Abstract
Phytostabilization is a green, cost-effective technique for mine rehabilitation and ecological restoration. In this study, the phytostabilization capacity of Erica australis L. and Nerium oleander L. was assessed in the climatic and geochemical context of the Riotinto mining district, southwestern Spain, where both plant species colonize harsh substrates of mine wastes and contaminated river banks. In addition to tolerating extreme acidic conditions (up to pH 3.36 for E. australis), both species were found to grow on substrates very poor in bioavailable nutrients (e.g., N and P) and highly enriched with potentially phytotoxic elements (e.g., Cu, Cd, Pb, S). The selective root absorption of essential elements and the sequestration of potentially toxic elements in the root cortex are the main adaptations that allow the studied species to cope in very limiting edaphic environments. Being capable of a tight elemental homeostatic control and tolerating extreme acidic conditions, E. australis is the best candidate for use in phytostabilization programs, ideally to promote early stages of colonization, improve physical and chemical conditions of substrates and favor the establishing of less tolerant species, such as N. oleander.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining activities generate serious environmental problems, from soil degradation to water pollution, from landscape disruption to biodiversity loss. The exploitation of metal-bearing ore minerals, such as oxides or sulfides, is commonly associated with acid mine drainage (AMD) that impacts on local ecosystems (Bonnail et al. 2019). In many historical pyrite mining areas of the world, abandoned mine lands are a continual source of soil pollution with the release of potentially toxic metals and metalloids (Sarmiento et al. 2009; Oliveira et al. 2012).
Soil contamination has gained considerable attention as potential source of human and ecological risk over a large area in the southern Portugal and southwestern Spain in correspondence to the Iberian Pyrite Belt (IPB), the largest volcanogenic massive sulfide ore of the world (Cánovas et al. 2008; Fernández-Caliani et al. 2008; Canha et al. 2010). In this region, Riotinto, about 90 km NW of Sevilla, emerged since the antiquity as the main center of a massive and prosper mining industry. In all likelihood, Riotinto was first in history to experience the deleterious impacts of mining operations on natural environment, which soon became manifest with the accumulation of pyrite-rich wastes and the production of AMD waters (Lottermoser 2010). In Riotinto, mining and mineral processing has left an extraordinary footprint on the territory, made of vastly disrupted lands and extensive tailing and waste rock dumps, which represent, to this day, a diffuse environmental and human health threat (Romero et al. 2006; Fernández-Caliani et al. 2008; Sánchez de la Campa et al. 2011).
Risk mitigation of hazardous substances (e.g., metals, metalloids, radioactivity, acids, process chemicals) in soils of abandoned mine sites requires monitoring, treatment and secure disposal. Conventional methods for contaminated soils reclamation are based on chemical and physical technologies for on-site management or disposal to landfill sites after excavation and eventual treatment. However, this approach is neither environmentally friendly nor cost-effective, especially in vast and unproductive areas, as it requires huge financial investments and labor (Venkateswarlu et al. 2016; Napoli et al. 2019).
Phytoremediation is a low-cost, green technology that uses vascular plants for environmental restoration and reclamation of contaminated soils, sludge and sediments (Salt et al. 1998; Rahman et al. 2016). Rehabilitation of abandoned mine spoils by phytostabilization technology is supported by several studies (Ernst 2005; Abreu and Magalhaes 2009; Mendez and Maier 2008; Dickinson et al. 2009; Napoli et al. 2019). Restoration of a vegetation cover can fulfill the objectives of stabilization, pollution control, visual improvement and removal of threats to humans (Freitas et al. 2004).
Autochthonous flora and endemic species play an essential role in phytostabilization programs (Doumas et al. 2018), especially in semiarid Mediterranean areas, whose distinctive climate and hydrological regime largely affect contaminant transport from mining wastes, mainly through eolian dispersion (Sims et al. 2013). Indeed, assisted or natural phytostabilization with native plants provides the foundation for primary succession on abandoned mining wastes, by improving the physical and chemical properties of the substrates, thus promoting the establishing of long-term self-sustaining vegetation on mine dumps and contaminated river banks (Ginocchio et al. 2017). For the purposes of a successful phytoremediation program, it is essential to investigate in situ soil–plant relationship of species which play a role in the early stages of colonization processes in contaminated and water-limited environment, such as that of Riotinto.
In the Riotinto area, communities of Erica andevalensis Cabezudo and Rivera, E. australis L., E. umbellata Loefl. ex L, E. scoparia L., Cistus ladanifer L., C. populifolius L., C. monspeliensis L., C. crispus L., Genista polyanthos R. Roem. ex Willk., Nerium oleander L., Securinega tinctoria L. and some Poaceae species spontaneously colonize metal-enriched substrata of mine tailings and the bank sediments of the River Tinto or other watercourses. Rufo et al. (2011) reported a total of 50 different species growing in the extremely acidic water of Tinto River, being the E. australis and N. oleander among the most important in terms of occurrence and cover. Studies about metal content in some plant species of this area and their relationship with their soils of growth have been published (Rodríguez et al. 2007; Rossini Oliva et al. 2009; de la Fuente et al. 2010; Monaci et al. 2011, Rossini-Oliva et al. 2018).
In this study, an interdisciplinary work was carried out to assess soil–plant relationships of two primary colonizer species in Riotinto: E. australis and N. oleander. The aim of this research was to determine the key features of substrates of the mining area and the associated plant responses in elemental partitioning and accumulation for assessing the potential use of the two selected species in phytostabilization programs.
