Abstract
The levels of 238U, 232Th, and 40K with gross alpha and beta values in soils collected from the Kahramanmaraş city center were measured in this study, and the annual effective dose equivalent (AEDE), excessive lifetime cancer risk (ELCR), and terrestrial absorbed gamma dose rates were calculated for gamma radiation from 238U, 232Th, and 40K radionuclides. The samples’ gross alpha and beta radioactivity concentrations, respectively, range from 0.06 ± 0.01 Bq/kg to 0.45 ± 0.04 Bq/kg and 0.14 ± 0.02 Bq/kg to 0.95 ± 0.09 Bq/kg. The Kahramanmaraş province’s soil samples have mean gross alpha and beta radiation values of 0.25 ± 0.03 Bq/kg and 0.52 ± 0.05 Bq/kg, respectively. 238U, 232Th, and 40K activity concentrations in soil samples range from 2.32 ± 0.2 Bq/kg to 40.10 ± 1.4 Bq/kg, 0.60 ± 0.03 Bq/kg to 10.47 ± 1.01 Bq/kg, and 11.60 ± 1.01 Bq/kg to 160.84 ± 4.6 Bq/kg, respectively. 238U, 232Th, and 40K each had average activity concentrations of 11.50 ± 1.1, 4.50 ± 0.4, and 62.20 ± 1.6 Bq/kg in soil, respectively. The annual effective dose equivalent (AEDE), excessive lifetime cancer risk (ELCR), and terrestrial absorbed gamma dose rate range from 0.01 ± 0.01 μSv y−1 to 0.03 ± 0.02 μSv y−1, and 0.01 ± 0.01 × 10−3 to 0.12 ± 0.03 × 10−3, 1.72 ± 0.01 nGy h−1 to 25.05 ± 0.21 nGy h−1, respectively. Additionally, the average annual effective dose equivalent (AEDE), average excessive lifetime cancer risk (ELCR), and average terrestrial absorbed gamma dose rates are 0.01 ± 0.01 μSv y−1, and 0.05 ± 0.02 × 10−3, 9.81 ± 0.09 nGy h−1, respectively. The acquired data were compared to both domestic and international standards.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The major source of soil radioactivity is rocks. The concentration of radioactivity contained in the soil; weather conditions, sedimentation, filtration, absorption, and precipitation as a result of the movement of groundwater, irrigation with other substances, and high degree of porosity may increase or decrease. The presence of natural radionuclides in the soil, such as 238U, 232Th, and 40K, makes the soil radioactive. The majority of naturally occurring radionuclides are concentrated in volcanic, phosphate, granite, and salt rocks. Depending on the weather, these rocks erode over time and become extremely minute fragments that mix with the soil when it rains or when water flows through them. By doing this, they raise the soil’s inherent radioactivity. Even certain human practices that are utilized in agriculture to boost soil productivity, such as artificial insemination and fertilization (artificial fertilizers include radioactive 32P), raise the soil’s localized surface radioactivity (Niewiadomski et al., 1980). Liden & Holm (1985) provide concentrations of 40K, 232Th, and 238U in various rocks and soils. As a result, it can be shown that different types of rocks have different activity concentration quantities of 238U, 232Th, and 40K. The highest 40K and 232Th activity concentration amounts are seen to be in erupted granite rock species, while the highest 238U activity concentration amount is seen to be in sedimentary clay skewers. From this point of view, we can say that granite rocks are rich in potassium and thorium, while clay-swollen rocks are rich in uranium. Therefore, according to the types of rocks under the soil on earth, variations can be seen in the amount and types of radioactivity contained in the soil. In alkaline rocks, another type of rock, uranium, and thorium may also tend to be abundant (Gascoyne, 1992). It is said that uranium and thorium have high concentrations due to the fact that they are rich in quartz in eruption and granite rocks (Valkovic, 2000). In the report published by UNSCEAR, it is estimated that gamma radiation levels are also quite high in places where thorium-rich granite rocks are found in many places (UNSCEAR, 1993).
When the geological structure of the earth is examined, it is seen that there are rock beds just below the soil layer of a certain thickness. These rock deposits are also presumed to cause terrestrial radioactivity. In particular, it is known that a significant part of gamma radiations originate from the surface layer at a depth of 0–25 cm (UNSCEAR, 1993). Significant levels of thorium are present in some regions’ granite rocks, which are dispersed across extremely broad areas. According to radiometric studies, the levels of gamma radiation observed in the vicinity of these rocks are relatively high. The radiation intensity measured in the air in the Colorada region of the USA is around 8–16 μR/hour, in Brazil it is 5–158 μR/hour, in Italy it is 7–50 μR/hour, in Sweden, it is 2–400 μR/hour, in India, it is around 2–110 μR/hour. As can be seen, natural radiation levels are quite high in these countries (UNSCEAR, 1993). The annual radiation doses that people living in these countries receive from natural sources are much higher than in other countries.
