Abstract
Lung function level and decline are each predictive of morbidity and mortality. Evaluation of the combined effect of these measurements may help further identify high-risk groups. Using Copenhagen City Heart Study longitudinal spirometry data (n = 10,457), 16–21 year risks of chronic obstructive pulmonary disease (COPD) morbidity, COPD or coronary heart disease mortality, and all-cause mortality were estimated from combined effects of level and decline in forced expiratory volume in one second (FEV1). Risks were evaluated using Cox proportional hazards models for individuals grouped by combinations of baseline predicted FEV1 and quartiles of slope. Hazard ratios (HR) and 95 % confidence intervals (CI) were estimated using stratified analysis by gender, smoking status, and baseline age (≤45 and >45). For COPD morbidity, quartiles of increasing FEV1 decline increased HRs (95 % CI) for individuals with FEV1 at or above the lower limit of normal (LLN) but below 100 % predicted, reaching 5.11 (2.58–10.13) for males, 11.63 (4.75–28.46) for females, and 3.09 (0.88–10.86) for never smokers in the quartile of steepest decline. Significant increasing trends were also observed for mortality and in individuals with a baseline age ≤45. Groups with ‘normal’ lung function (FEV1 at or above the LLN) but excessive declines (fourth quartile of FEV1 slope) had significantly increased mortality risks, including never smokers and individuals with a baseline age ≤45.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung function is a significant predictor of chronic obstructive pulmonary disease (COPD) and coronary heart disease (CHD) morbidity and mortality and all-cause mortality [1–19]. Although COPD is multifactorial by nature, it is generally caused by inflammatory response to inhaled hazardous exposures that may lead to chronic bronchitis, small airways disease, or emphysema [20, 21]. Changes leading to COPD are usually progressive, starting many years before abnormality can be detected with spirometry or radiology. Early markers suitable for detection of the disease processes are not yet available [22, 23]. Furthermore, there is also a strong association between COPD and CHD which is not fully understood [24].
Generally, the level of forced expiratory volume in one second (FEV1) in relation to a predicted value is an indicator of the presence and severity of airflow impairment, while an excessive rate of FEV1 decline is an indicator of ongoing adverse health effects (e.g. COPD progression, increasing abdominal obesity, decreasing fitness) [20]. Lung function decline has been assessed with regard to morbidity and mortality [25], but none of the published studies we are aware of have evaluated the combined effect of the level of lung function and the rate of lung function decline on morbidity and mortality. Evaluating the combined effect of the level and the rate of decline on morbidity or mortality risk provides information relevant in clinical and occupational settings where periodic spirometry may provide an opportunity for prevention of further excessive decline through intervention on preventable risk factors or medical treatment [26–29].
The purpose of this study was to evaluate the morbidity and mortality risks associated with the combined effect of the level and rate of lung function decline. Study participants were placed in lung function categories using baseline forced expiratory volume in one second (FEV1b) compared to predicted values [30], and quartiles of FEV1 slope measured over the subsequent years of follow-up. Within the categories, the prevalence rates of respiratory symptoms were also investigated. Because the purpose of our study was to evaluate the effect of lung function on morbidity and mortality outcomes, covariates known to be associated with decreasing lung function (e.g. smoking, diet, exposures, or obesity) were not included in the models, but the analysis were repeated in never smokers.
Methods
Study population
The Copenhagen City Heart Study is a 28-year cardiovascular disease study with four examinations of males and females age 20 years and older. The primary sample (n = 19,698) was drawn randomly from the Copenhagen Population Register in five-year age groups. Clinical examinations (with spirometry) and a self-administered questionnaire were conducted. An electronic spirometer (N 403 Monaghan, United States) was used during examinations one (1976–78) and two (1981–83). At each examination, three spirometric measurements were obtained with at least two within 5 % of one another. If airflow obstruction was identified (FEV1 < 80 % and/or the ratio of FEV1 and forced vital capacity [FVC] < 0.7), bronchial reversibility testing was performed and spirometric measurements were repeated after 30 min. The highest values of FEV1 and FVC were retained in the data set. Age and gender data were obtained at study enrollment from the Copenhagen Population Register and height was measured during the clinical examination. Smoking status and current respiratory symptoms of chronic bronchitis were ascertained by questionnaire at each examination, and shortness of breath was ascertained at examination two [31, 32].