Materials and Methods
Study area and sampling
The Iberian Pyrite Belt (IPB) is one of the largest metallic sulfide deposits in the world. It extends for about 250 km (between 25 and 70 km wide) between southern Portugal and southwestern Spain (Leistel et al. 1998). Riotinto area is included in the IPB and represents the most important European metallogenic and mining region, extending for about 640 km2. The mining and smelting activity in the district dates back to the Iberians and Tartessians (about 3000 BC). In 2002, the mines were closed down due to economic reasons (Chopin and Alloway 2007), although lately the possibility to restore Cu mining has been occasionally reconsidered (i.e., in Cerro Colorado). Riotinto is host of a massive deposit sulfur in the IPB, with about 5000 × 106 tons, containing 45% S, 40% Fe, 0.9% Cu, 0.8% Pb, 2.1% Zn, 26 mg kg−1 Ag and 0.5 mg kg−1 Au (García Palomero 1992). The area has a Mediterranean climate with a mean annual rainfall of 600 to 800 mm and mean annual temperature of 18 °C. Rainfall mostly occurs during autumn and winter (mean 70 mm/month), and summers are very hot and dry. Areas affected by past mining and smelting activities are devoid of vegetation or contain patches of simple plant communities dominated by E. andevalensis or by mixed communities of E. andevalensis and E. australis (Monaci et al. 2011). Vegetated soils are extremely acidic, enriched in metals/metalloids, such as As, Cu, Pb and Zn, and poor of nutrients (Rufo et al. 2007; Monaci et al. 2011). In some sites, E. andevalensis disappears and other species, such as E. australis, N. oleander and Cistus spp., can be found.
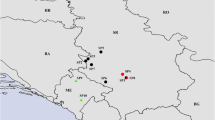
Different sampling sites (six for Erica and four for Nerium), representing different edaphic and environmental characteristics, were selected inside the mining area of Riotinto (Fig. 1). At each site, samples of soils and specimens of E. australis and N. oleander were collected. Two additional sampling sites were located in the undisturbed areas of Linares (30 km N of Riotinto) and Alanís (120 km NE of Riotinto) which acted as control areas for soil and plant material. Denomination and description of the sampling sites are reported in the following, while the corresponding geodata are listed in Table 1 (Electronic Supplementary Material).
-
Zarandas (Z): area not directly affected by past mining and smelting activities and characterized by some environmental recovery measures undertaken in the past, such as terrace planting of Pinus pinea.
-
Nerva (N): site distinguished by unstable mining and smelting waste accumulated as dry, coarse-textured piles and with dispersed patches of vegetation dominated by N. oleander, E. australis and E. andevalensis.
-
Tinto River (RT): area close to the springs of Tinto River mainly colonized by N. oleander, E. australis, E. andevalensis, C. monspeliensis and C. salvifolius with the inclusion of individuals of Ulex eriocladus, Phagnalon saxatile, Helichryisum stoechas and C. ladanifer.
-
Peña de Hierro (PH): an old mining spot, characterized by a flat area where mine spoils have recently been revegetated with P. pinaster.
-
Peña de Hierro Gossan (PHG): it is in the highest part of Peña de Hierro formed by gossan, an aggregate of goethite, hematite and jarosite–beudantite originated by sulfur superficial oxidation where the vegetation has a heather form by E. australis and E. umbellata.
-
Peña de Hierro hill (PHC): a flat area 90% covered for vegetation consisting mainly of E. australis and Halimium ocymoides.
-
Odiel River headwater (PO): an area with very scanty vegetation cover close to Odiel River.
-
Nerva stream (NA): a site close to Nerva municipality with a dense vegetation dominated by N. Oleander.
-
Non-contaminated sites (control; C): two areas far from the mining areas and other potential sources of contamination located in Linares de la Sierra (NE of the Provincia of Huelva) and Alanís (Natural Parque of Sierra Norte of Seville).
Within each site, a composite sample, consisting in at least 3–5 plants of the same species, and a soil sample under each plant were taken. Soil was collected up to 15 cm depth in order to prevent the loss of fine roots. At each site, a composite sample of topsoil (0–15 cm) was also taken at random around each plant. Soil samples were air-dried, sieved (< 2 mm) and stored until analysis.
Sample pre-treatment, analysis and quality control
The main soil physicochemical parameters were determined according to the following standard methods. The particle size distribution was determined by sieving and sedimentation, applying the Robinson’s pipette method. Bulk density was measured on undisturbed core samples taken by the cylinder method at moisture content near field capacity, and water-holding capacity (WHC) was obtained from water retention of disturbed soil samples using ceramic pressure plate (Soil Moisture Equipment Corp., Santa Barbara, CA, USA) at air pressures of 0.03 and 1.5 MPa. Soil pH and electrical conductivity (EC) were determined in soil/deionized water suspension of 1/2.5 (weight/volume). Total C and N content was determined by elemental analysis (TruSpec CN, LECO). Cation exchange capacity (CEC) was determined by a method based on the triethylenetetramine (Trien)–Cu complex (Meier and Kahr 1999). Exchangeable Ca, Mg, K and Na were determined in Trien–Cu extract by ICP-OES. Iron oxides (Fox) were extracted using sodium citrate–dithionite according to Holmgren (1967). Available P was estimated by the Bray-P method (Bray and Kurtz 1945).
Available concentrations of Cd, Cu, Fe, Pb and Zn were extracted with EDTA 0.05 M pH 7 (Quevauviller et al. 1998) and total metals extracted with aqua regia in a pressurized PFA digestion vessels in a microwave digester (1200 Mega, Milestone). In both cases, the elements were analyzed by ICP-OES. The Certified Reference Materials (CRMs), “Montana Soil” (NIST 2111) and “Amended Soil” (BCR 143) were used to check the accuracy and precision of the analytical procedures. The results obtained for certified materials show a recovery range from 90 to 100%. All analyses performed were done in duplicate, and all results were calculated on a dry weight basis.
Leaves of the same age and stage of development were selected in laboratory; live roots were carefully separated from soil and cleaned. The bark was detached from roots to be analyzed separately from the internal tissue (endodermis and vasculature). The selected material was then oven-dried and homogenized with a centrifugal ball mill (Retsch, model S100). About 0.4 g of each sample was digested with ultrapure-grade HNO3 in closed Teflon vessels in a microwave oven (Milestone, Ethos 1) under optimal time, temperature and pressure conditions. Analytical determinations of macro- and microelements concentrations were performed through ICP-OES (Optima 5300 DV; Perkin Elmer) for Al, Ca Cu, K, Fe, Mg, Mn, Na, S and Zn and by high-resolution continuum source atomic absorption spectrometry (ContrAA 700, Analytic Jena) for Cd and Pb. Procedural blanks and replicate determinations were performed to check sample homogeneity and uncertainties related to digestion and analysis of samples. The accuracy of analytical procedures was checked by routine determination of macro- and microelements in the CRMs “Tomato Leaves” (NIST 1573a) and “Apple Leaves” (NIST 1515a).