The radioactivity present in the soil changes slightly with biochemical processes. While the development of plant root systems provides balance in the soil, on the one hand, it also takes the water that the plant needs from the soil. The disintegration of rocks and their subsequent mixing with soil through water are both processes that depend heavily on humic acid. Oxidations in the soil’s bottom layers are where the breakdown of organic materials in the soil starts. Over time, the substrates lose some of their uranium content. Mobility in the soil continues with the formation of iron oxides and other elements. The acid formed in some soils prevents the retention of radionuclides by means of calcium carbonate in the environment. These stages of development in the soil also reduce radionuclide concentrations in rocks and therefore external radiation levels. People are exposed to natural radiation at those rates because of the natural radionuclides in the soil, which alter the natural radiation of the environment or environment according to their percentage in the soil (UNSCEAR, 1982a). Radionuclides that were once present in the earth’s crust have relatively low average amounts. Myrick et al., provided activity concentrations of naturally occurring radionuclides in soil (Myrick et al., 1983). As a result, the average activity concentration of 40K in soil is 370 Bq/kg, 232Th in activity is 35 Bq/kg, 238U in activity is 35 Bq/kg, and 226Ra in activity is 40 Bq/kg.
Many studies have been carried out in recent years to determine the level of natural radioactivity in soil and rock samples (Algattawi et al., 2019; Dina et al., 2022; Milena et al., 2022).
This research aims to determine the gross alpha and beta radioactivity values and the activity concentrations of radionuclides (238U, 232Th, and 40K) in soil samples collected in Kahramanmaraş. Additionally, this study aims to calculate terrestrial absorbed gamma dose rate and the annual effective dose equivalent (AEDE) in soil samples using the activity concentrations of gamma-ray emitting radionuclides (238U, 232Th, and 40K), and to calculate excessive lifetime cancer risk (ELCR). This is the first research to assess the radioactive level of soil samples in Kahramanmaraş’s city center. Thus, a basic level was identified in terms of the radioactive level of soil samples in Kahramanmaraş’s city center, and it is also added to the existing literature.
Study area
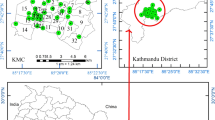
This research was carried out at Kahramanmaraş, Turkey. In terms of population, Kahramanmaraş is Turkey’s 11th biggest province. It is Turkey’s 18th biggest province with a surface area of 14,327 km2. It is situated between the 37–38 northern parallels and the 36–37 eastern meridian. Figure 1 depicts the geographical map of the province of Kahramanmaraş, Turkey, as well as the map of places acquired from the samples (https://www.google.com/maps, 2022). For this study, 14 soil samples were collected from 14 different locales of Kahramanmaraş. These locations are in the city center. The locations and geographic coordinates of the places where the samples were taken as in Table 1. A thorough understanding of geology, including bedrock and soil, is required for soil natural radioactivity mapping. So, the places where the samples were taken and the soil and rock structures of these places are given in Table 1.
Turkey, Kahramanmaras, and Study Area-Map (https://www.google.com/maps/)
Materials and methods
With consideration for the sample collection requirements, such as not dumping fertilizer or lime from the areas where soil samples would be taken in and around, and not burning stalks, roots, and weeds, samples were obtained with a garden shovel from a depth of 0–5 cm. While samples were taken in a neighborhood, samples were taken not only from one place but also from several points in the neighborhood and placed in disposable sterile bags, and soil samples of 2 kg weight taken from each neighborhood were mixed homogeneously. Without wasting any time, the soil samples collected as a result of the field investigations were brought to the lab environment, where they were laid out on single-use plastic bags to dry at room temperature for ten days. With this process, the amount of moisture present in the samples was reduced to a minimum. The soil samples wrapped in aluminum foils were then subjected to drying in an oven at 105 °C. It did not participate in the calculations because the amount of radioactivity that would be lost by evaporation during the drying process was negligible. As a result of this process, the soil samples that were completely dried were sifted using a special sieve with 100 mesh pores and labeled in clear plastic bags. Then, each soil sample was transferred to a tared planchette in a quantity that would not cause self-absorption in a way to exhibit a homogeneous distribution, and a quantity of pure water was added to it to prevent sample loss and dried on the electric stove. The samples in the 2-inch-diameter stainless steel planchettes were then dried for approximately 2 h at 105 °C before being kept in a suitable condition until counting. The soil samples were prepared for counting using the aforementioned techniques, and the gross alpha, gross beta, and radioactive contents were determined using the proper counting systems.
