Study), Arfan Ikram (PI Neurological diseases), Caroline Klaver (PI Ophthalmic diseases), Tamar Nijsten (PI Dermatological diseases), Robin Peeters (PI Internal Medicine), Bruno Stricker (PI Pharmaco-epidemiology), Henning Tiemeier (PI Psychiatric diseases), André Uitterlinden (PI Genomic studies), and Meike Vernooij (PI Population Imaging); and Jan Heeringa, MD, PhD, study coordinator, Eric Neeleman, head IT, and Frank van Rooij, MSc, head data-management. The study of respiratory diseases is conducted in close collaboration with Prof Guy Brusselle, Department of Respiratory Medicine, Ghent University Hospital, Ghent, Belgium. The study of pulmonary hypertension is conducted in close collaboration with Ardeshir Ghofrani, University of Giessen Lung Center, Giessen, Germany.
Emeritus principal investigators
The following persons are Principal Investigator Emeritus of the Rotterdam Study:
Frank van den Ouweland (PI Internal Medicine 1990–1992), Diederick Grobbee (PI Cardiovascular diseases 1990–1996), Albert Hofman (PI Neurological diseases 1990–1996), Paulus de Jong (PI Ophthalmic diseases 1990–2005), Huibert Pols (PI Internal Medicine 1993–2006), Monique Breteler (PI Neurological diseases 1997–2010), Gabriel Krestin (PI Population Imaging 1998–2010), Johannes Vingerling (PI Ophthalmic diseases 2005–2010), Jacqueline Witteman (PI Cardiovascular diseases 1997–2011), Ernst Kuipers (PI Internal Medicine 2007–2013), Harry Janssen (PI Hepatic diseases 2007–2013).
References
Oeppen J, Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296(5570):1029–31.
Peto R, Doll R. There is no such thing as aging. BMJ. 1997;315(7115):1030–2.
Hofman A, Grobbee DE, de Jong PT, van den Ouweland FA. Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. Eur J Epidemiol. 1991;7(4):403–22.
Hofman A, Breteler MM, van Duijn CM, Krestin GP, Pols HA, Stricker BH, et al. The Rotterdam Study: objectives and design update. Eur J Epidemiol. 2007;22(11):819–29.
Hofman A, Breteler MM, van Duijn CM, Janssen HL, Krestin GP, Kuipers EJ, et al. The Rotterdam Study: 2010 objectives and design update. Eur J Epidemiol. 2009;24(9):553–72.
Hofman A, van Duijn CM, Franco OH, Ikram MA, Janssen HLA, Klaver CCW, et al. The Rotterdam Study: 2012 objectives and design update. Eur J Epidemiol. 2011;26(8):657–86.
Almqvist C, Adami HO, Franks PW, Groop L, Ingelsson E, Kere J, et al. LifeGene-a large prospective population-based study of global relevance. Eur J Epidemiol. 2011;26(1):67–77.
Puddu PE, Menotti A, Tolonen H, Nedeljkovic S, Kafatos AG. Determinants of 40-year all-cause mortality in the European cohorts of the Seven Countries Study. Eur J Epidemiol. 2011;26(8):595–608.
Tong SL, Neale RE, Shen XM, Olsen J. Challenges for epidemiologic research on the verge of a new era. Eur J Epidemiol. 2011;26(9):689–94.
Mackenbach JP, Slobbe L, Looman CWN, van der Heide A, Polder J, Garssen J. Sharp upturn of life expectancy in the Netherlands: effect of more health care for the elderly? Eur J Epidemiol. 2011;26(12):903–14.
Janszky I, Bjorngaard JH, Romundstad P, Vatten L. A novel approach to quantify random error explicitly in epidemiological studies. Eur J Epidemiol. 2011;26(12):899–902.
Peters T, Brage S, Westgate K, Franks PW, Gradmark A, Diaz MJT, et al. Validity of a short questionnaire to assess physical activity in 10 European countries. Eur J Epidemiol. 2012;27(1):15–25.
Modig K, Drefahl S, Andersson T, Ahlbom A. The aging population in Sweden: can declining incidence rates in MI, stroke and cancer counterbalance the future demographic challenges? Eur J Epidemiol. 2012;27(2):139–45.
Leening MJG, Kavousi M, Heeringa J, van Rooij FJA, Verkroost-van Heemst J, Deckers JW, et al. Methods of data collection and definitions of cardiac outcomes in the Rotterdam Study. Eur J Epidemiol. 2012;27(3):173–85.
Eriksson AK, Ekbom A, Hilding A, Ostenson CG. The influence of non-response in a population-based cohort study on type 2 diabetes evaluated by the Swedish Prescribed Drug Register. Eur J Epidemiol. 2012;27(3):153–62.
Svensson M, Svensson T, Hansen AW, Lagerros YT. The effect of reminders in a web-based intervention study. Eur J Epidemiol. 2012;27(5):333–40.
Vernooij MW. Minimally invasive autopsy: the technological revival of autopsy? Eur J Epidemiol. 2012;27(7):487–8.
Jaddoe VWV, van Duijn CM, Franco OH, van der Heijden AJ, van Iizendoorn MH, de Jongste JC, et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol. 2012;27(9):739–56.
Larsen SB, Dalton SO, Schuz J, Christensen J, Overvad K, Tjonneland A, et al. Mortality among participants and non-participants in a prospective cohort study. Eur J Epidemiol. 2012;27(11):837–45.
de Torbal A, Boersma E, Kors JA, van Herpen G, Deckers JW, van der Kuip DA, et al. Incidence of recognized and unrecognized myocardial infarction in men and women aged 55 and older: the Rotterdam Study. Eur Heart J. 2006;27(6):729–36.
Leening MJG, Elias-Smale SE, Felix JF, Kors JA, Deckers JW, Hofman A, et al. Unrecognised myocardial infarction and long-term risk of heart failure in the elderly: the Rotterdam Study. Heart. 2010;96(18):1458–62.
Krijthe BP, Leening MJG, Heeringa J, Kors JA, Hofman A, Franco OH, et al. Unrecognized myocardial infarction and risk of atrial fibrillation: the Rotterdam Study. Int J Cardiol. 2013;168(2):1453–7.
Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure the Rotterdam Study. Eur Heart J. 2004;25(18):1614–9.
Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27(8):949–53.
Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746–5122.
van Vark LC, Kardys I, Bleumink GS, Knetsch AM, Deckers JW, Hofman A, et al. Lipoprotein-associated phospholipase A2 activity and risk of heart failure: the Rotterdam study. Eur Heart J. 2006;27(19):2346–52.
Nanchen D, Leening MJG, Locatelli I, Cornuz J, Kors JA, Heeringa J, et al. Resting heart rate and the risk of heart failure in healthy adults: the Rotterdam Study. Circ Heart Fail. 2013;6(3):403–10.
Heeringa J, van der Kuip DAM, Hofman A, Kors JA, van Rooij FJA, Lip GY, et al. Subclinical atherosclerosis and risk of atrial fibrillation: the Rotterdam Study. Arch Intern Med. 2007;167(4):382–7.
Heeringa J, Hoogendoorn EH, van der Deure WM, Hofman A, Peeters RP, Hop WC, et al. High-normal thyroid function and risk of atrial fibrillation: the Rotterdam Study. Arch Intern Med. 2008;168(20):2219–24.
Krijthe BP, de Jong FH, Hofman A, Franco OH, Witteman JCM, Stricker BHCh, et al. Dehydroepiandrosterone sulfate levels and risk of atrial fibrillation: the Rotterdam Study. Eur J Prev Cardiol. 2012;epub ahead of print.
Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2(2):e000102.
Vasan RS, Glazer NL, Felix JF, Lieb W, Wild PS, Felix SB, et al. Genetic variants associated with cardiac structure and function: a meta-analysis and replication of genome-wide association data. JAMA. 2009;302(2):168–78.
Pfeufer A, van Noord C, Marciante KD, Arking DE, Larson MG, Smith AV, et al. Genome-wide association study of PR interval. Nat Genet. 2010;42(2):153–9.
den Hoed M, Eijgelsheim M, Esko T, Brundel BJ, Peal DS, Evans DM, et al. Identification of heart rate-associated loci and their effects on cardiac conduction and rhythm disorders. Nat Genet. 2013;45(6):621–31.
Benjamin EJ, Rice KM, Arking DE, Pfeufer A, van Noord C, Smith AV, et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet. 2009;41(8):879–81.
Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44(6):670–5.
Hak AE, Pols HAP, Visser TJ, Drexhage HA, Hofman A, Witteman JCM. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med. 2000;132(4):270–8.
Rutten JH, Mattace-Raso FU, Steyerberg EW, Lindemans J, Hofman A, Wieberdink RG, et al. Amino-terminal pro-B-type natriuretic peptide improves cardiovascular and cerebrovascular risk prediction in the population: the Rotterdam study. Hypertension. 2010;55(3):785–91.
Kavousi M, Elias-Smale SE, Rutte JWH, Leening MJG, Vliegenthart R, Verwoert GC, et al. Evaluation of newer risk markers for coronary heart disease risk classification: a cohort study. Ann Intern Med. 2012;156(6):438–44.
Kardys I, de Maat MP, Uitterlinden AG, Hofman A, Witteman JC. C-reactive protein gene haplotypes and risk of coronary heart disease: the Rotterdam Study. Eur Heart J. 2006;27(11):1331–7.
Oei HH, van der Meer IM, Hofman A, Koudstaal PJ, Stijnen T, Breteler MMB, et al. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation. 2005;111(5):570–5.
van der Bom JG, de Knijff P, Haverkate F, Bots ML, Meijer P, de Jong PT, et al. Tissue plasminogen activator and risk of myocardial infarction. The Rotterdam Study. Circulation. 1997;95(12):2623–7.
Koller MT, Leening MJG, Wolbers M, Steyerberg EW, Hunink MGM, Schoop R, et al. Development and validation of a coronary risk prediction model for older US and European persons in the Cardiovascular Health Study and the Rotterdam Study. Ann Intern Med. 2012;157(6):389–97.
Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96(5):1432–7.
Mattace-Raso FUS, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113(5):657–63.
Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112(4):572–7.
van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation. 2004;109(9):1089–94.
Elias-Smale SE, Proenca RV, Koller MT, Kavousi M, van Rooij FJ, Hunink MG, et al. Coronary calcium score improves classification of coronary heart disease risk in the elderly: the Rotterdam study. J Am Coll Cardiol. 2010;56(17):1407–14.
Elias-Smale SE, Wieberdink RG, Odink AE, Hofman A, Hunink MGM, Koudstaal PJ, et al. Burden of atherosclerosis improves the prediction of coronary heart disease but not cerebrovascular events: the Rotterdam Study. Eur Heart J. 2011;32(16):2050–8.
Leening MJG, Elias-Smale SE, Kavousi M, Felix JF, Deckers JW, Vliegenthart R, et al. Coronary calcification and the risk of heart failure in the elderly: the Rotterdam Study. J Am Coll Cardiol Img. 2012;5(9):874–80.
