Abstract
The aim of the present study was to appraise the levels of heavy metal contamination (Zn and Pb) in sediment of the Langat River (Selangor, Malaysia). Samples were collected randomly from 15 sampling stations located along the Langat River. The parameters measured were pH, redox potential, salinity, electrical conductivity, loss of ignition, cation exchanges capacity (Na, Mg, Ca, K), and metal ions (Zn and Pb). The geo-accumulation index (I geo) and contamination factor (C f) were applied to determine and classify the magnitude of heavy metal pollution in this urban river sediment. Results revealed that the I geo of Pb indicated unpolluted to moderately polluted sediment at most of the sampling stations, whereas Zn was considered to be within background concentration. The I geo results were refined by the C f values, which showed Pb with very high C f at 12 stations. Zinc, on the other hand, had low to moderate C f values. These findings indicated that the sediment of the Langat River is severely polluted with Pb. The Zn concentration at most sampling points was well below most sediment quality guidelines. However, 40% of the sampling points were found to have a Pb concentration higher than the consensus-based probable effect concentration of 128 mg/kg (concentrations above this value are likely to cause harmful effects). This result not only highlights the severity of Pb pollution in the sediment of the Langat River, but also the potential risk it poses to the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing pollutant levels in surface water is a controversial issue in developing countries. Most of these pollutants originate from anthropogenic activities such as sand mining, construction, waste dumping, agriculture, and discharge from municipal, residential, or industrial activities (Çevik et al. 2009; Wei and Yang 2010; Balogh et al. 2016). Effluent-containing metals are discharged into the rivers in large quantities, causing high levels rates of contamination (Islam et al. 2015). The continuous release of large volumes of industrial and domestic waste into rivers has led to increasing levels of metals in river water, which eventually accumulate in the river sediment. The presence of metals at high concentrations can be toxic, because such metals are resistant to degradation, and could potentially remain for a long time in the environment. Heavy metals in the environment can also be contributed by natural phenomena such as rock weathering with subsequent dissolution of metals in rivers (Looi et al. 2013). However, the portions of metal released due to natural processes such as volatilization, deposition from mineral rock, and weathering of bed rock are small (Rodríguez Martín et al. 2015).
Uncontrolled discharge of contaminants from industrial activities and inadequate water management will directly affect the natural ecosystem (Mokhtar et al. 2009). Heavy metal pollution in the aquatic environment will result in detrimental effects on aquatic organisms in the river as well as other organisms that source food from the contaminated area (Zeitoun and Mehana 2014; Fatima et al. 2014). The availability of heavy metal ions in the water enhanced the bioaccumulation of the metals into aquatic organisms (Looi et al. 2014). The uptakes of such metals occur through ingestion and adsorption, because such ions are widely distributed along the river once discharged. The results from studies on the effect of such metals on the health of aquatic organism are widely established because a heavy metal significantly affects living organisms (such as fish, macrophytes, mollusks, and snails) once it enters their bodies. However, the excretion and natural processes of living organisms make it hard to use them to determine the exact concentration of the metals accumulated in the biota. Thus, great attention has been focused on the applicability of sediment as an indicator because metal ions are segregated into sediments, meaning that once deposited at a particular site, time series analysis could be conducted.
River sediment is broadly perceived as an appropriate indicator of the level of contamination of a river. There are a number of studies investigating the variability of metals in sediments (Lim et al. 2013; Yin Chi 2013) and soils (Thomasi et al. 2015; Praveena et al. 2015). Sediment acts as a metal reservoir and provides a sensible indicator of the background pollution of the river. Sediment also has a high retention capacity, as well as the potential to release accumulated contaminants back into the river (water) system (Shafie et al. 2014). The primary sources of heavy metals in the river sediments are from the chemical weathering of bedrocks, soil, and runoff over the riverbank. Sediment is known to affect the chemical composition of stream waters in various ways. Some minerals dissolve and may cause precipitation of certain dissolved ions, while others may help stabilize the composition of stream water due to their exchange (buffering) capacity. Furthermore, sediment analyses may provide a geochemical and quantitative approximation of the metal concentrations of associated water. Some previous studies conducted to assess the pollution level in the sediments in Malaysia river systems include Naji et al. (2010), Sherene (2010), Lim et al. (2012), Idriss and Ahmad (2013) and Shafie et al. (2014).