Measurement of gross alpha and gross beta activity concentrations
The samples were measured for their gross alpha radioactivity with 7286 low-level alpha counters, and their gross beta radioactivity with windowless scintillation counters. Calibration of counting systems was performed using the pertinent manuals (Bal et al, 2015). For each counting period, the counting time was 3000 s for gross alpha activities and 1000 s for gross beta activities. To calculate total counts for each gross alpha and gross beta activity, three counting periods were chosen. For the purpose of calculation, the background was subtracted from the gross count to produce the net counts. (Karahan, 1997; Doğru and Canbazoğlu, 2002; Canbazoğlu et al., 2000; Doğru et al., 2002). The concentrations of gross alpha and beta activity in the soil samples were determined using the equations in (3.1) and (3.2) below.
In these equations; Aα Aβ: The amount of activity in pCi, NS: is the net count in a minute, SS is: Standard deviation, VDF is: the yield correction factor, 2.22 is the conversion factor (1 pCi = 2.22 dpm), and E is the beta calculated from the corresponding calibration curve is the yield factor (cpm/dpm) (Karahan, 1997; Doğru and Canbazoğlu, 2002; Canbazoğlu et al., 2000; Doğru et al., 2002). It is given by the equation of yield correction factor (3.3) for the activity counted on the aluminum planchet,
The value of T in this equation is found from the self-absorption curves for U3O8 and KCl drawn according to certain standards as a function of thickness in mg/cm2 (Karahan, 1997; Tuncer, 1991). The parameter V in Eq. (3.3) is the yield and is calculated by Eq. (3.3). The following Eq. (3.4) is used to convert the counting rate into decays,
where G: Correction factor for geometry, B: Scatterback correction factor, and T: Self-absorption correction factor. 1/GBT is the yield correction factor and is VDF for short.
The thickness varies with the effective area with the mass of the sample and is expressed by the Eq. (3.5),
Measurement of radionuclides activity concentrations
A gamma-sensitive scintillation counter was used for concentration counts of the radionuclide activity of the samples. The energy of the uranium or thorium series that is most prevalent is measured along with the amounts of the radionuclides 238U, 232Th, and 40K in the samples. The most abundant radioisotopes of the uranium and thorium series in nature are as follows. 226Ra is a decay product of 238U. There is a photo peak energy with a 36% abundance at 186 keV, according to Degerlier et al. (2008). This photo peak energy does not appear in the spectrum as it is intertwined with the photo peak energy of 235U at 185.7 keV. But it is possible to find it by account. This radionuclide is evaluated separately from 238U in activity measurements (Karahan, 1997). 214Pb is the decay product of 238U and is detected at a photo peak energy of 351 keV at a 36% abundance, according to Degerlier et al. (2008).
214Bi is a decay product of the 238U series, seen at 609 keV with a 45% abundance. 228Ac is a decay product of the 232Th series, for example, there are many peaks at different energies with an abundance of 12% at 338 keV, 911 keV, and 5% at 28.964 keV (Degerlier, et al., 2008). The 208Tl is a decay product of the 232Th series and has the highest abundance at 583 keV with 30.7% despite having many peaks at different energies. 40K is found alone in nature. There is no decay series. It is the natural radioactive element that contributes the most to the natural gamma radiation dose, as it is abundant in soil. At the photo peak energy of 1460 keV, there is an abundance of 11%. In light of this information, for 238U, 238U has 214Bi 609 keV energy, which is a decay product of 45% in nature, and for 232Th, gamma rays with an energy of 583 keV of 208Tl, which is a decay product of the 232Th series and has the highest abundance of 30.7% in nature, and gamma rays with 1460 keV energy for 40K were used. Each sample was counted for a period of 10 h. Energy calibration of the detector was performed by using 60Co (37 kBq) and 226Ra (370 kBq) point sources. Photo peak efficiency was 24%.238U, 232Th, and 40 K activity concentrations. The concentrations of 238U, 232Th, and 40K radionuclides in the soil samples were determined using the equation in (3.7) below.
A is an activity in Bq/kg; Cn is the net number of counts per minute, Mn is the mass of the sample, V is the efficiency of the detector, and P is the absolute probability of transition of gamma decay (Baykara & Doğru, 2009).
Terrestrial absorbed gamma dose rate (D)
The amount of gamma radiation absorbed by the body each hour as a result of exposure to terrestrial radiation at a height of 1 m above the soil surface is known as the terrestrial absorbed gamma dose rate (D). The following calculation can be used to compute the terrestrial absorbed gamma dose ratio (Beck, 1972).
where the numbers 0.427, 0.662, and 0.043 are the 238U, 232Th, and 40K radionuclide dose coefficients, respectively. The radionuclides 238U, 232Th, and 40K’s respective activity concentrations are written as AU, ATh, and AK in Bq kg−1 units. The rate of terrestrial absorption gamma radiation for soil samples was estimated in the study and expressed in nGy h−1.
