Abstract
This study investigated 16 United States environmental protection agency priority PAHs profiles and their sources in 40 urban soils collected from two industrialised cities, Jamshedpur and Bokaro, in east India and assessed their health risk to humans. The results showed the predominance of high molecular weight (HMW) PAHs (4–5 rings). The total PAHs concentration in surface soils ranged from 2223 to 11,266 ng/g and 729 to 5359 ng/g (dw), respectively, for Jamshedpur and Bokaro. Higher concentrations of PAHs were recorded at the selected industrial areas and heavy traffic zones of both cities. In JSR city 4-ring PAHs contributed 43% of total PAHs trailed by 5-ring PAHs 27.2%. Similarly, in BKR city 4-ring PAHs contributed 34% of the total PAHs, followed by 3-ring PAHs 28.9% and 5-ring PAHs 22.9%. Total organic carbon in surface soils exhibited moderate correlation with the low molecular weight (ΣLMW) PAHs (R2 = 0.69) and a comparatively strong correlation with the ΣHMW PAHs (R2 = 0.89), suggesting strong adsorption of HMW PAHs to urban soils. The Diagnostic and PMF modelling analysis indicated that the major sources of PAHs contamination in soils were petroleum combustion, vehicular emissions, biomass, and coal combustion. The health risk assessment shows that the cumulative probability of carcinogenic risks was under the acceptable limits of 10–4 to 10–6. At some sampling areas in both cities, the maximum value of total exposure cancer risk slightly exceeded the acceptable limits indicating some carcinogenic risk for adults.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, more than half of the earth’s population lives in cities around the world, and two-thirds of the world’s total population is anticipated to become urban by 2050 (FAO, 2017). In the past few decades, as people migrated from rural areas to urban areas, urbanisation and industrialization have been developed rapidly and with the advancement of urbanization, industrialization and transportation, the domestic waste discharge has increasingly overwhelmed the ecological carrying capacity of urban soils (Chen, 2007). The soils under the influence of municipal background are impacted by serious anthropogenic activities like constructions, manufacturing operations and transport are known as urban soils (Rossiter, 2007; Norra & Stuben, 2003). Urban soils play essential roles for the healthy lives of persons living in town, the city development, and the sustainable growth of the urban ecosystem (Mielke et al., 1999; Pavao-Zuckerman & Byrne, 2009). However, due to fast economic growth in some developing countries, soil pollution in urban environments has become more significant. Polycyclic aromatic hydrocarbons (PAHs) are one type of persistent organic pollutants (POPs), with some being toxic and carcinogenic. Amongst the hundreds of PAHs, United States environmental protection agency (USEPA) has listed 16 PAHs as priority pollutants in the environment. PAHs may be transported far from their sources due to their volatile nature and accumulate in the soil which being their primary accumulation, and consequently, pose long-term health hazards to humans (Gan et al., 2009; Gao et al., 2018). Moreover, investigations have originated that the quantity of human exposure to PAHs through soils is more than water or air (Agarwal, 2009). The polycyclic aromatic hydrocarbons (PAHs) are universal in the city environment and have transformed classified as primary contaminants in towns (Wang et al., 2013, 2020a, 2020b). In view of thermal variation and incomplete combustion of biological carbon (C), biofuel, oil, and coals, the PAHs enter the environment (Abdel-shafy & Mansour, 2016; Cai et al., 2017). Based upon PAHs source, these come from natural and anthropogenic sources. However, in urban areas, anthropogenic activities contribute to maximum PAHs in soils (Buczynska et al., 2013; Wang et al., 2015). Polycyclic aromatic hydrocarbons (PAHs) pollution of urban soil poses important threat to resident welfare, such as children and adults who have been exposed for a long time to high pollution levels. Generally, the urban atmospheres produce more PAHs from production, transport, and human activities. Therefore, the PAH concentrations in urban soils are more compared to other soils. Thus, the PAHs in urban soils can influence human health through water, air, food, and another medium (Wang et al., 2020a, 2020b). In the last two decades, globally several studies reported about PAHs contamination and their significant sources of emission at several areas (Ducoulomber & Rychen, 2003; Ray et al., 2008; Skrbic et al., 2005; Xing-Hong et al., 2006), including the PAHs concentration in soils of various cities of India (Agarwal et al., 2009; Gupta & Kumar, 2019; Kumar et al., 2014; Ray et al., 2008). Jamshedpur (JSR) city is one of East India major industrial hubs located in one Indian state Jharkhand. The town is sheltered to industries, like Tata Steel, Tata Motors, and Tata energy. The city contains one of India’s biggest manufacturing sectors, famous as the Adityapur Industrial Area. This industrial belt includes the industries regulated under the Adityapur Industrial Area Development Authority—Special Economic Zone (AIADA-SEZ). Above 1200 minor and medium-range companies are present in this industrial belt, and the JSR city holds the country’s 36th largest urban agglomeration (JUA) (Ambade et al., 2020; Ambade & Sethi, 2021). Several studies have been reported recently regarding PAHs in air, water and sediments of JSR city (Ambade et al., 2020; Ambade & Sethi, 2021; Kumar et al., 2020). However, the soil contamination of PAHs is not reported. Like JSR city, Bokaro (BKR) city is also the fastest-growing industrial city of East India and a growing urban agglomeration. BKRis called the steel city of East India due to its large number of steel and iron industries. Apart from this BKRcity is home to oil, gas, and cement industries. Therefore, it is necessary to characterize the grade of the soil in JSR and BKRcities to ascertain the overall environmental quality due to PAH contamination.
The main aims of the current study were: (1) to determine the status and spatial distribution of PAHs in the two industrial cities (JSR and BKR); (2) to classify possible PAHs sources by with diagnostic ratio and PMF modelling; and (3) to measure the human health risk of PAHs in urban soils.