Kardys I, Klaver CC, Despriet DD, Bergen AA, Uitterlinden AG, Hofman A, et al. A common polymorphism in the complement factor H gene is associated with increased risk of myocardial infarction: the Rotterdam Study. J Am Coll Cardiol. 2006;47(8):1568–75.
Psaty BM, O’Donnell CJ, Gudnason V, Lunetta KL, Folsom AR, Rotter JI, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet. 2009;2(1):73–80.
Psaty BM, Hofman A. Genome-wide association studies and large-scale collaborations in epidemiology. Eur J Epidemiol. 2010;25(8):525–9.
Dehghan A, Kottgen A, Yang Q, Hwang SJ, Kao WL, Rivadeneira F, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372(9654):1953–61.
Kottgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet. 2009;41(6):712–7.
Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41(6):677–87.
Wain LV, Verwoert GC, O’Reilly PF, Shi G, Johnson T, Johnson AD, et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43(10):1005–11.
Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, et al. International Consortium for Blood Pressure Genome-Wide Association S. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 20116;478(7367):103–9.
Ganesh SK, Zakai NA, van Rooij FJ, Soranzo N, Smith AV, Nalls MA, et al. Multiple loci influence erythrocyte phenotypes in the CHARGE Consortium. Nat Genet. 2009;41(11):1191–8.
Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C, et al. Meta-analysis of genome-wide association studies in > 80,000 subjects identifies multiple loci for C-reactive protein levels. Circulation. 2011;123(7):731–8.
Smith NL, Chen MH, Dehghan A, Strachan DP, Basu S, Soranzo N, et al. Novel associations of multiple genetic loci with plasma levels of factor VII, factor VIII, and von Willebrand factor: the CHARGE (Cohorts for Heart and Aging Research in Genome Epidemiology) Consortium. Circulation. 2010;121(12):1382–92.
Bis JC, Kavousi M, Franceschini N, Isaacs A, Abecasis GR, Schminke U, et al. Meta-analysis of genome-wide association studies from the CHARGE consortium identifies common variants associated with carotid intima media thickness and plaque. Nat Genet. 2011;43(10):940–7.
O’Donnell CJ, Kavousi M, Smith AV, Kardia SL, Feitosa MF, Hwang SJ, et al. Genome-wide association study for coronary artery calcification with follow-up in myocardial infarction. Circulation. 2011;124(25):2855–64.
Murabito JM, White CC, Kavousi M, Sun YV, Feitosa MF, Nambi V, et al. Association between chromosome 9p21 variants and the ankle-brachial index identified by a meta-analysis of 21 genome-wide association studies. Circ Cardiovasc Genet. 2012;5(1):100–12.
Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43(4):333–8.
Consortium CAD, Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45(1):25–33.
Heine-Bröring RC, Brouwer IA, Proença RV, van Rooij FJA, Hofman A, Oudkerk M, et al. Intake of fish and marine n-3 fatty acids in relation to coronary calcification: the Rotterdam Study. Am J Clin Nutr. 2010;91(5):1317–23.
van Woudenbergh GJ, Kuijsten A, Tigcheler B, Sijbrands EJ, van Rooij FJA, Hofman A, et al. Meat consumption and its association with C-reactive protein and incident type 2 diabetes: the Rotterdam Study. Diabetes Care. 2012;35(7):1499–505.
Hofman A, van ‘t Veer P, et al. Dietary amino acids and the risk of hypertension in a Dutch older population: the Rotterdam Study. Am J Clin Nutr. 2013;97(2):403–10.
Engberink MF, Bakker SJ, Brink EJ, van Baak MA, van Rooij FJA, Hofman A, et al. Dietary acid load and risk of hypertension: the Rotterdam Study. Am J Clin Nutr. 2012;95(6):1438–44.
Altorf-van der Kuil W, Engberink MF, van Rooij FJA, Hofman A, van ‘t Veer P, Witteman JCM, et al. Dietary protein and risk of hypertension in a Dutch older population: the Rotterdam study. J Hypertens. 2010;28(12):2394–400.
Zillikens MC, van Meurs JB, Rivadeneira F, Hofman A, Oostra BA, Sijbrands EJ, et al. Interactions between dietary vitamin E intake and SIRT1 genetic variation influence body mass index. Am J Clin Nutr. 2010;91(5):1387–93.
Hruby A, Ngwa JS, Renström F, Wojczynski MK, Ganna A, Hallmans G, et al. Higher magnesium intake is associated with lower fasting glucose and insulin, with no evidence of interaction with select genetic loci, in a meta-analysis of 15 CHARGE Consortium Studies. J Nutr. 2013;143(3):345–53.
Nettleton JA, Hivert MF, Lemaitre RN, McKeown NM, Mozaffarian D, Tanaka T, et al. Meta-analysis investigating associations between healthy diet and fasting glucose and insulin levels and modification by loci associated with glucose homeostasis in data from 15 cohorts. Am J Epidemiol. 2013;177(2):103–15.
Kanoni S, Nettleton JA, Hivert MF, Ye Z, van Rooij FJA, Shungin D, et al. Total zinc intake may modify the glucose-raising effect of a zinc transporter (SLC30A8) variant: a 14-cohort meta-analysis. Diabetes. 2011;60(9):2407–16.
Dehghan A, Kardys I, de Maat MP, Uitterlinden AG, Sijbrands EJ, Bootsma AH, et al. Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes. 2007;56(3):872–8.
Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47(2):362–7.
Kardys I, Deckers JW, Stricker BH, Vletter WB, Hofman A, Witteman J. Distribution of echocardiographic parameters and their associations with cardiovascular risk factors in the Rotterdam Study. Eur J Epidemiol. 2010;25(7):481–90.
Klipstein-Grobusch K, Den Breeijen JH, Goldbohm RA, Geleijnse JM, Hofman A, Grobbee DE, et al. Dietary assessment in the elderly: validation of a semiquantitative food frequency questionnaire. Eur J Clin Nutr. 1998;52(8):588–96.
Goldbohm RA, Van den Brandt PA, Brants HA, Van’t Veer P, Al M, Sturmans F, et al. Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur J Clin Nutr. 1994;48(4):253–65.
Goldbohm RA, Van’t Veer P, Van den Brandt PA, Van’t Hof MA, Brants HA, Sturmans F. Reproducibility of a food frequency questionnaire and stability of dietary habits determined from five annually repeated measurements. Eur J Clin Nutr. 1995;49(6):420–9.
Feunekes GI, Staveren WAV, Vries JHD, Burema J, Hautvast JG. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am J Clin Nutr. 1993;58(4):489–96.
Siebelink E, Geelen A, De Vries JHM. Self-reported energy intake by FFQ compared with actual energy intake to maintain body weight in 516 adults. Br J Nutr. 2011;106(02):274–81.
Caspersen CJ, Bloemberg BP, Saris WH, Merritt RK, Kromhout D. The prevalence of selected physical activities and their relation with coronary heart disease risk factors in elderly men: the Zutphen Study, 1985. Am J Epidemiol. 1991;133(11):1078–92.
Stel VS, Smit JH, Pluijm SM, Visser M, Deeg DJ, Lips P. Comparison of the LASA Physical Activity Questionnaire with a 7-day diary and pedometer. J Clin Epidemiol. 2004;57(3):252–8.
Voorrips LE, Ravelli AC, Dongelmans PC, Deurenberg P, Van Staveren WA. A physical activity questionnaire for the elderly. Med Sci Sports Exerc. 1991;23(8):974–9.
Engeland A, Bjorge T, Daltveit AK, Skurtveit S, Vangen S, Vollset SE, et al. Risk of diabetes after gestational diabetes and preeclampsia. A registry-based study of 230,000 women in Norway. Eur J Epidemiol. 2011;26(2):157–63.
Joseph J, Svartberg J, Njolstad I, Schirmer H. Risk factors for type 2 diabetes in groups stratified according to metabolic syndrome: a 10-year follow-up of The Tromso Study. Eur J Epidemiol. 2011;26(2):117–24.
Faramawi MF, Caffrey JL, Amanzadeh J, Sharpa LD, Qualls-Hampton R. Cystatin C estimated renal dysfunction predicts T wave axis deviation in US adults: results from NHANES III. Eur J Epidemiol. 2011;26(2):101–7.
Autenrieth CS, Baumert J, Baumeister SE, Fischer B, Peters A, Doring A, et al. Association between domains of physical activity and all-cause, cardiovascular and cancer mortality. Eur J Epidemiol. 2011;26(2):91–9.
Behrens G, Leitzmann MF, Sandin S, Lof M, Heid IM, Adami HO, et al. The association between alcohol consumption and mortality: the Swedish women’s lifestyle and health study. Eur J Epidemiol. 2011;26(2):81–90.
Montonen J, Drogan D, Joost HG, Boeing H, Fritsche A, Schleicher E, et al. Estimation of the contribution of biomarkers of different metabolic pathways to risk of type 2 diabetes. Eur J Epidemiol. 2011;26(1):29–38.
Honjo K, Iso H, Inoue M, Tsugane S. Adult height and the risk of cardiovascular disease among middle aged men and women in Japan. Eur J Epidemiol. 2011;26(1):13–21.
Niedhammer I, Bourgkard E, Chau N, Lorhandicap Study G. Occupational and behavioural factors in the explanation of social inequalities in premature and total mortality: a 12.5-year follow-up in the Lorhandicap study. Eur J Epidemiol. 2011;26(1):1–12.
Liu JH, Blair SN, Teng YP, Ness AR, Lawlor DA, Riddoch C. Physical activity during pregnancy in a prospective cohort of British women: results from the Avon longitudinal study of parents and children. Eur J Epidemiol. 2011;26(3):237–47.
Tamakoshi A, Lin YS, Kawado M, Yagyu K, Kikuchi S, Iso H. Effect of coffee consumption on all-cause and total cancer mortality: findings from the JACC study. Eur J Epidemiol. 2011;26(4):285–93.
Borne Y, Engstrom G, Essen B, Sundquist J, Hedblad B. Country of birth and risk of hospitalization due to heart failure: a Swedish population-based cohort study. Eur J Epidemiol. 2011;26(4):275–83.
Grundtvig M, Hagen TP, Amrud ES, Reikvam A. Mortality after myocardial infarction: impact of gender and smoking status. Eur J Epidemiol. 2011;26(5):385–93.
Voko Z, de Brouwer S, Lubsen J, Danchin N, Otterstad JE, Dunselman P, et al. Long-term impact of secondary preventive treatments in patients with stable angina. Eur J Epidemiol. 2011;26(5):375–83.
Peeters A, Nusselder WJ, Stevenson C, Boyko EJ, Moon L, Tonkin A. Age-specific trends in cardiovascular mortality rates in the Netherlands between 1980 and 2009. Eur J Epidemiol. 2011;26(5):369–73.
Berard E, Bongard V, Arveiler D, Amouyel P, Wagner A, Dallongeville J, et al. Ten-year risk of all-cause mortality: assessment of a risk prediction algorithm in a French general population. Eur J Epidemiol. 2011;26(5):359–68.
