Abstract
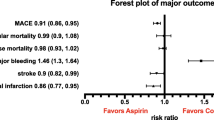
Randomized evidence for aspirin in the primary prevention of cardiovascular disease (CVD) among women is limited and suggests at most a modest effect for total CVD. Lack of compliance, however, can null-bias estimated effects. We used marginal structural models (MSMs) to estimate the etiologic effect of continuous aspirin use on CVD events among 39,876 apparently healthy female health professionals aged 45 years and older in the Women’s Health Study, a randomized trial of 100 mg aspirin every other day versus placebo. As-treated analyses and MSMs controlled for time-varying determinants of aspirin use and CVD. Predictors of aspirin use differed by randomized group and prior use and included medical history, CVD risk factors, and intermediate CVD events. Previously reported intent-to-treat analyses found small non-significant effects of aspirin on total CVD (hazard ratio (HR) = 0.91, 95 % confidence interval (CI) = 0.81–1.03) and CVD mortality (HR = 0.95, 95 % CI = 0.74–1.22). As-treated analyses were similar for total CVD with a slight reduction in CVD mortality (HR = 0.88, 95 % CI = 0.67–1.16). MSMs, which adjusted for non-compliance, were similar for total CVD (HR = 0.93; 95 % CI: 0.81, 1.07) but suggested lower CVD mortality with aspirin use (HR = 0.76; 95 % CI: 0.54, 1.08). Adjusting for non-compliance had little impact on the estimated effect of aspirin on total CVD, but strengthened the effect on CVD mortality. These results support a limited effect of low-dose aspirin on total CVD in women, but potential benefit for CVD mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aspirin has long been known to be effective in treating acute evolving myocardial infarction (MI) and in the secondary prevention of cardiovascular disease (CVD) [1]. A meta-analysis of secondary prevention trials suggested a relative risk reduction of 15 % in vascular mortality and of 30 % in nonfatal cardiovascular events among those assigned to aspirin use following a cardiovascular event. Trials of primary prevention with aspirin, however, have been less definitive, with some suggesting a strong benefit, especially for coronary heart disease (CHD) [2–4] and others suggesting little benefit for myocardial infarction, with conflicting results for stroke [5, 6]. A recent meta-analysis found an overall 12 % reduction in any serious vascular event [7].
The Women’s Health Study (WHS), the largest trial of aspirin for primary prevention among women to date, tested the effect of aspirin among 39,876 female health professionals and found no effect on the composite endpoint of major cardiovascular disease, which included MI, stroke, and CVD mortality. Based on accumulated evidence, the US Preventive Services Task Force strongly recommended in 2002 that clinicians consider aspirin use for all adults at increased risk of CHD or stroke, including postmenopausal women and those with CVD risk factors [8]. Development of less extreme indicators of cardiovascular risk, such as hypertension, diabetes, angina, transient ischemic attack (TIA), and revascularization procedures, may prompt some to begin aspirin use. Thus, women who take aspirin may be at higher risk of CVD due to a host of underlying risk factors and behaviors. Some of these intermediate events may also be in the causal pathway from aspirin to CVD, leading to complex associations among aspirin, intervening events, and CVD.
Confounding by such time-varying factors that are also affected by exposure can bias the estimated intent-to-treat effect, and usual as-treated analyses cannot correct for this type of bias. Marginal structural models (MSMs) [9] can be used to effectively adjust for time-varying confounding by nonfatal CVD events which are also affected by aspirin use. Such models can incorporate a host of intervening variables, and have been previously used to adjust for post-MI use of aspirin in an analysis of aspirin and CVD mortality in the Physician’s Health Study (PHS) [10]. This paper presents an analysis adjusting for time-varying noncompliance to aspirin use due to intervening events within the WHS. In contrast to the PHS, the primary endpoint of the WHS was incident CVD, with CVD mortality serving as a secondary endpoint. The aim of this analysis was to determine whether control for less serious time-varying CVD risk factors, such as angina, TIA, and revascularization procedures, could unmask a protective effect of aspirin on incident CVD as well as on CVD mortality.
Methods
Study population
The WHS was a randomized trial of aspirin (100 mg of aspirin taken every other day) and vitamin E (600 IU every other day) in the primary prevention of cardiovascular disease and cancer. Detailed descriptions of the trial and its results have been published elsewhere [6, 11, 12]. Briefly, women were eligible if they were aged 45 years or higher, had no history of CVD or cancer, agreed to avoid taking outside aspirin or vitamin E, and remained compliant to pill taking during a 3-month placebo run-in period. A total of 39,876 female health professionals were randomized in 1993–1996 to aspirin and/or vitamin E in a two-by-two factorial design with four study groups defined as aspirin only, vitamin E only, both, or neither. The trial was approved by the Institutional Review Board of Brigham and Women’s Hospital and monitored by an external Data and Safety Monitoring Board.