Annual effective dose equivalent (AEDE)
The biological impact of the gamma dose rate received in the air and the radiation dosage to which it is subjected over the course of a year is calculated using the annual effective dose equivalent (AEDE). The following equation is used to determine the annually effective dosage equivalent (UNSCEAR, 1982b, 1988):
It’s important to know how long gamma radiation is exposed when estimating how gamma rays affect living organisms. Exposure time is defined as the occupancy factor. When a year is taken as 365 days and there is radiation exposure for 24 h, people are exposed to 8760 h of radiation for a year. Assuming that an average person spends about 5 h a day outdoors, the busyness factor is taken as 0.2. The coefficient of 0.7 in equity is the coefficient of conversion of air-absorbed dose to effective dose for adults. For each soil sample used in the study, the yearly effective dosage equivalent was determined (Degerlier et al., 2008; UNSCEAR, 1982b, 1988).
Excessive lifetime cancer risk (ELCR)
When a population is exposed to ionizing radiation over the course of a lifetime, there is a chance that cancer may develop. This is referred to as excessive lifetime cancer risk. The formula for calculating excessive lifetime cancer risk (ELCR) is as follows: (Kolo et al., 2017; Taskin et al., 2009).
where the study’s annual active dosage equivalent, is denoted as AEDE, and the average life expectancy is denoted as DL (70 years). 0.057 Sv−1 is used as the value for RF, which stands for the risk of deadly cancer per risk factor Sievert (ICRP, 2007).
Results and discussion
In this section, the gross alpha and gross beta activity as well as the quantities of naturally occurring radioactive nuclei found in 14 soil samples taken from Kahramanmaraş’s city center are assessed. These observations identified the 40K radioisotope as well as the activity concentrations of the most prevalent components of the 238U and 232Th natural radioactive series. Annual effective dose equivalent (AEDE) for gamma radiation from 238U, 232Th, and 40K radionuclides, excess lifetime cancer risk (ELCR), and the calculated terrestrial absorbed gamma dose rates are also given in the following subheadings.
Gross alpha and beta activity concentrations in soil samples
Table 2 lists the gross alpha and gross beta radiation values of soil samples taken from various areas to describe the Kahramanmaraş city center. When the gross alpha and gross beta radiation levels of soil samples from the province center of Kahramanmaraş are analyzed, the samples’ gross alpha radioactivity levels vary from 0.06 ± 0.01 to 0.45 ± 0.04 Bq/kg, and their gross beta radioactivity levels range from 0.14 ± 0.02 to 0.95 ± 0.09 Bq/kg. Figures 2 and 3 display the frequency distribution of soil’s gross alpha and beta radioactivity.
Figure 2 shows that the range of gross alpha concentration in five samples was between 0.20 and 0.30 Bq/kg. Additionally, the range of gross alpha content in four samples was 0.10–0.20 Bq/kg. In eight samples, the concentration of gross beta ranged from 0.40 to 0.60 Bq/kg, as shown in Fig. 3. There are two samples with gross beta concentrations ranging from 0.20 to 0.40 to 0.80 to 1.00 Bq/kg.
The Hürriyet neighborhood sample has the greatest gross alpha radiation level at 0.45 ± 0.04 Bq/kg, while the Kazma Bağları location sample has the lowest gross alpha radioactivity level at 0.06 ± 0.01 Bq/kg, as can be noted. Furthermore, the Karamanlı neighborhood has the lowest gross beta radiation level (0.14 ± 0.02 Bq/kg), whereas the Gaffarlı area has the greatest gross beta radioactivity level (0.95 ± 0.09 Bq/kg). The average gross radioactivity values of the soil samples from the province of Kahramanmaraş are 0.25 ± 0.03 Bq/kg and 0.52 ± 0.05 Bq/kg, respectively.