Morbidity data were obtained from the National Patient Register with discharges from all Danish acute-care, non-psychiatric hospitals since 1977 [33]. Mortality data were from the Civil Registration System (where vital status is continuously updated) [34], and cause-specific mortality data were from the National Register of Causes of Death. In the current study, health outcomes are (1) primary and secondary COPD hospital diagnoses (International Classification of Diseases [ICD]-8 491–492, and ICD-10 J41–J44); (2) COPD or CHD mortality (ICD-8 410–414, and ICD-10 I20–I25) as the underlying or contributing cause; and (3) all-cause mortality. (Denmark transitioned directly from the 8th to the 10th revision of ICD in 1993.) Use of lung function measurements at examinations one and two (1976–78 and 1981–83) permitted a 16–21 year follow-up that lasted through 5/8/2009 for COPD morbidity, 12/31/2006 for cause-specific mortality, and 5/17/2009 for all-cause mortality. Additional Copenhagen City Heart Study information is available elsewhere [31, 35].
Statistical methods
We used Cox proportional hazards models to estimate morbidity and mortality risks associated with the combined effect of the FEV1b level and subsequent FEV1 slope (difference in FEV1 between the first two examinations, divided by time between the examinations) with an average follow-up of 5 years (Fig. 1). Baseline lung function results were divided into three groups: (1) FEV1b at or above 100 % predicted; (2) FEV1b at or above the lower limit of normal (LLN) but <100 % predicted; or (3) FEV1b below the LLN. The predicted values and LLN were derived using Quanjer’s published population-based reference equation [30]. The LLN approximates the one-sided 95 % confidence limit for the expected value, where 5 % of apparently healthy individuals who have never smoked would be identified as abnormal. Eight lung function categories were formed from combinations of FEV1b (groups 1 and 2 only) and quartiles of FEV1 slope (Fig. 2). The ninth category included individuals in group 3, whose FEV1b was below the LLN. The reference category included individuals with FEV1b at or above 100 % predicted and in the first (i.e. lowest) quartile of FEV1 decline. Analyses were based on FEV1 measurements because these are the most effective for evaluation of longitudinal lung function changes [36].
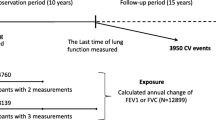
Sample size and morbidity and mortality follow-up. Above the timeline are the numbers of subjects who participated in spirometry testing by Copenhagen City Heart Study examination. Below the timeline is the number of subjects who participated in spirometry testing at examinations one and two and the numbers of health outcomes that occurred during the morbidity and mortality follow-up periods. Note that the follow-up periods are represented as dashed lines and that follow-up for COPD or CHD mortality ended in 2006
Hazard ratios (HR) and 95 % confidence intervals (CI) were estimated overall, by gender, for never smokers, and by baseline age (≤45 and >45). The stratification was in recognition of the importance of age, gender, and smoking as determinants of lung function and risk factors for morbidity and mortality. Although not all models were gender-specific, all models used gender-specific quartile values for the FEV1 slope to account for differences due to gender. All models were adjusted for baseline age and height to account for their potential effect on the level as well as decline in lung function and because these are essential covariates that would typically be available and taken into consideration in a clinical context [37]. However, smoking was not included in the models as a risk factor because the interest was to determine the effect of lung function and its decline on morbidity and mortality for application in prevention. Additional analyses were conducted in never smokers to demonstrate risk in the absence of smoking. The single cut point at age 45 was selected because it is an age related to COPD development [38, 39] and the 16–21 year morbidity and mortality follow-up permitted estimation of morbidity and mortality risks associated with lung function decline earlier in life, which is less well-known. Time to event (or censor) was from examination two until: (1) COPD hospital diagnosis, death, or end of follow-up for COPD morbidity; and (2) death or end of follow-up for mortality.
Population attributable risks (PAR) were calculated with Levin’s formula [40] for category 9 and jointly for categories 8 and 9 to demonstrate the potential contribution of an excessive FEV1 slope in those with a ‘normal’ FEV1b beyond that of FEV1b below the LLN. We also plotted the relationship between the nine categories and the overall prevalence rates of current respiratory symptoms of chronic bronchitis and shortness of breath at examination two. As in our previous study, we conducted sensitivity analyses to assess the effect of an adjustment to FEV1 measurements for 1981 by a mean difference with respect to years 1982 and 1983 [41]. Preliminary analysis of mean FEV1 values had revealed a slightly excessive increase in mean FEV1 in 1981, but not in 1982 or 1983, as compared to mean FEV1 for baseline examinations in 1976–78. It is unknown to the authors whether the increase was due to early difficulties with the Monaghan spirometer that was replaced with a dry wedge spirometer by examination three or to other issues [42]. To adjust for the increase, we reduced the individual FEV1 measurements for 1981 by a fixed value (289 ml for males and 201 ml for females) to align the 1981 values with the 1976–78 and 1982–83 values. The fixed values are the average difference in FEV1 values from examination one to two (for 1982 only), minus 30 ml to correct for annual loss from 1981 to 1982. Models excluding the 1981 values were conducted for a sensitivity analysis regarding the adjustment.