Data Analysis
Major and trace element datasets were tested for normality with the Shapiro–Wilk’s test (p > 0.05) and for homogeneity of variance with the Levene’s test (p > 0.05). Because of the asymmetric distribution of most datasets, logarithmic transformation was used to obtain a normal distribution. T test was performed to test the differences between plant species and collection points on log-transformed data.
Principal component analysis was applied for data reduction of elemental concentrations in soils and in leaves of E. australis and N. oleander from the Riotinto mining area and control areas of Linares and Alanís. The principal components (PCs) having eigenvalues greater than unity were included in the model. Interpretation of PCs was done in terms of factor coordinates (correlation between element data and factor axes) and relative contribution of different groups of data to the variance of factor axes.
Multivariate results were represented as a biplot projection of the original data in the vector space defined by the PCs. The statistical analyses were performed by R software (R Development Core Team).
A set of quantitative indexes was used to characterize soil contamination by elements and to recognize their preferential partitioning among different plant tissues. The contamination factor (CF) (Hakansson 1980), i.e., the ratio between concentrations in soil in the mining area with respect to those of the control area, was used to classify soils of this study according to an established scale of contamination (Liu et al. 2005). The pollution load index (PLI) was also calculated (Tomlinson et al. 1980) as:
where FC is the contamination factor for each contaminant and N is the number of contaminants. This value indicates the global soil contamination. A PLI < 1 indicates no contamination, PLI = unity indicates that soil contamination is the same than in control site, and PLI > unity means contaminated soils (Cabrera et al. 1999).
The exclusion coefficient (EC), i.e., the ratio between element concentrations in root cortex and internal vascular part, was calculated to identify the capacity of the species to retain elements to the root cortex and avoid absorption inside internal plant tissues. The bioaccumulation factor (BF; Monaci et al. 2011), i.e., the ratio between element concentrations in leaves and the soil, and the translocation factor (TF; McGrath and Zhao 2003), calculated as the ratio between the elemental concentration in leaves and that in the inner roots, were used to apportion the plants’ capacity of preferential element partitioning to (TF values > unity) and accumulation in (BC values > unity) the leaves. In general, plant species have a TF < 1 for most trace elements; in case of TF > unity, then plants can behave as accumulators (McGrath and Zhao 2003).
Results
Soil physical–chemical characteristics
Table 1 reports physical–chemical data on soils from Riotinto and the two control sites. Distinctive features of Riotinto soils were the high acidity and the very low P and CEC (p < 0.01). The pH ranged between 3.36 and 4.98 in E. australis soils and between 3.14 and 7.24 in N. oleander soils. For both plant species, Riotinto soils had significantly lower pH than the respective soils in control areas (p < 0.05). With respect to control, Riotinto soils were also characterized by significantly lower concentrations of total N and CEC, ranging in the mining area between 0. 2–4.4 g kg−1 and 0.29–6.0 cmol+ kg−1, respectively. The lowest values of pH, N concentration, available P content and CEC were found in substrates inhabited by E. australis to levels that are commonly associated with deficiency status in plants (Moreno 1978). Iron oxides were notably high in E. australis soils from both Linares and Riotinto, with the latter dataset being very dispersed (coefficient of variation = 122%; Table 1).
In Table 2, major and trace element concentrations in soils of this study are reported as total content, exchangeable (for Ca, K, Mg and Na) and EDTA-extractable (for Cd, Cu, Fe, Pb and Zn) fraction. Compared to control areas, Riotinto soils showed significantly higher (p < 0.05) total concentrations of Cu, Pb and S and lower concentration of Mn. Likewise, soil EDTA-extracted Cu and Pb were significantly higher in Riotinto (p < 0.05). In the mining areas, substrates inhabited by E. australis were also characterized by significantly lower levels of total and exchangeable Ca than those from the control site (p < 0.01) as well as Mg concentration, while N. oleander soils of Riotinto showed significantly lower Al and K concentrations than the control (p < 0.05).
As far as EDTA-extractable element concentrations concern, substrata of the E. australis in Riotinto did not show significant differences with respect to the soils of N. oleander (p < 0.05) being the variability very high. The pattern of total element concentrations of soils from the two plant species in Riotinto is shown in Fig. 2a and compared with geochemical European topsoil baselines (Salminen et al. 2005). Soils of this study had higher values of Cd, Cu, Fe, Pb and S content than European topsoil baselines. Soils of N. oleander were distinguished by significantly higher total Ca, Mg, Mn, P, S and Zn concentrations, with respect to those of E. australis. In both species, the contamination factors (CFs) for Cu, Cd, Pb and S were > 1 (Fig. 2b). Soils from N. oleander can be considered moderately contaminated with Cd and Fe (1 > CF > 3) and highly contaminated with Cu, Pb, S and Zn (CF > 6; Liu et al. 2005). Also, on the basis of the CFs, soils from E. australis appear moderately contaminated for Cd and S (1 > CF > 3), considerably contaminated for Cu (CF = 4) and highly contaminated for Pb (CF > 6). Values of PLI were > 1 only for N. oleander (Fig. 2b).
Pattern of total element concentrations in plant soils from Riotinto. Inset a median element concentrations and geochemical topsoil baselines for Europe (median values; Salminen et al. 2005). Inset b contamination factor (CF, means) and pollution load index (PLI) for E. australis and N. oleander
Element concentrations in plant compartments
Element concentrations (mean ± standard deviation) in the root cortex and exclusion coefficients (ECs, median) for the two plant species in the Riotinto area and the control areas of Linares and Alanís are shown in Fig. 3. The respective inner roots data are presented in Fig. 1 (Electronic Supplementary Material). Overall, root cortexes of N. oleander showed higher concentrations of the macronutrients Ca, K, Mg, Na and P (p < 0.01) and lower concentrations of Al, Mn and Pb (p < 0.01) than those of the E. australis. With respect to the control areas, only two metals, i.e., Cu and Pb, were significantly accumulated in the root cortex of E. australis and N. oleander from Riotinto (p < 0.01). Copper concentration, ranging very closely in specimens of E. australis and N. oleander from the control areas (7.40–7.70 mg kg−1), in Riotinto was remarkably enriched in roots of both species, by a factor 30 in E. australis and by a factor 53 in N. oleander. The mean Pb concentration in the root cortex of E. australis and N. oleander from Riotinto was 40.3 and 23.8 mg kg−1, respectively, showing a five–tenfold increase with respect to control sites (p < 0.01).