As seen in Table 2; when all of the samples are compared with each other, it is seen that the radioactivity levels of the soil samples Kazma Bağları position with 1 number and the sample Yenişehir neighborhood with 14 number, the sample Gaffarlı neighborhood with 5 number, and the sample Eyüp Sultan neighborhood with 8 number and the sample Oruç Reis neighborhood with 10 number and the sample Hürriyet neighborhood with 12 number and the sample Akif İnan neighborhood with 13 number are remarkable with each other. Gross alpha radioactivity levels of samples are distinct from one another with values of 0.06 ± 0.01 Bq/kg and 0.21 ± 0.02 Bq/kg; however, the beta radioactivity levels of samples with 1 and 14 numbers are quite near to one another with values of 0.56 ± 0.05 Bq/kg and 0.54 ± 0.05 Bq/kg, respectively. Additionally, despite the fact that samples with the numbers 5 and 8 had gross beta radioactivity levels that are quite similar to one another at 0.95 ± 0.09 Bq/kg and 0.92 ± 0.09 Bq/kg, respectively,
The samples’ respective gross alpha radioactivity values are 0.41 ± 0.04 Bq/kg and 0.27 ± 0.03 Bq/kg. Additionally, the gross alpha radioactivity levels of the samples with the 10, 12, and 13 numbers are different from one another, with values of 0.17 ± 0.02 Bq/kg, 0.45 ± 0.04 Bq/kg, and 0.22 ± 0.02 Bq/kg, respectively, despite the fact that the samples’ gross beta radioactivity levels are very similar to one another with values of 0.43 ± 0.04 Bq/kg, 0.45 ± 0.04 Bq/kg, and 0.44 ± 0.04 Bq/kg, respectively. In addition to the amount of radioactivity in the soils, which varies based on a number of factors, including weather, soil filtration, absorption, sedimentation produced by groundwater movement, degree of porosity, etc., diverse soil structures and rock structures also exist. In Table 1, information is given about the soil structure of the regions where soil samples are taken.
Despite having the same soil structure, samples with the numbers 1, 4, 5, 6, and 11 had different gross alpha and gross beta activity. The underlying rock structure and different soil profiles might both be to blame for this.
When samples with 2, 8, 9, and 10 numbers are examined, the soil structures are the same. Not only did Alivual soils cause the gross alpha radioactivity of the uranium-rich limestone and conglomerate soil sample to be high, but also the alluvial nature of the soil caused the highest gross beta radioactivity to be measured (Liden & Holm, 1985; Hansen & Stout, 1968). Samples with 3, 7, and 13 numbers have the same soil structure. They are at modest levels when measured against the gross alpha and gross beta radioactivities of other soil samples. The reason for this may be that the regions where samples with 3, 7, and 13 numbers were taken are dense with metamorphic marbles and sandy limestone, which are old units. (Valkovic, 2000). The soil structure of samples with 12 and 14 numbers is colluvial soil. Although the gross beta activities are close to each other, the difference in gross alpha activities may be due to the change in the free lime content of the soil structure.
Table 3 lists the gross alpha and beta values for soil samples obtained from the city center of Kahramanmaraş and from other investigations. The gross alpha and beta values in studies by Lee et al. (2014), Bose et al. (1993), Dimovska et al. (2012), Bal et al. (2015), and Canbazoğlu et al. (2012) are significantly higher than the values in our study when the gross alpha and beta values of soil samples taken from Kahramanmaraş city center are compared with those in other studies. However, the values of our study and the study by Chijioke et al., (2018) in Nigeria are in agreement. This may be due to the soil and rock structures in the study areas. The reason for the differences in the radioactivity concentration of the soils in other studies may have been due to the weather conditions in the study area. In the same way, it may have differed due to many effects such as sedimentation, filtration, absorption, sedimentation caused by the movement of groundwater, high porosity, etc. In addition to these, it may have differed due to the different soil rock structures.
The activity concentrations of radionuclides (238U, 232Th and 40K) in soil samples
Table 4 lists the activity concentrations of 238U, 232Th, and 40K in 14 soil samples collected from the Kahramanmaraş city center. When Table 4 is studied, the Pnarbaşı neighborhood sample has the lowest concentration of 238U at a value of 2.32 ± 0.2 Bq/kg, and the Gaffarlı neighborhood sample has the greatest concentration at a value of 40.10 ± 1.4 Bq/kg. Besides; the lowest concentration of 232Th is in the Hürriyet neighborhood sample with 0.60 ± 0.03 Bq/kg, and the highest concentration of 232Th is in the Göllü neighborhood sample with 10.47 ± 1.01 Bq/kg. Apart from these, the Oruç Reis neighborhood sample has the lowest 40K concentration (11.60 ± 1.01 Bq/kg), while the Pnarbaşı neighborhood sample has the highest 40K concentration (160.84 ± 4.6 Bq/kg). There are currently 11.50 ± 1.1, 4.50 ± 0.4, and 62.20 ± 1.6 Bq/kg, respectively, of 238U, 232Th, and 40K activity concentrations in soil.
Figures 4, 5, and 6 display the frequency distribution of soil’s 238U, 232Th, and 40K radioactivity concentrations. In ten samples, the quantity of 238U ranged from 0.00 to 10.00 Bq/kg, as shown in Fig. 4. Figure 5 shows that the concentration of 232Th ranged from 0.00 to 2.00 Bq/kg in six samples. Figure 6 shows that the content of 40K ranged from 0.00 to 50.00 Bq/kg in eight samples.
