Abstract
Food production in areas contaminated by industrial wastes poses a serious risk to farmers and consumers. Here, we evaluate Cd, Cr, Ni, and Pb concentrations in the soils and the edible parts of lettuce, chives, tomatoes, pepper, and cassava plants grown by small farmers in areas contaminated by slag from an abandoned steel plant in Havana, Cuba. The total, environmentally available, and bioavailable concentrations of metals in the soils and the metals bioconcentration factor in the plants were determined. The risks to human health from food and soil ingestion were estimated. The total and environmentally available concentrations of Cd, Cr, and Pb were above values considered safe by international standards, with likely adverse effect on human health. Cadmium was the most bioavailable metal, reflected in the highest accumulation in the crops' edible parts. Even with negligible DTPA-available Cr concentrations in soils, the Cr concentrations in edible parts of the crops exceeded regulatory levels, suggesting that rhizosphere mechanisms may increase Cr availability. The consumption of vegetables represented 70% of the daily intake dose for Cr, Cd, and Ni, while accidental ingestion of contaminated soil is the predominant human exposure route for Pb. Our results demonstrated the health risks associated with cultivating and consuming vegetables grown on metal contaminated soils in Havana and can assist public policies capable of guaranteeing the sustainability of urban agriculture and food security.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal contaminated soils are a relevant socio-environmental problem worldwide. The accumulation of heavy metals in soils can affect soil and crop quality and pose a risk to human health either through the direct exposure to contaminated soils or the entry of these contaminants into the food chain (Kumar et al., 2019; Margenat et al., 2019). Heavy metals have several adverse effects on human health, including renal dysfunction, damage to the central nervous system, and cancer (Skerfving et al., 2015; Vigneri et al., 2017). Therefore, studies that assess the impacts of the entry of heavy metals into soils and crops and their effects on populations exposed to contamination are fundamental for ensuring food security and human health.
Heavy metals in soils originate from natural and anthropogenic sources (Sidhu et al., 2018, 2020). Natural sources correspond to lithogenesis, weathering, erosion, and other geological processes (Kabata-Pendias, 2011), while anthropic sources include industrial activities (Antoniadis et al., 2019; Kasemodel et al., 2019), recycling of electronic waste (Jiang et al., 2019), disposal and incineration of solid urban waste (Figueiredo et al., 2019), vehicular emissions (Silva et al., 2017b), and agricultural activities, mainly the use of pesticides and chemical fertilizers (Silva et al., 2016). In general, the highest concentrations of heavy metals in soils are derived from industrial processes, notably mining and metallurgy (Shaheen et al., 2017; Silva et al., 2017a; Zhang et al., 2018).
Metallurgy poses a risk of heavy metal contamination due to the indiscriminate disposal of slag in soils and atmospheric deposition of particulate matter emitted by smokestack chimneys (Qing et al., 2015; Vareda et al., 2019). For instance, soils in the vicinity of an iron and steel plant in China had mean concentrations of As, Cd, Pb, Ni, Cr, and Zn above the background values for the region; besides, Cd and Zn concentrations were higher than the maximum permissible values for agricultural soils (Zhou et al., 2019a). The inadequate slag disposal by a Pb smelter plant in Santo Amaro, Brazil, led to the highest environmentally available concentrations of As, Cd, Pb, and Zn in soils surrounding smelting plants reported in the country (Silva et al., 2017a).
Food production in locations close to industrial areas occurs in several regions of the world and is directly related to urbanization, rapid population growth, and high demand for food in large cities (Feng et al., 2019; Saljnikov et al., 2019). Agriculture in areas that received industrial wastes can promote the transfer of heavy metals from the soil to edible parts of the crops (Sidhu et al., 2018). The consumption of contaminated food represents the main non-occupational pathways of exposure to heavy metals (Antoniadis et al., 2019; Hu et al., 2018; Sharma & Nagpal, 2019).
The accumulation of metals in crops varies with the metal availability in the soil and mobility in the plant (Bi et al., 2009; Pan et al., 2016). Zhuang et al. (2009) found that the consumption of vegetables was the most significant route of metal exposure for humans, with Cd posing the highest risk to human health due to the high transfer from the soil to the crops (Sidhu et al., 2018). Indeed, Cd was the metal with the greatest translocation from the soil to the edible tissues of 78 food crops grown in contaminated soils in China, with leafy and tuberous root species showing the highest Cd bioaccumulated (Zheng et al., 2020). In agricultural sites contaminated with As, Cd, Hg, and Pb nearing industrial areas, unacceptable human health risks were reported, with the consumption of vegetables being the main route of metal daily intake (Huang et al., 2018).
In this scenario, to assess both the metal bioavailability in contaminated soils and the accumulation of metals in edible parts of crops are essential for food security. Here, we evaluated an agricultural area influenced by slag deposition of an abandoned steel plant in Havana, Cuba, currently used for subsistence agriculture by people living in the vicinity of the plant. The work aimed (i) to determine the plant-available and the environmentally available concentrations of Cd, Cr, Ni, and Pb in the soils surrounding the steel plant, (b) to evaluate the metal accumulation and bioconcentration factor in the edible parts of different crops grown in the contaminated soils, and (c) to estimate the human health risks from vegetables consumption and ingestion of soil particles.
Material and methods
Study area and sampling of soils and crops
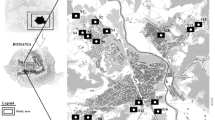
The study area lies in Havana, Cuba (23° 2′ 22.43″ N–82° 16′ 27.38″ W). The mean annual temperature, air humidity, and precipitation are 27 °C, 81%, and 1032 mm, respectively; winds have mean annual velocity of 7.6 mph and West-Southwest (WSW) predominant direction (NOAA, 2019). The site lies in the vicinity of an abandoned steel plant that received slags contaminated with metals. Currently, the area belongs to Cuba’s forest heritage and is mainly dedicated to forest production. However, small growers living in the region produce food in the contaminated soils, which poses a health risk to the local communities. Five of the areas under cultivation had samples of soils and foodstuff collected for analyses (Tables 1 and 2). The soils in the study area are Inceptisol and Entisol (Soil Survey Staff 2010) developed from hard limestones.