Morkedal B, Romundstad PR, Vatten LJ. Informativeness of indices of blood pressure, obesity and serum lipids in relation to ischaemic heart disease mortality: the HUNT-II study. Eur J Epidemiol. 2011;26(6):457–61.
Eryd SA, Smith JG, Melander O, Hedblad B, Engstrom G. Inflammation-sensitive proteins and risk of atrial fibrillation: a population-based cohort study. Eur J Epidemiol. 2011;26(6):449–55.
Hansen JL, Tolstrup JS, Jensen MK, Gronbaek M, Tjonneland A, Schmidt EB, et al. Alcohol intake and risk of acute coronary syndrome and mortality in men and women with and without hypertension. Eur J Epidemiol. 2011;26(6):439–47.
Fedorowski A, Hedblad B, Melander O. Early postural blood pressure response and cause-specific mortality among middle-aged adults. Eur J Epidemiol. 2011;26(7):537–46.
Faeh D, Braun J, Tarnutzer S, Bopp M. Obesity but not overweight is associated with increased mortality risk. Eur J Epidemiol. 2011;26(8):647–55.
Kowall B, Rathmann W, Heier M, Giani G, Peters A, Thorand B, et al. Categories of glucose tolerance and continuous glycemic measures and mortality. Eur J Epidemiol. 2011;26(8):637–45.
Nicolotti N, Chuang SC, Cadoni G, Arzani D, Petrelli L, Bosetti C, et al. Recreational physical activity and risk of head and neck cancer: a pooled analysis within the international head and neck cancer epidemiology (INHANCE) Consortium. Eur J Epidemiol. 2011;26(8):619–28.
Sonestedt E, Wirfalt E, Wallstrom P, Gullberg B, Orho-Melander M, Hedblad B. Dairy products and its association with incidence of cardiovascular disease: the Malmo diet and cancer cohort. Eur J Epidemiol. 2011;26(8):609–18.
Jacobsen BK, Knutsen SF, Oda K, Fraser GE. Parity and total, ischemic heart disease and stroke mortality. The Adventist Health Study, 1976–1988. Eur J Epidemiol. 2011;26(9):711–8.
Lagro J, Claassen J, Rikkert M. Early postural blood pressure response and cause-specific mortality among middle-aged adults: what is the role of diastolic blood pressure? Eur J Epidemiol. 2011;26(11):893–4.
Empana JP, Bean K, Guibout C, Thomas F, Bingham A, Pannier B, et al. Paris Prospective Study III: a study of novel heart rate parameters, baroreflex sensitivity and risk of sudden death. Eur J Epidemiol. 2011;26(11):887–92.
Bernstein AM, Rosner BA, Willett WC. Cereal fiber and coronary heart disease: a comparison of modeling approaches for repeated dietary measurements, intermediate outcomes, and long follow-up. Eur J Epidemiol. 2011;26(11):877–86.
Lehto HR, Lehto S, Havulinna AS, Ketonen M, Lehtonen A, Kesaniemi YA, et al. Sex differences in short- and long-term case-fatality of myocardial infarction. Eur J Epidemiol. 2011;26(11):851–61.
Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Wine, beer or spirit drinking in relation to fatal and non-fatal cardiovascular events: a meta-analysis. Eur J Epidemiol. 2011;26(11):833–50.
Thelle DS. Case fatality of acute myocardial infarction: an emerging gender gap. Eur J Epidemiol. 2011;26(11):829–31.
Marsh RW. Predicting cardiovascular events using three stage Discriminant Function is much more accurate than Framingham or QRISK. Eur J Epidemiol. 2011;26(12):915–8.
Abbasi A, Corpeleijn E, Peelen LM, Gansevoort RT, de Jong PE, Gans ROB, et al. External validation of the KORA S4/F4 prediction models for the risk of developing type 2 diabetes in older adults: the PREVEND study. Eur J Epidemiol. 2012;27(1):47–52.
Auger N, Harper S, Barry AD, Trempe N, Daniel M. Life expectancy gap between the Francophone majority and Anglophone minority of a Canadian population. Eur J Epidemiol. 2012;27(1):27–38.
Iversen LB, Strandberg-Larsen K, Prescott E, Schnohr P, Rod NH. Psychosocial risk factors, weight changes and risk of obesity: the Copenhagen City Heart Study. Eur J Epidemiol. 2012;27(2):119–30.
Vandenheede H, Deboosere P, Stirbu I, Agyemang CO, Harding S, Juel K, et al. Migrant mortality from diabetes mellitus across Europe: the importance of socio-economic change. Eur J Epidemiol. 2012;27(2):109–17.
Petersen CB, Gronbaek M, Helge JW, Thygesen LC, Schnohr P, Tolstrup JS. Changes in physical activity in leisure time and the risk of myocardial infarction, ischemic heart disease, and all-cause mortality. Eur J Epidemiol. 2012;27(2):91–9.
Kuhntopf S, Tivig T. Early retirement and mortality in Germany. Eur J Epidemiol. 2012;27(2):85–9.
Claessen H, Brenner H, Drath C, Arndt V. Repeated measures of body mass index and risk of health related outcomes. Eur J Epidemiol. 2012;27(3):215–24.
Juutilainen A, Kastarinen H, Antikainen R, Peltonen M, Salomaa V, Tuomilehto J, et al. Trends in estimated kidney function: the FINRISK surveys. Eur J Epidemiol. 2012;27(4):305–13.
Demakakos P, Marmot M, Steptoe A. Socioeconomic position and the incidence of type 2 diabetes: the ELSA study. Eur J Epidemiol. 2012;27(5):367–78.
Kramer HU, Muller H, Stegmaier C, Rothenbacher D, Raum E, Brenner H. Type 2 diabetes mellitus and gender-specific risk for colorectal neoplasia. Eur J Epidemiol. 2012;27(5):341–7.
Cook NR, Cole SR, Buring JE. Aspirin in the primary prevention of cardiovascular disease in the Women’s Health Study: effect of noncompliance. Eur J Epidemiol. 2012;27(6):431–8.
Park SY, Wilkens LR, Murphy SP, Monroe KR, Henderson BE, Kolonel LN. Body mass index and mortality in an ethnically diverse population: the Multiethnic Cohort Study. Eur J Epidemiol. 2012;27(7):489–97.
Galli L, Salpietro S, Pellicciotta G, Galliani A, Piatti P, Hasson H, et al. Risk of type 2 diabetes among HIV-infected and healthy subjects in Italy. Eur J Epidemiol. 2012;27(8):657–65.
van Oeffelen AAM, Agyemang C, Bots ML, Stronks K, Koopman C, van Rossem L, et al. The relation between socioeconomic status and short-term mortality after acute myocardial infarction persists in the elderly: results from a nationwide study. Eur J Epidemiol. 2012;27(8):605–13.
Lissner L, Lanfer A, Gwozdz W, Olafsdottir S, Eiben G, Moreno LA, et al. Television habits in relation to overweight, diet and taste preferences in European children: the IDEFICS study. Eur J Epidemiol. 2012;27(9):705–15.
Brinks R, Tamayo T, Kowall B, Rathmann W. Prevalence of type 2 diabetes in Germany in 2040: estimates from an epidemiological model. Eur J Epidemiol. 2012;27(10):791–7.
Hansson J, Galanti MR, Hergens MP, Fredlund P, Ahlbom A, Alfredsson L, et al. Use of snus and acute myocardial infarction: pooled analysis of eight prospective observational studies. Eur J Epidemiol. 2012;27(10):771–9.
Grau M, Sala C, Sala J, Masia R, Vila J, Subirana I, et al. Sex-related differences in prognosis after myocardial infarction: changes from 1978 to 2007. Eur J Epidemiol. 2012;27(11):847–55.
Kiefte-de Jong JC, Chowdhury R, Franco OH. Fish intake or omega-3 fatty acids: greater than the sum of all parts? Eur J Epidemiol. 2012;27(12):891–4.
Flohil SC, van der Leest RJ, Dowlatshahi EA, Hofman A, de Vries E, Nijsten T. Prevalence of actinic keratosis and its risk factors in the general population: the Rotterdam Study. J Invest Dermatol. 2013;133(8):1971–8.
Kiiski V, de Vries E, Flohil SC, Bijl MJ, Hofman A, Stricker BH, et al. Risk factors for single and multiple basal cell carcinomas. Arch Dermatol. 2010;146(8):848–55.
Jacobs LC, Wollstein A, Lao O, Hofman A, Klaver CC, Uitterlinden AG, et al. Comprehensive candidate gene study highlights UGT1A and BNC2as new genes determining continuous skin color variation in Europeans. Hum Genet. 2013;132(2):147–58.
Dowlatshahi EA, Kavousi M, Nijsten T, Arfan Ikram M, Hofman A, Franco OH, et al. Psoriasis is not associated with atherosclerosis and incident cardiovascular events: the Rotterdam Study. J Invest Dermatol. 2013;133(10):2347–54.
Oei L, Rivadeneira F, Ly F, Breda SJ, Zillikens MC, Hofman A, et al. Review of radiological scoring methods of osteoporotic vertebral fractures for clinical and research settings. Eur Radiol. 2013;23(2):476–86.
Makurthou AA, Oei L, Saddy SE, Breda SJ, Castaño-Betancourt MC, Hofman A, et al. Scheuermann’s disease: evaluation of radiological criteria and population prevalence. Spine (Phila Pa 1976). 2013.
Oei L, Zillikens MC, Dehghan A, Buitendijk GH, Castaño-Betancourt MC, Estrada K, et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam Study. Diabetes Care. 2013;36(6):1619–28.
Castaño-Betancourt MC, Rivadeneira F, Bierma-Zeinstra S, Kerkhof HJ, Hofman A, Uitterlinden AG, et al. Bone parameters across different types of hip osteoarthritis and their relationship to osteoporotic fracture risk. Arthr Rheum. 2013;65(3):693–700.
Castaño-Betancourt MC, Van Meurs JB, Bierma-Zeinstra S, Rivadeneira F, Hofman A, Weinans H, et al. The contribution of hip geometry to the prediction of hip osteoarthritis. Osteoarthr Cartil. 2013b.
van der Loos MJ, Haring R, Rietveld CA, Baumeister SE, Groenen PJ, Hofman A, et al. Serum testosterone levels in males are not associated with entrepreneurial behavior in two independent observational studies. Physiol Behav.
Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44(5):491–501.
arcOGEN Consortium; arcOGEN Collaborators, Zeggini E, Loughlin J. Identification of new susceptibility loci for osteoarthritis (arcOGEN): a genome-wide association study. Lancet. 2012;380(9844):815–23.
Castaño Betancourt MC, Cailotto F, Kerkhof HJ, Cornelis FM, Doherty SA, Hart DJ, et al. Genome-wide association and functional studies identify the DOT1L gene to be involved in cartilage thickness and hip osteoarthritis. Proc Natl Acad Sci USA. 2012;109(21):8218–23.