The ECs (Fig. 3) for Cd, Mg, Na, P or S were close to unity for the two species of this study. The ECs for Cu, Pb, Fe and Al were slightly higher in N. oleander (1.41, 1.46, 2.20 and 2.30, respectively) and very far apart from the unity in E. australis (e.g., EC for Cu in E. australis = 6.80). Erica australis in Riotinto was also distinguished by ECs for Cu, Pb and Zn significantly higher than the respective ECs of the controls (p < 0.05). For N. oleander, a significant increase in the EC in Riotinto with respect to the control was found for Ca (p < 0.01) and Zn (p < 0.05).
Leaf concentrations of Ca, K, P, S and Zn were significantly greater in N. oleander than in the E. australis (p < 0.01; Fig. 4) which in turn accumulated higher concentrations of Mn in the leaves (p < 0.01). The leaf concentration of Cd, Mn, Na and Zn in E. australis was lower in Riotinto than in the control; in contrast, the leaf Pb concentration was higher in the mining area with respect to the unpolluted area of Alanís (p < 0.05). Leaf Cu concentration in E. australis from Riotinto was not different from the values found in the control, despite the high content of this metal in Riotinto soils (Table 2). Leaves of N. oleander from the mining soils had higher concentrations of Cu (p < 0.05) and had lower concentrations of Ca (p < 0.01) than those of the control area.
Bioaccumulation factors (BFs) of E. australis and N. oleander were generally < unity for Al, Cd, Cu, Fe, Pb and Zn and > unity for P, in both mining and control areas (Fig. 4). N. oleander also showed a high value of BF for Ca in all the substrates of this study and for K in Riotinto. The BFs of E. australis in Riotinto were > unity for Ca, Mn and Mg and < unit for S of both species. Instead, both E. australis and N. oleander showed a high BF for the latter element in the control area.
The values of translocation factors (TFs) determined in this study are shown in Fig. 5. Translocation factors for E. australis were generally < unity with the exception of Mn in both mining and control areas and of Pb in Riotinto. Aluminum, Cd, Cu, Fe and Pb in N. oleander were generally characterized by TFs > unity in mining and control areas.
Multivariate analysis applied to the soil dataset showed four principal components (PCs) representing 80.0% of the original variance. The first Principal Component (PC1) was mainly correlated (loadings > 0.7) with total concentrations of Al, Cd, Fe, Mg, Mn, N and S, but also with Feox, exchangeable Ca and Na, as well as CEC and pH. Principal Component 2 (PC2) was associated with total Zn, EDTA-extractable Zn and total and available P (Bray) while PC3 mainly with K (loading = 0.76) and available water (0.77). The remaining PCs accounted for a residual amount of the original variance and were weakly associated (loadings > 0.5) with total Fe, clay content and total C. The observational data that mostly contributed (30%) to PC1 were those collected from the mining wastes of Peña de Hierro (PHC, PHG), while those from the River Tinto (NA, RT) were the largest contributors (35%) to PC2 and PC3. Principal Component 4 was mainly determined (28%) by N. oleander samples from the control area of Alanís. Principal component analysis of leaf data singled out three PCs having eigenvalues > unity which accounted for 76% of the original variance. The first PC was associated mainly with Ca (loading = 0.79), K (0.78), Mn (0.84) and S (0.71) concentrations. Principal Component 2 was correlated with Cu (loading = − 0.79), Mg (0.89) and Zn (− 0.73), while PC3 was mainly determined by Al (0.80). Lead showed a cross-loading among the three PCs included in the model. The biplots of leaf and soil data are shown in Fig. 6.
Discussion
Riotinto soil data delineate a pattern of very different edaphic environments, distinguished by highly spatially variable chemical features, reaching extreme values in the most impacted mining sites. The biogeochemistry of the plant substrate depicted in this study is coherent with previous reports from the same area (Soldevilla et al. 1992; Márquez-García et al. 2009; Monaci et al. 2011), pointing to the very low pH and infertility of soils (low N content, available P and CEC) as the main limiting factors for plant growth. A relevant attribute of the studied soils is also the limited macronutrient (Ca, P and K) and micronutrient supply and the high content of Pb and Cu (Table 2) largely exceeding the baselines for the geological domain of Riotinto (South Portuguese Zone; Galán et al. 2008).
The conditions of elevated soil acidity recurrently observed in soils of Riotinto not only represent edaphic conditions which are particularly hostile per se, but are also likely to play a major part in the availability of nutritive and toxic metals. Indeed, low soil pH may enhance mobilization of toxic metals, such as Cd and Pb, and constrain availability of elements, like Ca, Fe or P (Kabata-Pendias and Pendias 2011; Marschner 2011). In this study, the most acidic soils (often inhabited by E. australis) were characterized by the lowest levels of nutrients (i.e., Ca, Mg, Mn and P).
Soil contamination in the study area is not limited to the obvious anomaly of Pb and Cu, rather has an essentially polymetallic nature. This is a feature common to similar geological environments around the world dominated by sulfide minerals (Mendez and Maier 2007). The comparison of the present data with European geochemical topsoil baselines (Salminen et al. 2005; Fig. 2a) indicates a significant enrichment for various elements (e.g., Cd, Cu, Fe, Pb, S), which share a common origin in the mineralogical composition (sulfide metallic deposits) of the ores of the Iberian Pyrite Belt. Among the elements investigated in this study, the derived CFs values (Fig. 2b) pointed out as considerable (3 < CF < 6; Liu et al. 2005) or high (CF > 6) the soil contamination by Cd, Cu, Pb and S.
