Abstract
The current study was conducted to assess the level of potentially toxic elements (PTEs) contamination (Cu, Pb, Zn, Cr, As, Cd, and Ni) in surface soils from Arak city. Arak, which is an industrial city, is a prominent center of chemicals, metal/electric, manufacturing factories, and other industries. Forty-three surface soil samples were collected from 0–20 cm after removing the visible surface contamination in the dry season in June 2017. Metal concentrations were found highly variable, ranging from 174–3950 mg/kg for Cu, 181–3740 mg/kg for Pb, 48–186 mg/kg for Zn, 105–1721 mg/kg for Ni, 0.8–0.9 mg/kg for As, 114–1624 mg/kg for Cr, and 3.45–12.36 mg/kg for Cd. The results of geochemical fraction indicated that the main components of Pb, Cr, and Zn at most of the sampling sites are Fe–Mn bound/reducible. Meanwhile, the residual fraction is the dominant fraction of sequence extraction for Ni, Cu, and Cd. Higher values of reducible bound for Pb, Cr, and Zn, as well as a considerable percentage of Ni, Cu, and Cd, imply that the main source of the studied PTEs (except As) in the study area is both anthropogenic and geogenic inputs. The results of principal component analysis (PCA), correlation analysis, enrichment factor (EF), enrichment index (EI), and top enrichment factor (TEF) confirm that Pb, Ni, Cu, Cr, Cd, and Zn had a similar anthropogenic source which is confirmed by geochemical fractionation analysis. Carcinogenic risks (CR) of studied PTEs were estimated to be higher than the target limit of 1.0E-06, for adults and children except for Cr with values of 5.91E-04, and 3.81E-04 for children and adults, respectively. Higher CR values of Cr compared to other PTEs in Arak surface soil demonstrate that living target populations, including children and adults, particularly children, are more at risk of carcinogenic risks of PTEs. 206Pb/207Pb ratios of the collected samples indicated that Pb in Arak surface sample was derived from industrial inputs and deposition, as well as re-suspension vehicle exhaust emission from previously leaded gasoline. The findings concerning the applied end-member contribution of geogenic and industrial and vehicle emission represented that the contribution could vary from 68.0% to 15% (mean: 39.3) for industrial emission, 65% to 19% for vehicle exhaust (mean: 39), and 46% to 10% (mean: 21.6) for geogenic sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil is believed to be a dynamic and non-regenerate natural resource that is essential for human life and serves as the most important sink or absorber for toxic substances such as potential toxic elements (PTEs) (Ravankhah et al. 2018; Wang et al. 2020). Recently, PTEs in urban soils have drawn a great deal of scientific attention since its pollution may have a direct impact on public health (Fakhri et al. 2017; Kamunda et al. 2016; Shahsavani et al. 2017). This is due to the prevalence, large number of people close to the soil, suspended dust, or direct contact (Nazarpour et al. 2019; Turnbull et al. 2019).
Certain parameters, including the concentrations of PTEs in rocks and pedogenic materials, various soil processes, and human activities, which are known as anthropogenic inputs, determine the concentration of PTEs in the soil environment (Sun et al. 2010; Zhang et al. 2002). That is in view of the fact that PTEs could be transmitted to people through dust or direct contact. They also put human health in danger through different ways, such as polluting surface water and groundwater (Sun et al. 2010). Generally, soil PTEs contamination originates with a natural incidence (weathering of parent materials) and human activities (PTEs accumulation through greenhouse gas emissions, industrial waste, gasoline, lead disposal, pesticides, chemical fertilizer, atmospheric deposition, coal combustion residues, etc.) (Wei et al. 2010). PTEs contamination in agricultural soils not only disrupts the function of the soil and plant growth, but also damages human health through food chain contamination. PTEs can accumulate in living systems and increase their concentration as they pass from low-level organisms to higher levels of nutrition, known as biological absorption occurs (Kelepertzis et al. 2016; Nazarpour et al. 2017).
PTEs accumulate in soils and plants and are known as an ecological threat to plant physiological systems, including photosynthesis, gas exchange, and nutrient uptake, which results in reduced plant growth and dry matter accumulation (Fakhri et al. 2017; Mohammad et al. 2018). Animals grazing on such infected plants and drinking from contaminated waters, as well as marine animals that grow in waters contaminated with PTEs, are also significantly affected (Ghanavati et al. 2019a; Nazarpour et al. 2017). These metals can accumulate in their tissues and milk when lactating. Once PTEs are deposited in soil, they do not decompose and they accumulate in the ecosystem for an extended period of time, triggering severe environmental pollutions (Mtunzi et al. 2015).
In environmental researches, there are certain factors evaluated based on the type of contaminated metals, the severity of pollution, and the risk of human health (Jahandari et al. 2020). These factors are applied to compare, evaluate, and determine the source of contamination and also manage the effects of contaminated metals in surface soils. (Karim and Qureshi 2014) studied the potential ecological risk of PTEs in the urban soil of Karachi. They indicated that children compared to adults to non-carcinogenic health effects of PTEs, especially the risk of non-carcinogenic risk of Pb to children through ingestion, require special attention. In another research by (Oyeleke et al. 2016), the concentration of PTEs was studied around an abandoned battery company for the city of Ibadan in Nigeria. They showed that the average of Pb concentration was higher than the standard values. The main reasons for this increase are agricultural activities and the high volume of traffic in the above-mentioned region.
León-García et al. (2018) also investigated Sonora Rivers and Bacanuchi of Mexico to evaluate mobility, bioavailability, and pollution of heavy metal using enrichment factor (EF), potential ecological risk, and integrated pollution index (IPI). They implied that the EF and PER of Cu, Cr, Mn, Ni, Pb, and Zn in both rivers were high, which reveals the extreme pollution of the studied areas. The PTEs were mainly derived from mining activities in the upper stream. Besides, the IPI values were classified as non-contaminated to moderate contamination levels.
Accordingly, this study aimed to undertake 1) the investigation of the contamination level of the studied PTEs, enrichment factor (EF) indices, enrichment index (EI), and top enrichment factor (TEF), 2) source apportionment of metal pollution (natural or anthropogenic), utilizing multivariate analysis, assessment of geochemical fractionation of the studied PTEs, Pb isotopic ratio as well as an end-member model of isotopic ratios, 3) the evaluation of human health risk assessment of PTEs according to carcinogenicity risk index (RI) and non-cancer index (HI).
Material and methods
Study area
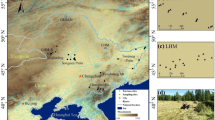
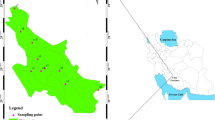
Arak city, the capital of Markazi Province, is located at 32° 50′ 40" N and 48° 20′ 21" E with approximately 443,000 inhabitants in 2016 in the center of Iran. Arak is an active industrial city, and due to various sources of diffusion, manufacturing factories, increasing number of vehicles, and population growth, it has been suffering from environmental pollution and PTEs for the past decades. The main manufacturing factories in this area, as shown in Figs. 1, 2, include Iranian Aluminum Company, Train Center (TC), HEPCO industry, Azarab Industries Company (AIC), Arak Industrial hub, Wagon Pars Company, Navrd Aluminum, Rolled Aluminum group, and so forth. Arak has a population of about 600,000 people and consists of six metropolitan areas. This city is situated in the center of Iran, and the total area of it is 98.71 km2, and its average elevation is 1748 m above the sea level. The city’s weather is relatively warm and dry and is cold and humid in winter (Vahedian et al. 2017). The total precipitation in different years is between 230 and 638 (mean: 341) mm per year. The mean annual temperature of Arak is 9 °C, and its climate is based on De Martone and semiarid classification based on aridity index, semiarid, and cold (Taghizadeh and Taghizadeh 2019).