All Copenhagen City Heart Study subjects gave informed consent to participate. The Study was performed in accordance with the 2nd Helsinki Declaration and approved by the regional ethics committee (100.2039/91). Current analyses were approved by the National Institute for Occupational Safety and Health Human Subjects Review Board (08-DRDS-03XP) and the West Virginia University Institutional Review Board (H-21909). All analyses were conducted with PROC PHREG (p < 0.05) using SAS version 9.2 (SAS Institute Inc, USA).
Results
The characteristics of the 10,457 individuals with spirometry testing at examinations one and two are summarized by subcohort (males, females, and never smokers) in Table 1. The table describes the sample sizes, duration of morbidity and mortality follow-up (16–21 years), gender-specific values for baseline FEV1 (FEV1b), the frequencies by the level of FEV1b and quartiles of FEV1 slope, the quartiles of FEV1 slope, and smoking status. For the combined COPD or CHD mortality events, the causes of death were CHD in 70 %, COPD in 25 %, and both causes in 5 % of these deaths. Of note are the high prevalence rates of current and former smoking. Individuals lost to follow-up by examination two (22.9 %) were older, had lower average height-adjusted FEV1, and higher prevalence of self-reported respiratory symptoms of chronic bronchitis, current asthma, and smoking at baseline.
Morbidity and mortality risks by gender, for never smokers, and by age at baseline
Morbidity and mortality risks by gender and for never smokers are presented in Table 2. For categories with FEV1b at or above 100 % predicted, only females in the fourth quartile of the slope had statistically significant morbidity and mortality risks. Categories with FEV1b below 100 % predicted showed increasing trends in morbidity and mortality risk with increasing quartiles of the slope, most often significant in females. Females demonstrated higher COPD or CHD mortality risks than males; this pattern was not as clearly seen for all-cause mortality. Among never smokers, there was a clear trend for increasing COPD morbidity risk with worsening lung function category, however the risk was only significant with category 9 (FEV1b below the LLN). Only all-cause mortality risk could be evaluated for never smokers by gender. There were no clear trends and only category 8 (FEV1b below 100 % predicted and the fourth quartile of the slope) was statistically significant with HRs of 2.58 (1.04–6.40) for males and 1.74 (1.12–2.70) for females (not shown).
Morbidity and mortality risks estimated by age categories (≤45 and >45) at baseline spirometry testing are shown in Table 3. The general increasing trend in the HRs across the lung function categories 1 to 9 was found in both age groups, but all-cause mortality risks were higher among the younger group. Among each subcohort (males, females, never smokers, and ages ≤45 and >45), cause-specific and all-cause mortality risks were significantly increased for those with ‘normal’ lung function (FEV1b at or above the LLN but below 100 % predicted) and with excessive FEV1 decline (fourth quartile of the slope).
Overall morbidity and mortality risks and attributable risk
Significant overall trends in the risk of morbidity and mortality outcomes across the nine categories for the combined effect of the lung function level and decline are demonstrated in Fig. 3a–c. Risks were higher for COPD morbidity as compared to the mortality outcomes. Statistically significant increases in the morbidity and mortality risks began at category 4 (FEV1b at or above 100 % predicted and the fourth quartile of the slope). By category 8 (FEV1b at or above the LLN but below 100 % predicted and the fourth quartile of the slope), HRs reached 7.29 (4.24–12.52) for COPD morbidity, 4.07 (2.70–6.13) for COPD or CHD mortality, and 2.13 (1.80–2.53) for all-cause mortality.