Materials and methods
Study area
The study area under observation comprises the urban agglomeration and industrial belts of both JSR and BKR cities. The JSR city (22°47’N 86°12’E) is one of East India well-planned industrial cities located in the southern part of Jharkhand State. Jamshedpur is a centre of commercial and industrial activities with high traffic density zones. According to the 2011 census, the population of Jamshedpur is around 1.3 million, with a population density of 6400 people per km2, making it one of the most crowded cities in East India. This city is also the home for many small and medium scale industries in Adityapur, and large enterprises, like Tata Iron and Steel Company (TISCO), TATA motors, and a cement company. BKR city (23.67˚N 86.15˚E) is also the fastest-growing industrial city of East India and a growing urban agglomeration. BKR is called the steel city of East India due to its large number of steel and iron industries. Apart from this, BKR city is home to oil, gas, and cement industries. BKR city has a population of around 0.6 million, a large population density compared to its area. The study sites were chosen due to their extensive industrial activities and the large urban agglomeration, which could be a more significant threat to these areas. Therefore, investigating the PAH contamination in the soils of these two cities is essential.
Sampling and pre-treatment
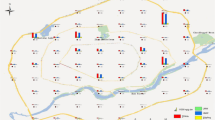
The sampling sites (industrial sites, traffic sites, and commercial sites) were chosen randomly from the two cities. A total of 40 samples were collected from both cities during January to mid-march 2020 based on a random pre-planned sampling process (Fig. 1). Twenty samples from JSR (J1–J20) and BKR (B1–B20) were collected using stainless steel scoops from 0 to 5 cm depth. The sample point coordinates were recorded using GPS (Garmin etrex-10). Each sample was a composite of 5 aliquots collected from a 6 × 6 m grid (4 samples from four corners of the 6 × 6 m grid and one from the centre). Each composite sample was thoroughly mixed for homogeneity, covered in aluminium foil and packed in airtight polythene bags and retained in the ice bags. Hand gloves were used during sampling to prevent impurities, and finally, the samples were transferred to the laboratory within 6 h of sampling. Then the samples were air-dried for 3 days at room temperature and after proper aeration, the samples were crushed into powder and passed through a 1 mm mesh sieve. After properly sieved, the samples were preserved in the refrigerator at ( – 4 °C) for further analysis.
Extraction, clean-up and analysis of PAHs from soil samples
The percentage of total organic carbon (TOC) matter in the soil was estimated using the titrimetric method (Jackson, 1973; Walkley & Black, 1934). The surrogate PAH standard consisting of deuterium-labelled PAHs (Naphthalene-D8, Phenanthrene-D10, Chrysene-D12 and Anthracene-D10) were spiked to 5 g of soil at a concentration of 200 µgL−1 for the recovery studies. The samples were then subjected to solvent extraction (Devi et al., 2016). To remove the elemental sulphur, copper granules were added before extraction. The samples were extracted using dichloromethane (DCM) in a Soxhlet extractor for 24 h. The solvent fractions were evaporated in a rotary evaporator (Hei-VAP Core—finger uplift model with G1 transverse glassware) and exchanged by n-hexane in a final volume of 2 ml. The concentrated samples were subjected to a silica gel column (30 cm long and 3 cm diameter glass column packed with 10 g activated silica gel) clean-up with DCM and 5 g anhydrous Na2SO4. Then 20 ml combination of DCM and n-hexane (1:1 v/v) was added as eluent to the final cleaned fraction. Finally, the eluent solvent was concentrated to 20 μL below the mild flow of pure nitrogen. To quantify all analytes, internal standard hexamethyl benzene was added at 5 µL prior to analysis. The USEPA’s 16 priority PAHs i.e. Naphthalene (NA), Acenaphthene (AC), Phenanthrene (Phen), Acenapthylene (ACY), Fluorene (Fluo), Anthracene (AN), Fluoranthene (Flur), Pyrene (Pye), Benzo(k)Flouranthene (BkF), Benzo(a)Anthracene (BaA), Chrysene (CHRY), Benzo(a)Pyrene(BaP), Benzo(b)Flouranthene (BbF), Benzo[g,h,i]perylene (BgP) Dibenzo [a,h]anthracene (DBA), and Indeno[1,2,3-cd]pyrene (IN) were analysed in an Agilent 7890B Gas Chromatograph (GC-FID) fitted with a HP-5 MS capillary column (30 m × 0.32 mm × 0.25 µm). In a splitless manner, 1 µL of each sample was injected. High purity nitrogen gas was used as the carrier gas. The carrier gas flowed with a flow rate of 1.83 mL/min. The initial oven temperature was set at 70 °C for 1 min and then raised to 300 °C at a rate of 5 °C min−1 by holding for 20 min. The temperatures of the injector and transmission route were kept at 290 °C and 320 °C, respectively.