Soil samples were collected at a depth of 0.0–0.2 m with a stainless steel auger. Ten samples were taken at each sampling site, within a 100 m radius, and mixed to obtain the composite samples. The soils were dried at room temperature, ground, and passed through a 2.0 mm mesh sieve for further analysis. Edible parts from five crops were sampled at the same soil collection sites: tomato (Lycopersicon esculentum) and pepper (Capsicum spp.) fruits, chives (Allium fistulosum), and lettuce (Lactuca sativa L.) leaves, and cassava (Manihot esculenta) tubers. Two samples composed of 15 single random samples from each edible part were obtained from each area. The vegetable samples were washed with running water, subjected to a triple wash with distilled water, dried in an oven at 65 ºC, and crushed in a Wiley mill.
Chemical analyses and quality control
The bioavailable concentrations of Cd, Cr, Ni, and Pb in the soils were assessed by DTPA (Lindsay & Norvell, 1978). Ten grams of soil were shaken for 2 h at 180 rpm in a 20 mL solution containing 0.005 mol L−1 DTPA + 0.1 mol L−1 TEA + 0.01 mol L−1 CaCl2 (pH 7.3).
The soil samples were macerated in an agate mortar and sieved in a 150 mm opening mesh to obtain the environmentally available concentrations of the metals. 0.5 g of soil in Teflon tubes was digested in an acid solution (9.0 mL HNO3 + 3.0 HCl) at 175 °C for 4′ 30″ using a microwave (Milestone–Ethos Easy) (USEPA 2007).
To determine the total metal concentrations, 0.5 g of the macerated and sieved soil (Ø < 150 mm) was digested in a Teflon beaker with an acid solution containing HF, HNO3, HClO4, and HCl at 190 °C in a hot plate (Alvarez et al., 2001). The total concentrations of heavy metals were determined to calculate the bioconcentration indexes in plants and to estimate the potential risk to human health through direct exposure to contaminated soil. 0.5 g of plant samples was digested with HNO3 + H2O2 solution (3:1) in a microwave oven (Milestone–Ethos Easy) at 180 °C for 10′ according to the modified 3050B methodology (USEPA, 1996).
All extracts obtained from the digestions were filtered (Ø < 2.0 µm), with the volume made up to 25 mL with ultrapure water in certified volumetric flasks. The Cd, Cr, Ni, and Pb concentrations in the extracts were determined by inductively coupled plasma optical emission spectrometry (ICP—OES Perkin Elmer Optima 7000 DV). Reference materials SRM 2709a (San Joaquin Soil) and SRM 1570a (Spinach Leaves) with metal concentrations certified by the National Institute of Standards and Technology—NIST, and blank samples were used for quality control of analyses.
The calibration solutions were prepared using elementary standards (Certipur Standard IV—Merck). All the material used in the analyzes was previously decontaminated with 5% HNO3 for 24 h and washed with distilled water. The analyzes were performed in duplicate, and the limits of detection (LOD) for the elements were determined according to the protocols of NT Technical Report 569 (Hovind et al., 2011). The recoveries of Cd, Cr, Ni, and Pb in the reference materials varied between 80 and 104% and 87–96% for SRM 2709a and SRM 1570a. The LOD were 0.007, 0.074, 0.068, and 0.073 mg kg−1 for Cd, Cr, Ni, and Pb, respectively.
Bioconcentration factors for metals (BCF)
The bioconcentration factor (BCF) evaluated the potential for metal accumulation in the crops edible parts related to the soil's metal total concentration (Sidhu et al., 2017). The BCF was calculated using Eq. (1):
where M—plant is the metal concentration (Cd, Cr, Ni or Pb) in the crop edible part (mg kg−1), and M—soil is the metal concentration (Cd, Cr, Ni e Pb) in the soil (mg kg−1).
Human health risk assessment
We estimated the non-carcinogenic risk to human health by exposure to Cd, Cr, Ni, and Pb in soils and crops edible parts according to the United States Environmental Protection Agency (USEPA, ). The carcinogenic risk was estimated and considered acceptable; hence, it was not discussed here. Two exposure routes were considered when modeling the non-carcinogenic risk: (i) ingestion of soil particles and (ii) ingestion of crops edible parts. Initially, the daily exposure doses (DED) (mg kg−1 day−1) were calculated for metals, taking into account the exposure routes (Eqs. 2 and 3). The description and values of the behavioral, toxicological, and exposure variables used in risk modeling are displayed in Table 3.
The hazard ratio (HQ) was calculated for each metal n using the relationship between the exposure dose of the metal n in the different routes and the reference dose (RfD) of the respective metal (Eq. 4). RfD is the maximum exposure dose at which there is no observable adverse effect on human health.
The multi-element interaction can have a synergistic effect on the receptor (Zhaoyong et al., 2018); therefore, the risks of individual elements are additive. We calculated the cumulative non-carcinogenic hazard expressed by the hazard index (HI) (Luo et al., 2012; Zhaoyong et al., 2018) to Eq. (5). HI values ≤ 1 indicate unlikely risk, while HI > 1 indicates a probable adverse effect on human health.
Statistical analysis
The minimum, maximum, mean, standard deviation, and coefficient of variation values were calculated for all variables analyzed. Pearson's linear correlation analysis (p < 0.05) was performed between variables. All statistical procedures were carried out using the STATISTICA software (v 10.0).