The within-site variation in soil compositional data was striking (Tables 1, 2), reflecting marked spatial differences in soil chemistry at a local scale. This variation might have been generated by topographically unequal erosion and weathering rates of parent material, as well as the widespread excavation and bulk material management during mining and post-mining operations. Moreover, in arid and semiarid environment, such as Riotinto, differences in soil composition could be explained also by water or eolian dispersion of slag particles (Chopin and Alloway 2007; Fernández-Remolar et al. 2011). Typical of Mediterranean areas, torrential rainfall and the scarce vegetation are key factors that enhance erosion of wastes and metal dispersion by surface runoff (Doumas et al. 2018). The semiarid climate and the lack of vegetation cover may also favor the eolian dispersion of metals from abandoned mine sites. It has been shown that southern Iberian peninsula mine wastes (mainly dry, unstable piles of crushed pyrite and roasted pyrite cinders) are a major source of toxic metals and metalloids, transported by wind, to local residents (Mendez and Maier 2008; Fernández-Caliani 2012; Castillo et al. 2013; Rivera et al. 2016; Doumas et al. 2018).
It may be worth considering that in highly contaminated and disturbed sites, such as Riotinto, not only the occurrence of extreme edaphic features, which may cause phytotoxicity and insufficient nutrient supply, but also the notable variability in substrate chemical composition can be very restrictive to plant colonization and survival. Patchily metal-contaminated soils cause microscale habitat fragmentation which exert an additional, strong selective pressure that can be very demanding in terms of ecophysiological plasticity of plant individuals to withstand to variable, excessive uptake of toxic metals. In such as conditions, plant adaptation to metalliferous soils, which often has been found genetically determined (Chen et al. 2015; Kuta et al. 2014), can be energetically very costly, thought it provides a competitive advantage (Maestri et al. 2010).
Metallophytes, i.e., plant species able to thrive on metalliferous soils, commonly exhibit a range of physiological and molecular mechanisms of metal exclusion and/or accumulation. By far the most common mechanism of tolerance of metallophytes is the physiological restriction of the entry into roots of the metals, which are in excessive concentration in the growth substrate, and/or the limitation of the transport of the absorbed toxic metals to the shoots, the most metabolic active plant parts (Rossini-Oliva et al. 2018). In this study, the existence of such as “excluding” behavior has been revealed by the analysis of different plant compartments. The elevated accumulation of Cu, Pb and other metals in the root cortex of E. australis and N. oleander specimens from the mining area (Fig. 3) suggests that these plants are able to colonize the harshest substrates of Riotinto by compartmentalizing specific elements in the cell walls or vacuoles (Sharma et al. 2016). By adopting this strategy, plants are constraining potentially harmful elements into limited sites (e.g., root cortex) where they cannot affect sensitive metabolic reactions. The restricted translocation of metals to aerial plant tissues is due to the presence of physical barrier (Casparian strip) in plant roots (Pourrut et al. 2011), precipitation in the intercellular space as insoluble metal-salts, or sequestration in the vacuoles of cortical or rhizodermal cells (Arias et al. 2010; Shahid et al. 2016). A range of gene families playing a key role in controlling metal uptake into cells, vacuolar sequestration and remobilization from the vacuole has been identified (Rascio and Navari-Izzo 2011). Ericaceae are known for being capable of absorbing essential macro- and micronutrients and sequestering excess toxic elements in the root bark and rhizosphere soil (Abreu et al. 2008; Monaci et al. 2011; Rossini Oliva et al. 2009; Rossini-Oliva et al. 2018). Similar excluder behavior has also been described for N. oleander from highly contaminated sites (de la Fuente et al. 2010; Franco et al. 2012; Trigueros et al. 2012). However, the two studied species differed in these regulating mechanisms. For example, compartmentalization of Al and Fe is performed at a remarkable strength (EC ≫ unity) in E. australis, while it is far more restrained in N. oleander (EC ≥ unity). Noteworthy differences can also be noticed in homeostasis of Mn, as it is excluded in roots of N. oleander (EC > 2) but is effectively uptaken from soil by E. australis and distributed to root internal tissues (EC < unit). The latter strategy appears advantageous in substrates, such as those of Riotinto, characterized by limited supply of Mn as plant micronutrient.
Because of the avoidance strategies of the two species of this study, the pattern of contamination of the Riotinto soils does not correspond to similar elemental enrichment in leaves. For example, despite the high concentrations of Pb in the EDTA fraction, indicating a high availability of this metal, both species of this study showed limited Pb accumulation in foliar tissues, which was reflected in the low BFs for this metal. Similarly, leaf Cu, Fe and Zn concentrations in E. australis specimens from the contaminated mining areas of Riotinto were well referable to normal values for plants (Kabata-Pendias and Pendias 2011) and comparable to foliar levels of controls. N. oleander also appear to be able to control Cu, Fe and Zn concentration in leaves (BFs < 1), although less effective than E. australis.
In this study, the only element actively accumulated in leaves, also against the limited availability in the substrate, was Mn. This feature is attributable only to E. australis, whose leaves showed high enrichment of this metal (overall average concentration: 438 mg kg−1) despite its very variable and especially low content (175 mg kg−1) in the mining substrates. In fact, E. australis is known to behave as Mn accumulator species (Abreu et al. 2008); Ericaceae are known to have intrinsic ability to tolerate high levels of and Mn (Markert 1996). The ecophysiological implications of Mn bioaccumulation in Ericacee have been discussed elsewhere (Monaci et al. 2011; Rossini-Oliva et al. 2018).
A comprehensive recognition of the multivariate plant–soil relationships for the two species investigated in this study can be drawn from the biplots of Fig. 6. Soil dataset was distinguished by the PCA according to the geochemical properties referable to the mineralogical composition (sulfide metallic deposits) of the ores of the IPB (i.e., Fe ox, Pb, Cu, S, CEC, pH, etc.). In particular, the PCA grouped sampling sites of this study according to the physical–chemical features of the growth substrate of E. australis and N. oleander. Multivariate analysis on leaf element datasets failed to discriminate between different sampling sites of E. australis within the mining areas, neither between the mining and the control areas. Instead, N. oleander revealed, in comparison with E. australis, a multi-elemental pattern of accumulation in leaves probably resulting from a less tightly controlled homeostatic regulation, at least in the range of the investigated edaphic environments. From this evidence, it can be inferred that N. oleander specimens from contaminated areas are more prone to be enriched of potentially toxic elements in the aerial plant parts. Concerning this feature, N. oleander does not appear ideal for use in phytostabilization programs, because of the potential risk of metal mobilization from mining waste to different components of the ecosystem, through the food chain. Nevertheless, considering the effectiveness of N. oleander in trapping metal-bearing atmospheric particulate (Fernández Espinosa and Rossini-Oliva 2006) and its considerable individual biomass with respect to other metallophytes of the IPB, this plant species may play a major role in mitigating the impact of wind and water erosion of mining waste dumps. In this respect, the contribution of N. oleander in phytostabilization of mining sites in semiarid Mediterranean areas is worth of further investigation.