In recent times, urbanization has severely impacted the Langat River, which is one of the main rivers in the state of Selangor. Pollution from activities occurring within the Langat River catchment has significantly contributed to metal pollution in the river sediment. Some of the more specific metal sources that can be found within the Langat River catchment include effluent from wastewater treatment plants, sand mining, and leachate from an illegal waste disposal site. Thus, there is a critical need to determine and monitor the concentration levels of heavy metals in the Langat River.
However, the metal contamination cannot just be assessed by analyzing metal concentrations alone because this would provide a biased interpretation of the results. Thus, it is crucial to differentiate and quantify the concentrations of the various metals to yield a better understanding of potential and actual environmental impacts from the contaminated sediments. Integration of additional tools such as the geo-accumulation index (I geo) and contamination factor (C f) can be applied for better understanding and a more comprehensive study about the possible effect of each metal. Therefore, complementary approaches that combine sediment quality guidelines and the geo-accumulation index are highly recommended in order to predict the ecological risk of particular areas. C f was also used to verify the possible risk associated with heavy metals, while I geo and sediment quality guidelines provide a precise evaluation of the fate and transport of metals from anthropogenic sources. However, there have been few studies to provide understanding of the interaction of metals in sediments considering the differences in chemical reactivity in tropical rivers. Therefore, this study aims to bridge information gaps on the dynamics of metals in different sediments characteristic of the Langat River. Moreover, this study is expected to update background contamination levels and the current status of heavy metal pollution, as well as illustrate and give a better understanding of the sediment exposure from the current pollution in this urbanized trans-boundary river.
Materials and methods
Study area
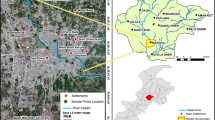
The Langat River is located in the south Klang Valley, which is in the central west coast area of Peninsular Malaysia. The Langat River lies within the latitude 2°40′152″–3°16′15″N and longitude 101°19′12″–102°1′10″E. The total catchment area of this basin is approximately 1815 km2 (Lim et al. 2013). The geological characteristics of the Langat River encompass mountainous zones (upstream, hilly areas that are urban, residential, and agricultural) and flat downstream areas (an alluvial zone that is mostly agricultural) (Shafie et al. 2013a; Mokhtar et al. 2015). Most of the mountainous area in the watershed is considered an undisturbed area. The Langat River is surrounded by agriculture. There is a permanent forest reserve in the upstream part, while the middle stream is used for residential areas and industrial activities and is the primary urbanized area. Agricultural, industrial, and mangrove areas are the main land uses in the downstream portion of the Langat River (Fig. 1).
The river flows through several urbanized areas (Kajang, Bangi, and Putrajaya). This catchment includes two reservoirs (Hulu Langat and Semenyih), which are critical for power and water supply for the citizens in Selangor. In addition, the Langat River is also used as a hydroelectricity generator for residential areas in Selangor. However, rapid development along the Langat River has become a threat to the water quality and the ecosystem of the river. The sampling was conducted from 9 to 10 September 2015 from the latitude 2°58.364′–3°12.882′ N and longitude 101°22.853′–101°52.995′ E. The sediment samples were collected from 15 different sampling points as shown in Fig. 1 and Table 1.
Field sampling and collection of samples
Fifteen sampling points were randomly chosen along the Langat River. The sediment samples (0–10 cm) were collected using plastic scoops that had been acid-washed and stored in pre-cleaned zip-lock bags. The locations of the sampling points were recorded using a global positing system (GPS) device (GARMIN GPS map 76 CSx, USA). A triplicate grab sample at each respective point was homogenized in the polyethylene zip-lock bag and stored in a cool box at 4 °C. Then, the sediment samples were immediately transported to the laboratory for further analysis.