Peters MJ, Broer L, Willemen HL, Eiriksdottir G, Hocking LJ, Holliday KL, et al. Genome-wide association study meta-analysis of chronic widespread pain: evidence for involvement of the 5p15.2 region. Ann Rheum Dis. 2013;72(3):427–36.
Stolk L, Perry JR, Chasman DI, He C, Mangino M, Sulem P, et al. Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat Genet. 2012;44(3):260–8.
Porcu E, Medici M, Pistis G, Volpato CB, Wilson SG, Cappola AR, et al. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet. 2013;9(2):e1003266.
Mayerle J, den Hoed CM, Schurmann C, Stolk L, Homuth G, Peters MJ, et al. Identification of genetic loci associated with Helicobacter pylori serologic status. JAMA. 2013;309(18):1912–20.
Lagiou P, Samoli E, Lipworth L, Lagiou A, Fang F, Rossi M, et al. Energy intake during pregnancy in relation to offspring gender by maternal height. Eur J Epidemiol. 2011;26(1):39–44.
Parker LA, Porta M, Lumbreras B, Lopez T, Guarner L, Hernandez-Aguado I, et al. Clinical validity of detecting K-ras mutations for the diagnosis of exocrine pancreatic cancer: a prospective study in a clinically-relevant spectrum of patients. Eur J Epidemiol. 2011;26(3):229–36.
Jorgensen L, Hansen JB, Brox J, Mathiesen E, Vik A, Jacobsen BK. Serum osteoprotegerin levels are related to height loss: the Tromso Study. Eur J Epidemiol. 2011;26(4):305–12.
Pearce MS, McNally RJQ, Day J, Korada SM, Turner S, Cheetham TD. Space-time clustering of elevated thyroid stimulating hormone levels. Eur J Epidemiol. 2011;26(5):405–11.
Golden SH, Wand GS, Malhotra S, Kamel I, Horton K. Reliability of hypothalamic-pituitary-adrenal axis assessment methods for use in population-based studies. Eur J Epidemiol. 2011;26(7):511–25.
Cerqueira C, Knudsen N, Ovesen L, Laurberg P, Perrild H, Rasmussen LB, et al. Doubling in the use of thyroid hormone replacement therapy in Denmark: association to iodization of salt. Eur J Epidemiol. 2011;26(8):629–35.
Feart C, Peres K, Samieri C, Letenneur L, Dartigues JF, Barberger-Gateau P. Adherence to a Mediterranean diet and onset of disability in older persons. Eur J Epidemiol. 2011;26(9):747–56.
Moebus S, Gores L, Losch C, Jockel KH. Impact of time since last caloric intake on blood glucose levels. Eur J Epidemiol. 2011;26(9):719–28.
Schottker B, Raum E, Rothenbacher D, Muller H, Brenner H. Prognostic value of haemoglobin A1c and fasting plasma glucose for incident diabetes and implications for screening. Eur J Epidemiol. 2011;26(10):779–87.
Tamakoshi K, Yatsuya H, Tamakoshi A, Grp JS. Early age at menarche associated with increased all-cause mortality. Eur J Epidemiol. 2011;26(10):771–8.
Jiang Y, Ben QW, Shen H, Lu WQ, Zhang Y, Zhu J. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2011;26(11):863–76.
Tamayo T, Jacobs DR, Strassburger K, Giani G, Seeman TE, Matthews K, et al. Race- and sex-specific associations of parental education with insulin resistance in middle-aged participants: the CARDIA study. Eur J Epidemiol. 2012;27(5):349–55.
Ma LL, Oei L, Jiang LD, Estrada K, Chen HY, Wang Z, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27(5):319–32.
Morseth B, Ahmed LA, Bjornerem A, Emaus N, Jacobsen BK, Joakimsen R, et al. Leisure time physical activity and risk of non-vertebral fracture in men and women aged 55 years and older: the Tromso Study. Eur J Epidemiol. 2012;27(6):463–71.
Nuesch E, Dale CE, Amuzu A, Kuper H, Bowling A, Ploubidis GB, et al. Inflammation, coagulation and risk of locomotor disability in elderly women: findings from the British Women’s Heart and Health Study. Eur J Epidemiol. 2012;27(8):633–45.
McNally RJQ, Blakey K, James PW, Pozo BG, Basta NO, Hale J. Increasing incidence of thyroid cancer in Great Britain, 1976–2005: age-period-cohort analysis. Eur J Epidemiol. 2012;27(8):615–22.
Bell GA, Kantor ED, Lampe JW, Shen DD, White E. Use of glucosamine and chondroitin in relation to mortality. Eur J Epidemiol. 2012;27(8):593–603.
Omsland TK, Holvik K, Meyer HE, Center JR, Emaus N, Tell GS, et al. Hip fractures in Norway 1999–2008: time trends in total incidence and second hip fracture rates. A NOREPOS study. Eur J Epidemiol. 2012;27(10):807–14.
Lagerros YT, Cnattingius S, Granath F, Hanson U, Wikstrom AK. From infancy to pregnancy: birth weight, body mass index, and the risk of gestational diabetes. Eur J Epidemiol. 2012;27(10):799–805.
Jacobsen BK, Knutsen SF, Oda K, Fraser GE. Obesity at age 20 and the risk of miscarriages, irregular periods and reported problems of becoming pregnant: the Adventist Health Study-2. Eur J Epidemiol. 2012;27(12):923–31.
Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102(12):2708–15.
Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29(12):1705–13.
Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5(10):1214–20.
Amiri M, Janssen F, Kunst AE. The decline in stomach cancer mortality: exploration of future trends in seven European countries. Eur J Epidemiol. 2011;26(1):23–8.
Coleman HG, Bhat S, Murray LJ, McManus D, Gavin AT, Johnston BT. Increasing incidence of Barrett’s oesophagus: a population-based study. Eur J Epidemiol. 2011;26(9):739–45.
Gao S, Yang WS, Bray F, Va P, Zhang W, Gao J, et al. Declining rates of hepatocellular carcinoma in urban Shanghai: incidence trends in 1976–2005. Eur J Epidemiol. 2012;27(1):39–46.
Marron M, Boffetta P, Moller H, Ahrens W, Pohlabeln H, Benhamou S, et al. Risk of upper aerodigestive tract cancer and type of alcoholic beverage: a European multicenter case-control study. Eur J Epidemiol. 2012;27(7):499–517.
Ott A, Breteler MM, van Harskamp F, Claus JJ, van der Cammen TJ, Grobbee DE, Hofman A. Prevalence of Alzheimer’s disease and vascular dementia: association with education. The Rotterdam study. BMJ. 1995;310(6985):970–3.
de Rijk MC, Breteler MM, Graveland GA, Ott A, Grobbee DE, van der Meché FG, Hofman A. Prevalence of Parkinson’s disease in the elderly: the Rotterdam Study. Neurology. 1995;45(12):2143–6.
Ruitenberg A, Ott A, van Swieten JC, Hofman A, Breteler MM. Incidence of dementia: does gender make a difference? Neurobiol Aging. 2001;22(4):575–80.
Hollander M, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Breteler MM. Incidence, risk, and case fatality of first ever stroke in the elderly population. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2003;74(3):317–21.
de Lau LM, Giesbergen PC, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MM. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology. 2004;63(7):1240–4.
Schrijvers EM, Verhaaren BF, Koudstaal PJ, Hofman A, Ikram MA, Breteler MM. Is dementia incidence declining?: Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology. 2012;78(19):1456–63.
Wieberdink RG, Ikram MA, Hofman A, Koudstaal PJ, Breteler MM. Trends in stroke incidence rates and stroke risk factors in Rotterdam, the Netherlands from 1990 to 2008. Eur J Epidemiol. 2012;27(4):287–95.
Ikram MA, Wieberdink RG, Koudstaal PJ. International epidemiology of intracerebral hemorrhage. Curr Atheroscler Rep. 2012;14(4):300–6.
Breteler MM, Claus JJ, Grobbee DE, Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam Study. BMJ. 1994;308(6944):1604–8.
Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349(9046):151–4.
Ott A, Slooter AJ, Hofman A, van Harskamp F, Witteman JC, Van Broeckhoven C, et al. Smoking and risk of dementia and Alzheimer’s disease in a population-based cohort study: the Rotterdam Study. Lancet. 1998;351(9119):1840–3.
Ruitenberg A, van Swieten JC, Witteman JC, Mehta KM, van Duijn CM, Hofman A, et al. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet. 2002;359(9303):281–6.
de Bruijn RF, Schrijvers EM, de Groot KA, Witteman JC, Hofman A, Franco OH, et al. The association between physical activity and dementia in an elderly population: the Rotterdam Study. Eur J Epidemiol. 2013;28(3):277–83.
in t’ Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med. 2001;345(21):1515–21.
Euser SM, Sattar N, Witteman JC, Bollen EL, Sijbrands EJ, Hofman A, et al. A prospective analysis of elevated fasting glucose levels and cognitive function in older people: results from PROSPER and the Rotterdam Study. Diabetes. 2010;59(7):1601–7.
Euser SM, van Bemmel T, Schram MT, Gussekloo J, Hofman A, Westendorp RG, et al. The effect of age on the association between blood pressure and cognitive function later in life. J Am Geriatr Soc. 2009;57(7):1232–7.
Hollander M, Bots ML, Del Sol AI, Koudstaal PJ, Witteman JC, Grobbee DE, et al. Carotid plaques increase the risk of stroke and subtypes of cerebral infarction in asymptomatic elderly: the Rotterdam study. Circulation. 2002;105(24):2872–7.
Wieberdink RG, Poels MM, Vernooij MW, Koudstaal PJ, Hofman A, van der Lugt A, et al. Serum lipid levels and the risk of intracerebral hemorrhage: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 2011;31:2982–9.
Wieberdink RG, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM, Ikram MA. Insulin resistance and the risk of stroke and stroke subtypes in the nondiabetic elderly. Am J Epidemiol. 2012;176(8):699–707.
Bos MJ, Schipper CM, Koudstaal PJ, Witteman JC, Hofman A, Breteler MM. High serum C-reactive protein level is not an independent predictor for stroke: the Rotterdam Study. Circulation. 2006;114(15):1591–8.
Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–22.
Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821–8.
Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303(18):1832–40.
Ikram MA, Seshadri S, Bis JC, Fornage M, DeStefano AL, Aulchenko YS, et al. Genomewide association studies of stroke. N Engl J Med. 2009;360(17):1718–28.
Schrijvers EM, Schurmann B, Koudstaal PJ, van den Bussche H, Van Duijn CM, Hentschel F, et al. Genome-wide association study of vascular dementia. Stroke. 2012;43(2):315–9.
Verhaaren BF, Vernooij MW, Koudstaal PJ, Uitterlinden AG, van Duijn CM, Hofman A, et al. Alzheimer’s disease genes and cognition in the nondemented general population. Biol Psychiatry. 2013;73(5):429–34.
Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JH, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44(7):1246–52.
Ikram MA, van der Lugt A, Niessen WJ, Krestin GP, Koudstaal PJ, Hofman A, et al. The Rotterdam Scan Study: design and update up to 2012. Eur J Epidemiol. 2011;26(10):811–24.