Conclusions
The plant species of this study, E. australis and N. oleander, presented large tolerance to a wide range of potential hazardous elements concentrations in soils, strongly impoverished in essential macro- and micronutrients, as well as to other unfavorable edaphic conditions for survival and growth. Being native plants of the IPB, E. australis and N. oleander are naturally endowed with traits that make them capable to withstand to selective pressures exerted by such as water-limited, metalliferous and unstable habitats. Overall, our results revealed that E. australis did not show nutrient unbalance, critical load of phytotoxic elements, or any breakdown in its elemental signature, even under the harshest conditions of the Riotinto mining area. This behavior indicates an effective and controlled homeostasis that makes E. australis the ideal candidate to promote primary colonization of mine tailings, dumps and other mine wastes. Not secondarily, E. australis do not accumulate high concentration of toxic elements in the aerial parts, and this accomplishes a main requirement for plant species uses in phytostabilization programs that is impeding the potential transfer of pollutants to consumers through the food chain. It can be assumed that primary colonization by E. australis and other Ericaceae at bare and exposed mining sites can initiate a relatively more stable and diverse vegetation cover so contributing to the mitigation of the most restrictive physical disturbances or stresses that made that original substrate unsuitable for plant growth. Further studies devoted to creating an accurate scientific knowledge of the biogeochemical factors that determine such as successional changes is a main prerequisite to create favorable conditions for phytostabilization programs on metal-enriched soils and abandoned mining dumps of semiarid Mediterranean regions.
Abbreviations
- BF:
-
Bioaccumulation factor
- CF:
-
Contamination factor
- EC:
-
Exclusion coefficient
- TF:
-
Translocation factor
References
Abreu, M. M., & Magalhaes, M. C. F. (2009). Phytostabilization of soils in mining areas. Case studies from Portugal. In L. Aachen & P. Eichmann (Eds.), Soil remediation (pp. 297–344). New York: Nova Science Publishers.
Abreu, M. M., Tavares, M. T., & Batista, M. J. (2008). Potential use of Erica andevalensis and Erica australis in phytoremediation of sulphide mine environments: São Domingos. Portugal. Journal of Geochemical Exploration,96(2–3), 210–222.
Arias, J. A., Peralta-Videa, J. R., Ellzey, J. T., Ren, M., Viveros, M. N., & Gardea-Torresdey, J. L. (2010). Effects of Glomus deserticola inoculation on Prosopis: Enhancing chromium and lead uptake and translocation as confirmed by X-ray mapping, ICP-OES and TEM techniques. Environmental and Experimental Botany,68(2), 139–148.
Bonnail, E., Macías, F., & Osta, V. (2019). Ecological improvement assessment of a passive remediation technology for acid mine drainage: Water quality biomonitoring using bivalves. Chemosphere,219, 695–703.
Bray, R. H., & Kurtz, L. T. (1945). Determination of total, organic, and available forms of phosphorus in soils. Soil Science,59, 39–45.
Cabrera, F., Clemente, L., Díaz-Barrientos, E., López, R., & Murillo, J. M. (1999). Heavy metal pollution of soils affected by the Guadiamar toxic flood. The Science of Total Environment,242, 117–129.
Canha, N., Freitas, M. C., Anawar, H. M., Dionísio, I., Dung, H. M., Pinto-Gomes, C., et al. (2010). Characterization and phytoremediation of abandoned contaminated mining area in Portugal by INAA. Journal of Radioanalytical and Nuclear Chemistry,286(2), 577–582.
Cánovas, C. R., Hubbard, C. G., Olías, M., Nieto, J. M., Black, S., & Coleman, M. L. (2008). Hydrochemical variations and contaminant load in the Río Tinto (Spain) during flood events. Journal of Hydrology,350(1–2), 25–40.
Castillo, S., de la Rosa, J. D., Sánchez de la Campa, A. M., González-Castanedo, Y., Fernández-Caliani, J. C., Gonzalez, I., et al. (2013). Contribution of mine wastes to atmospheric metal deposition in the surrounding area of an abandoned heavily polluted mining district (Rio Tinto mines, Spain). The Science of the Total Environment,449, 363–372.
Chen, C., Zhang, H., Wang, A., Lu, M., Shen, Z., & Lian, C. (2015). Phenotypic plasticity accounts for most of the variation in leaf manganese concentrations in Phytolacca americana growing in manganese-contaminated environments. Plant and Soil,396(1–2), 215–227.
Chopin, E. I. B., & Alloway, B. J. (2007). Trace element partitioning and soil particle characterization around mining and smelting areas at Tharsis, Riotinto and Huelva, SW Spain. Science of Total Environment,373, 488–500.
de la Fuente, V., Rufo, L., Rodríguez, N., Amils, R., & Zuluaga, J. (2010). Metal accumulation screening of the Río Tinto flora (Huelva, Spain). Biological Trace Element Research,134(3), 318–341.
Dickinson, N. M., Baker, A. J. M., Doronilla, A., Laidlaw, S., & Reeves, R. D. (2009). Phytoremediation of inorganics: Realism and synergies. International Journal of Phytoremediation,11, 97–114.
Doumas, P., Munoz, M., Banni, M., Becerra, S., Bruneel, O., Casiot, C., et al. (2018). Polymetallic pollution from abandoned mines in Mediterranean regions: A multidisciplinary approach to environmental risks. Regional Environmental Change,18(3), 677–692.
Ernst, W. H. O. (2005). Phytoextraction of mine wastes—Options and impossibilities. Chemie der Erde,65, 29–42.
Fernández Espinosa, A. J., & Rossini-Oliva, S. (2006). The composition and relationships between trace element levels in inhalable atmospheric particles (PM10) and in leaves of Nerium oleander L. and Lantana camara L. Chemosphere,62, 1665–1672.