Quality assurance
All glassware was washed with deionized water and n-hexane (CAS Number-110-54-3) before use. The reagents and chemicals used in this study were purchased from Merck and JT Baker (HPLC grade solvents). We used the standard solution of 16 PAHs in acetonitrile (ID-3697900), deuterium-labelled PAHs (HPLC grade solvents), and hexamethyl benzene (CAS Number-87-85-4, make: Sigma Aldrich, USA). The procedural blank samples, sample duplicates, samples spiked with surrogate standards [Phenanthrene-D10 (CAS Number-1517-22-2), Naphthalene-D8 (CAS Number-1146-65-2), Anthracene-D10 (CAS Number-1719-06-8), and Chrysene-D12 (CAS Number-1719-03-5)] were used in this study. A set of 9 PAH calibration standards (1–15 µg/L) were prepared from the stock solution by successive dilution with acetonitrile (HPLC grade JT Baker) and a 9 point calibration curve was plotted. The calibration curve was plotted for all standard solutions containing 16 PAHs and certified periodically with the medium level of calibration standard to check the instrument stability. The curve linearity ranged from 0.9932 to 0.9980, and all values were found within permissible limits of r2 ≥ 0.990 (Adeniji et al., 2017). The deuterated PAHs recoveries in soil samples were 61 ± 12% Naphthalene-D8, 82 ± 11% for Phenanthrene-D10, 81 ± 12% for Chrysene-D12, and 74 ± 10% for Anthracene-D10 and, respectively. The laboratory and field blanks were retrieved and investigated in a similar technique as the samples. The exposure limits of the target PAHs (MDLs) were estimated as three times the standard deviation (SD) plus the average concentrations of targeted amalgam in spiked blank samples, and the limit of quantifications (LOQs) was measured as ten times the standard deviation. The LOD and LOQ evaluation was in the range of 0.02–0.08 µg/L and 0.03–0.15 µg/L, respectively. The LOQ of each PAH was: NA, 0.05 µg/L; AC, 0.03 µg/L; Phen, 0.06 µg/L; ACY, 0.04 µg/L; Fluo, 0.07 µg/L; AN, 0.03 µg/L; Flur, 0.15 µg/L; Pye, 0.11 µg/L BkF, 0.08 µg/L; BaA, 0.06 µg/L; CHRY, 0.04 µg/L; BaP, 0.05 µg/L; BbF, 0.03 µg/L; BgP. 0.08 µg/L; DBA, 0.04 µg/L; and IN, 0.04 µg/L. The concentration less than the detection limit is considered as below detection limit (< DL). The standard deviation was < 12% for the instrument.
Health risk assessment
The USEPA (2005) health risk model is used in this study for health risk assessment. Humans are exposed to the PAHs in soil via three major pathways: consumption, inhalation and epidermal. However, three other equations were used for calculation of risk (Zhang et al., 2020) as follows: Eq. (1) is used to analyse the toxicity equivalency factor (TEF), BaP is used as the reference PAH for other PAHs (Yang et al., 2017) to estimate the BaP equivalent for other PAHs with Eq. (2). In Eq. (1) Ci, PAHs concentration in (ng/g) and TEFi is the corresponding toxic equivalency factor of the respective PAHs. The TEFi of 16 priority PAHs are shown in Table 1 (Collins et al., 1998).
For the calculation of incremental lifetime cancer risks (ILCR) by the three exposure pathways such as ingestion, inhalation and dermal Eqs. (3), (4) and (5) were used. For the calculation of total carcinogenic risks, Eq. (6) is used. According to the USEPA, there is a chance of additional human cancer over a 70-year lifespan, one in a million. The acceptable limit of CR is < 10–6 (USEPA, 2001). For these equations, CPAHs represents the sum of toxic equivalent concentrations (mg/kg) of the PAHs in soil. The CPAHs can be calculated by using Eqs. (1, 2). The values of these parameters were obtained from the study (Wang et al., 2011; Zhou & Lu, 2017). The exposure paths were calculated for adults by using the data. The parameters are:
Results and discussion
PAHs concentration and their composition in soil
The statistical evaluation of USEPA 16 priority pollutant PAHs in soil samples of two East Indian cities was summarized (Table 1). The total 16 PAHs Σ16PAHs concentration in the JSR soil varied from 2223.1 to 11,265.9 ng/g and for BKR from 729.6 to 5358.9 ng/g respectively. The Σ16PAHs mean concentration in JSR is 5867.4 ng/g and in BKR is 2233.7 ng/g. PAH concentrations were also determined at the selected industrial areas and heavy traffic zones of the two cities. Higher PAH concentrations in JSR were detected at sampling sites J16, J17, J18, J19 and J20 and in BKR at B1, B2, B3, and B5. The soil samples with a higher concentration of PAHs were found in the industrial belts of both cities. The concentration of individual 16 PAHs of both the cities was given in Fig. 2A. The outcome showed that PAHs in JSR soils had higher concentrations than the soils of BKR. The concentration of 4–5 ring PAHs was dominant in both cities. The 4-ring PAH Flur, Pye and BaA were detected at higher levels in the soils of both towns. In JSR city, the mean concentration of Pye was 830.7 ± 330.2 ng/g, followed by Flur 826.3 ± 439.8 ng/g and 5-RING PAH DBA 490.9 ± 205.1 ng/g. However, in BKR the concentration of PAHs in the soil was lower than that of JSR. The mean concentration of Flur was higher 223.6 ± 173.1 ng/g followed by Pye 219.3 ± 159.4 ng/g and 5-ring PAH DBA 167.2 ± 54.1 ng/g, respectively. The level of PAHs contamination in urban soil from the two cities is compared with the previously reported PAHs concentration in different urban soils worldwide (Table 2). The total 16 PAHs mean concentration observed in this study is less than reported in Kathmandu, Nepal 1172.8 ng/g (Pokhrel et al., 2018), Xi’an, China 1246 ng/g (Bao et al., 2018), Orlando and Tampa, USA 3227 ng/g and 4562 ng/g (Liu et al., 2019), Cape Town, South Africa 4080 ng/g (Omores et al., 2017), Lisbon, Portugal 2717 ng/g (Marinho et al., 2016) and Dhanbad, India 3488 ng/g (Suman et al., 2016). However, 16 PAHs mean concentration detected in this study is higher than reported in South of Italy 84.85 ng/g (Matar et al., 2019) and Pokhara, Nepal 273.7 ng/g (Pokhrel et al., 2018). Whereas, 16 PAHs mean concentration observed in this study is similar to reported in Novi Sad, Serbia 363 ng/g (Skrbic et al., 2017) and Shenzhen, China 360 ng/g (Zhang et al., 2016). Both the city soils were dominated by HMW PAHs, exclusively 4-ring PAHs, similar to the soil of Orland and Tampa, USA (Liu et al., 2019), which indicates pyrogenic sources in both cities (Chen et al., 2016). (Fig. 2) shows the compositional pattern of PAHs in the soil of both towns. In JSR city 4-ring PAHs contributed 43% of total PAHs, followed by 5-ring PAHs 27.2% and 3-ring PAHs 17.5%. Similar trends of compositional trends were observed in the BKR city, where 4-ring PAHs contributed 34% of the total PAHs, followed by 3-ring PAHs 28.9% and 5-ring PAHs 22.9%. The sum of ΣLMW PAHs in this study was relatively lower than ΣHMW PAHs (Table 1). In JSR city, the sum of ΣLMW PAHs ranged from 526.4 to 2179 ng/g, and ΣHMW PAHs ranged from 1697.6 to 9086.9 ng/g. However, in BKR city, the concentration was significantly lower, ΣLMW PAHs ranged from 277.4 to 1923.5 ng/g, and the ΣHMW PAHs ranged from 452.2 to 3435.4 ng/g. The predominance of 3, 4 and 5-ring PAHs and the lower level detection of 2 and 6-ring PAHs might be related to PAHs different ring properties. This might be due to the lower degradation and mobility of HMW PAHs than the LMW PAHs known to transform and degrade quickly (Aziz et al., 2014).