Cox proportional hazards models for overall (a) morbidity and (b and c) mortality risks by lung function category. Models adjusted for baseline age and height. The scales for the hazard ratio differ for the morbidity and mortality outcomes and are not directly comparable. Sensitivity analyses, excluding the adjusted 1981 FEV1 values, indicate there may be an underestimation of risk in the last three lung function categories for COPD morbidity and where FEV1b < LLN for COPD or CHD mortality. FEV 1b baseline forced expiratory volume in one second; LLN lower limit of normal; pred. predicted value; Q1 first quartile of FEV1 slope
To illustrate the effect of excessive decline on morbidity and mortality in a population, we estimated the PARs for the increased risk in category 9 and then jointly for categories 8 and 9 to quantify the additional increase in risk due to excessive decline in those with ‘normal’ lung function, using estimated HRs from the overall analysis. Table 4 shows that the additional contribution of category 8 is 12.0 % for COPD morbidity (49.6–37.6 %), 9.6 % for COPD or CHD mortality, and 7.1 % for all-cause mortality. The increasing categories of lung function impairment were also associated with increasing prevalence of symptoms of chronic bronchitis and shortness of breath (Fig. 4).
Overall prevalence rates of self-reported respiratory symptoms (filled square chronic bronchitis; filled triangle shortness of breath) at examination two by lung function category. FEV 1b baseline forced expiratory volume in one second; LLN lower limit of normal; pred., predicted value; Q1 first quartile of FEV1 slope
Given that individuals with abnormal baseline lung function were included in the models, several additional Cox models were conducted post hoc. These models excluded individuals with: (1) a ratio of FEV1b to baseline FVC (FEV1b/FVCb) below the LLN, (2) FEV1b and FEV1b/FVCb below the LLN, and (3) FVC below the LLN and FEV1b/FVCb above the LLN. Differences occurred mainly in category 9, with lower HRs in the first two models and a higher HR for COPD morbidity in the last model, but overall patterns were not affected.
Discussion
This study shows that the combined effect of the baseline FEV1 and excessive rate of FEV1 decline is associated with increased morbidity and mortality risks, even with a ‘normal’ baseline FEV1 (at or above the LLN). The effect of an excessive decline was seen in males, females, never smokers, and with a baseline age of 45 or under or above 45, although all-cause mortality risks were higher in those 45 or under. The contribution of excessive FEV1 decline in those with ‘normal’ FEV1 has public health significance as illustrated by the additional contribution to the PAR of 12.0 % for COPD morbidity, 9.6 % for COPD or CHD mortality, and 7.1 % for all-cause mortality.
Previous studies have demonstrated associations between morbidity and mortality and FEV1 and the rate of decline [1–19]. Our results confirm the well-recognized finding that FEV1 is an important marker of risk for cardiovascular and respiratory outcomes. Similar to prior studies, we also identified higher morbidity and mortality risks among females [11, 17], which may result from increased susceptibility to the effects of smoking [43], and increased COPD morbidity risk with lower lung function at younger ages [44].
Evaluating the combined effect of the level and rate of decline is a new approach and adds to current knowledge regarding prevention. Because FEV1 is considered the most useful of the spirometry tests for the evaluation of longitudinal changes in lung function, it was of interest to determine the combination of the level and the rate of decline on the future morbidity and mortality outcomes. Especially in individuals with normal levels of lung function, but excessive decline, who may not be considered at risk because their lung function level is within the normal limits. The three-tier classification of the level of lung function permits examination of results for individuals with FEV1b at or above the LLN but less than 100 % predicted, a group of potential concern if rates of decline are excessive. The LLN cut point was used to initially delineate ‘normal’ versus ‘abnormal’ levels of lung function because the LLN is based on population-based reference values that account for age, height, gender, and race. For example, using the LLN for the FEV1/FVC ratio was shown to be a better predictor of excessive rate of decline than a fixed ratio [25].
Our results indicate that prevention of an excessive lung function decline has public health significance even in individuals who have normal levels of FEV1. The results are particularly relevant to prevention in occupational settings where periodic spirometry is often conducted on relatively healthy workers to maintain workers’ fitness to wear respirators and to prevent occupational injury. Generally, in occupational settings most of the workers have ‘normal’ levels of lung function and prevention of excessive decline in lung function by intervening on the preventable risk factors [17, 18, 20, 44] would be of public health significance in at risk worker populations. Also, of clinical significance is the increased prevalence of symptoms of chronic bronchitis and shortness of breath with increasing decline in lung function starting from the fourth quartile in those with FEV1 at or above the predicted value.
Despite this study’s strengths, such as a large, age-stratified population and long outcome follow-up, there were limitations including the adjustment of FEV1 values for 1981 [41]. Sensitivity analyses excluding 1981 data indicated under- and overestimation of COPD morbidity and COPD or CHD mortality risks but was not possible for COPD or CHD mortality risk in never smokers because there were no events in the reference category of the comparison model. Underestimation of risk could also have occurred through self-selection of healthier subjects into the Copenhagen City Heart Study and survivor bias.