Fernández-Caliani, J. C. (2012). Risk-based assessment of multimetallic soil pollution in the industrialized peri-urban area of Huelva. Spain. Environmental Geochemistry and Health,34(1), 123–139.
Fernández-Caliani, J. C., Barba-Brioso, C., González, I., & Galán, E. (2008). Heavy metal pollution in soils around the abandoned mine sites of the Iberian pyrite belt (southwest Spain). Water, Air, and Soil pollution,200(1–4), 211–226.
Fernández-Remolar, D. C., Prieto-Ballesteros, O., Gómez-Ortíz, D., Fernández-Sampedro, M. P., Sarrazin, P., Gailhanou, M., et al. (2011). Río Tinto sedimentary mineral assemblages: A terrestrial perspective that suggests some formation pathways of phyllosilicates on Mars. Icarus,211, 114–138.
Franco, A., Rufo, L., & de la Fuente, V. (2012). Metal concentration and distribution in plant tissues of Nerium oleander (Apocynaceae, Plantae) from extremely acidic and less extremely acidic water courses in the Río Tinto area (Huelva, Spain). Ecological Engineering,47, 87–91.
Freitas, H., Prasad, M. N. V., & Pratas, J. (2004). Plant community tolerant to trace elements growing on the degraded soils of São Domingos mine in the south east of Portugal: Environmental implications. Environment International,30, 65–72.
Galán, E., Fernández-Caliani, J. C., González, I., Aparicio, P., & Romero, A. (2008). Influence of geological setting on geochemical baselines of trace elements in soils. Application to soils of South-West Spain. Journal of Geochemical Exploration,98, 89–106.
García Palomero, F. (1992). Mineralizaciones de Riotinto (Huelva): Geología, génesis y modelos geológicos para su explotación y evaluación de reservas mineras. In J. García Guinea & J. Martínez Frías (Eds.), Recursos minerales de España (pp. 1325–1352). Madrid: CSIC.
Ginocchio, R., León-Lobos, P., Arellano, E. C., Anic, V., Ovalle, J. F., & Baker, A. J. M. (2017). Soil physicochemical factors as environmental filters for spontaneous plant colonization of abandoned tailing dumps. Environmental Science and Pollution Research,24(15), 13484–13496.
Hakansson, L. (1980). An ecological risk index for aquatic pollution control. A sedimentological approach. Water Resources,14, 975–1001.
Holmgren, G. G. S. (1967). A rapid dithionite–citrate extractable iron procedure. Soil Science Society of America Proceedings,31, 210–211.
Kabata-Pendias, A., & Pendias, H. (2011). Trace elements in soils and plants. Boca Raton: CRC Press.
Kuta, E., Jedrzejczyk-Korycińska, M., Cieślak, E., Rostański, A., Szczepaniak, M., Migdałek, G., et al. (2014). Morphological versus genetic diversity of Viola reichenbachiana and V. riviniana (sect. Viola, Violaceae) from soils differing in heavy metal content. Plant Biology,16(5), 924–934.
Leistel, J. M., Marcoux, E., Thiéblemont, D., Quesada, C., Sánchez, A., Almodovar, G. R., et al. (1998). The volcanic-hosted massive sulphidic deposits of the Iberian Pyritic Belt. Mineralium Deposita,33, 2–30.
Liu, W. H., Zhao, J. Z., Ouyang, Z. Y., Söderlund, L., & Liu, G. H. (2005). Impacts of sewage irrigation on heavy metal distribution and contamination in Beijing, China. Environment International,31(6), 805–812.
Lottermoser, B. G. (2010). Mine wastes: Characterization, treatment and environmental impacts (p. 400). Berlin: Springer.
Maestri, E., Marmiroli, M., Visioli, G., & Marmiroli, N. (2010). Metal tolerance and hyperaccumulation: Costs and trade-offs between traits and environment. Environmental and Experimental Botany,68(1), 1–13.
Markert, B. (1996). Instrumental element and multi-element analysis of plant samples methods and applications. Chichester: Wiley.
Márquez-García, B., Hidalgo, P. J., & Córdoba, F. (2009). Effect of different media composition on the micropropagation of Erica andevalensis, a metal accumulator species growing in mining areas (SW Spain). Acta Physiologiae Plantarum,31(3), 661–666.
Marschner, P. (2011). Marschner’s mineral nutrition of higher plants. Amsterdam: Academic Press.
McGrath, S. P., & Zhao, F. J. (2003). Phytoextraction of metals and metalloids from contaminated soils. Current Opinion in Biotechnology,14, 277–282.
Meier, L. P., & Kahr, G. (1999). Determination of the cation exchange capacity (CEC) of clay minerals using the complexes of copper(II) ion with triethylenetetramine and tetraetylenepentamine. Clays and Clay Minerals,47, 386–388.
Mendez, M. O., & Maier, R. M. (2007). Phytoremediation of mine tailings in temperate and arid environments. Reviews in Environmental Science and Bio/Technology,7(1), 47–59.
Mendez, M. O., & Maier, R. M. (2008). Phytoremediation of mine tailings in arid and semiarid environments-and emerging remediation technology. Environmental Health Perspectives,116, 278–283.
Monaci, F., Leidi, E. O., Mingorance, M. D., Valdés, B., Oliva, S. R., & Bargagli, R. (2011). Selective uptake of major and trace elements in Erica andevalensis, an endemic species to extreme habitats in the Iberian Pyrite Belt. Journal of Environmental Sciences,23(3), 444–452.
Moreno, D. R. (1978). Clasificación de pH del suelo, contenido de sales y nutrientes asimilables. México, D.F.: INIA-SARH.
Napoli, M., Cecchi, S., Grassi, C., Baldi, A., Zanchi, C. A., & Orlandini, S. (2019). Phytoextraction of copper from a contaminated soil using arable and vegetable crops. Chemosphere,219, 122–129.
Oliveira, M. L. S., Ward, C. R., Izquierdo, M., Sampaio, C. H., de Brum, I., Kautzmann, A. S., et al. (2012). Chemical composition and minerals in pyrite ash of an abandoned sulfuric acid production plant. The Science of the Total Environment,430, 34–47.