Soil sampling and analysis
To study the concentration of PTEs in surface soils of Arak city, the sampling and measurement were performed using standard methods provided in published literature (Ghanavati and Nazarpour 2018; Ghanavati et al. 2019b). Sampling locations were selected based on pollutant inputs (hot spots) and their proximity to sources of pollutants and industrial spots. According to the area of the study range, 43 surface soil samples (from 0–20 com) were collected in Arak city, which covers the entire urban area (Fig. 1). The sampling was performed in June 2017. Figure 1 shows the distribution map of the hot spots of anthropogenic inputs of PTEs, from which some samples were collected. Surface soil samples were gathered from high-traffic areas, city squares, main streets, industrial and residential areas, sidewalks, asphalted streets, and places where soil had accumulated for some time. Approximately 500 g of a composite soil sample was collected from an area of 2 × 2 m2, air-dried and passed through a 200 mesh, and stored in plastic bags. Then, all the collected samples were transferred and stored in the laboratory until the measurement of the amount of PTEs was carried out at 4 °C temperature in the refrigerator.
The collected samples were analyzed in the Advanced Analysis Laboratory of the University of Ahvaz. To measure the PTEs, the soil samples were digested by using nitric acid (HNO3) 65%, hydrofluoric acid (HF) 40%, and hydrochloric acid (HCL) 37%. The digested samples were crossed through a membrane 8 μm filter and washed out utilizing deionized water. Subsequently, the PTEs, including Cu, Pb, Zn, As, Ni, Cr, and Cd, were measured employing the atomic absorption spectroscopy (AAS) (PG990 Model). We measured the control and duplicate samples with a precision of 4–6%, and reference materials NIST 2710 with an accuracy of 100 ± 8% (n = 10), for quality assurance (QA) and quality control (QC). The duplicate soil samples’ precision was 4–6% and less than 5%.
Thermo-Finnigan Neptune™ Series High-Resolution Multi-Collector measured Pb isotopes of collected samples Inductively coupled plasma mass spectrometry MC-ICP-MS in Actlabs-Canada. Álvarez-Iglesias et al. (2012) procedure was used to determine the isotopic ratios. Precision of isotopic ratios was: 0.09% for 206Pb/204Pb, 0.05% for 207Pb/204Pb, 0.08% for 208Pb/204Pb, and 0.04% for 208Pb/206Pb.
Enrichment factor (EF) and enrichment index (EI)
The enrichment factor (EF) was determined employing the measured and background concentration value of the studied PTEs to evaluate the comparative input of anthropogenic and geogenic origins of the target metals (Sutherland 2000). The EF of each toxic metal (i) can be calculated as follows:
where Ci and CRef represent the concentrations of metal i and the reference metal, respectively. According to Chen et al. (2004) and Xu et al. (2012), Fe was chosen as the reference element. The ratio in the dust sample is normalized to the background value, which is the upper continental crustal (UCC) as indicated by Wedepohl (1995) and McLennan (2001). Based on the EFs, elemental origins could be divided into seven categories: elements with EF < 2 with no enrichment, 2–5 indicated a moderate enrichment, 5–20 with significant enrichment, EF values with 20–40 with high to severe enrichment > 50 extremely severe enrichments (Yongming et al. 2006).
Enrichment index (EI) was applied to determine the contamination level of PTEs of topsoils (Eq. 2) (Kříbek et al. 2016, 2014), in which the mMe is the mean concentration value of the analyzed PTEs. Enrichment index (EI) values higher than 1 for every defined sample show that the surface soil was severely affected by anthropogenic inputs.
In order to estimate the percentage of anthropogenic form geogenic sources of PTEs, especially for the elements with a mixture of sources of geogenic and anthropogenic, Eq. 3 was applied (Eby 2004).
where \(\left[ {M_{{{\text{Total}}}} } \right]\) and \(\left[ M \right]_{{{\text{Reference}}}}\) represent the total concentration (mg/kg) of the target metal in a soil sample and reference material (UCC), respectively.\(Al_{{{\text{sample}}}}\) and \(Al_{{{\text{Reference}} }}\) are the concentration value of Al in a soil sample and reference material, respectively.
Human health risk assessment
Human health risk assessment of carcinogenic or non-carcinogenic toxic metals was conducted as a multistage process carried out in two parts based on the US Environmental Protection Agency (USEPA) (Singh et al. 2010). The average daily dose value (mg/kg/day), the risk of exposure to a contaminant by ingestion, inhalation, and dermal contact were estimated using Eqs. 4, 5 and 6 (Bennett et al. 2001):
where ADDing, ADDinh, ADDdermal are the average daily metal intake (mg/kg-day) by ingestion, inhalation, and dermal contact, respectively. C is the concentrations of metals in the dust (mg/kg); IngR and InhR are the ingestion rate and inhalation rate of dust (mg/day and m3/day), respectively; EF represents the exposure frequency to metals (day/year); ED is the exposure duration to metals (year); BW is the bodyweight of the person exposed to metals (Kg); AT demonstrates the averaging time (the period in which exposure is averaged-days) to any amount of metals on a daily basis; PEF is the particle emission factor for metals from dust to air (m3/kg); SA shows the skin area exposed to metals (cm2); AF presents adherence factor (mg/cm2-day); and ABF is the dermal absorption factor (unitless). The details of each parameter and its values in the risk assessment equations (Chabukdhara and Nema 2013; Qing et al. 2015; Wei et al. 2015) are given in Table 1. In the following, the calculation of the average daily dose value of the metals via the three routes of ingestion, inhalation, and dermal contact, hazard quotient (HQ) was calculated based on reference daily intake (\({{\mathbf{R}}_{\mathbf{f}}\mathbf{D}}_{\mathbf{i}}\)) using Eq. (7).
where HQi is the hazard quotient in each intake path, ADDi shows the average daily dose value of metal intake by each of the three mentioned routes (mg/kg/day), and \(R_{f} D_{i}\) is the reference daily intake that estimates the maximum risk in the human population exposed daily to heavy metals considering sensitive groups (adults and children) (Man et al. 2010). The values of \(R_{f} D_{i}\) were collected from the US Department of Energy's Risk Assessment Information System (RAIS) (Ferreira-Baptista and De Miguel 2005). There will be no adverse effects on human health provided that the acceptable average daily intake (ADDi) is less than the reference daily intake; otherwise, if ADDi is higher than \(R_{f} D_{i}\), it is likely to have an adverse effect on human health (Kurtz et al. 2001). When the HQ value is ≤ 1, there will not be an adverse effect, but when HQ > 1, an adverse effect is expected on human health (Man et al. 2010). Summing up the hazard quotient in each intake path (HQi), the HI can be generated to estimate the risk of all contaminated metals according to Eq. (8):
Here, the calculated HI for all elements indicates the severity of undesirable effects in all pathways of human exposure (Wei et al. 2015). For HI ≤ 1, there are no apparent adverse health effects of heavy metals, whereas concerning HI > 1, there will be hostile health effects for residential populations (Bennett et al. 2001). The health risk assessment for carcinogenic heavy metal exposures for both adults and children was calculated through each of the three routes of ingestion, inhalation, and dermal contact employing Eq. (9).
In the above equation, CR is the cancer risk, and SF is the carcinogenic slope factor (mg/kg/day). SF captures the assessed daily contaminant intake during a lifetime of being exposed to the growing risk of an individual developing cancer (Hu et al. 2011). In general, according to the US Environmental Protection Agency, if the carcinogenic risk (CR) is less than 1 × 106 (the probability of one’s cancer in every one million people), this risk can be negligible, while if the CR is more than 1 × 104, it will be harmful and hazardous to human health. CR between the range of 1 × 10–6 and 1 × 10–4 represents an acceptable risk which is under control (Wei et al. 2015).
Result and discussion
PTEs concentration
Descriptive statistics of PTEs concentration in the collected surface soils of Arak city are presented in Table 2. The range and average value of the studied PTEs were as follows: Pb 181–3740 mg/kg (mean: 947.40), Zn 48–586 mg/kg (mean: 290.50), Cu 174–3950 mg/kg (mean: 2839.77), Ni 105–1721 mg/kg (mean: 792.87), As 0.08–0.90 mg/kg (mean: 0.17), Cr 114–1624 mg/kg (mean: 733.67), and Cd 3.45–12.36 mg/kg (mean: 7.68). Our results revealed that the mean concentration value of all of the studied PTEs in the study area was higher than the local baseline, upper continental crust (UCC), world soil, Canadian, Dutch, and Chinese soil quality guidelines (Table 3). However, this suggests the anthropogenic source of PTEs, such as vehicle traffic emission, industrial inputs, construction, and steel industry (Babaei et al. 2018; Sadeghdoust et al. 2020).