Results and discussion
Bioavailable and environmentally available concentrations of heavy metals in soils
Both the total and the environmentally available concentrations of metals in the soils followed the order Cr > Pb > Ni > Cd (Fig. 1). The environmentally available concentrations of Pb (in all cases), Cd (in three out of five sites), and Cr (in one site) were higher than the quality reference values for metals in the soils of Cuba, which are 50.0, 0.6, and 153.0 mg kg−1 for Pb, Cd, and Cr, respectively; on the other hands, Ni concentrations were below the soil quality reference value (170.0 mg kg−1) (Alfaro et al., 2015). Besides, the concentrations of metals in soils, in general, were higher than the limits regarded as toxic to crops: 72.0, 75.0, 30.0, and 1.3 mg kg−1 for Pb, Cr, Ni, and Cd, respectively (CONAMA, 2009).
Available and environmentally available mean concentrations (± standard deviation) of Cd, Cr, Ni, and Pb in soils contaminated by steel slag and cultivated with food crops in Havana, Cuba. Pearson’s linear coefficient, p < 0.05 significant at 5% probability. The available Cr contents were below the detection limit (0.074 mg kg−1)
The mean environmentally available concentrations of Cd, Cr, and Ni in the soils were threefold higher than reported to similar studies elsewhere (Table 4). The high Cr concentrations resulted mainly from the deposition of slag in the cultivation areas. Chromium has a high affinity for oxygen and is one of the elements added during the steel deoxidation process (Fisher & Barron, 2019). Also, there is an input of Cr through other urban-industrial wastes used in the area and the atmospheric deposition of industrial activities. The use of agricultural inputs, especially solid urban wastes, has also been reported as a significant Cd input source in cultivated soils in Cuba (Alfaro et al., 2017).
The available concentrations of Pb, Ni, and Cd in the soils varied between 16.8 and 104.0, 1.1–1.8, and 0.2–0.5 mg kg−1, respectively (Fig. 1). We found positive correlations between the available and environmentally available concentrations of Pb, Ni, and Cd in soils. The mean available Pb concentration (52.0 ± 37.3 mg kg−1) was 37- and 130-fold higher than Ni and Cd, respectively. The high Pb concentration in all sites (> 70.0 mg kg−1) is likely due to Pb anthropic sources, as 80% of the total Pb was readily available for plant uptake. Other studies have also found high concentrations of available Pb in soils that received industrial residues, especially foundry and steel slag (150.0–2485.0 mg kg−1) (Pelfrêne et al., 2011; Silva et al., 2017a).
The available Cr concentrations were below the LOD (0.074 mg kg−1). The low bioavailability of Cr is because most of the Cr in the soil is associated with the immobile residual fraction (> 80% of the total), mainly adsorbed onto clay minerals and, to a lesser extent to iron oxides and organic matter (Kabata-Pendias, 2011). Besides, Cr has high chemical stability in steel slags as it is an alkaline material with a high concentration of phosphates and carbonates. The pH also governs the bioavailability of Cr (III and VI) in the soil (Ertani et al., 2017; Fisher & Barron, 2019; Guo et al., 2018). At pH > 5, Cr is in the form of a stable precipitate [Cr(OH)3nH2O] of low solubility, which reduces bioavailability (Ahmed 2016). The use of hard water in irrigation, which tends to alkalinize soils (Aguilar et al., 2015), also decreases Cd bioavailability in the studied sites (Table 2).
Other studies evaluating the availability of Cr in soils found results similar to ours (Alexakis et al., 2019; Sun et al., 2019). Gattullo et al. (2020), evaluating the bioavailability and fractionation of Cr in polluted agricultural soils (3807.0–5160.0 mg kg−1 Cr), found that despite the high total concentrations, the Cr availability estimated by DTPA in soils wase negligible (< 0.3 mg kg−1 Cr), and that 99% of the total Cr accumulated in the most recalcitrant soil fractions (organic matter and sulfides) and residual fraction.
Heavy metal concentrations in the edible parts of vegetables
The concentrations of Cd, Cr, Ni, and Pb in the crops varied between < LD (0.007) and 1.3, 1.3 and 46.9, 0.9 and 23.1, and < LD (0.073) and 5.9 mg kg−1, respectively. The highest levels were observed in the leafy vegetables (chives and lettuce), while the cassava tubers showed the lowest metal concentrations (Fig. 2a and d). The mean concentrations (mg kg−1) of Cr (31.6), Ni (16.9), Pb (4.9), and Cd (1.0) in leafy vegetables were up to 2000%, 1308%, 145%, and 150%, respectively, larger than in the edible parts of the other species. Similar results were found by Fan et al. (2017) and Zheng et al. (2020), who reported higher concentrations of As, Cd, Cr, Cu, Ni, Pb, and Zn in leafy vegetables than fruits and tubers.
Mean concentrations (± standard deviation) of Cd, Cr, Ni and Pb (A–D) and values of bioconcentration factors (E–H) of food crops grown in soil contaminated by slag from an abandoned steel plant in Havana, Cuba. LOD detection limit (0.007 and 0.073 mg kg−1 for Cd and Pb, respectively); nc not calculated
Several studies have shown that leafy species, such as cabbage, mustard, spinach, chicory, and lettuce grown on contaminated soils had a high concentration of metals, representing a significant route for human metal exposure (Pelfrêne et al., 2019; Zhou et al., 2016). The bioaccumulation of heavy metals in leafy vegetables is probably due to the high transpiration rate inherent in the large mass of leaves, which increases the absorption of water and metals by mass flow (Marchiol et al., 2004). Such crops can also uptake metals via leaf by resuspension of fine soil particles and atmospheric deposition in areas with high metal concentrations in the soil (Zhou et al., 2016).