The literature reports that the average activity concentrations of 238U, 232Th, and 40K in soil are 25 Bq/kg, 25 Bq/kg, and 370 Bq/kg, respectively, on a global scale (Liden & Holm, 1985). When these values are compared to the mean activity concentrations of 238U, 232Th, and 40K in soil samples taken from the heart of Kahramanmaraş, it is found that the concentrations do not go over these limits. The remaining samples, with the exception of the sample with the number 5, don’t appear to surpass these levels for the concentration of 238U. When a sample with five numbers is analyzed, it becomes clear that the 40K activity concentration is also larger than the concentrations in the other samples. This results from the sample with the 5 number also having greater gross alpha and gross beta activity levels than the other samples, that is, the soil structure of this sample (lime-rich claystone potassium-rich) and the rock structure on which it is located (the middle eocene, formed in 38–54 million years, sandstone, mudstone, pebble, spilite, serpentine, limestone, and conglomerate). The main reason why soil samples have a higher concentration of 238U than 232Th concentration is that the samples are taken from a depth of 0–5 cm. In scientific studies, it has been reported that the upper part of the soil has a higher concentration of uranium than thorium (Hansen & Stout, 1968). The fact that the top section of the soil is rich in organic matter is frequently thought to be the cause of the concentrations of 238U and 232Th in soil samples changing in an inverse proportion. This is due to the fact that uranium’s mobility is less and thorium’s mobility is stronger in soils with a high level of organic matter (Hansen & Huntington, 1969; Schulz, 1965).
Table 5 lists the activity levels of 238U, 232Th, and 40K in soil samples obtained from Kahramanmaraş’s city center and from other investigations. When compared to previous studies, the activity concentrations of 238U, 232Th, and 40K in soil samples from the center of Kahramanmaraş are much greater in research by Baykara & Doğru (2009), Al-Hamarneh and Awadallah (2009), UNSCEAR (2000a), Kova’cs et al., (2013), Tsai et al., (2008), and Hannan et al., (2013). The reason for the differences in the radioactivity concentration of the soils; may have been due to the weather conditions in the study area. In the same way, it may have differed due to many effects such as sedimentation, filtration, absorption, sedimentation caused by the movement of groundwater, high porosity, etc. In addition to these, it may have differed due to the different soil rock structures.
AEDE, ELCR, and terrestrial absorbed gamma dose rate
The computed annual effective dose equivalent (AEDE), excessive lifetime cancer risk (ELCR), and terrestrial absorbed gamma dose rate are all shown in Table 6. Accordingly to this, the terrestrial absorbed gamma dose rate, annual effective dose equivalent (AEDE), and excessive lifetime cancer risk (ELCR) range from 1.72 ± 0.01 nGy h−1 to 25.05 ± 0.21 nGy h−1, 0.01 ± 0.01 μSv y−1 to 0.03 ± 0.02 μSv y−1, and 0.01 ± 0.01 × 10−3 to 0.12 ± 0.03 × 10−3, respectively. İn addition, the average terrestrial absorbed gamma dose rate, average annual effective dose equivalent (AEDE), and average excessive lifetime cancer risk (ELCR) are 9.81 ± 0.09 nGy h−1, 0.01 ± 0.01 μSv y−1, and 0.05 ± 0.02 × 10−3, respectively.
The global average rate of terrestrial gamma radiation absorption is 60 nGy h−1 (UNSCEAR, 2000b). The current study’s mean terrestrial absorbed gamma radiation rate is lower than the global average value. Outdoor terrestrial gamma radiation has an average annual effective dose equivalent (AEDE) of 70 mSv worldwide (UNSCEAR, 2000b). As a result, the Kahramanmaraş city center’s average yearly effective dosage is lower than the global average value. UNSCEAR reports that the average ELCR value throughout the globe is 0.29 × 10−3 (UNSCEAR, 2000b). The average ELCR for the current research is thus lower than the global average value.
Conclusion
With the exception of the 238U concentration of the sample with the number 5, it appears that the other samples do not exceed these values when the activity concentrations of 238U, 232Th, and 40K in soil samples from the Kahramanmaraş city center are compared to global average activity concentrations values. Additionally, the average excessive lifetime cancer risk (ELCR), average annual effective dose equivalent (AEDE), and average terrestrial absorbed gamma radiation rate in Kahramanmaraş city center are lower than the global average values.