A Kolmogorov–Smirnov results showed that the concentration of the studied PTEs had non-normal distribution behavior (sig > 0.05) except for As (sig < 0.05). Skewness values of the studied PTEs ranging from 1.54 to 21.36 were higher than zero, which indicates the non-normal distribution and mean value of the studied PTEs are higher than median value. They also imply the existence of highly contaminated areas and polluted hot spots which cover the entire study area.
According to the coefficient of variance (CV), the studied PTEs indicate that the elements with CV values higher than > 0.5 include Ni, Cr, Pb, Cu, and As and Cd with (CV < 0.5) (Borojerdnia et al. 2020). Metals with lower CV demonstrate that geogenic sources of PTEs and inputs of metals in the soil are limited. In addition, metals with higher CV indicate a mixture of geogenic and anthropogenic sources (Ghadimi et al. 2013; Ghanavati et al. 2019b).
In general, there are no standard guidelines for soil sampling and geochemical analysis in the urban environment, especially in Iran. Therefore, a comparison between the average of PTEs concentration in surface soil and other urban areas in the world could be more useful rather than defined threshold values and local baseline. Table 4 presents the mean concentration value of the studied PTEs associated with other selected metropolises in the world, local background, and baseline values in the study area. All of the studied PTEs are of higher values compared to the world soil and baseline values (Table 4).
Table 4 presents the mean concentration (in mg/kg) value of the studied PTEs associated with other selected metropolises in the world, local background, and baseline values in the study area. All of the studied PTEs are of higher values compared to the world soil and baseline values (Table 4). Accordingly, the concentrations of Ni, Cr, Pb, Zn, Cu, As, and Cd in Arak soil samples are 33, 10, 36, 1.55, 72, 1.58, and 18.5 times higher than those in world soils, respectively (Kabata-Pendias 2011; Kabata-Pendias and Pendias 2001), while the levels of these PTEs in studied samples are 15.8, 10.4, 27, 1.21, 94, 1.58, and 21.9 background value in the world (Du et al. 2013). The concentrations of Ni, Cr, Pb, Cu, and Cd in Arak urban soils are 12.98, 9.16, 5.4, 30, and 3.5 times higher than Isfahan (Iran) urban soils, while the concentrations of Zn and As are lower than those in Isfahan (Iran) (Mehr et al. 2017). The concentrations of Ni, Cr, Pb, and Cu in Arak urban soils are 22.7, 21.8, 3.8, and 12.6 times higher than Tehran (Iran) urban soils, while the concentrations of Zn and Cd are lower than those in Tehran (Iran) (Saeedi et al. 2012). The concentrations (in mg/kg) of Ni (4.2), Cr (1.8), Pb (7.4), Zn (2.8), Cu (3.2), As (2), and Cd (3.5) are lower than those in the corresponding recommended values, while the levels of Cr and Ni in sampled soils are 3.3 and 2.12 times higher than Napoli (Italy), respectively (Cicchella et al. 2008). The concentrations (in mg/kg) of Ni (17.6, 13.9), Cr (1.02, 14), Pb (12, 17), Cu (41, 25), and Cd (1.75, 14) are lower than those in the corresponding recommended values, while the levels of Zn and As in sampled soils are lower than Ahvaz (Iran) (Ghanavati et al. 2019a) and Xiangyang (China) (Das Sharma 2019), respectively. The mean concentration of studied PTEs in Arak surface soil is estimated to be higher than Ottawa (Canada) (Rasmussen et al. 2001), Shiraz (Iran) (Jahandari et al. 2020), and Andhra Pradesh (India) (Das Sharma 2019) (Table 4).”
Obtained results indicate that high concentration values of the studied PTEs are attributed to anthropogenic inputs, for instance, human activities regarding industrial contributions and vehicle emission in the study area. The main potential sources of PTEs in the study area include Arak refinery and petrochemical complex, vehicle emission, and manufacturing factories. The main manufacturing factories are Iranian Aluminum Company, Train Center (TC), HEPCO industry, Azarab Industries Company (AIC), Arak Industrial hub, Wagon Pars Company, Navrd Aluminum, and Rolled Aluminum group (Fig. 2).
Dehghani et al. (2017) reported that concentration of PTEs in surface soil of Tehran was triggered by anthropogenic inputs, such as vehicle emission, tire weathering and braking, and combustion of fossil fuels. Jien et al. (2011) demonstrated the presence of PTEs concentration in street dust in downtown areas, tunnels, parking's, and residential areas, and that the higher and lower concentration of PTEs was observed in the tunnels and residential area, respectively. Abdel-Latif and Saleh (2012) showed that the most important source of Pb in street dust is car-fueled additives, and other PTEs such as Cr, Cu, and Zn, which come from the erosion of alloys used in vehicles and other materials. Industrial activities might also be sources of emission of these metals in the urban soil environment (Al-Khashman 2007; Charlesworth et al. 2003). Fossil fuels combustion and lubricants used in cars are the main sources of Ni in an urban environment (Wei et al. 2010). The most remarkable source of As is from parent materials, yet as their accumulation in urban environments is due to geogenic sources, such as fossil fuel combustion, iron steel processing industries, and mineral activity (Brown and Peake 2006). The Cr is utilized in the manufacture of batteries, plastics, and building materials (Wei et al. 2010). In the study area, there was abundant high dense of residential buildings around the street; so tire and battery erosion would be a significant source of Cd. Fuel combustion is a possible source of Cr, but its anthropogenic origins in the city and the severity of contamination are determined.
The results of EF mean values of the studied elements indicate the following order of enrichment: Cd > Pb > Ni > Cr > Cu > Zn > As. According to (Yongming et al. 2006), Cd, Pb, Ni, and Cr, with EF values higher than 10, were released from anthropogenic emission into surface soil in the study area. Cu and Zn have EF values between 5 and 10, which is a significant enrichment, with a mixture of geogenic and anthropogenic source. As with EF value (2.1) indicates moderate enrichment and geogenic sources.
Anthropogenic from the geogenic contribution of the studied elements was calculated, and the findings indicated the following order of anthropogenic contribution: Cd (89%) > Ni (74%) > Pb (72%) > Cr (52%) > Cu (48%) > Zn (39%) > As (11%), which exhibits that the main source of enrichment is the anthropogenic sources, including residential and industrial hubs, like Iran combine manufacturing company, manufacturing factories, aluminum rolling, HEPCO industry, vehicle traffic, residential, and commercial zones in Arak city. To investigate the amount of anthropogenic contribution of contamination, EI value was calculated. The results of EI values demonstrated that EI > 3 in the sampling locations (S3A, S4A, S1A, S8, s9, S10, S12, S13, S14, and S19), which are situated in the heavy industrial hub and manufacturing companies.
Possible source identification
Table 5 represents the Spearman correlation among PTEs concentrations in Arak surface soil samples. Kolmogorov–Smirnov test was used to normalize the data. Following the normalization, Spearman correlation was applied to investigate the inter-elements relations among PTEs, finding possible sources and pathways of PTEs (Babaei et al. 2018; Ghanavati et al. 2019b; Huang et al. 2009). The correlation coefficient among PTEs indicated that there are two main groups: The first group, containing Cr, Ni, Pb, and Cu, shows a significant correlation at p < 0.01: Ni–Cr (0.96), Ni–Pb (0.52), Ni–Cu (0.54), Cr–Pb (0.54), Cr–Cu (0.58), Pb–Cu (0.83), which suggests that the studied PTEs are originated with common sources. The second group shows a significant correlation among Cr, Zn, and Cd at p < 0.01: Cr–Cd (0.44), Cr–Zn (0.42), Zn–Cd (0.51), which have a different sources/mixing compared to the first group with other sources. Arsenic is also not correlated with other PTEs, which reveals the existence of various sources for the release of PTEs in the surface soils of Arak city.