Sample preparation and analysis
All the apparatus and glassware were preliminarily cleaned by an overnight soak in 5% nitric acid (HNO3). The glassware was then rinsed several times with distilled water and dried in an oven to remove all contaminants and traces of cleaning agent on the glassware (APHA 2005). The sediment samples were air-dried for 2 weeks until the sediment weight was constant. The air-dried sediment samples were crushed using a mortar and pestle and then sieved through 2-mm and 63-μm sieves before use in the analyses. The pH, salinity, and conductivity were determined via electrical measurements recorded using electrodes from a mixture of sediment and water in the ratio of 1:2 (Radojevic and Bashkin 2006). In order to prepare the sediment for measurement, approximately 10 g of sediment samples (2 mm) was weighed and then put in a 50-mL centrifuge tube. Then, about 20 mL of distilled water was added to the sediment samples at the ratio of 1:2 (sediment to distilled water). The mixtures were shaken continuously in the orbital shaker for 30 min. Next, the mixtures were allowed to stand for about an hour to stabilize (Radojevic and Bashkin 2006). The pH was measured using a pH meter (Thermo Orion 230 A pH meter), while salinity and conductivity were recorded using a multi-parameter probe (Multiparameter probe YSI 556). Meanwhile, the organic matter was determined using the loss of ignition (LOI) method (Radojevic and Bashkin 2006) and the cation exchange capacity (CEC) was determined based on the method proposed by Appelo and Postma (2005), with some enhancement by Aris et al. (2010).
Prior to the determination of total metals, the samples were digested in aqua regia solution and heated in a block digester. The sediment sample (≤63 μm) was pre-digested by mixing 15 mL of aqua regia into the sample in an acid block digester and was left to stand overnight. The extraction process was then continued by heating the sediment at 50 °C for 30 min and then raised to 120 °C for 2 h. The digested samples were cooled and filtered through a 0.45-μm cellulose nitrate membrane filter. The extracts were then diluted with 0.25 M of HNO3 in a 100-mL volumetric flask and sent for analysis. The sediment samples were analyzed for CEC (Na, Mg, K, Ca) and metals ions (Pb and Zn) using a flame atomic absorption spectrometer (FAAS, Shimadzu, Japan). The recovery and accuracy of the extraction method and analysis were compared to the Standard Reference Material 1646a (Estuarine Sediment) from the National Institutes of Standards and Technology (NIST). The percentage recovery for Zn and Pb was 100.2 and 99.9%, respectively.
Instrumental analysis
The concentration of heavy metals (Pb and Zn) and four major cations (Ca, Mg, K, and Na) in the sediment was analyzed using atomic absorption spectrometry (AAS) (Shimadzu AA-6800F, Japan). The AAS is widely used in environmental analysis because this instrument is reliable and simple to operate. It detects the presence and concentration of elements by analyzing the spectrum produced when an element is vaporized and absorbs certain frequencies of light. The techniques make use of the wavelengths of light specifically absorbed by an element, as can be seen in Table 2. The highest purity of Mili-Q water (18 MΩ/cm, Milipore, MA, USA) was used for the preparation of standard and diluted samples. The calibration standard solution used for heavy metal determination in the sediment samples was prepared from standard 1000 mg/L stock solution in a 100-mL volumetric flask.
Geo-accumulation index
The geo-accumulation index (I geo) was applied to determine the metal pollution in the sediment samples. This determination equation was introduced by Müller (1979) as a quantitative measure of the intensity of contamination in aquatic sediments. The expression is recommended to determine the metal concentrations in the Langat River by comparing the current concentration with the average values in the continental crust or in shale, as previously reported (Shaw et al. 1976; Wedepohl 1995). The equation used for the calculation of I geo was as follows:
where C n , the measured concentration of heavy metals in Langat River sediment and B n , the geochemical background value in average shale. Because the natural background values of Pb and Zn were not available, their background concentrations (Zn = 52 and Pb = 17 mg/kg) were adopted from Wedepohl (1995) for the calculation of I geo and C f. The I geo is typically divided into six grades: I geo = 0: background concentration; I geo = (0–1): unpolluted; I geo = (1–2): moderately polluted to unpolluted; I geo = (2–3): moderately polluted; I geo = (3–4): moderately to highly polluted; and I geo > 5: very highly polluted (Wedepohl 1995). The factor 1.5 is the background matrix factor due to lithogenic effects.
Contamination factor
The contamination factor (C f) is used to quantify the contamination status of pollutants in the sediment based on their concentrations in the sample and their background concentration. The C f is calculated as follows:
where ‘C m sample’ refers to metal content in the sediment and ‘C m background’ is the metal content from a natural reference, such as those reported in Wedepohl (1995). When C f < 1, it is described as low C f; 1 ≤ C f < 3 is moderate C f; 3 ≤ C f < 6 is considerable C f, and C f ≥ 6 is a very high C f. The measured results in the sediment were used to portray the classification of metal contamination of Pb and Zn in the urban river sediment.