As a result; although the level of natural radioactivity caused by terrestrial radionuclides is low in the city center of Kahramanmaraş, people’s living environments and eating habits will determine the dose they will be exposed to. It turns out that it is impossible to escape from natural radiation. However, the fact that we are surrounded by natural radiation does not mean that we are in great danger, and the human body needs natural radiation as well as all the elements in nature. It is in our hands to reduce natural radioactivity levels in the environments we live in. For example, good ventilation in our homes and workplaces reduces the level of radiation we breathe, or filtering drinking water can prevent the radionuclides in the body from being taken into the body. In this regard, the radon gas emissions of our samples should be evaluated in the next few days to determine the amount of natural radioactivity in the Kahramanmaraş city center. In addition, these works should be carried out in the city center of Kahramanmaraş, in the local well waters used as drinking and utility water, and in the dam lakes that provide aquaculture products to the city. With this study, the natural radiation level of the Kahramanmaraş city center, which had not been done before, is determined. Thus, these study results can be used as a reference value for future differences.
Data availability
The corresponding author can provide you with the information needed to access the data that support this study’s conclusions.
References
Algattawi, A. A. A., Fayez-Hassan, M., Khalil, E. I., & Elez, H. A. (2019). Radiological effects of soil and rock samples of different Libyan regions. Engineering, 11(5), 247–259.
Al-Hamarneh, I. F., & Awadallah, M. I. (2009). Soil radioactivity levels and radiation hazard assessment in the highlands of northern Jordan. Radiation Measurements, 44(1), 102–110.
Bal, S. Ş, Kuluöztürk, M. F., & Doğru, M. (2015). The gross alpha and beta radioactivity concentration on the Sivrice (Elazığ) fault zone. Bitlis Eren University Journal of Science and Technology, 2(2), 49–51.
Baykara, O., & Doğru, M. (2009). Determination of terrestrial gamma, 238U, 232Th and 40K in soil along fracture zones. Radiation Measurements, 44(1), 116–121.
Beck, H. (1972). Physics of environmental gamma radiation fields (No. CONF-720805--P1).
Bose, S. R., Williamson, T. G., Mulder, R. U., & Molla, M. R. (1993). Impact of a 2 MWth research reactor on radioactivity in sediments. Health Physics, 65(2), 200–208.
Canbazoğlu, C., Doğru, M., Çelebi, N., & Kopuz, G. (2012). Assessment of natural radioactivity in Elazığ region, eastern Turkey. Journal of Radioanalytical and Nuclear Chemistry, 292(1), 375–380.
Canbazoğlu, C., Doğru, M., Yalçın, M., Baykara, O., & Külahcı, F. (2000). Elazığ ili içme ve kullanım sularındaki toplam alfa ve beta radyoaktivite seviyelerinin belirlenmesi. Fırat Üniversitesi Fen Ve Mühendislik Bilimleri Dergisi, 12(2), 125–133.
Chijioke, M. A., Chikwendu, E. O., Benedict, C. E., Chinedu, I., & Bridget, A. O. (2018). Gross alpha and beta activity concentrations in soil and some selected Nigerian food crops. International Journal of Physical Sciences, 13(11), 183–186.
Degerlier, M., Karahan, G., & Ozger, G. (2008). Radioactivity concentrations and dose assessment for soil samples around Adana Turkey. Journal of Environmental Radioactivity, 99(7), 1018–1025.
Dimovska, S., Stafilov, T., & Šajn, R. (2012). Radioactivity in soil from the city of Kavadarci (Republic of Macedonia) and its environs. Radiation Protection Dosimetry, 148(1), 107–120.
Dina, N. T., Das, S. C., Kabir, M. Z., Rasul, M. G., Deeba, F., Rajib, M., & Ali, M. I. (2022). Natural radioactivity and its radiological implications from soils and rocks in Jaintiapur area, North–east Bangladesh. Journal of Radioanalytical and Nuclear Chemistry, 331(11), 4457–4468.
Doğru, M., Canbazoğlu, C., & Şahin, S. (2002). Determination of the gross alpha and beta radioactivity in Kangal curative water. Balkan Physics Letters, 10, 130–134.
Gascoyne, M. (1992). Geochemistry of the actinides and their daughters. In Uranium-series disequilibrium: applications to earth, marine, and environmental sciences. 2 edn.
Hannan, M. A., Nguyen, N., & Rivas, M. (2013). Natural radioactivity and its gamma dose rate in mission (Texas) soils. Journal of Radioanalytical and Nuclear Chemistry, 295, 729–736.
Hansen, R. O., & Stout, P. R. (1968). Isotopic distributions of uranium and thorium in soils. https://www.google.com/maps Date of access: 11 July 2022
Hansen, R. O., & Huntington, G. L. (1969). Thorium movements in morainal soils of the high Sierra California. Soil Science, 108(4), 257–265.
Karahan, G. (1997). İstanbul’un çevresel doğal radyoaktivitesinin tayini ve doğal radyosyonların yıllık etkin doz eşdeğeri (Doctoral dissertation, Enerji Enstitüsü).