Principal components analysis (PCA) was applied to recognize the relation among PTEs in urban surface soil and determine their possible sources (Facchinelli et al. 2001; Rodríguez-Seijo et al. 2015). Kaiser–Meyer–Olkin index and Bartlett’s test of sphericity were significant at p < 0.001 and 0.499, respectively. This conveyed that the studied PTEs in Arak urban surface soil were more appropriate for applying PCA test (Fig. 3). The results PCA with varimax rotation showed that the three first components accounted for 81.14% of the total variance (Table 6). The PC1 accounted for 38.39% of the total difference, which was remarkably higher than Ni (0.762), Cr (O.860), Pb (0.786), and Cu (0.896). PC1 suggests that the studied PTEs may be derived from anthropogenic sources or a mixture of geogenic and anthropogenic sources. These findings are also confirmed by the top enrichment factor (TEF) introduced by (Facchinelli et al. 2001). The top enrichment factor (TEF) as an indicator of the ratio of top concentration and deep soil layer concentration value of these metals was higher than other metals, which confirms that these metals also have the same geochemical behavior and origins from same anthropogenic sources. Higher TEF for each of the elements indicates a possible anthropogenic source of metal. In general, TEF value for metals originated with lactogenic and pedogenic sources is < 2 (Facchinelli et al. 2001). The PTEs associated with PC1 have TEF > 2, which confirms the anthropogenic sources of these elements in Arak city.
The second component (PC2) accounted for 28% of the total variance and included Cd (0.690) and Zn (0.612) (Table 7). Regarding high EF value of Cd and Zn as well as higher EF, separation of Cd, and Zn for other PTEs, PC2 indicated a different geochemical behavior and mixture of different sources of Cd and Zn in the surface soil in the study area. With regard to the corresponding significant coefficient of correlation between Cd and Zn in the studied soil samples, the anthropogenic inputs of these elements are applicable. TEF values of Cd and Zn are higher than 2, which reveals an anthropogenic input into soils mainly from steel manufacturing companies, wear of vehicle tires, corrosion of metals, burning of garbage, and industrial gases (Ghanavati and Nazarpour 2018; Kong et al. 2011). The component (PC3), with 14.92% of the total variance, had a positive loading only for As metal revealing the origin from a different source of other metals such as geogenic origins (Amiri et al. 2016).
Chemical speciation of the studied PTEs
To investigate the bioavailability, possible source, and mobility of PTEs the geochemical fractionation is applicable (Bird et al. 2005). For this purpose, some of the seven species fractionations include water-soluble, acid-soluble/exchangeable, carbonate bound, humic acid bound, Fe–Mn oxide bound (reducible), organic matter bound, and residual fractions (Gope et al. 2017; Khan et al. 2014). The main partitions of Pb are Fe–Mn oxide bound (mean: 63.7%), residual (mean: 14.5%), organic matter bound (mean: 7%), carbonate bound (mean: 8.6%), and humic acid bound (mean: 4.2%). Our results indicated that the highest percentage of fractions belonged to a reducible bound and residual fraction. A higher rate with reducible bound is associated with the high tendency of Pb to absorb on clay minerals (Pavlović et al. 2018). The association of Pb with reducible bound causes more abortion of other PTEs in soil, particularly in pH > 7, as was the case with the soil samples of the studied area (Marwa et al. 2012; Tawiah 2018). The obtained results concerning a moderate to high concentration level and mobility of Pb also exhibited that Pb could be able to remobilize and be a potential threat to the environment (Pavlović et al. 2018). Pavlović et al. (2018) suggested an association of Pb with the reducible fraction in the Serbian industrial cities’ surface soil samples. Our findings are also confirmed by other published previous literature, such as Forghani et al. 2015 and Ramos et al. 1994.
The main partitions of Cu are residual (mean: 31%), organic matter bound (mean: 27%), Fe–Mn bound/reducible (mean: 24%), acid-soluble/exchangeable (mean: 4.5%). The high value of organic matter bound fraction is attributed to the tendency of Cu to sulfide the compounds in soil, and also to the stable condition of organic complexes of Cu(II) which has a high affinity with organic matter bounds (Feizi et al. 2019; Pavlović et al. 2018; Wang et al. 2020).
The main partitions of Ni are Fe–Mn bound/reducible (mean: 48.2%), residual (mean: 31.2%), organic matter bound (mean: 11%), acid-soluble/exchangeable (mean: 6.2%). Soil geochemical characteristics, including soil salinity, pH, together with redox conditions, could release Ni into Fe–Mn oxide bounds, and this bound could be regarded as a threat for the ecosystem (Jain et al. 2007). The results of chemical fractionation of all of the soil samples indicate that the highest percentage of fractions belonged to reducible bound and residual fraction, which suggested that Ni sources are a mixture of geogenic and anthropogenic inputs (Ghrefat et al. 2012).
The main partitions of Cr are Fe–Mn bound/reducible (mean: 62.6%), residual (mean: 19.2%), acid-soluble/exchangeable (mean: 11.8%). The collected samples in the studied area, which is an area with fairly high industrial activities or high-traffic area, implied a higher fraction of Fe–Mn bound, which suggests that Cr originates from anthropogenic inputs into this area. Meanwhile, the samples from the newly developed areas and lower traffic zones indicate a higher percent of residual fraction, which confirms both of geogenic and anthropogenic source of Cr in the collected samples (Pavlović et al. 2018).
The main partitions of As are residual (mean: 75.7%), Fe–Mn bound/reducible (mean: 11.2%), organic matter bound (mean: 6%), carbonate bound (mean: 4.5%). In the following, the limit of detection for the exchangeable portion indicates that As has a stable condition under anthropogenic alter induced situation with lower mobility, which is in agreement with previously published literature (Ghrefat et al. 2012; Osakwe 2013; Pavlović et al. 2018). Lower concentrations of As in the collected samples are a distinct class of PCA, and a higher fraction of residual for As confirms the geogenic source of As in the studied area.
The main partitions of Zn are Fe–Mn bound/reducible (mean: 48.3%), residual bound/reducible (mean: 22.6%), acid-soluble/exchangeable (mean: 16%). The higher percentage of the reducible and residual fractions of Zn indicates that certain portions of Zn are available for plants and biota uptakes, which causes higher bioavailability as well as potential ecosystem risk in the surface soil (Jain et al. 2007; Li et al. 2001).
The main partitions of Cd fractions are as follows: residual fraction (mean: 32%), humic acid bound (24.3%), carbonate bound (18%), soluble/exchangeable (11.5%), organic matter bound (5.4%), Fe–Mn bound/reducible (mean: 4.3%). The findings indicated that acid-soluble/exchangeable, carbonate bound, humic acid bound are attributed to more than 50% of the total Cd in the soil samples, which confirms that Cd bioavailability is affected by Alkaline pH (López Arias and Grau Corbí 2005).
Human health risks and carcinogenic risk (CR) of PTEs
The evaluation of human health risk caused by PTEs in a city through dermal contact, inhalation, and ingestion routes was implemented for both children and adults. The slope parameters and reference dose values of PTEs are presented in Table 1. Human health risk and cancer risk factors, including carcinogenic and non-carcinogenic, the hazard quotient (HQ), the hazard index (HI), and cancer risk (CR) for both children and adults were determined and are presented in Table 7. According to HQ value, the order of the exposure routes for PTEs in Arak surface soil was as follows: Ingestion > Inhalation > Dermal. According to US EPA (1996), HQ < 1 indicates no adverse of non-carcinogenic risk of the studied PTEs, and HQ > 1 signifies contrary health of PTEs (USEPA 1997). The results of HQ of all the studied PTEs were < 1 confirming no non-carcinogenic risk for both target populations. This finding is in accordance with the results of the literature (Egorova and Ananikov 2017; Wei et al. 2015; Wu et al. 2018). Cheng et al. (2018) and Huang et al. (2020) reported that ingestion is the main way of exposure for both children and adults, which is related to the suspension of soil particles and exposure of residents and hand-to-mouth contact.