Ikram MA, van Oijen M, de Jong FJ, Kors JA, Koudstaal PJ, Hofman A, et al. Unrecognized myocardial infarction in relation to risk of dementia and cerebral small vessel disease. Stroke. 2008;39(5):1421–6.
Ikram MA, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, Breteler MM. Kidney function is related to cerebral small vessel disease. Stroke. 2008;39(1):55–61.
Ikram MA, Vrooman HA, Vernooij MW, van der Lijn F, Hofman A, van der Lugt A, et al. Brain tissue volumes in the general elderly population. The Rotterdam Scan Study. Neurobiol Aging. 2008;29(6):882–90.
Ikram MK, de Jong FJ, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, et al. Retinal vascular calibers associate differentially with cerebral gray matter and white matter atrophy. Alzheimer Dis Assoc Disord. 2013.
Poels MM, Zaccai K, Verwoert GC, Vernooij MW, Hofman A, van der Lugt A, et al. Arterial stiffness and cerebral small vessel disease: the Rotterdam Scan Study. Stroke. 2012;43(10):2637–42.
Poels MM, Steyerberg EW, Wieberdink RG, Hofman A, Koudstaal PJ, Ikram MA, et al. Assessment of cerebral small vessel disease predicts individual stroke risk. J Neurol Neurosurg Psychiatry. 2012;83(12):1174–9.
Verhaaren BF, Vernooij MW, Dehghan A, Vrooman HA, de Boer R, Hofman A, et al. The relation of uric acid to brain atrophy and cognition: the Rotterdam Scan Study. Neuroepidemiology. 2013;41(1):29–34.
Saavedra Perez HC, Direk N, Hofman A, Vernooij MW, Tiemeier H, Ikram MA. Silent brain infarcts: a cause of depression in the elderly? Psychiatry Res. 2013;211(2):180–2.
Ikram MA, Vernooij MW, Vrooman HA, Hofman A, Breteler MM. Brain tissue volumes and small vessel disease in relation to the risk of mortality. Neurobiol Aging. 2009;30(3):450–6.
Ikram MA, Vrooman HA, Vernooij MW, den Heijer T, Hofman A, Niessen WJ, et al. Brain tissue volumes in relation to cognitive function and risk of dementia. Neurobiol Aging. 2010;31(3):378–86.
Ikram MA, Fornage M, Smith AV, Seshadri S, Schmidt R, Debette S, et al. Common variants at 6q22 and 17q21 are associated with intracranial volume. Nat Genet. 2012;44(5):539–44.
Bis JC, DeCarli C, Smith AV, van der Lijn F, Crivello F, Fornage M, et al. Common variants at 12q14 and 12q24 are associated with hippocampal volume. Nat Genet. 2012;44(5):545–51.
Stein JL, Medland SE, Vasquez AA, Hibar DP, Senstad RE, Winkler AM, et al. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet. 2012;44(5):552–61.
Verhaaren BF, de Boer R, Vernooij MW, Rivadeneira F, Uitterlinden AG, Hofman A, et al. Replication study of chr17q25 with cerebral white matter lesion volume. Stroke. 2011;42(11):3297–9.
Fornage M, Debette S, Bis JC, Schmidt H, Ikram MA, Dufouil C, et al. Genome-wide association studies of cerebral white matter lesion burden: the CHARGE consortium. Ann Neurol. 2011;69(6):928–39.
Debette S, Bis JC, Fornage M, Schmidt H, Ikram MA, Sigurdsson S, et al. Genome-wide association studies of MRI-defined brain infarcts: meta-analysis from the CHARGE Consortium. Stroke. 2010;41(2):210–7.
Ikram MA, DeCarli C. Next frontiers in the genetic epidemiology of Alzheimer’s disease. Eur J Epidemiol. 2012;27(11):831–6.
Verlinden VJ, van der Geest JN, Hoogendam YY, Hofman A, Breteler MM, Ikram MA. Gait patterns in a community-dwelling population aged 50 years and older. Gait Posture. 2013;37(4):500–5.
Bos MJ, van Rijn MJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Incidence and prognosis of transient neurological attacks. JAMA. 2007;298(24):2877–85.
Killgore WD, Glahn DC, Casasanto DJ. Development and validation of the design organization test (DOT): a rapid screening instrument for assessing visuospatial ability. J Clin Exp Neuropsychol. 2005;27(4):449–59.
Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145(2):205–11.
Gulsvik AK, Gulsvik A, Svendsen E, Maehle BO, Thelle DS, Wyller TB. Diagnostic validity of fatal cerebral strokes and coronary deaths in mortality statistics: an autopsy study. Eur J Epidemiol. 2011;26(3):221–8.
Bos MJ, Alberts VP, Koudstaal PJ, Breteler MMB. Increased risk of stroke after the diagnosis of heart failure: is it a paradox of initiating heart failure treatment? Reply. Eur J Epidemiol. 2011;26(5):430.
Yetkin E. Increased risk of stroke after the diagnosis of heart failure: is it a paradox of initiating heart failure treatment? Eur J Epidemiol. 2011;26(5):429–30.
Reuser M, Willekens FJ, Bonneux L. Higher education delays and shortens cognitive impairment. A multistate life table analysis of the US Health and Retirement Study. Eur J Epidemiol. 2011;26(5):395–403.
Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;26:S1–58.
Wang A, Costello S, Cockburn M, Zhang XB, Bronstein J, Ritz B. Parkinson’s disease risk from ambient exposure to pesticides. Eur J Epidemiol. 2011;26(7):547–55.
Elbaz A. In search of the causes of Parkinson’s disease, seasons 1 to 4. Eur J Epidemiol. 2011;26(7):505–9.
Gordon PH, Artaud F, Aouba A, Laurent F, Meininger V, Elbaz A. Changing mortality for motor neuron disease in France (1968–2007): an age-period-cohort analysis. Eur J Epidemiol. 2011;26(9):729–37.
Broer L, Koudstaal PJ, Amin N, Rivadeneira F, Uitterlinden AG, Hofman A, et al. Association of heat shock proteins with Parkinson’s disease. Eur J Epidemiol. 2011;26(12):933–5.
Creavin ST, Gallacher J, Pickering J, Fehily A, Fish M, Ebrahim S, et al. High caloric intake, poor cognition and dementia: the Caerphilly Prospective Study. Eur J Epidemiol. 2012;27(3):197–203.
Chowdhury R, Stevens S, Ward H, Chowdhury S, Sajjad A, Franco OH. Circulating vitamin D, calcium and risk of cerebrovascular disease: a systematic review and meta-analysis. Eur J Epidemiol. 2012;27(8):581–91.
Larsson SC, Orsini N, Wolk A. Long-chain omega-3 polyunsaturated fatty acids and risk of stroke: a meta-analysis. Eur J Epidemiol. 2012;27(12):895–901.
Consortium AG, Fritsche LG, Chen W, et al. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45(4):433–9.
Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98(8):1310–5.
Varma R, Paz SH, Azen SP, et al. The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology. 2004;111(6):1121–31.
Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology. 1995;102(10):1450–60.
van Koolwijk LM, Ramdas WD, Ikram MK, et al. Common genetic determinants of intraocular pressure and primary open-angle glaucoma. PLoS Genet. 2012;8(5):e1002611.
Ramdas WD, Wolfs RC, Kiefte-de Jong JC, et al. Nutrient intake and risk of open-angle glaucoma: the Rotterdam Study. Eur J Epidemiol. 2012;27(5):385–93.
Springelkamp H, Lee K, Ramdas WD, et al. Optimizing the information yield of 3-D OCT in glaucoma. Invest Ophthalmol Vis Sci. 2012;53(13):8162–71.
Solouki AM, Verhoeven VJ, van Duijn CM, et al. A genome-wide association study identifies a susceptibility locus for refractive errors and myopia at 15q14. Nat Genet. 2010;42(10):897–901.
Verhoeven VJ, Hysi PG, Wojciechowski R, et al. Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia. Nat Genet. 2013;45(3):314–8.
Claessen H, Genz J, Bertram B, Trautner C, Giani G, Zollner I, et al. Evidence for a considerable decrease in total and cause-specific incidences of blindness in Germany. Eur J Epidemiol. 2012;27(7):519–24.
Tiemeier H, van Dijck W, Hofman A, Witteman JCM, Stijnen T, Breteler MMB. Atherosclerosis and late life depression are related. The Rotterdam Study. Arch Gen Psychiatry. 2004;61:369–76.
Tiemeier H, van Tuijl HR, Hofman A, Kiliaan AJ, Breteler MMB. Plasma fatty acid composition and depression in the elderly are associated: the Rotterdam Study. Am J Clin Nutr. 2003;78:40–6.
Dekker MJ, Koper JW, van Aken MO, Pols HA, Hofman A, de Jong FH, Kirschbaum C, Witteman JC, Lamberts SW, Tiemeier H. Salivary cortisol is related to atherosclerosis of carotid arteries. J Clin Endocrinol Metab. 2008;93:3741–7.
Hek K, Demirkan A, Lahti J, Terracciano A, Teumer A, Cornelis MC, et al. A genome-wide association study of depressive symptoms. Biol Psychiatry. 2013;73:667–78.
Velders FP, Kuningas M, Kumari M, Dekker MJ, Uitterlinden AG, Kirschbaum C, et al. Genetics of cortisol secretion and depressive symptoms: a candidate gene and genome wide association approach. Psychoneuroendocrinology. 2011;36:1053–61.
Saavedra Perez HC, Direk N, Hofman A, Vernooij MW, Tiemeier H, Ikram MA. Silent brain infarcts: a cause of depression in the elderly? Psychiatry Res. 2013;211:180–2.
Krijthe BP, Walter S, Newson RS, Hofman A, Hunink MG, Tiemeier H. Is positive affect associated with survival? A population-based study of elderly persons. Am J Epidemiol. 2011;173:1298–307.
Luijendijk HJ, van den Berg JF, Dekker MJHJ, van Tuijl HR, Otte W, Smit F, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65:1394–401.
Wittchen HU, Lachner G, Wunderlich U, Pfister H. Test–retest reliability of the computerized DSM-IV version of the Munich-Composite International Diagnostic Interview (M-CIDI). Soc Psychiatry Psychiatr Epidemiol. 1998;33:568–78.
van den Berg JF, van Rooij FJ, Vos H, Tulen JH, Hofman A, Miedema HM, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;17:295–302.
Prigerson HG, Maciejewski PK, Reynolds CF 3rd, Bierhals AJ, Newsom JT, Fasiczka A, et al. Inventory of Complicated Grief: a scale to measure maladaptive symptoms of loss. Psychiatry Res. 1995;59:65–79.
Naarding P, Tiemeier H, Breteler MM, Schoevers RA, Jonker C, Koudstaal PJ, et al. Clinically defined vascular depression in the general population. Psychol Med. 2007;37:383–92.