Kolo, M. T., Amin, Y. M., Khandaker, M. U., & Abdullah, W. H. B. (2017). Radionuclide concentrations and excess lifetime cancer risk due to gamma radioactivity in tailing enriched soil around Maiganga coal mine, Northeast Nigeria. International Journal of Radiation Research, 15(1), 71–80.
Kovács, T., Szeiler, G., Fábián, F., Kardos, R., Gregorič, A., & Vaupotič, J. (2013). Systematic survey of natural radioactivity of soil in Slovenia. Journal of Environmental Radioactivity, 122, 70–78.
Lee, S. K., Wagiran, H., & Ramli, A. T. (2014). A survey of gross alpha and gross beta activity in soil samples in Kinta District, Perak. Malaysia. Radiation Protection Dosimetry, 162(3), 345–350.
Lıden, K., & Holm, E. (1985). Measurement and dosimetry of radioactivity in the environment. The Dosimetry of Ionizing Radiation, 1, 291–367.
Milena, Z., Nenad, Z., Tijana, Z., Milan, S., Vesna, M., Dragana, K., & Dragoslav, N. (2022). Ecological studies of the naturally occurring radionuclides, 137Cs and heavy metals in soil, plants and milk in surrounding of Kragujevac city, Serbia. Journal of Radioanalytical and Nuclear Chemistry, 331(3), 1285–1298.
Myrick, T. E., Berven, B. A., & Haywood, F. F. (1983). Determination of concentrations of selected radionuclides in surface soil in the US. Health Physics, 45(3), 631–642.
Niewiadomski, T., Koperski, J., & Ryba, E. (1980). Natural radiation in Poland and its disturbance in an urban environment. Health Physics, 38(1), 25–32.
Schulz, R. K. (1965). Soil chemistry of radionuclides. Health Physics, 11(12), 1317–1324.
Taskin, H., Karavus, M., Ay, P., Topuzoglu, A., Hidiroglu, S., & Karahan, G. (2009). Radionuclide concentraons in soil and lifeme cancer risk due to gamma radioacvity in Kirklareli Turkey. Journal of Environmental Radioactivity, 100(1), 49–53.
ICRP (International Commission on Radiological Protection), (2007). The 2007 recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 37(2–4). Elsevier Ltd.
Tsai, T. L., Lin, C. C., Wang, T. W., & Chu, T. C. (2008). Radioactivity concentrations and dose assessment for soil samples around nuclear power plant IV in Taiwan. Journal of Radiological Protection, 28(3), 347.
Tuncer, S. (1991). Çeşitli İçme Sularında Radyoaktivite Seviyelerinin Tayinin (Doctoral dissertation, Yüksek Lisans Tezi, İTÜ Nükleer Bilimler Enstitüsü, İstanbul).
UNSCEAR, (1982a). United Nations Scientific Committee on the Effects of Atomic Radiation. Ionizing radiation: sources and biological effects. 1982a report to the general assembly, with annexes.
UNSCEAR, (1982b). United Nations Scientific Committee on the Effects of Atomic Radiation, Sources and Biological Effects of Ionizing Radiation. United Nations, New York.
UNSCEAR, (1988). United Nations Scientific Committee on the Effects of Atomic Radiation, Sources and Biological Effects of Ionizing Radiation. United Nations, New York.
UNSCEAR, (1993). Report, United Nations scientific committee on the effects of atomic radiation sources, effects and risks of ıonizing radiations. New York.
UNSCEAR (2000a) Source and effects of ionizing radiation. United Nations Scientific Committee on the Effects of Atomic Radiation, United Nations, New York
UNSCEAR,. (2000b). United Nations Scientific Committee on the Effects of Atomic Radiation, Sources and Biological Effects of Ionizing Radiation. United Nations, New York
Valkovic, V. (2000). Radioactivity in the environment: Physicochemical aspects and applications. Elsevier.
Acknowledgements
We would like to thank Doç. Dr. Sultan Şahin Bal, Doç. Dr. Şule Karatepe Çelik for support.
Funding
This research was not supported by any organization.
Author information
Authors and Affiliations
Contributions
Study conception, design, and data collection are done by Erdal Küçükönder and Serdar Gümbür. Serdar Gümbür wrote the main manuscript text and prepared figures. Ömer Söğüt and Mahmut Doğru helped analyze and interpret of results, and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they have no interest in conflict. All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Küçükönder, E., Gümbür, S., Söğüt, Ö. et al. Natural radioactivity in soil samples taken from Kahramanmaraş provincial center. Environ Geochem Health 45, 5245–5259 (2023). https://doi.org/10.1007/s10653-023-01577-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-023-01577-w