Spatial distribution of PAHs
The distribution characteristics of LMW PAHs and HMW PAHs in the soils of two cities were given in the form of ΣLMW PAHs and ΣHMW PAHs (Fig. 3). The concentration of ΣLMW PAHs was found to be lower than ΣHMW PAHs in all the soils sampled from both cities. The spatial distribution pattern of PAHs in urban soils can help to understand the background sources of soil contamination in soils. From the spatial distribution map, the high concentration of ΣHMW PAHs in JSR city was detected at sampling sites J18 (8284.9 ng/g), J19 (7981.9 ng/g), J20 (7250.5 ng/g), and J17 (5703.2 ng/g); whereas the higher concentration of ΣLMW PAHs was detected at sampling sites J18 (2132.3 ng/g), J17 (1585.4 ng/g), J8 (1548.9 ng/g) and J5 (1337.3 ng/g). In BKR city, high concentration of ΣHMW PAHs was detected at sampling sites B1 (2298.7 ng/g), B2 (2056 ng/g), B5 (1999.8 ng/g), and B10 (2167.9 ng/g). Whereas the ΣLMW PAHs were detected at sampling sites B2 (1819.9 ng/g), B4 (988.9 ng/g) and B6 (844.6 ng/g). The higher concentrations of HMW PAHs were detected in the samples from the urbanized belts of both cities. The dominance of the HMW PAHs in the presently studied soils can be attributed to the PAHs molecular properties. The HMW PAHs were mostly united with particulate matter, and the LMW PAHs were diffused primarily on the gaseous form (Harner & Bidleman., 1998).
Source apportionment
Diagnostic ratios
The diagnostic ratio is a comprehensive application to determine the potential sources of PAH contamination in soil and reduce uncertainties in the environment (Tobiszewski & Namiesnik, 2012; Cai et al., 2007). In this study, the same types of anthropogenic activities and a similar trend of PAHs were observed in both cities. Therefore, for source apportionment, combined concentration from both towns was used for diagnostic ratios. The ratios of Flur/(Flur + Pye), AN/(AN + Phen), BaA/(BaA + Chry) and IN/(IN + BgP) were used to detect the potential sources of PAHs in soils of both the cities. The ratios of Flur/(Flur + Pye) < 0.4 suggests a source of petroleum, proportions Flur/(Flur + Pye) > 0.5 gives a source of biofuel and petroleum combustion and the ratios of Flur/(Flur + Pye) between 0.4 and 0.5 suggests a source of petroleum combustion; ratios of IN/(IN + BgP) < 0.2, > 0.5 and between 0.2 and 0.5 advises the origins of petroleum, biomass and coal combustion, and petroleum combustion. BaA/(BaA + Chry) ratio < 0.2 indexes a petroleum source, while > 0.35 gives a source of petroleum combustion and ratios between 0.2 and 0.35 suggest a mixed source. Meanwhile, the proportions of AN/(AN + Phen) > 0.1 imply a source of combustion, and ratios < 0.1 indicate a petroleum source (Tobiszewski & Namiesnik. 2012). As shown in Fig. 4, 48% ratios of IN/(IN + BgP) were between 0.2 and 0.5, and 52% ratios were more than 0.5 suggested a source of petroleum, biofuel, and coal combustion. The 70% ratios of BaA/(BaA + Chry) were more than 0.35, suggesting a source of combustion, 17% ratios were between 0.2 and 0.35 and suggest a mixed source and 13% ratios indicates a source of petroleum. The soil samples with the AN/(AN + Phen) ratio of more than 0.1 accounted for 86% implies a source of combustion, and less than 0.1 accounted for 13%, suggest a source of petroleum. However, the 40% ratios of Flur/(Flur + Pye) were < 0.4 and between 0.4 and 0.5 indicates a source of oil and petroleum combustion, while 20% ratios indicate a source of biofuel and coal combustion. Diagnostic ratios showed the source of PAH contamination in both cities surface soils is mostly due to pyrogenic sources. However, the diagnostic ratio fails to describe the influence rate of every source involved. So the data sets were subjected to positive matrix factorization (PMF) analysis for identifying the specific sources and their contribution.