Results and discussion
Descriptive statistics of the urban river sediment
Descriptive statistics were used to summarize the physicochemical parameters of the river sediment (such as pH, salinity, conductivity, CEC organic matter, and heavy metals: Pb and Zn) according to the minimum, maximum, standard deviation, and coefficient of variance of each one (Table 3). The analysis of mean, standard deviation (SD), and coefficient of variance (CV) is necessary to represent the accuracy and precision of each parameter measured. Most of the CV analyses were >30%, which indicates high variation among the stations. The variation was due to the different environmental conditions and sediment characteristics along the Langat River. In general, it was found that the Pb concentration range (15.29–157.82 mg/kg) in the sediment was higher than that of Zn (23.70–90.02 mg/kg). Analysis of variance (ANOVA) was applied to determine whether the differences in the variables between stations were significant. The result revealed that there were significant differences (p < 0.05) in all variables between the sampling stations.
The pH in the Langat River sediment varied from 5.86 to 7.04, indicating a slightly acidic condition. The pH was slightly acidic due to several different land use activities including industrial, construction, mining, and palm oil silviculture along the Langat River. The lowest pH was recorded at SP (sampling point) 10 due to the use of fertilizer, which may contain monosodium and disodium methylarsonate, by the oil palm plantations (Sarmani 1989). The salinity of the sediment ranged from 0 to 6.85 ppt. The highest salinity was found at SP15, which was located in the downstream area near the estuary. The salinity was higher due to the intrusion of seawater into the river (Lim et al. 2013). Meanwhile, the electrical conductivity is defined as the tendency of dissolved material in solution to conduct electricity (Lim et al. 2013). Higher conductivity (5.70–11,150.00 μs/cm) was also recorded at SP05. This is because of the presence of mineral salts in the sediment that provide good conduction of electricity (Shafie et al. 2013b). Salinity influences electrical conductivity (i.e., the conductivity increases with salinity). This can be supported by the positive correlation between salinity and conductivity (r = 0.560 at p < 0.05). The range of organic matter recorded ranged from 0.02 to 0.54%, while the amount of CEC in the sediment ranged from 1.00 to 14.40 meq/100 g. The CEC is used to determine the capacity of sediments to absorb cations (Shafie et al. 2013a).
The exchangeability of CEC in sediments represents the amount of cations that can be absorbed. Pearson’s correlation analysis showed that there was a positive correlation between Ca and CEC in the Langat River sediment. Ca also showed a positive correlation with CEC in the Langat River (r = 0.536 at p < 0.05). Furthermore, Ca was the predominant metal in the sediment. The most exchangeable cation in this study was Ca due to its higher charge, which made it more likely to be absorbed (Shafie et al. 2013b). In this study, Mg and K also showed a strong positive correlation with CEC with r = 0.867 and 0.900 at p < 0.05, respectively. K was found to be high in the Langat River because this element is an important component in the fertilizer used in agricultural areas. The surface runoff and accumulation of this fertilizer increased the amount of K in the sediment (Lim et al. 2013).
Pb and Zn contamination in sediment of the Langat River
The highest concentration of Zn was recorded at SP09 (90.01 mg/kg) (Fig. 2). The sand mining activities and alteration of the riverbank through various human activities along the Langat River caused soil erosion and resulted in the increase in Zn in the sediment at these sites. Leaching from building materials is also a major source of Zn in the river (Sorme and Lagerkvist 2002). The increase in Zn entering the Langat River could affect the geochemistry of the sediment and cause adverse impacts on the biota (Pillay et al. 2014).
The mean Zn concentration (53.00 ± 19.13 mg/kg) in this study was found to be comparable to the mean Zn concentration reported for other rivers in Malaysia [i.e., the Penang River (Ong et al. 2016), Sedili Kecil River (Shuhaimi-Othman et al. 2012), Balok River (Mohd Zahari et al. 2015), Perlis River (Jamil et al. 2014), Sepang Besar River (Ramsie et al. 2014), and the Kelantan River (Ahmad et al. 2009)]. However, the same cannot be said about the Pb concentration in this study because the highest mean concentration (118.47 ± 35.60 mg/kg) in this study was higher than that reported for all the other rivers mentioned. A comparison between the results obtain in this study with previously reported Pb and Zn in Langat River sediment has revealed a surge in the concentration of these two heavy metals. Judging by the magnitude of the increase, there is a high probability that the concentrations could reach levels that will cause serious toxicity to aquatic organisms in the near future. This is cause for concern because the communities living along the river depend on the river for their income and food. Table 4 summarizes the range of Pb and Zn in other Malaysian rivers and the previously reported concentrations for the Langat River, compared with the results obtained in this study.