The metal concentrations in the plant tissues were compared with the maximum permissible concentrations of metals in plants and vegetables (0.2, 1.3, 3.0 and 10.0 mg kg−1 for Cd, Cr, Pb, and Ni, respectively) intended for human and animal nutrition (WHO, 1996, FAO/WHO, 2001). In chives and lettuce, the mean concentrations of Cr, Cd, and Ni were 24-, 5- and twofold higher than the food safety regulatory levels, respectively. The Cr concentrations in the fruits of tomatoes and pepper exceeded the allowable values, for the latter, the Cd concentrations were four times higher than allowed. Pb concentrations exceeded the allowable limits for chives (98%), lettuce (27%), and pepper (22%). For cassava, the metals concentrations were within safety standards.
The BCFs of heavy metals in vegetables were < 1 and followed the order Cd > Ni > Cr > Pb (Fig. 2e–h). The highest BCF was found for Cd in lettuce; it was 63%, 57%, and 42% higher than chives, tomatoes, and pepper, respectively. Lettuce is a Cd accumulator species and is considered a model plant for metal phytotoxicity studies (Kolahi et al., 2020; Oteef et al., 2015; Zorrig et al., 2019). The high Cd BCF may be related to the higher bioavailable concentration of Cd in the soil (61% of the total) compared to the other metals evaluated. Cd has high availability and mobility in the soil solution because it is found mainly in free ion (Cd2+) form. It is also found in minerals with a low resistance to weathering, such as sphalerite, biotite, and amphiboles, favoring the increase of Cd solubility in the soil (Kabata-Pendias, 2011). Because of the chemical similarity between Cd and Zn, Cd uptake occurs through the Zn transporters (ZIP family), facilitating Cd accumulation in plants (Asgher et al., 2015; Kramer et al., 2007).
The BCF for Pb in the crops was much lower (< 0.06) than the other metals, indicating the low translocation and restricted mobility of Pb in plants. The plant Pb uptake correlates positively with the Pb concentrations in the soil solution, but only 0.005% to 0.13% of Pb in the soil can be absorbed by plants (Ashraf et al., 2020; Monferran & Wunderlin, 2013). Blaylock et al. (1997) showed that in soils with a pH between 5.6 and 7.5, Pb solubility is controlled by phosphates or carbonate precipitates, which dramatically decreases Pb solubility.
Pb adsorption by the oxides of Fe and Mn is considered the primary process of retaining this metal in the soil and explains Pb low availability for plants (Kabata-Pendias, 2011). The absorbed Pb preferentially accumulates in the root system; it has been estimated that only 3% of the Pb in the roots is translocated to the aerial part (Ashraf et al., 2020). The Pb retention in the roots occurs by precipitation of Pb with anions and Pb adsorption on negatively charged sites on the cell wall. This extracellular mechanism leads mainly to the formation of Pb carbonate precipitates. Meyers et al. (2008) found that Pb can be stored in the vacuoles after root absorption. The authors also stated that the primary mechanism responsible for accumulating Pb in the roots is the Pb deposition on the cell wall in the form of Pb—pyrophosphate.
The largest BCFs for Cr occurred in chives and lettuce. Despite the apparent low availability in the soils, the Cr concentrations exceeded by up to 20 times the Cr regulatory levels allowed for these crops. A similar result was observed by Fan et al. (2017), who identified Cr concentrations above the allowable limits in leafy vegetables, fruits, and tubers, despite the exchangeable pool of Cr being less than 1%. The accumulation and distribution of Cr in the plant do not depend only on the soil characteristics and metal concentration but also on the plant species (Golovatyj et al., 1999). For example, the production of root exudates, mainly citric and oxalic acid, can increase the lability of the insoluble species of Cr in the soil, thus boosting Cr uptake and accumulation in the plant (Srivastava et al., 1998). Communities of rhizospheric microorganisms can also influence metals availability and transfer metals from the microbial cell to the plant cell by releasing low molecular weight organic acids (Kumar et al., 2016; Pii et al., 2015).
Ni showed a behavior similar to that of Cr, with the largest BCFs found in leafy vegetables. Nickel and Cr have similar geochemical characteristics in the soil, although Ni usually has higher availability (Fig. 1b and c) (Vithanage et al., 2019). Nickel is a micronutrient absorbed by active processes and has high mobility in the phloem, these factors explain Ni absorption and distribution in plants (Kabata-Pendias, 2011).
Human health risk assessment
Average daily intakes of Cd, Cr, Ni, and Pb, considering exposure to soil ingestion and vegetable consumption, varied between 0.57 and 13.8, 50.3–186.9, 18.6–110.9, and 25.8–54.3 µg day−1, respectively (Fig. 3). In general, the daily intakes of Cd, Cr, Ni, and Pb were lower than the maximum tolerable doses established by the World Health Organization and the United States Environmental Protection Agency (10.0, 2500.0, 5000.0, and 232.0 µg day−1 for Cd, Cr, Ni, and Pb, respectively).
Daily intake mean values (± standard deviation) of Cd, Cr, Ni, and Pb from accidental ingestion of soil and consumption of food crops grown in soil contaminated by slag from an abandoned steel plant in Havana, Cuba. The daily intake by the consumption of food crops for Cd at site 3 and Pb at site 2 was not calculated because the contents these metals were below the detection limit (0.007 and 0.073 mg kg−1 for Cd and Pb, respectively)
The contribution of vegetables to the daily amount of metal ingested was greater than the intake of contaminated soil, except for lead. On average, vegetables consumption accounted for 80, 69, and 60% of Cd, Cr, and Ni daily intake values, respectively. Lettuce, chives, and tomatoes were the foodstuffs that most contributed to Cd, Cr, and Ni intakes; for Pb, the intake of contaminated soil was the main route of exposure (74% of the daily Pb intake). The mean values of the hazard index (HI) followed the order Cr > Pb > Cd > Ni. The HI for each metal was < 1, but the synergistic effect of exposure to metals exceeded the target value (in two out of the five locations), indicating a likely adverse effect on human health (Fig. 4). Cadmium, Cr, Ni, and Pb contributions to the ΣHI varied by 1–25%, 51–75%, 3–6%, and 14–41%, respectively. Lettuce had the highest cumulative HI (1.71), followed by chives (1.10), tomatoes (0.64), cassava (0.49), and pepper (0.47).