Positive matrix factorization (PMF)
The 16 PAH concentration data sets of 40 soil samples and their uncertainty values were loaded to the PMF model (5.0) for source analysis. PMF is widely used for source identification in the atmosphere. (Callén et al., 2014). The uncertainties of individual PAHs for different soil samples were measured by using the formula as:
And for concentration < MDL the uncertainty can be estimated as:
where concentration and MDL refer to the concentration of PAHs and their method detection limits. The error fraction is calculated from the replicate measurements of each sample. The total PAH PMF results were bootstrapped using 35 base runs and the base random seed was 66, the S/N ratio was minimum for NA (1.4) and maximum for BbF (5.4) and, except the PAH NA and AC all other PAHs are having an S/N ratio > 2. The number of bootstrap runs was 20 and the minimum correlation of PMF analysis was R = 0.6, and by using the random seed, four factors amongst 3–6 factors were examined based on molecular markers in different sources (Li et al., 2016). The Qtrue value ranged from 231.8 to 814.3. Similarly, the Qrobust ranged from 231.8 to 801.2, and from BS-DISP diagnostics, it found that the largest decrease in Q value was – 1.404 whereas the dQ% was – 0.18 respectively. The source profiles and contributions of 16 PAHs received from PMF analysis are shown in Fig. 5. Factor 1, accounts for 48% of the total PAHs measured and is heavily laden with the PAHs, like NA, AC, ACY, Phen, AN, and Fluo. The LMW PAHs were mostly weighted in factor 1. The PAHs AC, ACY, Phen, Fluo and AN were typically discharged from fossil fuel combustion, coal and coke production (Duval & Friedlander, 1981; Khalili et al., 1995; Ravindra et al., 2008; Wang et al., 2013). Similarly, factor 1 was labelled as coal combustion sources. Factor 2 accounts for 32% of the total PAHs measured and was predominated by the 6-ring PAH BgP. The PAH BgP can be linked to a source of vehicular emissions (Wang et al., 2017). So, factor 2 is attributed as emission sources like traffic emissions. Factor 3 contributed 18% of the total PAHs measured and is heavily loaded with the 4-ring PAHs, such as Flur, Pye, BaA and Chry. The diesel emission sources can be characterized by PAHs Pye, BaA and Chry (Khalili et al., 1995; Wang et al., 2013; Yang et al., 2013). So, factor 3 can be regarded as the source of petroleum combustion. Factor 4 accounted for 12% of the total PAHs and is heavily loaded with BkF, BaP, DBA, and IN. BKF and BaP are the markers of fossil fuel combustion (Kavouras et al., 2001; Park et al., 2002). Besides, the PAH like DBA and IN are the markers of heavy diesel emissions (Wang et al., 2013; Yang et al., 2013). So, overall, Factor 4 suggests a source of petroleum combustion. The diagnostic and PMF results showed that petroleum combustion, automobile emissions, and coal combustion were significant sources of contamination of both cities’ urban soils.
Pearson correlation between PAHs and TOC
The ΣLMW PAHs and ΣHMW PAHs at various sampling sites and the TOC percentage at the respective areas were analysed for correlation using Pearson correlation method. To predict the soil contamination with PAHs TOC plays a vital role because it can predict PAHs adsorption to soil (Wang et al., 2013). In this study, Pearson’s correlation studies indicate that the ΣLMW PAHs and TOC (%) were positively reciprocal with a coefficient of (R2 = 0.69). In contrast, ΣHMW PAHs and TOC (%) were more strongly associated with a coefficient of (R2 = 0.89) (Fig. 6). This implies TOC plays a significant role in PAHs adsorption to soils of this area.
Human health risk assessment
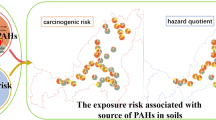
The carcinogenic risks of the USEPA 16 PAHs in soils to adult humans via the three exposure pathways (consumption, inhalation, and epidermal contact) were calculated for the two cities (Table 3). The probability of carcinogenic risks upon adults of JSR city by the dermal contact ranged from 1.36 × 10–7 to 2.16 × 10–6 with a mean value of 1.87 × 10–7. Similarly, for BKR city, the dermal exposure carcinogenic risk on adults ranged from 1.12 × 10–7 to 2.81 × 10–6 with a mean value of 1.46 × 10–7. Amongst the three exposure pathways, the dermal pathway had the highest carcinogenic chance in both cities. The consumption pathway is the second most contributor to carcinogenic risk for both cities. The carcinogenic risk contributor value of consumption pathway for adults ranged from 1.17 × 10–8 to 1.75 × 10–6 for JSR city and 0.56 × 10–8 to 1.29 × 10–6 for BKR city. The third exposure pathway, inhalation carcinogenic contribution value, ranged from 1.85 × 10–11 to 3.42 × 10–11 for JSR city and 0.28 × 10–11 to 1.62 × 10–11 for BKR city. Therefore, the epidermal and consumption exposure pathways were the most effective carcinogenic risk contributors for adults. The risk assessment showed that the JSR city adults are at a higher carcinogenic risk due to PAHs contamination in soils than BKR city. The results showed that the cumulative expectancy carcinogenic risks were under the acceptable limits of 10–4 to 10–6 (USEPA, 2001). However, at some soil sampling areas in both cities, the maximum value of total exposure (CR) slightly exceeded adult’s limits, indicating a negligible carcinogenic risk. This study highlights usefulness of PAHs analysis in soils for estimating human health risk.