Newson RS, Hek K, Luijendijk HJ, Hofman A, Witteman JC, Tiemeier H. Atherosclerosis and incident depression in late life. Arch Gen Psychiatry. 2010;67:1144–51.
Direk N, Koudstaal PJ, Hofman A, Ikram MA, Hoogendijk WJ, Tiemeier H. Cerebral hemodynamics and incident depression: the Rotterdam Study. Biol Psychiatry. 2012;72:318–23.
van den Berg JF, Knvistingh Neven A, Tulen JH, Hofman A, Witteman JC, Miedema HM, et al. Actigraphic sleep duration and fragmentation are related to obesity in the elderly: the Rotterdam Study. Int J Obes. 2008;32:1083–90.
van den Berg JF, Miedema HM, Tulen JH, Hofman A, Neven AK, Tiemeier H. Sex differences in subjective and actigraphic sleep measures: a population-based study of elderly persons. Sleep. 2009;32:1367–75.
Hek K, Tiemeier H, Newson R, Luijendijk HJ, Hofman A, Mulder CL. Anxiety disorders and comorbid depression in community dwelling older adults. Int J Methods Psychiat Res. 2011;20:157–68.
The Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behaviour. Nat Genet. 2010;42:441–7.
Omidvar M, Stolk L, Uitterlinden AG, Hofman A, Van Duijn CM, Tiemeier H. The effect of catechol-O-methyltransferase Met/Val functional polymorphism on smoking cessation: retrospective and prospective analyses in a cohort study. Pharmacogenet Genomics. 2009;19:45–51.
Newson R, Boelen PA, Hek K, Hofman A, Tiemeier H. The prevalence and characteristics of complicated grief in older adults. J Affect Disord. 2011;132:231–8.
Rietveld CA, Cesarini D, Benjamin DJ, Koellinger PD, De Neve JE, Tiemeier H, et al. Molecular genetics and subjective well-being. Proc Natl Acad Sci USA. 2013.
Walter S, Mackenbach J, Vokó Z, Lhachimi S, Ikram MA, Uitterlinden AG, et al. Genetic, physiological, and lifestyle predictors of mortality in the general population. Am J Public Health. 2012;102:e3–10.
Timmermans S, Bonsel GJ, Steegers-Theunissen RPM, Mackenbach JP, Steyerberg EW, Raat H, et al. Individual accumulation of heterogeneous risks explains perinatal inequalities within deprived neighbourhoods. Eur J Epidemiol. 2011;26(2):165–80.
Gudmundsson P, Andersson S, Gustafson D, Waern M, Ostling S, Hallstrom T, et al. Depression in Swedish women: relationship to factors at birth. Eur J Epidemiol. 2011;26(1):55–60.
Ferrante G, Baldissera S, Moghadam PF, Carrozzi G, Trinito MO, Salmaso S. Surveillance of perceptions, knowledge, attitudes and behaviors of the Italian adult population (18–69 years) during the 2009–2010 A/H1N1 influenza pandemic. Eur J Epidemiol. 2011;26(3):211–9.
Regidor E, Astasio P, Ortega P, Martinez D, Calle ME, de la Fuente L. Healthy and unhealthy migrant effect on the mortality of immigrants from wealthy countries residing in Spain. Eur J Epidemiol. 2011;26(4):265–73.
Cnattingius S, Svensson T, Granath F, Iliadou A. Maternal smoking during pregnancy and risks of suicidal acts in young offspring. Eur J Epidemiol. 2011;26(6):485–92.
Strand BH, Cooper R, Hardy R, Kuh D, Guralnik J. Lifelong socioeconomic position and physical performance in midlife: results from the British 1946 birth cohort. Eur J Epidemiol. 2011;26(6):475–83.
Rothman KJ, Stein Z, Susser M. Rebuilding bridges: what is the real role of social class in disease occurrence? Eur J Epidemiol. 2011;26(6):431–2.
Danziger PD, Silverwood R, Koupil I. Fetal growth, early life circumstances, and risk of suicide in late adulthood. Eur J Epidemiol. 2011;26(7):571–81.
Pasta L, Marchese G. North African refugees, fleeing the fighting, admitted to Sicilian hospitals from January 1st to June 10th, 2011. Eur J Epidemiol. 2011;26(8):687.
Johansen A, Holmen J, Stewart R, Bjerkeset O. Anxiety and depression symptoms in arterial hypertension: the influence of antihypertensive treatment. The HUNT study, Norway. Eur J Epidemiol. 2012;27(1):63–72.
Brattstrom O, Larsson E, Granath F, Riddez L, Bell M, Oldner A. Time dependent influence of host factors on outcome after trauma. Eur J Epidemiol. 2012;27(3):233–41.
Blom V, Bergstrom G, Hallsten L, Bodin L, Svedberg P. Genetic susceptibility to burnout in a Swedish twin cohort. Eur J Epidemiol. 2012;27(3):225–31.
Franco OH, Wong YL, Kandala NB, Ferrie JE, Dorn JM, Kivimaki M, et al. Cross-cultural comparison of correlates of quality of life and health status: the Whitehall II Study (UK) and the Western New York Health Study (US). Eur J Epidemiol. 2012;27(4):255–65.
Mitchell KR, Ploubidis GB, Datta J, Wellings K. The Natsal-SF: a validated measure of sexual function for use in community surveys. Eur J Epidemiol. 2012;27(6):409–18.
Da Silva MA, Singh-Manoux A, Brunner EJ, Kaffashian S, Shipley MJ, Kivimaki M, et al. Bidirectional association between physical activity and symptoms of anxiety and depression: the Whitehall II study. Eur J Epidemiol. 2012;27(7):537–46.
Smithers LG, Golley RK, Mittinty MN, Brazionis L, Northstone K, Emmett P, et al. Dietary patterns at 6, 15 and 24 months of age are associated with IQ at 8 years of age. Eur J Epidemiol. 2012;27(7):525–35.
Niederkrotenthaler T, Rasmussen F, Mittendorfer-Rutz E. Perinatal conditions and parental age at birth as risk markers for subsequent suicide attempt and suicide: a population based case-control study. Eur J Epidemiol. 2012;27(9):729–38.
Stickley A, Leinsalu M, Kunst AE, Bopp M, Strand BH, Martikainen P, et al. Socioeconomic inequalities in homicide mortality: a population-based comparative study of 12 European countries. Eur J Epidemiol. 2012;27(11):877–84.
Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65.
Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60.
Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368(9537):804–13.
Hanania NA, King MJ, Braman SS, Saltoun C, Wise RA, Enright P, et al. Asthma in the elderly: current understanding and future research needs—a report of a National Institute on Aging (NIA) workshop. J Allergy Clin Immunol. 2011;128(3 Suppl):S4–24.
van Durme YM, Verhamme KM, Stijnen T, van Rooij FJ, Van Pottelberge GR, Hofman A, et al. Prevalence, incidence, and lifetime risk for the development of COPD in the elderly: the Rotterdam study. Chest. 2009;135(2):368–77.
Lahousse L, Vernooij MW, Darweesh SK, Akoudad S, Loth DW, Joos GF, et al. Chronic obstructive pulmonary disease and cerebral microbleeds. The Rotterdam Study. Am J Respir Crit Care Med. 2013;188(7):783–8.
van Durme YM, Verhamme KM, Aarnoudse AJ, Van Pottelberge GR, Hofman A, Witteman JC, et al. C-reactive protein levels, haplotypes, and the risk of incident chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179(5):375–82.
Lahousse L, van den Bouwhuijsen QJ, Loth DW, Joos GF, Hofman A, Witteman JC, et al. Chronic obstructive pulmonary disease and lipid core carotid artery plaques in the elderly: the Rotterdam Study. Am J Respir Crit Care Med. 2013;187(1):58–64.
Hancock DB, Eijgelsheim M, Wilk JB, Gharib SA, Loehr LR, et al. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet. 2010;42:45–52. doi:10.1038/ng.500.
Artigas MS, Loth DW, Wain LV, Gharib SA, Obeidat M, et al. Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet. 2011;43(11):1082–91.
Brusselle GG. Matrix metalloproteinase 12, asthma, and COPD. N Engl J Med. 2009;361(27):2664–5.
Van Durme YM, Eijgelsheim M, Joos GF, Hofman A, Uitterlinden AG, Brusselle GG, et al. Hedgehog-interacting protein is a COPD susceptibility gene: the Rotterdam Study. Eur Respir J. 2010;36(1):89–95.
Wilk JB, Shrine NR, Loehr LR, Zhao JH, Manichaikul A, Lopez LM, Smith AV, Heckbert SR, Smolonska J, et al. Genome-wide association studies identify CHRNA5/3 and HTR4 in the development of airflow obstruction. Am J Respir Crit Care Med. 2012;186(7):622–32.
Hancock D, Artigas M, Gharib SA, Henry A, Manichaikul A, et al. Genome-wide joint meta-analysis of SNP and SNP-by-smoking interaction identifies novel loci for pulmonary function. Plos Genetics. 2012;8(12), e1003098.
Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–35.
Gimeno D, Delclos GL, Ferrie JE, De Vogli R, Elovainio M, Marmot MG, et al. Association of CRP and IL-6 with lung function in a middle-aged population initially free from self-reported respiratory problems: the Whitehall II study. Eur J Epidemiol. 2011;26(2):135–44.
Slachtova H, Gehring U, Hoek G, Tomaskova H, Luttmann-Gibson H, Moshammer H, et al. Parental education and lung function of children in the PATY study. Eur J Epidemiol. 2011;26(1):45–54.
Hertel S, Viehmann A, Moebus S, Mann K, Brocker-Preuss M, Mohlenkamp S, et al. Influence of short-term exposure to ultrafine and fine particles on systemic inflammation (vol 25, pg 581, 2010). Eur J Epidemiol. 2011;26(2):181–2.
Nicoll A, Sprenger M. Learning lessons from the 2009 pandemic: putting infections in their proper place. Eur J Epidemiol. 2011;26(3):191–4.
Beyerlein A, Ruckinger S, Toschke AM, Rosario AS, von Kries R. Is low birth weight in the causal pathway of the association between maternal smoking in pregnancy and higher BMI in the offspring? Eur J Epidemiol. 2011;26(5):413–20.
Bakker H, Jaddoe VWV. Cardiovascular and metabolic influences of fetal smoke exposure. Eur J Epidemiol. 2011;26(10):763–70.
Duijts L. Fetal and infant origins of asthma. Eur J Epidemiol. 2012;27(1):5–14.
Firoozi F, Lemiere C, Beauchesne MF, Perreault S, Forget A, Blais L. Impact of maternal asthma on perinatal outcomes: a two-stage sampling cohort study. Eur J Epidemiol. 2012;27(3):205–14.
Pekkanen J, Lampi J, Genuneit J, Hartikainen AL, Jarvelin MR. Analyzing atopic and non-atopic asthma. Eur J Epidemiol. 2012;27(4):281–6.
Gabriele C, Silva LM, Arends LR, Raat H, Moll HA, Hofman A, et al. Early respiratory morbidity in a multicultural birth cohort: the Generation R Study. Eur J Epidemiol. 2012;27(6):453–62.