Other studies have also reported high carcinogenic and non-carcinogenic risks for exposure to soil and food contaminated by As, Cd, Pb, and Zn in areas under the influence of steel slag (Cai et al., 2019; Silva et al., 2017a; Wei et al., 2020). The contributions of smelting activities to the risk reached up to 94%, indicating the high impact of this activity on agricultural sites and reinforcing the need for soil metals monitoring to guarantee food security.
Conclusion
The agricultural use of areas contaminated by industrial wastes poses a serious risk to farmers and consumers. The total and environmentally available concentrations of Cd, Cr, and Pb in agricultural soils contaminated by steel slags in Havana were above values considered safe by international standards. Despite having the lowest total and environmentally available concentration in the studied soils, Cd was the most bioavailable metal, reflected in the highest accumulation in the crops' edible parts, especially the leafy vegetables. Even with negligible DTPA-available Cr concentrations in soils, the Cr concentrations in edible parts of the crops exceeded regulatory levels, suggesting that rhizosphere mechanisms could increase Cr availability. The hazard index values in lettuce and chives cultivation systems indicate a likely adverse effect on human health. The consumption of vegetables posed 70% of the daily intake dose for Cr, Cd, and Ni. On the other hands, accidental ingestion of contaminated soil particles was the Pb predominant human exposure route.
References
Aguilar, Y., Calero, B., Rodriguez, D., & Muniz, O. (2015). Cuba’s polygon program — Agricultural land rehabilitation. Current Opinion in Environmental Sustainability, 15, 72–78.
Ahmed, F., Hossain, M., Abdullah, A. T., Akbor, M., & Ahsan, M. (2016). Public health risk assessment of chromium intake from vegetable grown in the wastewater irrigated site in Bangladesh. Pollution, 2, 425–432.
Alexakis, D., Gamvroula, D., & Theofili, E. (2019). Environmental availability of potentially toxic elements in an agricultural mediterranean site. Environmental and Engineering Geoscience, 25, 169–178.
Alfaro, M. R., Montero, A., Ugarte, O. M., Nascimento, C. W. A., Accioly, A. M. A., Biondi, C. M., et al. (2015). Background concentrations and reference values for heavy metals in soils of Cuba. Environmental Monitoring and Assessment, 187, 4198–4208.
Alfaro, M. R., Nascimento, C. W. A., Ugarte, O. M., Alvarez, A. M., Accioly, A. M. A., Martin, B. C., et al. (2017). First national-wide survey of trace elements in Cuban urban agriculture. Agronomy for Sustainable Development. https://doi.org/10.1007/s13593-017-0437-7
Alsaleh, K. A. M., Meuser, H., Usman, A. R. A., Al-Wabel, M. I., & Al-Farraj, A. S. (2018). A comparison of two digestion methods for assessing heavy metals level in urban soils influenced by mining and industrial activities. Journal of Environmental Management, 206, 731–739.
Alvarez, J. R. E., Montero, A. A., Jimenéz, N. H., Muñiz, U. O., Padilla, A. R., & Molina, R. J. (2001). Nuclear and related analytical methods applied to the determination Cr, Ni, Cu, Zn, Cd and Pb in a red ferralitic soil and Sorghum samples. Journal of Radioanalytical and Nuclear Chemistry, 247, 479–486.
Antoniadis, V., Golia, E. E., Liu, Y., Wang, S., Shaheen, S. M., & Rinklebe, J. (2019). Soil and maize contamination by trace elements and associated health risk assessment in the industrial area of Volos, Greece. Environmental International, 124, 79–88.
Asgher, M., Khan, M. I. R., Anjum, N. A., & Khan, N. A. (2015). Minimising toxicity of cadmium in plants—Role of plant growth regulators. Protoplasma, 252, 399–413.
Ashraf, U., Mahmood, M. H., Hussain, S., Abbas, F., Anjum, S. A., & Tang, X. (2020). Lead (Pb) distribution and accumulation in different plant parts and its associations with grain Pb contents in fragrant rice. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.126003
ATSDR - Toxicological Profile for Cadmium U.S. Department of Health and Human. (2012). Toxicological profile for Cadmium. In U.S. Department of Health and Human Services - Public Health Service (Ed.). Agency for Toxic Substances and Disease Registry.
Bi, X., Feng, X., Yang, Y., Li, X., Shin, G. P. Y., Li, F., et al. (2009). Allocation and source attribution of lead and cadmium in maize (Zea mays L.) impacted by smelting emissions. Environmental Pollution, 157, 834–839.
Blaylock, M. J., Salt, D. E., Dushenkov, S., Zakharova, O., Gussman, C., Kapulnik, Y., et al. (1997). Enhanced accumulation of Pb in Indian Mustard by soil-applied chelating agents. Environmental Science and Technology, 31, 860–865.
Boente, C., Matanzas, N., García-González, N., Rodríguez-Valdés, E., & Gallego, J. R. (2017). Trace elements of concern affecting urban agriculture in industrialized areas: A multivariate approach. Chemosphere, 183, 546–556.
Cai, L., Wang, Q., Luo, J., Chen, L., Zhu, R., Whang, S., et al. (2019). Heavy metal contamination and health risk assessment for children near a large Cu-smelter in central China. Science of the Total Environment, 650, 725–733.