Conclusion
The 16 USEPA priority PAHs concentrations, possible sources, spatial distribution, and health risk in surface soils of two industrialized cities were investigated. The total concentration of PAHs in soils of JSR and BKR cities ranged from 2223.1 to 11,265.9 ng/g and 729.6 to 5358.9 ng/g (dw), respectively. The higher concentrations of PAHs were generally recorded at the industrial belts of the two cities. The HMW PAHs were dominant in urban soil at all sampling sites of both cities. The soils of JSR city were more contaminated as compared to BKR city. The 4-ring PAH Flur, Pye and BaA were predominant in all soil samples. Diagnostic ratio and PMF analysis specified that the chief origin of PAH contamination in soil was mostly of pyrogenic origin, involving petroleum combustion, vehicular emissions, and coal combustion. TOC in surface soils were moderately correlated with the ΣLMW PAHs (R2 = 0.69) and a comparatively strong correlation with the ΣHMW PAHs (R2 = 0.89), suggesting a significant role of TOC in the adsorption of PAHs in urban soils of the study area. The health risk study implies that the JSR city adults have a higher carcinogenic risk than BKR city. The results showed that the cumulative probability of carcinogenic risks was under the acceptable limits of 10–4 to 10–6. However, at some soil sampling areas in both cities, the maximum value of total exposure (CR) slightly exceeded adult limits, indicating a negligible carcinogenic risk. The study suggested that the PAH accumulation in urban soils can be controlled by reducing traffic emissions and biofuel and coal combustion sources.
References
Abdel-shafy, H. I., & Mansour, M. S. M. (2016). A review on polycyclic aromatic hydrocarbons : Source, environmental impact, effect on human health and remediation. Egyptian Journal of Petroleum, 25, 107–123.
Adeniji, A. O., Okoh, O. O., & Okoh, A. I. (2017). Petroleum hydrocarbon fingerprints of water and sediment samples of Buffalo River Estuary in the Eastern Cape Province, South Africa. Journal of Analytical Methods in Chemistry. https://doi.org/10.1155/2017/2629365
Agarwal, T. (2009). Concentration level, pattern and toxic potential of PAHs in traffic soil of Delhi India. Journal of Hazardous Materials., 171, 894–900.
Agarwal, T., Khillare, P. S., Shridhar, V., & Ray, S. (2009). Pattern, sources and toxic potential of PAHs in the agricultural soils of Delhi, India. Journal of Hazardous Materials, 163, 1033–1039.
Ambade, B., & Sethi, S. S. (2021). Health risk assessment and characterization of Polycyclic aromatic hydrocarbon from the hydrosphere. Journal of Hazardous, Toxic and Radioactive Waste, 25(2), 05020008. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000586
Ambade, B., Sethi, S. S., Kumar, A., Sankar, T. K., & Kurwadkar, S. (2020). Health risk assessment, composition, and distribution of polycyclic aromatic hydrocarbons (PAHs) in drinking water of Southern Jharkhand, East India. Archives of Environmental Contamination and Toxicology. https://doi.org/10.1007/s00244-020-00779-y
Aziz, F., Syed, J. H., Malik, R. N., Katsoyiannis, A., Mahmood, A., Li, J., et al. (2014). Occurrence of polycyclic aromatic hydrocarbons in the Soan River, Pakistan: Insights into distribution, composition, sources and ecological risk assessment. Ecotoxicology and Environmental Safety, 109, 77–84.
Bao, H., Hou, S., Niu, H., Tian, K., Liu, X., & Wu, F. (2018). Status, sources, and risk assessment of polycyclic aromatic hydrocarbons in urban soils of Xi’an, China. Environmental Science and Pollution Research, 25(19), 18947–18959.
Buczynskaa, J., Geypens, B., Van Grieken, R., & De Wael, K. (2013). Stable carbon isotopic ratio measurement of polycyclic aromatic hydrocarbons as a tool for source identification and apportionmentea review of analytical methodologies. Talanta, 105, 435–450.
Cai, C., Li, J., Wu, D., Wang, X., Tsang, D. C. W., Li, X., Sun, J., Zhu, L., Shen, H., Tao, S., & Liu, W. (2017). Spatial distribution, emission source and health risk of parent PAHs and derivatives in surface soils from the Yangtze River Delta, eastern China. Chemosphere, 178, 301–308.
Cai, Q. Y., Mo, C. H., Li, Y. H., Zeng, Q. Y., Katsoyiannis, A., Wu, Q. T., & Ferard, J. F. (2007). Occurrence and assessment of polycyclic aromatic hydrocarbons in soils from vegetable fields of the Pearl River Delta, south China. Chemosphere, 68, 159–168.
Callén, M. S., Iturmendi, M., & López, J. M. (2014). Source apportionment of atmospheric PM2.5-bound polycyclic aromatic hydrocarbons by a PMF receptormodel. Assessment of potential risk for human health. Environmental Pollution, 195, 167–177.
Chen, J. (2007). Rapid urbanization in China: A real challenge to soil protection and food security. CATENA, 69(1), 1–15.
Chen, Y., Tian, C., Li, K., Cui, X., Wu, Y., & Xia, Y. (2016). Influence of thermal maturity on Carbon isotopic composition of individual aromatic hydrocarbons during anhydrous closed-system pyrolysis. Fuel, 186, 466–475. https://doi.org/10.1016/j.fuel.2016.08.102
Collins, J. F., Brown, J. P., Alexeeff, G. V., & Salmon, A. G. (1998). Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives. Regulatory Toxicology and Pharmacology, 28, 45–54.
Devi, N. L., Yadav, I. C., Shihua, Q., Dan, Y., Zhang, G., & Raha, P. (2016). Environmental carcinogenic polycyclic aromatic hydrocarbons in soil from Himalayas, India: Implications for spatial distribution, sources apportionment and risk assessment. Chemosphere, 144, 493–502. https://doi.org/10.1016/j.chemosphere.2015.08.062
Ducoulomber, C. C., & Rychen, G. (2003). Assessment of soil and grass polycyclic aromatic hydrocarbon (PAH) contamination levels in agricultural fields located near a motorway and an airport. Agronomie, 23, 345–348.