Every 12 months, women were sent an annual supply of study pills supplied in monthly calendar packs, along with a study questionnaire on compliance, side effects, risk factors and medical events. Self-reported height and weight were collected, as well as blood pressure and cholesterol level. Hypertension was defined as a blood pressure of at least 140/90 mmHg or on anti-hypertensive medication. High cholesterol was defined as total cholesterol of at least 240 or self-reported physician-diagnosed high cholesterol. Family history of MI was defined as parental history prior to age 60. Follow-up continued until the scheduled end of the trial in March, 2004. Morbidity and mortality follow-up were 97.2 and 99.4 % complete, respectively.
On each questionnaire, women were asked about study pill use (aspirin or aspirin placebo) since the last questionnaire. The amount of reported aspirin use tended to have a U-shaped distribution, with most participants taking it either nearly every other day as assigned or taking very little if any. For these analyses, compliance to aspirin use was defined as use on at least 120 days per year.
Study endpoints were confirmed by a blinded Endpoints Committee of physicians following review of medical records. The primary CVD endpoint for the trial was a composite of first major cardiovascular event that included MI, stroke, or CVD death. Individual components of this served as secondary endpoints for the trial. Information was also collected and reviewed on coronary revascularization procedures, (bypass surgery (CABG) or percutaneous coronary angioplasty (PTCA)), transient ischemic attacks (TIA) and total mortality. Written informed consent was requested to obtain related medical records following a report of one of these events by the participant, a family member, or postal authorities. Reports were confirmed if they passed established clinical criteria.
Statistical analysis
As the first step of analysis, logistic models predicting observed aspirin use, including both study and outside use, were fit as a function of past aspirin use, intervening factors, and baseline variables. The data were broken up by time (year), and pooled over time using the counting process method [13]. We considered as candidates intermediate cardiovascular conditions, such as angina, TIA, or revascularization; intermediate risk factors for cardiovascular disease, such as blood pressure and cholesterol levels; markers for healthy behavior, such as exercise, smoking, alcohol use and diet; and side-effects of aspirin or other medical factors, such as gastrointestinal symptoms. Because the aim was to reduce bias due to variables in the causal pathway, we included in the final models only those that were known determinants of CVD or that were observed to be associated with CVD among women in the placebo group. Predictors of aspirin use differed by randomized aspirin assignment and aspirin use in the previous year; we thus fit separate models for groups defined by these variables.
The final MSM used weighted pooled logistic regression to estimate the hazard ratio [14–16]. Additional detail may be found in the supplementary material. The predicted probability of aspirin use was computed from the model for observed aspirin use described above and used as the denominator of the weights in the MSMs. To stabilize the weights and reduce their variability, the numerator of the weights consisted of predicted probability from a second logistic model for observed aspirin as a function of past aspirin use and a subset of baseline variables, without including intervening factors [17]. Because the weights can induce correlation between the observations in a person over time, robust standard errors were computed using the SAS procedure GENMOD.
The primary analysis used the main study endpoint of major cardiovascular disease, including MI, stroke, and CVD mortality. Subsequent analyses were conducted using the secondary component endpoints. Intervening events were defined differently for each endpoint (for example, MI and stroke are intervening events in the analysis of CVD mortality), and weights were reconstructed for each endpoint separately. All models for CVD adjusted for age at randomization and race/ethnicity with terms in the model, except in analyses of cardiovascular mortality which adjusted only for age due to small numbers of CVD deaths within racial groups. For comparison with these models we also conducted intent-to-treat and as-treated analyses using unweighted pooled logistic models. SAS version 9.2 (SAS Institute, Cary, North Carolina) was used for all analyses.
Results
At the time of randomization, women were aged 55 years on average, 54 % were postmenopausal, 26 % reported hypertension at baseline, and 30 % reported elevated cholesterol. A total of 19,934 women were assigned to active aspirin and 19,942 to placebo aspirin. Baseline characteristics were balanced over aspirin intervention groups as reported previously [6]. Over an average 10 year follow-up, 999 women reported a major cardiovascular event, which included 391 MIs and 487 strokes. There were 246 deaths with a confirmed cause of cardiovascular disease.