Christou, A., Aguera, A., Bayona, J. M., Cytryn, E., Fotopoulos, V., Lambropoulou, D. A., et al. (2017). The potential implications of reclaimed wastewater reuse for irrigation on the agricultural environment: The knowns and unknowns of the fate of antibiotics and antibiotic resistant bacteria and resistance genes - A review. Water Research, 123, 448–467.
CONAMA - Conselho Nacional do Meio Ambiente (2009). Resolução n° 420/2009. http://www.mma.gov.br/port/conama/legiabre.cfm?codlegi=620
Dala-Paula, B. M., Custódio, F. B., Knupp, E. A. N., Palmieri, H. E. L., Silva, J. B. B., & Glória, M. B. A. (2018). Cadmium, copper and lead levels in different cultivars of lettuce and soil from urban agriculture. Environmental Pollution, 242, 383–389.
Ertani, A., Mietto, A., Borin, M., & Nardi, S. (2017). Chromium in agricultural soils and crops: A review. Water, Air, & Soil Pollution, 228, 1–12.
Fan, Y., Li, H., Xue, Z., Zhang, Q., & Cheng, F. (2017). Accumulation characteristics and potential risk of heavy metals in soil-vegetable system under greenhouse cultivation condition in Northern China. Ecological Engineering, 102, 367–373.
FAO/WHO. (2001). Joint FAO/WHO Food standards Programme. http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX_711-33%252FAl0112ae.pdf
Feng, W., Guo, Z., Xiao, X., Peng, C., Shi, L., & Ran, H. (2019). Atmospheric deposition as a source of cadmium and lead to soil-rice system and associated risk assessment. Ecotoxicology and Environmental Safety, 180, 160–167.
Figueiredo, C. C., Chagas, J. K. M., Silva, J., & Paz-Ferreiro, J. (2019). Short-term effects of a sewage sludge biochar amendment on total and available heavy metal content of a tropical soil. Geoderma, 344, 31–39.
Fisher, L. V., & Barron, A. R. (2019). The recycling and reuse of steelmaking slags — A review. Resources, Conservation & Recycling, 146, 244–255.
Gattullo, C. E., Allegretta, I., Porfido, C., Rascio, I., Spagnuolo, M., & Terzano, R. (2020). Assessing chromium pollution and natural stabilization processes in agricultural soils by bulk and micro X-ray analyses. Environmental Science and Pollution Research, 27, 1–13.
Golovatyj, S. E., Bogatyreva, E. N., Golovatyi, S. E. (1999). Effect of levels of Cr content in a soil on its distribution in organs of corn plants. Soil Research and use of Fertilizers, 197–204.
Guo, J., Bao, Y., & Wang, M. (2018). Steel slag in China: Treatment, recycling, and management. Waste Management, 78, 318–330.
Harada, Y., Whitlow, T. H., Russell-Anelli, J., Walter, M. T., Bassuk, N. L., & Rutzke, M. A. (2019). The heavy metal budget of an urban rooftop farm. Science of the Total Environment, 660, 115–125.
Hovind, H., Magnusson, B., Krysell, M., Lund, U., Makinen, I. (2011). Internal Quality Control - Handbook for Chemical laboratories. Oslo, OS: Nordic Innovation.
Hu, W. Y., Wang, H. F., Dong, L. R., Huang, B., Ole, K. B., & Hans, C. B. H. (2018). Source identification of heavy metals in peri-urban agricultural soils of southeast China: An integrated approach. Environmental Pollution, 237, 650–661.
Huang, Z., Pan, X. D., Wu, P. G., Han, J. L., & Chen, Q. (2018). Heavy metals in vegetables and the health risk to population in Zhejiang, China. Food Control, 36, 248–252.
Jiang, G., Adebayo, A., Jia, J., Xing, Y., Deng, S., & Guo, L. (2019). Impacts of heavy metals and soil proprieties at a Nigerian e-waste site on soil microbial community. Journal of Hazardous Materials, 362, 187–195.
Kabata-Pendias, A. (2011). Trace elements in soils and plants (4th ed.). CRC Press.
Kasemodel, M. C., Sakamoto, I. K., Varesche, M. B. A., & Rodrigues, V. G. S. (2019). Potentially toxic metal contamination and microbial community analysis in an abandoned Pb and Zn mining waste deposit. Science of the Total Environment, 675, 367–379.
Kolahi, M., Kazemi, E. M., Yazdi, M., & Barnaby, A. (2020). Oxidative stress induced by cadmium in lettuce (Lactuca sativa Linn.): Oxidative stress indicators and prediction of their genes. Plant Physiology and Biochemistry, 146, 71–79.
Kramer, U., Talke, I. N., & Hanikenne, M. (2007). Transition metal transport. FEBS Letters, 581, 2263–2272.
Kumar, V., Kumar, M., Shrivastava, N., Bisht, S., Sharma, S., & Varma, A. (2016). Interaction among rhizospheric microbes, soil, and plant roots: Influence on micronutrient uptake and bioavailability. Plant, Soil and Microbe. https://doi.org/10.1007/978-3-319-29573-2_8
Kumar, V., Parihar, R. D., Sharma, A., Bakshi, P., Sidhu, G. P. S., Bali, A. S., Gyasi-Agyei, Y., & Rodrigo-Comino, J. (2019). Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere, 236, 124364. https://doi.org/10.1016/j.chemosphere.2019.124364
Lindsay, W. L., & Norvell, W. A. (1978). Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Science Society of America Journal, 42, 421–428.
Luo, X. S., Ding, J., Xu, B., Wang, Y. J., Li, H. B., & Yu, S. (2012). Incorporating bioaccessibility into human health risk assessments of heavy metals in urban park soils. Science of the Total Environment, 424, 88–96.
Marchiol, L., Assolari, S., Sacco, P., & Zerbi, G. (2004). Phytoextraction of heavy metals by canola (Brassica napus) and radish (Raphanus sativus) grown on multicontaminated soil. Environmental Pollution, 132, 21–27.