Pourrut, B., Shahid, M., Dumat, C., Winterton, P., & Pinelli, E. (2011). Lead uptake, toxicity, and detoxification in plants. Reviews of Environmental Contamination and Toxicology,213, 113–136.
Quevauviller, P., Lachica, M., Barahona, E., Gómez, A., Rauret, G., Ure, A., et al. (1998). Certified reference material for the quality control of EDTA- and DTPA extractable trace metal contents in calcareous soil (CRM 600). Fresenius’ Journal of Analytical Chemistry,360, 505–511.
Rahman, M. A., Reichman, S. M., De Filippis, L., Sany, S. B. T., & Hasegawa, H. (2016). Phytoremediation of toxic metals in soils and wetlands: Concepts and applications. In Hiroshi Hasegawa, et al. (Eds.), Environmental remediation technologies for metal-contaminated soils (pp. 161–195). Tokyo: Springer.
Rascio, N., & Navari-Izzo, F. (2011). Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Science,180(2), 169–181.
Rivera, M. B., Giráldez, M. I., & Fernández-Caliani, J. C. (2016). Assessing the environmental availability of heavy metals in geogenically contaminated soils of the Sierra de Aracena Natural Park (SW Spain). Is there a health risk? Science of the Total Environment,560–561, 254–265.
Rodríguez, N., Amils, R., Jiménez-Ballesta, R., Rufo, L., & De La Fuente, V. (2007). Heavy metal content in Erica andevalensis: An endemic plant from the extreme acidic environment of tinto river and its soils. Arid Land Research and Management,21(1), 51–65.
Romero, A., González, I., & Galán, E. (2006). Estimation of potential pollution of waste mining dumps at Peña del Hierro (Pyrite Belt, SW Spain) as a base for future mitigation actions. Applied Geochemistry,21(7), 1093–1108.
Rossini Oliva, S., Bargagli, R., Monaci, F., Valdés, B., Mingorance, M. D., & Leidi, E. (2009a). Stress responses of Erica andevalensis Cabezudo & Rivera plants induced by polluted water from Tinto River (SW Spain). Ecotoxicology,18, 1058–1067.
Rossini Oliva, S., Mingorance, M. D., Valdés, B., & Leidi, E. O. (2009b). Uptake, localisation and physiological changes in response to copper excess in Erica andevalensis. Plant and Soil,328(1–2), 411–420.
Rossini-Oliva, S., Abreu, M. M., & Leidi, E. O. (2018). A review of hazardous elements tolerance in a metallophyte model species: Erica andevalensis. Geoderma,319, 43–51.
Rufo, L., Nuria Rodríguez, N., Amils, R., de la Fuente, V., & Jiménez-Ballesta, R. (2007). Surface geochemistry of soils associated to the Tinto River (Huelva, Spain). Science of the Total Environment,378, 223–227.
Rufo, L., Rodríguez, N., & de la Fuente, V. (2011). Plant communities of extreme acidic waters: The Rio Tinto case. Aquatic Botany,95(2), 129–139.
Salminen, R., Batista, M. J., Bidovec, M., Demetriades, A., De Vivo, B., De Vos, B., et al. (2005). FOREGS geochemical atlas of Europe. Part 1. Background information, methodology, and maps. Espoo: Geological Survey of Finland.
Salt, D. E., Smith, R. D., & Raskin, I. (1998). Phytoremediation. Annual Review of Plant Physiology and Plant Molecular Biology,49, 643–668.
Sánchez de la Campa, A. M., de la Rosa, J. D., Fernández-Caliani, J. C., & González-Castanedo, Y. (2011). Impact of abandoned mine waste on atmospheric respirable particulate matter in the historic mining district of Rio Tinto (Iberian Pyrite Belt). Environmental Research,111(8), 1018–1023.
Sarmiento, A. M., Nieto, J. M., Casiot, C., Elbaz-Poulichet, F., & Egal, M. (2009). Inorganic arsenic speciation at river basin scales: The Tinto and Odiel rivers in the Iberian Pyrite Belt, SW Spain. Environmental Pollution,157(4), 1202–1209.
Shahid, M., Dumat, C., Khalid, S., Schreck, E., Xiong, T., & Niazi, N. K. (2016). Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. Journal of Hazardous Materials,325, 36–58.
Sharma, S. S., Dietz, K. J., & Mimura, T. (2016). Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant, Cell and Environment,39(5), 1112–1126.
Sims, D. B., Hooda, P. S., & Gillmore, G. K. (2013). Mining activities and associated environmental impacts in arid climates: A literature review. Environment and Pollution,2(4), 22–43.
Soldevilla, M., Maranon, T., & Cabrera, F. (1992). Heavy metal content in soil and plants from a pyrite mining area in southwest Spain. Communication in Soil and Plant Analysis,23, 1301–1319.
Tomlinson, D. L., Wilson, J. G., Harris, C. R., & Jeffrey, D. W. (1980). Problems in the assessments of heavy-metal levels in estuaries and formation of a pollution index. Helgol Meeresunters,33, 566–575.
Trigueros, D., Mingorance, M. D., & Rossini Oliva, S. (2012). Evaluation of the ability of Nerium oleander L. to remediate Pb-contaminated soils. Journal of Geochemical Exploration,114, 126–133.
Venkateswarlu, K., Nirola, R., Kuppusamy, S., Thavamani, P., Naidu, R., & Megharaj, M. (2016). Abandoned metalliferous mines: Ecological impacts and potential approaches for reclamation. Reviews in Environmental Science and Bio/Technology,15(2), 327–354.
Acknowledgements
The authors thank Dr. Eduardo O. Leidi of the Spanish National Research Council (CSIC), Institute for Natural Resources and Agrobiology of Sevilla, for discussions and comments on the manuscript. This work was partially granted by MICINN contract CGL2006/02860 and by Fundación Areces.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Monaci, F., Trigueros, D., Mingorance, M.D. et al. Phytostabilization potential of Erica australis L. and Nerium oleander L.: a comparative study in the Riotinto mining area (SW Spain). Environ Geochem Health 42, 2345–2360 (2020). https://doi.org/10.1007/s10653-019-00391-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-019-00391-7