Heikkinen SAM, Quansah R, Jaakkola JJK, Jaakkola MS. Effects of regular exercise on adult asthma. Eur J Epidemiol. 2012;27(6):397–407.
Redlberger-Fritz M, Aberle JH, Popow-Kraupp T, Kundi M. Attributable deaths due to influenza: a comparative study of seasonal and pandemic influenza. Eur J Epidemiol. 2012;27(7):567–75.
Patel S, Henderson J, Jeffreys M, Smith GD, Galobardes B. Associations between socioeconomic position and asthma: findings from a historical cohort. Eur J Epidemiol. 2012;27(8):623–31.
Madsen C, Rosland P, Hoff DA, Nystad W, Nafstad P, Naess OE. The short-term effect of 24-h average and peak air pollution on mortality in Oslo, Norway. Eur J Epidemiol. 2012;27(9):717–27.
Broms K, Norback D, Sundelin C, Eriksson M, Svardsudd K. A nationwide study of asthma incidence rate and its determinants in Swedish pre-school children. Eur J Epidemiol. 2012;27(9):695–703.
Vinceti M, Rothman KJ, Crespi CM, Sterni A, Cherubini A, Guerra L, et al. Leukemia risk in children exposed to benzene and PM10 from vehicular traffic: a case-control study in an Italian population. Eur J Epidemiol. 2012;27(10):781–90.
Figarska SM, Boezen HM, Vonk JM. Dyspnea severity, changes in dyspnea status and mortality in the general population: the Vlagtwedde/Vlaardingen study. Eur J Epidemiol. 2012;27(11):867–76.
Baughman P, Marott JL, Lange P, Martin CJ, Shankar A, Petsonk EL, et al. Combined effect of lung function level and decline increases morbidity and mortality risks. Eur J Epidemiol. 2012;27(12):933–43.
Psaty BM, O’Donnell CJ, Gudnason V, Lunetta KL, Folsom AR, Rotter JI, et al; CHARGE Consortium. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet. 2009;2(1):73–80.
Boomsma DI, Wijmenga C, Slagboom EP, Swertz MA, Karssen LC, Abdellaoui A. The genome of the Netherlands: design, and project goals. Eur J Hum Genet. 2013.
Altmaier E, Kastenmuller G, Romisch-Margl W, Thorand B, Weinberger KM, Illig T, et al. Questionnaire-based self-reported nutrition habits associate with serum metabolism as revealed by quantitative targeted metabolomics. Eur J Epidemiol. 2011;26(2):145–56.
Janssens A, Ioannidis JPA, Bedrosian S, Boffetta P, Dolan SM, Dowling N, et al. Strengthening the reporting of genetic risk prediction studies (GRIPS): explanation and elaboration. Eur J Epidemiol. 2011;26(4):313–37.
Kundu S, Aulchenko YS, van Duijn CM, Janssens A. PredictABEL: an R package for the assessment of risk prediction models. Eur J Epidemiol. 2011;26(4):261–4.
Janssens A, Ioannidis JPA, van Duijn CM, Little J, Khoury MJ, Grp G. Strengthening the reporting of genetic risk prediction studies: the GRIPS statement. Eur J Epidemiol. 2011;26(4):255–9.
Liu GF, Zhu HD, Dong YB, Podolsky RH, Treiber FA, Snieder H. Influence of common variants in FTO and near INSIG2 and MC4R on growth curves for adiposity in African- and European-American youth. Eur J Epidemiol. 2011;26(6):463–73.
Ding B, Kallberg H, Klareskog L, Padyukov L, Alfredsson L. GEIRA: gene-environment and gene–gene interaction research application. Eur J Epidemiol. 2011;26(7):557–61.
Pedersen GS, Mortensen LH, Andersen AMN. Ethnic variations in mortality in pre-school children in Denmark, 1973–2004. Eur J Epidemiol. 2011;26(7):527–36.
Gallo V, Egger M, McCormack V, Farmer PB, Ioannidis JPA, Kirsch-Volders M, et al. STrengthening the reporting of OBservational studies in Epidemiology-Molecular Epidemiology (STROBE-ME): an extension of the STROBE statement. Eur J Epidemiol. 2011;26(10):797–810.
Lin YL, Chen SY, Lai LC, Chen JH, Yang SY, Huang YL, et al. Genetic polymorphisms of clusterin gene are associated with a decreased risk of Alzheimer’s disease. Eur J Epidemiol. 2012;27(1):73–5.
Johnson PCD, Logue J, McConnachie A, Abu-Rmeileh NME, Hart C, Upton MN, et al. Intergenerational change and familial aggregation of body mass index. Eur J Epidemiol. 2012;27(1):53–61.
Gallo V, Egger M, McCormack V, Farmer PB, Ioannidis JPA, Kirsch-Volders M, et al. STrengthening the reporting of OBservational studies in Epidemiology-Molecular Epidemiology (STROBE-ME): an extension of the STROBE statement (vol 26, pg 797, 2011). Eur J Epidemiol. 2012;27(2):151.
Schelzke G, Kretzschmar HA, Zerr I. Clinical aspects of common genetic Creutzfeldt-Jakob disease. Eur J Epidemiol. 2012;27(2):147–9.
Costanza MC, Beer-Borst S, James RW, Gaspoz JM, Morabia A. Consistency between cross-sectional and longitudinal SNP: blood lipid associationss. Eur J Epidemiol. 2012;27(2):131–8.
Ken-Dror G, Humphries SE, Kumari M, Kivimaki M, Drenos F. A genetic instrument for Mendelian randomization of fibrinogen. Eur J Epidemiol. 2012;27(4):267–79.
Nan C, Guo BL, Warner C, Fowler T, Barrett T, Boomsma D, et al. Heritability of body mass index in pre-adolescence, young adulthood and late adulthood. Eur J Epidemiol. 2012;27(4):247–53.
Chen YC, Chen SY, Lai LC, Chen JH. Clusterin polymorphisms and Alzheimer’s disease Reply. Eur J Epidemiol. 2012;27(9):758.
Yu JT, Tan L. Clusterin polymorphisms and Alzheimer’s disease. Eur J Epidemiol. 2012;27(9):757.
Jansen L, Gondos A, Eberle A, Emrich K, Holleczek B, Katalinic A, et al. Cancer survival in Eastern and Western Germany after the fall of the iron curtain. Eur J Epidemiol. 2012;27(9):689–93.
Strandberg TE, Strandberg AY, Saijonmaa O, Tilvis RS, Pitkala KH, Fyhrquist F. Association between alcohol consumption in healthy midlife and telomere length in older men. The Helsinki Businessmen Study. Eur J Epidemiol. 2012;27(10):815–22.
Van Calster B, Vergouwe Y, Looman CWN, Van Belle V, Timmerman D, Steyerberg EW. Assessing the discriminative ability of risk models for more than two outcome categories. Eur J Epidemiol. 2012;27(10):761–70.
Panagopoulou P, Gogas H, Dessypris N, Maniadakis N, Fountzilas G, Petridou ET. Survival from breast cancer in relation to access to tertiary healthcare, body mass index, tumor characteristics and treatment: a Hellenic Cooperative Oncology Group (HeCOG) study. Eur J Epidemiol. 2012;27(11):857–66.
Spallek J, Arnold M, Razum O, Juel K, Rey G, Deboosere P, et al. Cancer mortality patterns among Turkish immigrants in four European countries and in Turkey. Eur J Epidemiol. 2012;27(12):915–21.
Ekberg S, Ploner A, de Faire U, Pedersen NL, Bennet AM. Familial effects on survival after myocardial infarction: a registry-based sib-pair study. Eur J Epidemiol. 2012;27(12):911–4.
Avery CL, Sitlani CM, Arking DE, Arnett DK, Bis JC, Boerwinkle E, et al. Drug-gene interactions and the search for missing heritability: a cross-sectional pharmacogenomics study of the QT interval. Pharmacogenomics J. 2013.
Teichert M, Eijgelsheim M, Rivadeneira F, Uitterlinden AG, van Schaik RH, Hofman A, et al. A genome-wide association study of acenocoumarol maintenance dosage. Hum Mol Genet. 2009;18:3758–68.
Teichert M, Eijgelsheim M, Uitterlinden AG, Buhre PN, Hofman A, De Smet PA, et al. Dependency of phenprocoumon dosage on polymorphisms in the VKORC1, CYP2C9, and CYP4F2 genes. Pharmacogenet Genomics. 2011;21:26–34.
Danese E, Montagnana M, Johnson JA, Rettie AE, Zambon CF, Lubitz SA, et al. Impact of the CYP4F2 p. V433 M polymorphism on coumarin dose requirement: systematic review and meta-analysis. Clin Pharmacol Ther. 2012;92:746–56.
van Schie RM, Wessels JA, Verhoef TI, Schalekamp T, le Cessie S, van der Meer FJ, et al. Evaluation of the effect of genetic variations in GATA-4 on the phenprocoumon and acenocoumarol maintenance dose. Pharmacogenomics. 2012;13:1917–23.
Teichert M, Visser LE, Uitterlinden AG, Hofman A, Buhre PJ, Straus S, et al. Selective serotonin re-uptake inhibiting antidepressants and the risk of overanticoagulation during acenocoumarol maintenance treatment. Br J Clin Pharmacol. 2011;72:798–805.
Teichert M, van Noord C, Uitterlinden AG, Hofman A, Buhre PN, De Smet PA, et al. Proton pump inhibitors and the risk of overanticoagulation during acenocoumarol maintenance treatment. Br J Haematol. 2011;153:379–85.
Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. Interaction between polymorphisms in the OCT1 and MATE1 transporter and metformin response. Pharmacogenet Genomics. 2010;20:38–44.
Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. Genetic variation in the multidrug and toxin extrusion 1 transporter protein influences the glucose-lowering effect of metformin in patients with diabetes: a preliminary study. Diabetes. 2009;58:745–9.
Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. Genetic variation in the organic cation transporter 1 is associated with metformin response in patients with diabetes mellitus. Pharmacogenomics J. 2009;9:242–7.
van Leeuwen N, Nijpels G, Becker ML, Deshmukh H, Zhou K, Stricker BH, et al. A gene variant near ATM is significantly associated with metformin treatment response in type 2 diabetes: a replication and meta-analysis of five cohorts. Diabetologia. 2012;55:1971–7.
Becker ML, Elens LL, Visser LE, Hofman A, Uitterlinden AG, van Schaik RH, et al. Genetic variation in the ABCC2 gene is associated with dose decreases or switches to other cholesterol-lowering drugs during simvastatin and atorvastatin therapy. Pharmacogenomics J. 2013;13:251–6.
Rodenburg EM, Eijgelsheim M, Geleijnse JM, Amin N, van Duijn CM, Hofman A, et al. CYP1A2 and coffee intake and the modifying effect of sex, age, and smoking. Am J Clin Nutr. 2012;96:182–7.