Compliance to white study pills (aspirin or aspirin placebo) tended to diminish as follow-up continued. Compliance to study pills was excellent during the first year for both agents, with approximately 88 % taking at least two-thirds of their study aspirin or aspirin placebo at the end of the first year. Use of study aspirin or placebo declined to 76 % at five years of follow-up and to 67 % by ten years of follow-up, with an average of 73 % throughout the trial. Compliance was slightly but significantly lower in the active versus placebo aspirin groups, with proportions averaging about one percent lower from 24 months onward. While participants were asked to avoid use of any other aspirin or aspirin-containing medications, outside use aspirin for four or more days per month averaged 12 % over the length of follow-up, with no significant difference by randomized aspirin assignment.
Predictors of aspirin use
Several factors strongly predicted aspirin use, including demographics, epidemiologic risk factors, intervening cardiovascular risk factors, and other medical conditions. The strongest predictors were randomized assignment and aspirin use in previous years. Overall 94 % of those in the randomized aspirin group who had used aspirin in the previous year (ASAt−1) continued to use it in the current year (ASAt), while only 18 % of those who had stopped using it began to take aspirin. In the placebo group, among those who were not taking outside aspirin in the previous year, only 3 % began to take outside aspirin in the current year. Among those who were taking it already, 71 % continued to do so. In addition, aspirin use two years ago (ASAt−2) had a continued effect on use in the current year.
The associations of standard epidemiologic risk factors with aspirin use are shown in Table 1. These tended to differ by randomized group as well as previous use of aspirin. In the placebo group, the use of aspirin generally increased with age. Among those in the active aspirin group, age had a nonlinear association with compliance and was estimated using a quadratic term. The peak age of compliance was near age 60 years. Among those randomized to placebo, those who were taking multivitamins, were current smokers, or had hypertension or high cholesterol tended to take outside aspirin regardless of prior use. In contrast, among those randomized to active aspirin, those who were using aspirin already were less likely to continue if they were smokers or had hypertension or high cholesterol. Those who had stopped using study aspirin were more likely to begin taking aspirin if they were taking multivitamins, used alcohol at least once a week, or had hypertension, high cholesterol or a family history of MI prior to age 60.
Aspirin use was strongly affected by intervening cardiovascular conditions. In the placebo group, those who experienced intervening CHD, such as angina and revascularization, a TIA, or other cardiovascular condition were more likely to go on or stay on outside aspirin, with stronger influence among those not taking aspirin already. In the active aspirin group, the effects differed strongly by previous use. Those using aspirin were more likely to stop when these conditions occurred; those not using aspirin were more likely to start using it. The same patterns tended to be true for diabetes and migraine.
Aspirin and cardiovascular disease
Intent-to-treat analyses using pooled logistic regression found no significant effect of randomized aspirin assignment on incidence of the composite endpoint of major CVD ((hazard rate ratio (RR) = 0.91, 95 % confidence interval (CI) = 0.80, 1.03, P = 0.12; Table 2). When adjustment was made for cardiovascular risk factors and the intervening events shown in Table 1, there was no change in the estimate. As-treated analyses using actual observed aspirin use during the trial showed an effect closer to the null. Adjusting for the intermediate risk factors and events had little impact.
Treatment weights for the major CVD endpoint were constructed using the logistic regression results shown in Table 1, which served as the denominators of the weights. The numerators were created from similar models including year, age, race and previous aspirin use only. Censoring weights were constructed that predicted the probability of censoring prior to the administrative end of the study in March 2004. These two sets of weights were multiplied and accumulated over time to form the inverse probability weights. Censoring weights were constructed using the same terms, but showed little variability, ranging only from 0.83 to 1.39. After multiplication of treatment and censoring weights, the mean weight was 1.005, with a median of 0.999 (interquartile range of 0.967–1.009). The weights were truncated at the 0.01th and 99.99th percentiles, representing values of 0.022 and 6.914. The marginal structural model also found no overall association of aspirin use with incidence of major CVD. The RR was 0.93 with a CI based on the robust standard error of 0.81, 1.07 (P = 0.32).
The secondary endpoints of MI, stroke and cardiovascular mortality were analyzed separately. New weights were constructed using intervening events that were not part of the outcome. In the analysis of MI, stroke was an intervening event, and vice versa. For CVD mortality, both MI and stroke were considered intervening events. The distributions of weights for these outcomes were similar to those for major CVD. The pooled logistic intent-to-treat analysis showed no effect of randomized aspirin on MI, but an 18 % reduction in stroke (P = 0.03; Table 3). The estimated effects in the MSMs tended to replicate this, but the latter analysis tended to be more variable and less significant for stroke, likely due to the variability introduced by the weighting.