Higher HI values, rather than threshold safe value limit (HI > 1), were observed for Cu, Pb, Cr, and Ni, suggesting non-carcinogenic risks of the studied PTEs for both target populations. Regarding the target populations, the non-carcinogenic risks in children were at an upper limit compared to adults, which indicated that children were relatively more at risk, especially by Ni, Cu, Pb, and Cr in Arak surface soil (Table 7).
Having studied PTEs in a kindergarten in Yerevan indicated that the HI value of children is higher than the acceptable threshold and adults (Tepanosyan et al. 2017). Zhaoyong et al. (2018) investigated the health risk of PTEs in the green land of China, and they suggested that ingestion, inhalation, and dermal contact are the main causes of non-carcinogenic risk of PTEs. Diami et al. (2016) indicated that higher values of HI for children makes them more sensitive to the non-carcinogenic risk of PTEs.
Carcinogenic risk (CR) from Cu, Cr, Ni, and Pb exposure was evaluated via the ingestion exposure pathways. The CR values were 6.26E-06 (Ni), 5.91E-04 (Cr), 3.90E-06 (Pb), 2.80E-09 (As), and 14.57E-07 (Cd) for children, and 4.49E-06 (Ni), 3.81E-04 (Cr), 3.23E-06 (Pb), 3.73E-10 (As), and 6.10E-08 (Cd) for adults (Table 7).
Carcinogenic risks (CR) of the studied PTEs were more significant than the limit value 1.0E-04, for adults and children, except for Cr with values of 5.91E-04, and 3.81E-04 for children and adults, respectively. Higher CR values of Cr rather than other PTEs in the surface soil of Arak showed that the target population, including children and adults, particularly children, are more at risk of carcinogenic risks of PTEs. Nazarpour et al. (2019) indicated the higher carcinogenic risk of PTEs in children rather than adults in Ahvaz surface soil. Similar studies have also confirmed the same results (Adimalla et al. 2019; Ghanavati et al. 2019b; Ihedioha et al. 2017; Tongo et al. 2017; Wongsasuluk et al. 2014).
Isotopic ratios
There are four isotopes for lead including 204Pb, 206Pb, 207Pb, and 208Pb. Pb isotopes, except 204Pb, are from radioactive decay of 238U, 235U, and 232Th. Their frequency is different depending on different sources. Various anthropogenic sources and ore minerals are of different isotopic characteristics (Cheng and Hu 2010). Pb isotopic ratios do not change through industrial or environmental/anthropogenic process, and their ore ratios are constant. Therefore, isotopic ratios are more functional for source identification and pathway of Pb in pollution studies (Han et al. 2015). Soil pollution source identification using Pb isotopes is based on the theory that believes natural materials (unpolluted soils and rocks) and pollutions containing Pb from anthropogenic sources have different isotopic characteristics (Nazarpour et al. 2019). Lead ores (mostly Galen (PbSO4)) have higher Pb/Th and Pb/U ration in comparison with other ores, and their isotopic ratios are constant during ages (Hansmann and Köppel 2000). Accordingly, lead ore minerals have a specific isotopic composition which is independent of age with low 206Pb/204Pb values (Doe and Delevaux 1972). On the contrary, Pb in unpolluted soils is the result of the weathering of bedrocks from which the isotopic composition of Pb has evolved and reflective U/Pb and Th/Pb in parent materials.
Isotopic ratios of Pb for 13 collected surface soil samples are presented in Table 8. These data indicate that samples from the industrial areas have isotopic compositions distinct from other samples. Generally, polluted soil samples have lower 206Pb/204Pb, 207Pb/204Pb, 208Pb/204Pb, and higher 208Pb/206Pb ratios, which is in agreement with calculated ratios in the collected samples (Table 8 and Fig. 4). Isotopic ratios of Pb in unpolluted soils in the studied area (such as S6A, S13A, and S12A) are more radiogenic, and their values are similar to the average of continental crust (206Pb/207Pb = 1.20) (Galušková et al. 2014). Therefore, these samples could be taken into consideration as natural soils with more geogenic isotopic ratios rather than anthropogenic sources. On the other hand, lower 206Pb/204Pb rates of samples around industrial areas (including S1A, S2A, S3A, and S4A) indicate the anthropogenic source of Pb in the surface soils (Komárek et al. 2008). Other Pb isotopic compositions are compatible with a mixture of anthropogenic and geogenic sources.
The plot of 208Pb/206Pb and 208Pb/206Pb sheds light to a nonlinear relation among the studied sampling sites, which is a result of a distinct Pb isotope characteristics in the studied area. Additionally, Fig. 4 shows that samples related to industrial areas (including S1A, S2A, S3A, and S4A) have higher isotopic ratios than other land uses in the studied area and vehicle leaded exhaust. Higher isotopic ratios of industrial samples are indirectly related to active companies such as Iranian Aluminum Company, Azarab Industries Company, and Arak industrial hub, as indicated in Fig. 2. Agriculture and park samples (S13A and S6A) showed two distinct isotopic ratios. S13A, with a higher Pb content and isotopic ratios, is located in the agriculture area, which is located adjacent to active industrial sites. The samples from a residential area with high-traffic volume (S8A, S9A, S11A, and S12A) are scattered adjacent to leaded vehicle exhaust and Mississippi ore isotopic ratio, which clearly indicates the role of traffic on pollution of Arak. Sample A9A with a higher value than other residential samples is located in the intersection of the main square and the ring road of Arak city with high-traffic volume.
In order to distinct different sources (geogenic and anthropogenic) of Pb, different equations and formula were suggested. The primary strategy of most studies is binary models that evaluate the contribution of end-members (for example, geogenic Pb vs. leaded gasoline) (Nazarpour et al. 2019). Three end-member contribution models could be applied to define the percent part of geogenic sources (F1), industrial emission (F2), and vehicle traffic (F3) employing Pb concentration and isotopic ratios (Huang et al. 2020; Yu et al. 2016).
Herein, F1, F2, and F3 are the contribution of geogenic, industrial input, and vehicle emission in soil, respectively. R1 represents 206Pb/207Pb in geogenic sources (1.2252), and R2, R2, and Rsoil are 206Pb/207Pb of industrial sources (1.1427–1.1567), and vehicle exhaust (1.097) and soil samples (Yu et al., 2016), respectively. C1, C2, C3, and Csoil are the concentration of Pb in background soil, industrial (6681 mg/kg), vehicle exhaust (2379 mg/kg), and soil sample concentration, respectively (Rodríguez-Seijo et al. 2015).
The calculated contributions of Pb sources, including geogenic, industrial, and vehicle exhausts, are presented in Table 8. The result indicated that regarding land use sampling site, the F as a factor of contribution varies from 68.0% to 15% (mean: 39.3) for industrial emission, 65% to 19% for vehicle exhaust (mean: 39), and 46% to 10% (mean: 21.6) for geogenic sources. In the studied area, there are several manufacturing companies as well as high-traffic volume, which make the main sources of Pb pollution as a mixture of industrial and traffic emission.
Conclusions and remarks
The results of the current study indicated that the mean concentrations of all of the PTEs, with the exception of As, have higher mean values compared to the world soil and baseline values in the study area. The surface soil of Arak city, in terms of As and Zn metals, showed high EF values for Cd, Pb, Ni and Cr, and significant to moderate values for Cr, Zn, and As with an anthropogenic contribution order as follows: Cd (89%) > Ni (74%) > Pb (72%) > Cr (52%) > Cu (48%) > Zn (39%) > As (11%). This implies that the main source of enrichment of PTEs in the study area is the anthropogenic sources. In the samples, EI > 3 values were observed to be approximately higher in traffic and manufacturing companies spots. Based on the correlation coefficients and principal component analysis (PCA), there is a similar relation between PTEs such as Pb, Cr, Cd, Ni, Cu, and Zn in terms of sources of contamination. The main sources of PTEs, except for As, were mainly the industrial hubs and manufacturing industries located in the studied area. The results of geochemical fraction indicated that the main partitions of Pb, Cr, and Zn at most of the sampling sites are Fe–Mn bound/reducible, while the residual fraction is the dominant fraction of sequence extraction for Ni, Cu, and Cd. Higher values of reducible bound for Pb, Cr, and Zn, as well as a considerable percentage of Ni, Cu, and Cd, demonstrated that the main source of the studied PTEs (except As) in the studied area is both anthropogenic and geogenic inputs. Health risk of the studied PTEs revealed the following order of exposure: inhalation > ingestion > dermal contact. The findings regarding HQ of all of the studied PTEs were lower than 1, confirming that there is no non-carcinogenic risk for children and adults. The value of HI for Cu, Pb, Ni, and Cr was HI > 1, which suggests the non-carcinogenic risk for both children and adults. Carcinogenic risks (CR) of the studied PTEs were higher than the acceptable threshold of 1.0E-06, for adults and children, except for Cr with values of 5.91E-04, and 3.81E-04 for children and adults, respectively. Higher CR values of Cr rather than other PTEs in the surface soil of Arak exhibited that the target population, including children and adults, especially children, could be more affected by carcinogenic risks of PTEs. 206Pb/207Pb ratios of the collected samples indicated that Pb in Arak surface sample derived from industrial and deposition as well as re-suspension vehicle exhaust emission from previously leaded gasoline. Our findings concerning the applied end-member contribution of geogenic, industrial, and vehicle emission indicated that the contribution could be different ranging from 68.0% to 15% (mean: 39.3) for industrial emission, 65–19% for vehicle exhaust (mean: 39), and 46–10 (mean: 21.6) for geogenic sources.