Margenat, A., Matamoros, V., Diez, S., Canameras, N., Comas, J., & Bayona, J. M. (2019). Occurrence and human health implications of chemical contaminants in vegetables grown in peri-urban agriculture. Environment International, 124, 49–57.
Meyers, D. E. R., Auchterlonie, G. J., Webb, R. I., & Wood, B. (2008). Uptake and localization of lead in the root system of Brassica juncea. Environmental Pollution, 153, 323–332.
Monferran, M. V., & Wunderlin, D. A. (2013). Biochemistry of metals/metalloids toward remediation process. In D. Gupta, F. Corpas, & J. Palma (Eds.), Trace metal stress in plants (pp. 43–72). Springer.
NOAA - National Oceanic and Atmospheric Administration (2019). https://www.ncdc.noaa.gov/. Accessed 21 June 2020.
Oka, G. A., Thomas, L., & Lavkulich, L. M. (2014). Soil assessment for urban agriculture: A vancouver case study. Journal of Soil Science and Plant Nutrition, 14, 657–669.
ONN: Oficina Nacional de Normalización. (2009). NC 32: 2009. Calidad de suelos. Determinación del pH y la conductividad eléctrica en el extracto de saturación. Edición 2 Oficina Nacional de Normalización (p. 11).
ONN: Oficina Nacional de Normalización. (2017). NC 51: 2017. Calidad de suelos. Análisis químico – Determinación del porcentaje de materia orgánica. Edición 3 Oficina Nacional de Normalización (p. 11).
Oteef, M. D. Y., Fawy, K. F., Abd-Rabboh, H. S. M., & Idris, A. M. (2015). Levels of zinc, copper, cadmium, and lead in fruits and vegetables grown and consumed in Aseer Region, Saudi Arabia. Environmental Monitoring and Assessment, 187, 1–11.
Pan, X., Wu, P., & Jiang, X. (2016). Levels and potential health risk of heavy metals in marketed vegetables in Zhejiang China. Scientific Reports, 6(1), 1–7.
Pasquini, M. W. (2006). The use of town refuse ash in urban agriculture around Jos, Nigeria: Health and environmental risks. Science of the Total Environment, 354, 43–59.
Pelfrêne, A., Sahmer, K., Waterlot, C., & Douay, F. (2019). From environmental data acquisition to assessment of gardeners’ exposure: Feedback in an urban context highly contaminated with metals. Environmental Science and Pollution Research, 26, 107–120.
Pelfrêne, A., Waterlot, C., Mazzuca, M., Nisse, C., Bidar, G., & Douay, F. (2011). Assessing Cd, Pb, Zn human bioaccessibility in smelter-contaminated agricultural topsoils (northern France). Environmental Geochemistry and Health, 33, 477–493.
Pii, Y., Mimmo, T., Tomasi, N., Terzano, R., Cesco, S., & Crecchio, C. (2015). Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biology and Fertility of Soils, 51, 403–415.
Qing, X., Yutong, Z., & Shenggao, L. (2015). Assessment of heavy metal pollution and human health risk in urban soils of steel industrial city (Anshan), Liaoning, Northeast China. Ecotoxicology and Environmental Safety, 120, 377–385.
Saljnikov, E., Mrvić, V., Čakmak, D., Jaramaz, D., Perović, V., Antić-Mladenović, S., & Pavlović, P. (2019). Pollution indices and sources appointment of heavy metal pollution of agricultural soils near the thermal power plant. Environmental Geochemistry and Health, 41, 2265–2279.
Shaheen, S. M., Know, E. E., Biswas, J. K., Tack, F. M. G., Ok, Y. S., & Rinklebe, J. (2017). Arsenic, chromium, molybdenum, and selenium: Geochemical fractions and potential mobilization in riverine soil profiles originating from Germany and Egypt. Chemosphere, 180, 553–563.
Sharma, A., & Nagpal, A. K. (2019). Contamination of vegetables with heavy metals across the globe: Hampering food security goal. Journal of Food Science and Technology, 57, 391–403.
Sidhu, G. P. S., Bali, A. S., Singh, H. P., Batish, D. R., & Kohli, R. K. (2020). Insights into the tolerance and phytoremediation potential of Coronopus didymus L.(Sm) grown under zinc stress. Chemosphere, 244, 125350. https://doi.org/10.1016/j.chemosphere.2019.125350
Sidhu, G. P. S., Bali, A. S., Singh, H. P., Batish, D. R., & Kohli, R. K. (2018). Ethylenediamine disuccinic acid enhanced phytoextraction of nickel from contaminated soils using Coronopus didymus (L.) Sm. Chemosphere, 205, 234–243. https://doi.org/10.1016/j.chemosphere.2018.04.106
Sidhu, G. P. S., Singh, H. P., Batish, D. R., & Kohli, R. K. (2017). Appraising the role of environment friendly chelants in alleviating lead by Coronopus didymus from Pb-contaminated soils. Chemosphere, 182, 129–136. https://doi.org/10.1016/j.chemosphere.2017.05.026
Silva, F. B. V., Nascimento, C. W. A., Araújo, P. R. M., Silva, F. L., & Lima, L. H. V. (2017a). Soil contamination by metals with high ecological risk in urban and rural areas. International Journal of Environmental Science and Technology, 14, 553–562.
Silva, F. B. V., Nascimento, C. W. A., Araújo, P. R. M., Silva, L. H. V., & Silva, R. F. (2016). Assessing heavy metals sources in sugarcane Brazilian soils: An approach using multivariate analysis. Environmental Monitoring and Assessment, 188, 1–12.
Silva, W. R., Silva, F. B. V., Araújo, P. R. M., & Nascimento, C. W. A. (2017b). Assessing human health risks and strategies for phytoremediation in soils contaminated with As, Cd, Pb, and Zn by slag disposal. Ecotoxicology and Environmental Safety, 144, 522–530.