Ruiter R, Visser LE, Rodenburg EM, Trifirò G, Ziere G, Stricker BH. Adverse drug reaction-related hospitalizations in persons aged 55 years and over: a population-based study in the Netherlands. Drugs Aging. 2012;29:225–32.
Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ. Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med. 2013;126:256–63.
Rodenburg EM, Hoorn EJ, Ruiter R, Lous JJ, Hofman A, Uitterlinden AG, et al. Thiazide-associated hyponatremia: a population-based study.
Schoofs MW, van der Klift M, Hofman A, de Laet CE, Herings RM, Stijnen T, et al. Thiazide diuretics and the risk for hip fracture. Ann Intern Med. 2003;139:476–82.
Ruiter R, Oei L, Visser LE, Peltenburg HG, Hofman A, Zillikens MC, et al. The effect of thiazide and loop diuretics on urinary levels of free deoxypyridinoline: an osteoclastic bone-resorption marker. J Clin Pharm Ther. 2013;38:225–9.
Marcus MW, Müskens RP, Ramdas WD, Wolfs RC, De Jong PT, Vingerling JR, et al. Corticosteroids and open-angle glaucoma in the elderly: a population-based cohort study. Drugs Aging. 2012;29:963–70.
Marcus MW, Müskens RP, Ramdas WD, Wolfs RC, de Jong PT, Vingerling JR, et al. Antithrombotic medication and incident open-angle glaucoma. Invest Ophthalmol Vis Sci. 2012;53:3801–5.
Marcus MW, Müskens RP, Ramdas WD, Wolfs RC, De Jong PT, Vingerling JR, et al. Cholesterol-lowering drugs and incident open-angle glaucoma: a population-based cohort study. PLoS ONE. 2012;7:e29724.
Clockaerts S, Van Osch GJ, Bastiaansen-Jenniskens YM, Verhaar JA, Van Glabbeek F, Van Meurs JB, et al. Statin use is associated with reduced incidence and progression of knee osteoarthritis in the Rotterdam study. Ann Rheum Dis. 2012;71:642–7.
Lahousse L, Loth DW, Joos GF, Hofman A, Leufkens HG, Brusselle GG, et al. Statins, systemic inflammation and risk of death in COPD: the Rotterdam study. Pulm Pharmacol Ther. 2013;26:212–7.
Stricker BH, Stijnen T. Analysis of individual drug use as a time-varying determinant of exposure in prospective population-based cohort studies. Eur J Epidemiol. 2010;25:245–51.
Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–64.
Chain A, Dieleman J, van Noord C, Hofman A, Stricker B, Danhof M, et al. Not-in-trial simulation I: bridging cardiovascular risk from clinical trials to real life conditions. Br J Clin Pharmacol. 2013.
Dominioni L, Poli A, Mantovani W, Rotolo N, Imperatori A. Volunteer effect and compromised randomization in the Mayo Project of screening for lung cancer. Eur J Epidemiol. 2011;26(1):79–80.
Knol MJ, VanderWeele TJ, Groenwold RHH, Klungel OH, Rovers MM, Grobbee DE. Estimating measures of interaction on an additive scale for preventive exposures. Eur J Epidemiol. 2011;26(6):433–8.
Groenwold RHH, Klungel OH, Grobbee DE, Hoes AW. Selection of confounding variables should not be based on observed associations with exposure. Eur J Epidemiol. 2011;26(8):589–93.
Adami HO, Lagiou P, Trichopoulos D. Breast cancer following diethylstilbestrol exposure in utero: insights from a tragedy. Eur J Epidemiol. 2012;27(1):1–3.
Platt RW, Delaney JAC, Suissa S. The positivity assumption and marginal structural models: the example of warfarin use and risk of bleeding. Eur J Epidemiol. 2012;27(2):77–83.
Adami HO, Lagiou P, Trichopoulos D. Breast cancer following diethylstilbestrol exposure in utero: a tragedy? Reply. Eur J Epidemiol. 2012;27(4):318.
Bluming AZ. Breast cancer following diethylstilbestrol exposure in utero: a tragedy? Eur J Epidemiol. 2012;27(4):317–8.
Tournaire M, Devouche E, Epelboin S, Cabau A. Diethylstilbestrol exposure: evaluation of the doses received in France. Eur J Epidemiol. 2012;27(4):315–6.
Ravera S, van Rein N, de Gier JJ, de Jong-van den Berg LTW. A comparison of pharmacoepidemiological study designs in medication use and traffic safety research. Eur J Epidemiol. 2012;27(6):473–81.
Morois S, Fournier A, Clavel-Chapelon F, Mesrine S, Boutron-Ruault MC. Menopausal hormone therapy and risks of colorectal adenomas and cancers in the French E3 N prospective cohort: true associations or bias? Eur J Epidemiol. 2012;27(6):439–52.
Keogh RH, Park JY, White IR, Lentjes MAH, McTaggart A, Bhaniani A, et al. Estimating the alcohol-breast cancer association: a comparison of diet diaries, FFQs and combined measurements. Eur J Epidemiol. 2012;27(7):547–59.
Heuvers ME, Wisnivesky J, Stricker BH, Aerts JG. Generalizability of results from the national lung screening trial. Eur J Epidemiol. 2012;27(9):669–72.
Chiolero A, Santschi V, Burnand B, Platt RW, Paradis G. Meta-analyses: with confidence or prediction intervals? Eur J Epidemiol. 2012;27(10):823–5.
Million M, Raoult D. Publication biases in probiotics. Eur J Epidemiol. 2012;27(11):885–6.
Lytsy P, Berglund L, Sundstrom J. A proposal for an additional clinical trial outcome measure assessing preventive effect as delay of events. Eur J Epidemiol. 2012;27(12):903–9.
Vrooman HA, Cocosco CA, van der Lijn F, et al. Multi-spectral brain tissue segmentation using automatically trained k-nearest-neighbor classification. NeuroImage. 2007;37(1):71–81.
van der Lijn F, den Heijer T, Breteler MMB, Niessen WJ. Hippocampus segmentation in MR images using atlas registration, voxel classification, and graph cuts. NeuroImage. 2008;43(4):708–20.
Heijer T den, Lijn F van der, Koudstaal PJ, et al. A 10-year follow-up of hippocampal volume on magnetic resonance imaging in early dementia and cognitive decline. Brain. 2010;6.
Vernooij MW, van der Lugt A, Ikram MA, et al. Total cerebral blood flow and total brain perfusion in the general population: the Rotterdam Scan Study. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2008;28(2):412–9.
Poels MMF, Ikram MA, Vernooij MW, et al. Total cerebral blood flow in relation to cognitive function: the Rotterdam Scan Study. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2008;28(10):1652–5.
Vernooij MW, Ikram MA, Wielopolski PA, et al. Cerebral microbleeds: accelerated 3D T2*-weighted GRE MR imaging versus conventional 2D T2*-weighted GRE MR imaging for detection. Radiology. 2008;248(1):272–7.
Poels MMF, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke J Cereb Circ. 2010;41(10 Suppl):S103–6.
Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam scan study. Neurology. 2008;70:1208–14.
Poels MMF, Ikram MA, Lugt A van der, et al. Incidence of Cerebral Microbleeds in the General Population: The Rotterdam Scan Study. Stroke J Cereb Circ. 2011.
Vernooij MW, Haag MDM, van der Lugt A, et al. Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol. 2009;66(6):714–20.
Lovelock CE, Cordonnier C, Naka H, et al. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke J Cereb Circ. 2010;41(6):1222–8.
Vernooij MW, de Groot M, van der Lugt A, et al. White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. NeuroImage. 2008;43(3):470–7.
Vernooij MW, Ikram MA, Vrooman HA, et al. White matter microstructural integrity and cognitive function in a general elderly population. Archiv Gen Psychiatry. 2009;66(5):545–53.
de Groot M, Vernooij MW, Klein S, Ikram MA, Vos FM, Smith SM, et al. Improving alignment in tract-based spatial statistics: evaluation and optimization of image registration. Neuroimage. 2013;1(76):400–11.
Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32.
Odink AE, Lugt AVD, Hofman A, et al. Association between calcification in the coronary arteries, aortic arch and carotid arteries: the Rotterdam study. Current. 2007;193:408–13.
Odink AE, Lugt AVD, Hofman A, et al. Risk factors for coronary, aortic arch and carotid calcification; the Rotterdam Study. J Hum Hypertens. 2009;24(2):86–92.
Elias-Smale SE, Odink AE, Wieberdink RG, et al. Carotid, aortic arch and coronary calcification are related to history of stroke: the Rotterdam Study. Atherosclerosis. 2010;212(2):656–60.
Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI—initial results. Stroke J Cereb Circ. 2006;37(3):818–23.
Yuan C, Mitsumori LM, Ferguson MS, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001;104(17):2051–6.
No authors listed. North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke J Cereb Circ. 1991;22(6):711–20.
Krille L, Jahnen A, Mildenberger P, Schneider K, Weisser G, Zeeb H, et al. Computed tomography in children: multicenter cohort study design for the evaluation of cancer risk. Eur J Epidemiol. 2011;26(3):249–50.
Gaillard R, de Ridder MAJ, Verburg BO, Witteman JCM, Mackenbach JP, Moll HA, et al. Individually customised fetal weight charts derived from ultrasound measurements: the Generation R Study. Eur J Epidemiol. 2011;26(12):919–26.
Gaensbacher S, Waldhoer T, Berzlanovich A. The slow death of autopsies: a retrospective analysis of the autopsy prevalence rate in Austria from 1990 to 2009. Eur J Epidemiol. 2012;27(7):577–80.
Gudmundsson EF, Gudnason V, Sigurdsson S, Launer LJ, Harris TB, Aspelund T. Coronary artery calcium distributions in older persons in the AGES-Reykjavik study. Eur J Epidemiol. 2012;27(9):673–87.
Smits C, Kapteyn TS, Houtgast T. Development and validation of an automatic speech-in-noise screening test by telephone. Int J Audiol. 2004;43(1):15–28.
Weber KP. Head impulse test in unilateral vestibular loss. Neurology. 2008;454–463.
Cox RM, Alexander GC. The International Outcome Inventory for Hearing Aids (IOI-HA): psychometric properties of the English vesion. Int J Audiol. 2002;41(1):30–5.
Newman CW, Sandridge SA, Bolek L. Development and psychometric adequacy of the screening version of the tinnitus handicap inventory. Otol Neurotol. 2008;29:276–81.
Acknowledgments
The Rotterdam Study is supported by the Erasmus MC University Medical Center and Erasmus University Rotterdam; The Netherlands Organisation for Scientific Research (NWO); The Netherlands Organisation for Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly (RIDE); The Netherlands Genomics Initiative (NGI); the Ministry of Education, Culture and Science; the Ministry of Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam. The contribution of inhabitants, general practitioners and pharmacists of the Ommoord district to the Rotterdam Study is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hofman, A., Murad, S.D., van Duijn, C.M. et al. The Rotterdam Study: 2014 objectives and design update. Eur J Epidemiol 28, 889–926 (2013). https://doi.org/10.1007/s10654-013-9866-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-013-9866-z