Chronic obstructive pulmonary disease (COPD) is generally thought to be under-diagnosed and mortality underreported, such misclassification could have biased the results toward the null and decreased associations [45]. Thus, the COPD or CHD mortality outcome was used [19, 46, 47]. COPD morbidity defined as a hospital diagnosis limits the generalizability of the results to individuals with more severe disease. The FEV1 slope calculated from only two measurements is vulnerable to regression to the mean, but adjustment for height may have lessened this effect. The duration of follow-up in the study was sufficient to estimate the slope, although the precision may have been somewhat improved by more frequent measurements [48].
Throughout these models, our method of stratification by combinations of lung function level and rate of decline created the potential for a relatively small sample size in the reference category that could influence the results. For example, this may partially explain the gender-specific differences in the morbidity and mortality risks. To explore this issue, we created a new reference category by combining categories 1 and 2. The addition of category 2 narrowed the gender gap in risk slightly and reduced the HRs.
In conclusion, this study provides evidence that individuals with excessive longitudinal decline are at increased risk of morbidity and mortality even before the point when their spirometry values would be interpreted as abnormal. Recognition of this provides an earlier opportunity for prevention. These study results may be useful to health care providers who evaluate individuals at-risk for lung function impairment.
References
Beaty TH, Cohen BH, Newill CA, Menkes HA, Diamond EL, Chen CJ. Impaired pulmonary function as a risk factor for mortality. Am J Epidemiol. 1982;116:102–13.
Beaty TH, Newill CA, Cohen BH, Tockman MS, Bryant SH, Spurgeon HA. Effects of pulmonary function on mortality. J Chronic Dis. 1985;38:703–10.
Krzyzanowski M, Jedrychowski W, Wysocki M. Factors associated with the change in ventilatory function and the development of chronic obstructive pulmonary disease in a 13-year follow-up of the Cracow Study. Risk of chronic obstructive pulmonary disease. Am Rev Respir Dis. 1986;134:1011–9.
Persson C, Bengtsson C, Lapidus L, Rybo E, Thiringer G, Wedel H. Peak expiratory flow and risk of cardiovascular disease and death. A 12-year follow-up of participants in the population study of women in Gothenburg, Sweden. Am J Epidemiol. 1986;124:942–8.
Lange P, Nyboe J, Jensen G, Schnohr P, Appleyard M. Ventilatory function impairment and risk of cardiovascular death and of fatal or non-fatal myocardial infarction. Eur Respir J. 1991;4:1080–7.
Bang KM, Gergen PJ, Kramer R, Cohen B. The effect of pulmonary impairment on all-cause mortality in a national cohort. Chest. 1993;103:536–40.
Rodriguez BL, Masaki K, Burchfiel C, et al. Pulmonary function decline and 17-year total mortality: the Honolulu heart program. Am J Epidemiol. 1994;140:398–408.
Tockman MS, Pearson JD, Fleg JL, et al. Rapid decline in FEV1. A new risk factor for coronary heart disease mortality. Am J Respir Crit Care Med. 1995;151:390–8.
Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313:711–6.
Neas LM, Schwartz J. Pulmonary function levels as predictors of mortality in a national sample of US adults. Am J Epidemiol. 1998;147:1011–8.
Ryan G, Knuiman MW, Divitini ML, James A, Musk AW, Bartholomew HC. Decline in lung function and mortality: the Busselton health study. J Epidemiol Community Health. 1999;53:230–4.
Schunemann HJ, Dorn J, Grant BJ, Winkelstein W Jr, Trevisan M. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo health study. Chest. 2000;118:656–64.
Beeckman LA, Wang ML, Petsonk EL, Wagner GR. Rapid declines in FEV1 and subsequent respiratory symptoms, illnesses, and mortality in coal miners in the United States. Am J Respir Crit Care Med. 2001;163:633–9.
Pelkonen M, Notkola IL, Tukiainen H, Tervahauta M, Tuomilehto J, Nissinen A. Smoking cessation, decline in pulmonary function and total mortality: a 30 year follow up study among the Finnish cohorts of the Seven Countries study. Thorax. 2001;56:703–7.
Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the first national health and nutrition examination survey follow up study. Thorax. 2003;58:388–93.
Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–9.
Mannino DM, Reichert MM, Davis KJ. Lung function decline and outcomes in an adult population. Am J Respir Crit Care Med. 2006;173:985–90.