The analysis of cardiovascular mortality, however, found different results by type of analysis (Table 3). There was no effect of randomized aspirin on CVD mortality in the pooled logistic intent-to-treat analysis (RR = 0.95, 95 % CI: 0.74, 1.22, P = 0.67). The as-treated analysis found a small non-significant 12 % reduction after controlling for other intervening factors. The marginal structural model found a sizeable 24 % reduction in risk that remained non-significant with a wide confidence interval (95 % CI: 0.54, 1.08, P = 0.13).
Additional analyses considered the amount of aspirin use rather than treating it as a dichotomous exposure. Weights were formed using polychotomous logistic regression with aspirin use categories of none, 1–166 days per year, and at least 167 days per year. The latter corresponds to nearly complete use of the study aspirin which is taken every other day. The results remained null in both as-treated and marginal structural models (Table 4), although there was a suggested dose–response effect for CVD mortality in the MSM.
Finally, pooled logistic intent-to-treat analyses suggested a difference in effect by age at randomization, with a significant 23 % reduction in the composite endpoint of major CVD among those aged 65 or older at randomization (RR = 0.77, 95 % CI: 0.62, 0.96, P = 0.02; Table 5). This result was replicated in both the as-treated and marginal structural analyses, with a significant interaction with age. For those aged 65 or older, the estimated effect in the MSM was a 27 % reduction in major CVD. Similar effect modification was seen in the analysis of CVD mortality. Although the interaction with age was not statistically significant, the estimated reduction in CVD mortality was also stronger in those over age 65.
Discussion
Since the completion of trials in secondary prevention [1], aspirin has been in wide use following a cardiovascular event. Following the results of the PHS and other trials [18], it has been used increasingly in primary prevention. Indications for aspirin have continued to expand following release of the US Preventive Services Task Force reports advocating use for those with risk factors for CVD [8, 19]. Such use, however, can serve as a confounder of continued aspirin use even in trials where initial aspirin use is assigned randomly. As in the PHS, in the WHS we found that prior use, cardiovascular risk factors and, especially, intervening cardiovascular conditions strongly influenced aspirin use during the trial in both active and placebo aspirin groups.
The published intent-to-treat analysis of the WHS data found no significant effect of randomized aspirin assignment on incidence of the composite endpoint of major CVD ((RR = 0.91, 95 % CI = 0.80, 1.03, P = 0.13) [6]. As-treated and marginal structural analyses generally tended to replicate the intent-to-treat analysis for incidence of major CVD in these data. Results remained null regardless of the strong effects of intermediate variables such as angina and TIA on aspirin use. The same was true for the secondary outcome of MI, while results for stroke continued to suggest a reduction in risk. When CVD mortality was the outcome, however, a decreased risk was seen in the MSM analysis, although not statistically significant. Results were strikingly similar to those seen in the PHS (Table 6). In the male physicians, the intent-to-treat effect on CVD mortality was null, but a 26 % reduction in CVD mortality was seen using a MSM [10], similar to that seen in the WHS. Combining these two trials suggests an overall 25 % reduction in CVD mortality with aspirin use (RR = 0.75, 95 % CI = 0.57–0.99, P = 0.04).
It is possible that the MSM results are stronger for CVD mortality than for CVD incidence due to the nature and impact of the intervening events. To be a time-varying confounder in the causal pathway, the factor has to (a) be a predictor of subsequent aspirin use, (b) be affected by past use, and c) be an independent determinant of the outcome. In the analysis of incident CVD most of the factors in Table 1 predict subsequent aspirin use and are also known or suspected risk factors for incident cardiovascular disease. They may not, however, be affected by previous aspirin use. While TIA showed some association with randomized aspirin in the trial (RR = 0.78, 95 % CI = 0.64–0.94, P = 0.01) [6], revascularization procedures [6], diabetes [20], and migraine [21] did not. Many of the other intervening cardiovascular conditions are likely not strongly affected by aspirin use, including angina and other cardiovascular surgeries. Thus the link between prior aspirin use and CV risk factors is not present for CVD incidence, and the MSM analysis leads to results similar to the intent-to-treat and as-treated analyses.
In the analysis of CV mortality, however, the intervening events of MI or stroke are included in the weights and can be heavily influenced by previous aspirin use. In the PHS, aspirin strongly influenced the outcome of MI, and in the WHS, aspirin influenced the outcome of stroke. Both events strongly affect CVD mortality as well as subsequent use of aspirin. They are thus time-varying confounders influenced by exposure. Adjusting for intermediate MI or stroke in the as-treated analyses does not lead to as strong an effect. Using IPW more completely adjusts for differences in aspirin use following MI or stroke that may be unbalanced by other CV risk factors. The strengthening of results for CVD mortality in the MSM analysis is due to both intervening events and subsequent non-compliance.