Duval, M. M., & Friedlander, S. K. (1981). Source resolution of polycyclic aromatic hydrocarbons in the Los Angeles atmospheres application of a CMB with first order decay. U.S. EPA Report EPA-600/2–81–161. U.S. Government Printing Office.
FAO. (2017). The future of food and agriculture. Food and Agriculture Organisation of the United Nations, (p. 163).
Gan, S., Lau, E. V., & Ng, H. K. (2009). Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). Journal of Hazardous Materials, 172, 532–549.
Gao, P., da Silva, E., Hou, L., Denslow, N. D., Xiang, P., & Ma, L. Q. (2018). Human exposure to polycyclic aromatic hydrocarbons: Metabolomics perspective. Environment International, 119, 466–477.
Gupta, H., & Kumar, R. (2019). Distribution of some polycyclic aromatic hydrocarbons in urban soils of Delhi, India. Environmental Technology and Innovation. https://doi.org/10.1016/j.eti.2019.100500
Harner, T., & Bidleman, T. F. (1998). Octanoleair partition coefficient for describing particle/gas partitioning of aromatic compounds in urban air. Environmental Science and Technology, 32, 1494–1502.
Hui, W. C., Hua, W. S., Zhou, S. L., Wang, H., Jie, L. B., Chen, H., Yu, Y. N., & Shi, Y. X. (2015). Polycyclic aromatic hydrocarbons in soils from urban to rural areas in Nanjing: Concentration, source, spatial distribution, and potential human health risk. Science of the Total Environment, 527–528, 375–383.
Jackson, M. L. (1973). Soil chemical analysis. Prentice Hall of India Pvt. Ltd.
Kavouras, I. G., Koutrakis, P., Tsapakis, M., Lagoudaki, E., Stephanou, E. G., Von Baer, D., et al. (2001). Source apportionment of urban particulate aliphatic and polynuclear aromatic hydrocarbons (PAHs) using multivariate methods. Environmental Science and Technology, 35(11), 2288–2294.
Khalili, N. R., Scheff, P. A., & Holsen, T. M. (1995). PAH source fingerprints for coke ovens, diesel and gasoline engines, highway tunnels, and wood combustion emissions. Atmospheric Environment, 29, 533–542.
Kumar, A., Ambade, B., Sankar, T. K., Sethi, S. S., & Kurwadkar, S. (2020). Source identification and health risk assessment of atmospheric PM2.5- bound polycyclic aromatic hydrocarbons in Jamshedpur, India. Sustainable Cities and Society, 52, 101–801.
Kumar, V., Kothiyal, N. C., Saruchi Mehra, R., Prakash, A., Sinha, R. R., Tyagi, S. K., & Gaba, R. (2014). Determination of some carcinogenic PAHs with toxic equivalency factor along roadside soil within a fast developing northern city of India. Journal of Earth System Science, 123(3), 479–489.
Li, J., Zheng, Y., Luo, X., Lin, Z., Zhang, W., & Wang, X. (2016). PAH contamination in Beijing’s topsoil: a unique indicator of the megacity’s evolving energy consumption and overall environmental quality. Scientific Report, 6, 33245. https://doi.org/10.1038/srep33245
Liu, Y. G., Gao, P., Su, J., Da Silva, E. B., de Oliveira, L. M., Townsend, T., et al. (2019). PAHs in urban soils of two Florida cities: Background concentrations, distribution, and sources. Chemosphere, 214, 220–227.
Marinho, R. A. P., Shepherd, T., Nowell, G., Cachada, A., Duarte, A. C., Cave, M., et al. (2016). Source and pathway analysis of lead and polycyclic aromatic hydrocarbons in Lisbon urban soils. Science of the Total Environment, 573, 324–336.
Matar, T., Stefano, A., Marcello, D. B., Annamaria, L., Daniela, Z., Roberto, R., et al. (2019). Source patterns and contamination level of polycyclic aromatic hydrocarbons (PAHs) in urban and rural areas of Southern Italian soils. Environmental Geochemistry and Health, 41(2), 507–528.
Mielke, H. W., Gonzales, C. R., Smith, M. K., & Mielke, P. W. (1999). The urban environment and children’s health: Soils as an indicator of lead, zinc, and cadmium in New Orleans, Louisiana, USA. Environmental Research, 81, 117–129.
Norra, S., & Stüben, D. (2003). Urban soils. Journal of Soil Sediment, 3, 230–233.
Omores, R. A., Wewers, F., Ikhide, P. O., Farrar, T., & Giwa, A. (2017). Spatio-temporal distribution of polycyclic aromatic hydrocarbons in urban soils in Cape Town, South Africa. International Journal of Environmental Research, 11(2), 189–196.
Park, S. S., Kim, Y. J., & Kang, C. H. (2002). Atmospheric polycyclic aromatic hydrocarbons in Seoul, Korea. Atmospheric Environment, 36(17), 2917–2924.
Pavao-Zuckerman, M. A., & Byrne, L. B. (2009). Scratching the surface and digging deeper: Exploring ecological theories in urban soils. Urban Ecosystems, 12, 9–20.
Pokhrel, B., Gong, P., Wang, X., Chen, M., & Gao, S. (2018). Distribution, sources, and air-soil exchange of OCPs, PCBs and PAHs in urban soils of Nepal. Chemosphere, 200, 532–541.
Ravindra, K., Sokhi, R., & Van-Grieken, R. (2008). Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmospheric Environment, 42, 2895–2921.
Ray, S., Khillare, P. S., Agarwal, T., & Shridhar, V. (2008). Assessment of PAHs in soil around the International Airport in Delhi, India. Journal of Hazardous Materials, 156, 9–16.
Rossiter, D. G. (2007). Classification of urban and industrial soils in the world reference base for soil resources. Journal of Soils and Sediments, 7, 96–100.