Mannino DM, Davis KJ. Lung function decline and outcomes in an elderly population. Thorax. 2006;61:472–7.
Lash TL, Johansen MB, Christensen S, et al. Hospitalization rates and survival associated with COPD: a nationwide Danish cohort study. Lung. 2011;189:27–35.
American Thoracic Society/European Respiratory Society Task Force. Standards for the diagnosis and management of patients with COPD, version 1.2. 2004. www.thoracic.org/go/copd. Accessed 17 Feb 2011.
Hogg JC, Pierce RA. Remodelling of peripheral lung tissue in COPD. Eur Respir J. 2008;31:913–4.
Allen TC. Pathology of small airways disease. Arch Pathol Lab Med. 2010;134:702–18.
Cornwell WD, Kim V, Song C, Rogers TJ. Pathogenesis of inflammation and repair in advanced COPD. Semin Respir Crit Care Med. 2010;31:257–66.
Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514–9.
Akkermans RP, Berrevoets MA, Smeele IJ, et al. Lung function decline in relation to diagnostic criteria for airflow obstruction in respiratory symptomatic subjects. BMC Pulm Med. 2012;12:12.
Hankinson JL, Wagner GR. Medical screening using periodic spirometry for detection of chronic lung disease. Occup Med. 1993;8:353–61.
Townsend MC. Spirometry in the occupational health setting–2011 update. J Occup Environ Med. 2011;53:569–84.
Townsend MC. Evaluating pulmonary function change over time in the occupational setting. J Occup Environ Med. 2005;47:1307–16.
Lange P. Spirometric findings as predictors of survival. Thorax. 2011;66:1–2.
Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, European community for steel and coal. Official statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5–40.
Schnohr P, Jensen G, Lange P, Scharling H, Appleyard M. The Copenhagen City Heart Study. Eur Heart J. 2001;3:H1–83.
Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ. 1968;56:1–188.
Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish national hospital register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46:263–8.
Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39:22–5.
Lange P, Mogelvang R, Marott JL, Vestbo J, Jensen JS. Cardiovascular morbidity in COPD: a study of the general population. COPD. 2010;7:5–10.
Burrows B, Lebowitz MD, Camilli AE, Knudson RJ. Longitudinal changes in forced expiratory volume in one second in adults. Methodologic considerations and findings in healthy nonsmokers. Am Rev Respir Dis. 1986;133:974–80.
Miller MR, Pedersen OF. New concepts for expressing forced expiratory volume in 1 s arising from survival analysis. Eur Respir J. 2010;35:873–82.
Ferguson GT, Enright PL, Buist AS, Higgins MW. Office spirometry for lung health assessment in adults: a consensus statement from the National Lung Health Education Program. Chest. 2000;117:1146–61.
Doherty DE. A review of the role of FEV1 in the COPD paradigm. COPD. 2008;5:310–8.
Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9:531–41.
Baughman P, Marott JL, Lange P, Andrew M, Hnizdo E. Health outcomes associated with lung function decline and respiratory symptoms and disease in a community cohort. COPD. 2011;8:103–13.
Lokke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax. 2006;61:935–9.
Camp PG, O’Donnell DE, Postma DS. Chronic obstructive pulmonary disease in men and women: myths and reality. Proc Am Thorac Soc. 2009;6:535–8.
Kohansal R, Martinez-Camblor P, Agusti A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180:3–10.
Jensen HH, Godtfredsen NS, Lange P, Vestbo J. Potential misclassification of causes of death from COPD. Eur Respir J. 2006;28:781–5.
Sin DD. Is COPD really a cardiovascular disease? Chest. 2009;136:2.
Schneider C, Bothner U, Jick SS, Meier CR. Chronic obstructive pulmonary disease and the risk of cardiovascular diseases. Eur J Epidemiol. 2010;25:253–60.
Hnizdo E, Sircar K, Glindmeyer HW, Petsonk EL. Longitudinal limits of normal decline in lung function in an individual. J Occup Environ Med. 2006;48:625–34.
Acknowledgments
The National Institute for Occupational Safety and Health funded this research. The authors thank Dr. Michael Andrew, of the National Institute for Occupational Safety and Health, for his helpful comments.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Rights and permissions
About this article
Cite this article
Baughman, P., Marott, J.L., Lange, P. et al. Combined effect of lung function level and decline increases morbidity and mortality risks. Eur J Epidemiol 27, 933–943 (2012). https://doi.org/10.1007/s10654-012-9750-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-012-9750-2