References
Álvarez-Iglesias, P., Rubio, B., & Millos, J. (2012). Isotopic identification of natural vs. anthropogenic lead sources in marine sediments from the inner Ría de Vigo (NW Spain). Science of the total environment, 437, 22–35.
Abdel-Latif, N. M., & Saleh, I. A. (2012). Heavy metals contamination in roadside dust along major roads and correlation with urbanization activities in Cairo. Egypt Journal of American Science, 8, 379–389.
Adimalla, N., Li, P., & Qian, H. (2019). Evaluation of groundwater contamination for fluoride and nitrate in semi-arid region of Nirmal Province, South India: A special emphasis on human health risk assessment (HHRA). Human and Ecological Risk Assessment: an International Journal, 25(5), 1107–1124. https://doi.org/10.1080/10807039.2018.1460579.
Al-Khashman, O. A. (2007). Determination of metal accumulation in deposited street dusts in Amman. Jordan Environmental geochemistry and health, 29, 1–10.
Amiri, M., Sarikhani, R., Ghasem Dehnavi, A., & Moradpour, A. (2016). The assessment of pollution and distribution of heavy metals applying geochemical data and statistical analysis in soils surrounded kermanshah refinery quaternary. Journal of Iran, 2, 257–269.
Babaei, H., Ghanavati, N., & Nazarpour, A. (2018). Contamination level of mercury in the street dust of ahvaz city and its spatial distribution. JWSS-Isfahan University of Technology, 22, 249–259.
Bennett, P. M., et al. (2001). Exposure to heavy metals and infectious disease mortality in harbour porpoises from England and Wales. Environmental pollution, 112, 33–40.
Bird, G., Brewer, P. A., Macklin, M. G., Serban, M., Balteanu, D., & Driga, B. (2005). Heavy metal contamination in the Arieş river catchment, western Romania: Implications for development of the Roşia Montană gold deposit. Journal of Geochemical Exploration, 86, 26–48.
Borojerdnia, A., Rozbahani, M. M., Nazarpour, A., Ghanavati, N., & Payandeh, K. (2020). Application of exploratory and Spatial Data Analysis (SDA), singularity matrix analysis, and fractal models to delineate background of potentially toxic elements: A case study of Ahvaz (p. 140103). Science of The Total Environment: SW Iran.
Brown, J. N., & Peake, B. M. (2006). Sources of heavy metals and polycyclic aromatic hydrocarbons in urban stormwater runoff. Science of the total environment, 359, 145–155.
CEPA. (2007). Canadian soil quality guidelines for the protection of environmental and human health.
Chabukdhara, M., & Nema, A. K. (2013). Heavy metals assessment in urban soil around industrial clusters in Ghaziabad, India: Probabilistic health risk approach. Ecotoxicology and environmental safety, 87, 57–64.
Charlesworth, S., Everett, M., McCarthy, R., Ordonez, A., & De Miguel, E. (2003). A comparative study of heavy metal concentration and distribution in deposited street dusts in a large and a small urban area: Birmingham and coventry West Midlands, UK. Environment International, 29, 563–573.
Chen, T., Zheng, Y., Chen, H., & Zheng, G. (2004). Background concentrations of soil heavy metals in Beijing. Huan jing ke xue=Huanjing kexue, 25, 117–122.
Cheng, H., & Hu, Y. (2010). Lead (Pb) isotopic fingerprinting and its applications in lead pollution studies in China: A review. Environmental pollution, 158, 1134–1146.
Cheng, X., et al. (2018). Soil heavy metal pollution and risk assessment associated with the Zn-Pb mining region in Yunnan Southwest China. Environmental monitoring and assessment, 190, 194.
Cicchella, D., De Vivo, B., Lima, A., Albanese, S., McGill, R., & Parrish, R. R. (2008). Heavy metal pollution and Pb isotopes in urban soils of Napoli Italy. Geochemistry: Exploration, Environment, Analysis, 8, 103–112.
Das Sharma, S. (2019). Risk assessment and mitigation measures on the heavy metal polluted water and sediment of the Kolleru Lake in Andhra Pradesh India. Pollution, 5, 161–178.
Dehghani, S., Moore, F., Keshavarzi, B., & Beverley, A. H. (2017). Health risk implications of potentially toxic metals in street dust and surface soil of Tehran Iran. Ecotoxicology and environmental safety, 136, 92–103.
Diami, S. M., Kusin, F. M., & Madzin, Z. (2016). Potential ecological and human health risks of heavy metals in surface soils associated with iron ore mining in Pahang Malaysia. Environmental science and pollution research, 23, 21086–21097.
Doe, B. R., & Delevaux, M. H. (1972). Source of lead in southeast Missouri galena ores. Economic Geology, 67, 409–425.
Du, Y., Gao, B., Zhou, H., Ju, X., Hao, H., & Yin, S. (2013). Health risk assessment of heavy metals in road dusts in urban parks of Beijing China. Procedia Environmental Sciences, 18, 299–309.
Eby, G. (2004). Principles of environmental geochemistry. Brooks: Cole Publishing Company, Cole, CA.
Egorova, K. S., & Ananikov, V. P. (2017). Toxicity of metal compounds: knowledge and myths. Organometallics, 36, 4071–4090.
Facchinelli, A., Sacchi, E., & Mallen, L. (2001). Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environmental pollution, 114, 313–324.
Fakhri, Y., Mousavi Khaneghah, A., Hadiani, M. R., Keramati, H., Hosseini Pouya, R., Moradi, B., & da Silva, B. S. (2017). Non-carcinogenic risk assessment induced by heavy metals content of the bottled water in Iran. Toxin Reviews, 36, 313–321.
Feizi, M., Jalali, M., Antoniadis, V., Shaheen, S. M., Ok, Y. S., & Rinklebe, J. (2019). Geo-and nano-materials affect the mono-metal and competitive sorption of Cd, Cu, Ni, and Zn in a sewage sludge-treated alkaline soil. Journal of hazardous materials, 379, 120567.
Ferreira-Baptista, L., & De Miguel, E. (2005). Geochemistry and risk assessment of street dust in Luanda, Angola: A tropical urban environment. Atmospheric environment, 39, 4501–4512.
Forghani, G., Mokhtari, A. R., Kazemi, G. A., & Fard, M. D. (2015). Total concentration, speciation and mobility of potentially toxic elements in soils around a mining area in central Iran. Geochemistry, 75, 323–334.
Galušková, I., Mihaljevič, M., Borůvka, L., Drábek, O., Frühauf, M., & Němeček, K. (2014). Lead isotope composition and risk elements distribution in urban soils of historically different cities Ostrava and Prague, the Czech Republic. Journal of Geochemical Exploration, 147, 215–221.
Ghadimi, F., Ghomi, M., Ranjbar, M., & Hajati, A. (2013). Statistical analysis of heavy metal contamination in urban dusts of Arak Iran Iranica. Journal of Energy and Environment, 4, 406–418.