Other analyses of these data suggest that the effect may be somewhat dose-related, at least in terms of days taken. Although not significant, the reduction seemed stronger in those who were taking aspirin at least 167 days per year. We were unable to directly analyze the effects of aspirin dose, however. The PHS used a dose of 325 mg every other day, while the WHS used a dose of 100 mg every other day. It is thus impossible to separate whether any differences in effects seen in the two trials are due to gender or dose. It is also possible that dose played a role in the present analyses. When individuals experience an MI or stroke, they are likely to take aspirin at a higher dose than that used in the WHS. Because confounding by dose is nearly complete, however, it is impossible to separate the effects of differing doses even using MSM models.
Other limitations of these analyses must be considered. These include self-reported measures of compliance as well as many of the intermediate risk factors. The main analyses assume proportionality of effects over time in the survival models and thus report the average effects over the study period. In addition, any attempts to suggest causality rely on the assumption of no unmeasured confounding and on the correct modeling of the treatment and censoring weights.
These analyses thus support the limited effects found in the intent-to-treat analysis for CVD morbidity. The effects of intermediate variables on aspirin use, or more likely, the effects of aspirin on the intermediate variables, were not sufficient to alter conclusions. Results for CVD mortality, however, were strengthened in the adjusted analyses. As for men, a serious nonfatal event such as an MI or stroke could be affected by previous aspirin use and could also lead to future aspirin use. Adjusting for such effects suggests an approximate 25 % reduction in CVD mortality with continued aspirin use versus continued nonuse. The analyses of CVD mortality reflect the effects of aspirin in both primary prevention for incident CVD and in secondary prevention for CVD mortality, where it has been shown to be effective in women as well as men. Lingering questions concern the effects of duration of use versus effects in secondary prevention. Whether a strategy of starting aspirin only after a CVD event, or the development of serious risk factors, is the most appropriate one for women remains to be determined.
References
Antiplatelet Trialists’ Collaboration. Secondary prevention of vascular disease by prolonged antiplatelet therapy. Brit Med J. 1988;296:320–31.
Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–62.
The Medical Research Council’s General Practice Research Framework. Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet. 1998;351:233–41.
The Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group [see comments]. N Engl J Med. 1989;321:129–35.
Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Brit Med J. 1988;296:313–6.
Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–304.
Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomized trials. Lancet. 2009;373:1849–60.
U.S. Preventive Services Task Force. Aspirin for the primary prevention of cardiovascular events: recommendation and rationale. Ann Intern Med. 2002;136:157–60.
Robins JM, Hernan M, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60.
Cook NR, Cole SR, Hennekens CH. Use of a marginal structural model to determine the effect of aspirin on cardiovascular mortality in the Physicians’ Health Study. Am J Epidemiol. 2002;155:1045–53.
Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women’s Health Study. J Womens Health Gend Based Med. 2000;9:19–27.
Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer. J Am Med Assoc. 2005;294:47–55.
Therneau TM, Grambsch PM. Modeling survival data: extending the cox model. New York: Springer; 2000.
Robins JM. Association, causation, and marginal structural models. Synthese. 1999;121:151–79.
Hernán M, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–70.
D’Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med. 1990;9:1501–15.
Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–64.
Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. Brit Med J. 2002;324:71–86.
U.S. Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396–404.
Pradhan AD, Cook NR, Manson JE, Ridker PM, Buring JE. A randomized trial of low-dose aspirin in the prevention of clinical type 2 diabetes in women. Diab Care. 2009;32:3–8.
Benseñor IM, Cook NR, Lee IM, Chown MJ, Hennekens CH, Buring JE. Low-dose aspirin for migraine prophylaxis in women. Cephalalgia. 2001;21:175–83.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute [grant numbers HL-43851 and HL-84609]; and the National Cancer Institute [grant number CA-47988]. Aspirin and aspirin placebo were provided by Bayer HealthCare. The authors would like to thank Marilyn Chown, Rimma Dushkes, and Gregory Kotler for expert data management and programming assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cook, N.R., Cole, S.R. & Buring, J.E. Aspirin in the primary prevention of cardiovascular disease in the Women’s Health Study: Effect of noncompliance. Eur J Epidemiol 27, 431–438 (2012). https://doi.org/10.1007/s10654-012-9702-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-012-9702-x