Singh, D. P., Gadi, R., & Mandal, T. K. (2012). Levels, sources, and toxic potential of polycyclic aromatic hydrocarbons in urban soil of Delhi, India. Human Ecological Risk Assessment: An International Journal, 18, 393–411.
Skrbic, B., Cvejanov, J., & Durisic-Mladenovic, N. (2005). Polycyclic aromatic hydrocarbons in surface soils of Novi Sad and bank sediment of the Danube river. Journal of Environmental Science and Health Part a: Environmental Science Engineering, 40, 29–42.
Skrbic, B. D., Ðurisˇic´-Mladenovic´, N., Tadic´, Ð. J., & Cvejanov, J. Ð. (2017). Polycyclic aromatic hydrocarbons in urban soil of Novi Sad, Serbia: Occurrence and cancer risk assessment. Environmental Science and Pollution Research, 24, 16148–16159.
Suman, S., Sinha, A., & Tarafdar, A. (2016). Polycyclic aromatic hydrocarbons (PAHs) concentration levels, pattern, source identification and soil toxicity assessment in urban traffic soil of Dhanbad, India. Science of Total Environment, 545–546, 353–360. https://doi.org/10.1016/j.scitotenv.2015.12.061
Tobiszewski, M., & Namiesnik, J. (2012). PAH diagnostic ratios for the identification of pollution emission sources. Environmental Pollution, 162, 110–119.
USEPA. (2001). Risk assessment guidance for superfund: Volume III part A, process for conducting probabilistic risk assessment. US Environmental Protection Agency.
USEPA. (2005). Guidelines for carcinogen risk assessment, EPA/630/P- 03/001F. Risk assessment forum. United States Environmental Protection Agency.
Walkley, A., & Black, C. A. (1934). An estimation method for determination of soil organic matter and a proposed modification of the chromic acid titration method. Soil Science, 37, 29–33.
Wang, C., Wang, J., Zhou, S., Tang, J., Jia, Z., Ge, L., Li, Y., & Wu, S. (2020). Polycyclic aromatic hydrocarbons and heavy metals in urban environments: Concentrations and joint risks in surface soils with diverse land uses. Land Degradation and Development, 31, 383–391.
Wang, D., Zhu, S., Wang, L., Zhen, Q., Han, F., & Zhang, X. (2020). Distribution, origins and hazardous E_ects of polycyclic aromatic hydrocarbons in Topsoil Surrounding Oil Fields: A case study on the Loess Plateau, China. International Journal of Environmental Research and Public Health, 17, 1390.
Wang, J. Z., Cao, J. J., Dong, Z. B., Guinot, B. J. M., Gao, M. L., Huang, R. J., et al. (2017). Seasonal variation, spatial distribution and source apportionment for polycyclic aromatic hydrocarbons (PAHs) at nineteen communities in Xi’an, China: The effects of suburban scattered emissions in winter. Environmental Pollution, 231, 1330–1343.
Wang, W., Huang, M. J., Kang, Y., et al. (2011). Polycyclic aromatic hydrocarbons (PAHs) in urban surface dust of Guangzhou, China: Status, sources and human health risk assessment. Science of the Total Environment, 409(21), 4519. https://doi.org/10.1016/j.scitotenv.2011.07.030
Wang, Y., Miao, Y., Zhang, Y. C., Li, M. H., & Wu Yu, G. (2013). Polycyclic aromatic hydrocarbons (PAHs) in urban soils of the megacity Shanghai: Occurrence, source apportionment and potential human health risk. Science of the Total Environment, 447, 80–89.
Xing-Hong, L., Ling-Ling, M. A., Xiu-Fen, L., Shan, F., Hang-Xin, C., & Xiao-Bai, X. (2006). Polycyclic aromatic hydrocarbon in urban soil from Beijing. Journal of Environmental Science, 18(5), 944–950.
Yang, B., et al. (2013). Source apportionment of polycyclic aromatic hydrocarbons in soils of Huanghuai Plain, China: Comparison of three receptor models. Science of the Total Environment, 443(31), 39.
Yang, J. Y., Yu, F., Yu, Y. C., Zhang, J. Y., Wang, R. H., Srinivasulu, M., & Vasenev, V. I. (2017). Characterization, source apportionment, and risk assessment of polycyclic aromatic hydrocarbons in urban soil of Nanjing, China. Journal of Soils Sediments, 17, 1116–1125.
Zhang, D., Wang, J., & Zeng, H. (2016). Soil polycyclic aromatic hydrocarbons across urban density zones in Shenzhen, China: Occurrences, source apportionments, and spatial risk assessment. Pedosphere, 26, 676–686.
Zhang, H., Wang, J., Bao, H., Li, J., & Wu, F. (2020). Polycyclic aromatic hydrocarbons in urban soils of Zhengzhou city, China: Occurrence, source and human health evaluation. Bulletin of Environment Contamination and Toxicology, 105(3), 446–452. https://doi.org/10.1007/s00128-020-02982-y
Zhou, Y., & Lu, X. (2017). Assessment of pollution, sources, and risks of polycyclic aromatic hydrocarbons in soil from urban parks in Xi’an city. China Environmental Science, 38, 4800–4808.
Funding
Not available.
Author information
Authors and Affiliations
Contributions
BA, SSS managed the project; BA and SSS collected the samples; CMR and SSS prepared and analysed the samples; BA and SSS wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors wish to declare that there is no conflict of interest regarding the publication of this manuscript.
Consent to publish
Yes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ambade, B., Sethi, S.S. & Chintalacheruvu, M.R. Distribution, risk assessment, and source apportionment of polycyclic aromatic hydrocarbons (PAHs) using positive matrix factorization (PMF) in urban soils of East India. Environ Geochem Health 45, 491–505 (2023). https://doi.org/10.1007/s10653-022-01223-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-022-01223-x