Ghanavati, N., & Nazarpour, A. (2018). Environmental investigation of heavy metals concentration in Ahvaz city street dust, by using Geographical Information Systems (GIS). Journal of Environmental Studies, 44(3), 393–410.
Ghanavati, N., Nazarpour, A., & De Vivo, B. (2019). Ecological and human health risk assessment of toxic metals in street dusts and surface soils in Ahvaz Iran. Environmental geochemistry and health, 41, 875–891.
Ghanavati, N., Nazarpour, A., & Watts, M. J. (2019). Status, source, ecological and health risk assessment of toxic metals and polycyclic aromatic hydrocarbons (PAHs) in street dust of Abadan Iran. CATENA, 177, 246–259.
Ghrefat, H. A., Yusuf, N., Jamarh, A., & Nazzal, J. (2012). Fractionation and risk assessment of heavy metals in soil samples collected along Zerqa River. Jordan Environmental Earth Sciences, 66, 199–208.
Gope, M., Masto, R. E., George, J., Hoque, R. R., & Balachandran, S. (2017). Bioavailability and health risk of some potentially toxic elements (Cd, Cu, Pb and Zn) in street dust of Asansol India. Ecotoxicology and Environmental Safety, 138, 231–241.
Han, L., Gao, B., Wei, X., Gao, L., Xu, D., & Sun, K. (2015). The characteristic of Pb isotopic compositions in different chemical fractions in sediments from three gorges reservoir China. Environmental Pollution, 206, 627–635.
Hansmann, W., & Köppel, V. (2000). Lead-isotopes as tracers of pollutants in soils. Chemical Geology, 171, 123–144.
Hu, X., Zhang, Y., Luo, J., Wang, T., Lian, H., & Ding, Z. (2011). Bioaccessibility and health risk of arsenic, mercury and other metals in urban street dusts from a mega-city Nanjing, China. Environmental Pollution, 159, 1215–1221.
Huang, S., et al. (2009). Multivariate analysis of trace element concentrations in atmospheric deposition in the Yangtze River Delta East China. Atmospheric Environment, 43, 5781–5790.
Huang, Y., et al. (2020). Tracing Pb and possible correlated Cd contamination in soils by using lead isotopic compositions. Journal of hazardous materials, 385, 121528.
IDOE (Iran Department of Environment). (2014). Iranian soil quality guidelines for the protection of environmental and human health. Tehran: Iranian Department of Environment. http://www.doe.ir/Portal/file/?692345/1395-standards.pdf.
Ihedioha, J., Ukoha, P., & Ekere, N. (2017). Ecological and human health risk assessment of heavy metal contamination in soil of a municipal solid waste dump in Uyo Nigeria. Environmental geochemistry and health, 39, 497–515.
Jahandari, A., Abbasnejad, A., & Jamasb, R. (2020). Concentration, likely sources, and ecological risk assessment of potentially toxic elements in urban soils of Shiraz City SW Iran: A preliminary assessment. Arabian Journal of Geosciences, 13, 1–10.
Jain, C., Malik, D., & Yadav, R. (2007). Metal fractionation study on bed sediments of Lake Nainital Uttaranchal, India. Environmental Monitoring and Assessment, 130, 129–139.
Jien, S.-H., Tsai, C.-C., Hseu, Z.-Y., & Chen, Z.-S. (2011). Baseline concentrations of toxic elements in metropolitan park soils of Taiwan terrestrial and aquatic. Environmental Toxicology, 5, 1–7.
Kabata-Pendias, A. (2011). Trace elements in soils and plants. Taylor and Francis Group: CRC Press.
Kabata-Pendias, A., & Pendias, H. (2001). Trace elements in soils and plants (3rd ed.). FL, USA: CRC Press Boca Raton.
Kamunda, C., Mathuthu, M., & Madhuku, M. (2016). Health risk assessment of heavy metals in soils from witwatersrand gold mining basin South Africa. International Journal of Environmental Research and Public Health, 13, 663.
Karim, Z., & Qureshi, B. A. (2014). Health risk assessment of heavy metals in urban soil of Karachi Pakistan. Human and ecological risk assessment: an international journal, 20, 658–667.
Kelepertzis, E., Komárek, M., Argyraki, A., & Šillerová, H. (2016). Metal (loid) distribution and Pb isotopic signatures in the urban environment of Athens Greece. Environmental pollution, 213, 420–431.
Khan, S., Reid, B. J., Li, G., & Zhu, Y.-G. (2014). Application of biochar to soil reduces cancer risk via rice consumption: a case study in Miaoqian village Longyan, China. Environment international, 68, 154–161.
Komárek, M., Ettler, V., Chrastný, V., & Mihaljevič, M. (2008). Lead isotopes in environmental sciences: A review. Environment international, 34, 562–577.
Kong, S., et al. (2011). Potential threat of heavy metals in re-suspended dusts on building surfaces in oilfield city. Atmospheric Environment, 45, 4192–4204.
Kříbek, B., et al. (2016). Contamination of soil and grass in the Tsumeb smelter area, Namibia: Modeling of contaminants dispersion and ground geochemical verification. Applied Geochemistry, 64, 75–91.
Kříbek, B., Majer, V., Knésl, I., Nyambe, I., Mihaljevič, M., Ettler, V., & Sracek, O. (2014). Concentrations of arsenic, copper, cobalt, lead and zinc in cassava (Manihot esculenta Crantz) growing on uncontaminated and contaminated soils of the Zambian Copperbelt. Journal of African Earth Sciences, 99, 713–723.
Kurtz, J. C., Jackson, L. E., & Fisher, W. S. (2001). Strategies for evaluating indicators based on guidelines from the environmental protection agency’s office of research and development. Ecological indicators, 1, 49–60.
León-García, G. J., Meza-Figueroa, D. M., Valenzuela-García, J. L., Encinas-Romero, M. A., Villalba-Atondo, A. I., Encinas-Soto, K. K., et al. (2018). Study of heavy metal pollution in arid and semi-arid regions due to mining activity: Sonora and Bacanuchi Rivers. International Journal of Environmental Sciences and Natural Resources. https://doi.org/10.19080/IJESNR.2018.11.555804.
Li, X., Poon, C.-s, & Liu, P. S. (2001). Heavy metal contamination of urban soils and street dusts in Hong Kong. Applied geochemistry, 16, 1361–1368.
López Arias, M., & Grau Corbí, J. (2005). Metales pesados, materia orgánica y otros parámetros de la capa superficial de los suelos agrícolas y de pastos de la España Peninsular. Resultados por provincias INIA-Ministerio de Educación y Ciencia, Madrid-Spain: II.
Man, Y. B., et al. (2010). Health risk assessment of abandoned agricultural soils based on heavy metal contents in Hong Kong, the world’s most populated city. Environment international, 36, 570–576.
Marwa, E. M., Meharg, A. A., & Rice, C. M. (2012). Risk assessment of potentially toxic elements in agricultural soils and maize tissues from selected districts in Tanzania. Science of the Total Environment, 416, 180–186.
McLennan, S. M. (2001). Relationships between the trace element composition of sedimentary rocks and upper continental crust Geochemistry (p. 2). Geosystems: Geophysics.
Mehr, M. R., Keshavarzi, B., Moore, F., Sharifi, R., Lahijanzadeh, A., & Kermani, M. (2017). Distribution, source identification and health risk assessment of soil heavy metals in urban areas of Isfahan province Iran. Journal of African Earth Sciences, 132, 16–26.
Mohammad, A. F., Panahpour, E., & Nazarpour, A. (2018). The study on the level of pollution and spatial distribution of concentrations of heavy metals (Cr, Ni, Cu, Pb and Zn) in the Industrial City of MahShahr. JNE, 71(3), 399–412.
Mtunzi, F., Dikio, E., & Moja, S. (2015). Evaluation of heavy metal pollution on soil in Vaderbijlpark South Africa. International Journal of Environmental Monitoring and Analysis, 3, 44–49.