Skerfving, S., Lofmark, L., Lundh, T., Mikoczy, Z., & Stromberg, U. (2015). Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology, 49, 114–120.
Srivastava, S., Srivastava, S., Prakash, S., & Srivastava, M. M. (1998). Fate of trivalent chromium in presence of organic acids. Chemical Speciation and Bioavailability, 10, 147–150.
Sun, G., Feng, X., Yin, R., Zhao, H., Zhang, L., Sommar, J., Li, Z., & Zhang, H. (2019). Corn (Zea mays L.): A low methylmercury staple cereal source and an important biospheric sink of atmospheric mercury, and health risk assessment. Environment International. https://doi.org/10.1016/j.envint.2019.104971
Swartjes, F. A., Versluijs, K. W., & Otte, P. F. (2013). A tiered approach for the human health risk assessment for consumption of vegetables from with cadmium-contaminated land in urban areas. Environmental Research, 126, 223–231.
USEPA - United States Environmental Protection Agency (1989). Risk assessment guidance for superfund, Vol. 1: Human health evaluation manual. EPA/540/1–89/002. https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part. Accessed 14 May 2020.
USEPA - United States Environmental Protection Agency (1996). Method 3050B: Acid digestion of sediments sludges and soils. Available at: https://www.epa.gov/sites/production/files/2015-06/documents/epa-3050b.pdf. Accessed 14 May 2020.
USEPA - United States Environmental Protection Agency (2000). Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=20533. Accessed 14 May 2020.
USEPA - United States Environmental Protection Agency (2001). Risk assessment guidance for superfund: Volume III–Part A, process for conducting probabilistic risk assessment. https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-volume-iii-part. Accessed 14 May 2020.
USEPA - United States Environmental Protection Agency (2007). Method 3051A: Microwave assisted acid digestion of sediments, sludges, soils, and oils. Available at: https://www.epa.gov/sites/production/files/2015-12/documents/3051a.pdf. Accessed 14 May 2020.
USEPA - United States Environmental Protection Agency (2013). Reference dose (RfD): Description and use in health risk assessments, background document 1A, integrated risk information system (IRIS). http://www.epa.gov/iris/rfd.html. Accessed 21 May 2020.
Vareda, J. P., Valente, A. J. M., & Durães, L. (2019). Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. Journal of Environmental Management, 246, 101–118.
Vigneri, R., Malandrino, P., Giani, F., Russo, M., & Vigneri, P. (2017). Heavy metals in the volcanic environment and thyroid cancer. Molecular and Cellular Endocrinology, 457, 73–80.
Vithanage, M., Kumarathilaka, P., Oze, C., Karunatilake, S., Seneviratne, M., Hseu, Z., et al. (2019). Occurrence and cycling of trace elements in ultramafic soils and their impacts on human health: A critical review. Environment International. https://doi.org/10.1016/j.envint.2019.104974
WBG - World Bank Group (2020). Life expectancy at birth. http://datatopics.worldbank.org/world-development%20indicators/themes/people.html#population. Accessed 18 August 2020.
Wei, X., Zhou, Y., Jiang, Y., Tsang, D. C. W., Zhang, C., Liu, J., et al. (2020). Health risks of metal(loid)s in maize (Zea mays L.) in an artisanal zinc smelting zone and source fingerprinting by lead isotope. Science of the Total Environment, 742, 1–10.
WHO - World Health Organization (1996). Permissible limits of heavy metals in soil and plants. WHO guidelines for assessing quality of herbal medicines with reference to contaminants and residues. https://www.omicsonline.org/articles-images/2161-0525-5-334-t011.html. Accessed 1 August 2020.
Zhang, X., Wei, S., Sun, Q., Wadood, S. A., & Guo, B. (2018). Source identification and spatial distribution of arsenic and heavy metals in agricultural soils around Hunan industrial estate by positive matrix factorization model, principle components analysis and geo statistical analysis. Ecotoxicology and Environmental Safety, 159, 354–362.
Zhaoyong, Z., Xiaodong, Y., Simay, Z., & Mohammed, A. (2018). Health risk evaluation of heavy metals in green land soils from urban parks in Urumqi, northwest China. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-017-0737-0
Zheng, S., Wang, Q., Yuan, Y., & Sun, W. (2020). Human health risk assessment of heavy metals in soil and food crops in the Pearl River Delta urban agglomeration of China. Food Chemistry. https://doi.org/10.1016/j.foodchem.2020.126213
Zhou, H., Yang, W., Zhou, X., Liu, L., Gu, J., Wang, W., et al. (2016). Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. International Journal of Environmental Research and Public Health, 13(3), 289.
Zhou, T., Bo, X., Qu, J., Wang, L., Zhou, J., & Li, S. (2019a). Characteristics of PCDD/Fs and metals in surface soil around an iron and steel plant in north China plain. Chemosphere, 216, 413–418.
Zhuang, P., MacBride, M. B., Xia, H., Li, N., & Li, Z. (2009). Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Science of the Total Environment, 407, 1551–1561.
Zorrig, W., Cornu, J. W., Maisonneuve, B., Rouached, A., Sarrobert, C., Shahzad, Z., et al. (2019). Genetic analysis of cadmium accumulation in lettuce (Lactuca sativa). Plant Physiology and Biochemistry. https://doi.org/10.1016/j.plaphy.2019.01.011
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed equally for the conception and writing of the manuscript. All authors critically revised the manuscript and approved of the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alfaro, M.R., Ugarte, O.M., Lima, L.H.V. et al. Risk assessment of heavy metals in soils and edible parts of vegetables grown on sites contaminated by an abandoned steel plant in Havana. Environ Geochem Health 44, 43–56 (2022). https://doi.org/10.1007/s10653-021-01092-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-01092-w