Nazarpour, A., Ghanavati, N., & Babaenejad, T. (2017). Evaluation of the level of pollution and potential ecological risk of some heavy metals in surface soils in the Ahvaz oil-field Iranian. Journal of Health and Environment, 10, 391–400.
Nazarpour, A., Watts, M. J., Madhani, A., & Elahi, S. (2019). Source, spatial distribution and pollution assessment of Pb Zn, Cu, and Pb, isotopes in urban soils of Ahvaz City, a semi-arid metropolis in southwest. Iran Scientific reports, 9, 1–11.
NEPAC (National Environmental Protection Agency of China). (1995). Environmental quality standard for soils (GB 15618–1995) (in Chinese).
Osakwe, S. (2013). Chemical partitioning of iron, cadmium, nickel and chromium in contaminated soils of south-eastern Nigeria. Chemical Speciation & Bioavailability, 25, 71–78.
Oyeleke, P. O., Abiodun, O. A., Salako, R. A., Odeyemi, O. E., & Abejide, T. B. (2016). Assessment of some heavy metals in the surrounding soils of an automobile battery factory in Ibadan Nigeria African. Journal of Environmental Science and Technology, 10, 1–8.
Pavlović, D., et al. (2018). Fractionation, mobility, and contamination assessment of potentially toxic metals in urban soils in four industrial Serbian cities. Archives of environmental contamination and toxicology, 75, 335–350.
Qing, X., Yutong, Z., & Shenggao, L. (2015). Assessment of heavy metal pollution and human health risk in urban soils of steel industrial city (Anshan) Liaoning, Northeast China. Ecotoxicology and environmental safety, 120, 377–385.
Ramos, L., Hernandez, L. M., & Gonzalez, M. J. (1994). Sequential fractionation of copper, lead, cadmium and zinc in soils from or near donana National Park. Journal of environmental Quality, 23, 50–57.
Rasmussen, P., Subramanian, K., & Jessiman, B. (2001). A multi-element profile of house dust in relation to exterior dust and soils in the city of Ottawa. Canada Science of the total environment, 267, 125–140.
Ravankhah, N., Mirzaei, R., & Masoum, S. (2018). Assessment of accumulation and human health risk of trace elements in the vicinity of industrial estates. Central Iran Archives of Hygiene Sciences, 7, 118–125.
Rodríguez-Seijo, A., Arenas-Lago, D., Andrade, M. L., & Vega, F. A. (2015). Identifying sources of Pb pollution in urban soils by means of MC-ICP-MS and TOF-SIMS. Environmental Science and Pollution Research, 22, 7859–7872.
Sadeghdoust, F., Ghanavati, N., Nazarpour, A., Babaenejad, T., & Watts, M. J. (2020). Hazard, ecological, and human health risk assessment of heavy metals in street dust in Dezful Iran. Arabian Journal of Geosciences, 13, 1–14.
Saeedi, M., Li, L. Y., & Salmanzadeh, M. (2012). Heavy metals and polycyclic aromatic hydrocarbons: pollution and ecological risk assessment in street dust of Tehran. Journal of hazardous materials, 227, 9–17.
Shahsavani, A., et al. (2017). Risk assessment of heavy metals bioaccumulation: fished shrimps from the Persian Gulf. Toxin reviews, 36, 322–330.
Singh, A., Sharma, R. K., Agrawal, M., & Marshall, F. M. (2010). Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food and chemical toxicology, 48, 611–619.
Sun, Y., Zhou, Q., Xie, X., & Liu, R. (2010). Spatial, sources and risk assessment of heavy metal contamination of urban soils in typical regions of Shenyang China. Journal of hazardous materials, 174, 455–462.
Sutherland, R. (2000). Bed sediment-associated trace metals in an urban stream Oahu, Hawaii. Environmental geology, 39, 611–627.
Taghizadeh, M., & Taghizadeh, M. (2019). Investigation of bioaccumulation of heavy metals concentration in arak metropolitan. Journal of Research in Environmental Health, 5, 31–42. https://doi.org/10.22038/jreh.2019.37780.1273.
Tawiah, R. (2018). Bioaccessibility studies of potential toxic elements (ptes) in medicinal plants from different locations in accra metropolis. Ghana: University of Ghana.
Tepanosyan, G., Maghakyan, N., Sahakyan, L., & Saghatelyan, A. (2017). Heavy metals pollution levels and children health risk assessment of Yerevan kindergartens soils. Ecotoxicology and environmental safety, 142, 257–265.
Tongo, I., Ogbeide, O., & Ezemonye, L. (2017). Human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in smoked fish species from markets in Southern Nigeria. Toxicology reports, 4, 55–61.
Turnbull, R., Rogers, K., Martin, A., Rattenbury, M., & Morgan, R. (2019). Human impacts recorded in chemical and isotopic fingerprints of soils from Dunedin City New Zealand. Science of the total environment, 673, 455–469.
USEPA U (1997) Exposure factors handbook office of research and development, Washington.
Vahedian, M., Khanjani, N., Mirzaee, M., & Koolivand, A. (2017). Associations of short-term exposure to air pollution with respiratory hospital admissions in Arak Iran. Journal of environmental health science and engineering, 15, 17.
VROM. (2000). Circular on target values and intervention values for soil remediation annex a: Target values, soil remediation intervention values and indicative levels for serious contamination. Dutch Ministry of Housing, Spatial Planning and Environment (VROM).
Wang, C., et al. (2020). Polycyclic aromatic hydrocarbons and heavy metals in urban environments: Concentrations and joint risks in surface soils with diverse land uses. Land Degradation & Development, 31, 383–391.
Wedepohl, K. H. (1995). The composition of the continental crust. Geochimica et cosmochimica Acta, 59, 1217–1232.
Wei, B., Jiang, F., Li, X., & Mu, S. (2010). Heavy metal induced ecological risk in the city of Urumqi NW China. Environmental monitoring and assessment, 160, 33.
Wei, X., Gao, B., Wang, P., Zhou, H., & Lu, J. (2015). Pollution characteristics and health risk assessment of heavy metals in street dusts from different functional areas in Beijing. China Ecotoxicology and environmental safety, 112, 186–192.
Wongsasuluk, P., Chotpantarat, S., Siriwong, W., & Robson, M. (2014). Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province Thailand. Environmental geochemistry and health, 36, 169–182.
Wu, W., Wu, P., Yang, F., Sun, D.-l, Zhang, D.-X., & Zhou, Y.-K. (2018). Assessment of heavy metal pollution and human health risks in urban soils around an electronics manufacturing facility. Science of the Total Environment, 630, 53–61.
Xu, P., et al. (2012). Use of iron oxide nanomaterials in wastewater treatment: A review. Science of the Total Environment, 424, 1–10.
Yongming, H., Peixuan, D., Junji, C., & Posmentier, E. S. (2006). Multivariate analysis of heavy metal contamination in urban dusts of Xi’an Central China. Science of the total environment, 355, 176–186.
Yu, R., Zhang, W., Hu, G., Lin, C., & Yang, Q. (2016). Heavy metal pollution and Pb isotopic tracing in the intertidal surface sediments of Quanzhou Bay, southeast coast of China. Marine pollution bulletin, 105, 416–421.
Zhang, X. P., Deng, W., & Yang, X. M. (2002). The background concentrations of 13 soil trace elements and their relationships to parent materials and vegetation in Xizang (Tibet) China. Journal of Asian Earth Sciences, 21, 167–174.
Zhaoyong, Z., Xiaodong, Y., Simay, Z., & Mohammed, A. (2018). Health risk evaluation of heavy metals in green land soils from urban parks in Urumqi, northwest China. Environmental Science and Pollution Research, 25, 4459–4473.
Acknowledgements
The authors would like to sincerely thank Prof. Jaume Bech as Advisory Board in Environmental Geochemistry and Health for handling this manuscript, valuable comments and suggestions, and two anonymous reviewers, which have improved the quality of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bineshpour, M., Payandeh, K., Nazarpour, A. et al. Status, source, human health risk assessment of potential toxic elements (PTEs), and Pb isotope characteristics in urban surface soil, case study: Arak city, Iran. Environ Geochem Health 43, 4939–4958 (2021). https://doi.org/10.1007/s10653-020-00778-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00778-x