The highest total Pb concentration in the Langat River was at SP15 (157.82 mg/kg). This sampling point was located in the downstream area, near the estuary of the Langat River, where there are hauling areas for fishing boats, jetties, and industrial activities. The mangrove area can act as a substantial sink of heavy metals from the river system (Haris and Aris 2013, 2015; Haris et al. 2017). The anthropogenic sources of Pb could have been from spillage of leaded petrol from the boats and leaching from marine paint and undercoating. The Langat River is also an important route for transfer via shipping of the merchandise from factories (Lim et al. 2012).
The Kendall’s tau-b result shows that Pb only had a significant positive correlation (r = 0.435; p < 0.05) with sediment salinity, while Zn was found to be positively correlated with K (r = 0.402), Ca (r = 0.771), CEC (r = 0.429), and organic matter (i.e., LOI) (r = 0.486). These results suggest that the Pb concentration in the sediment may be more stable because only the sediment salinity has a significant influence on its concentration. Zinc, on the other hand, is influenced by four different sediment parameters. Based on this result, it can be assumed that Zn is most likely to be adsorbed at exchange sites occupied by K and especially Ca (due to the stronger positive correlation), and also organic matter, rather than at exchange sites occupied by Na and Mg.
Geo-accumulation index and contamination factor for Langat River sediment
Table 5 shows the I geo value for Zn and Pb in the Langat River. The geo-accumulation index (I geo) for Zn in Langat River sediment ranged from −1.72 to 0.21. Most stations were in class 0 (background concentration) with the exception of SP06 and SP09 which were in class 1 (unpolluted) (Table 5; Fig. 3). This indicates that the Langat River was practically uncontaminated with anthropogenic sources of Zn. The negative I geo values of Zn indicated that there was no contamination in the sediment and the Zn found in the sediment was mostly from natural processes such as soil and rock weathering.
The contamination factor (C f) was calculated to determine the contamination status in the sediment of the Langat River. Table 5 shows the C f values of Pb and Zn at each sampling site. The pollution intensity of Zn at most sampling points showed C f < 1 (low C f) except for SP06, SP08, SP09, SP14, and SP15, which were classified as moderate C f (1 ≤ C f < 3; see Fig. 4).

(Data from: Long and Morgan 1991; EC & MENVIQ 1992; Persaud et al. 1993; Smith et al. 1996; US EPA 1996; MacDonald et al. 2000)
Comparison of a Pb and b Zn concentration in river sediment with freshwater sediment quality guidelines: PEL (Pb = 91.3 mg/kg; Zn = 315 mg/kg); PEL-HA28 (Pb = 82 mg/kg; Zn = 540 mg/kg); ERM (Pb = 110 mg/kg; Zn = 270 mg/kg); TEL (Pb = 35; Zn = 123 mg/kg); ERL (Pb = 35 mg/kg; Zn = 120 mg/kg); LEL (Pb = 31 mg/kg; Zn = 120 mg/kg); MET (Pb = 42 mg/kg; Zn = 150 mg/kg); TEL-HA28 (Pb = 37 mg/kg; Zn = 98 mg/kg); consensus-based PEC (Pb = 128 mg/kg; Zn = 459 mg/kg); consensus-based TEC (Pb = 35.8 mg/kg; Zn = 121 mg/kg).
The overall distribution of Pb in the Langat River was observed to be >2, which indicates moderate pollution (2 < I geo < 3). The I geo for Zn was below ‘0’ because the sampling points were located upstream, far from anthropogenic and urbanization activities. The sources of Pb contamination in the Langat River can be related to the leakage of diesel from shipping, boat painting, and industrial activities, which could increase the level of Pb in the sediment (Shafie et al. 2013b). The sampling points with moderate C f values indicated the role of human activities such as sand mining, oil palm plantations, industrial areas, and waste dumping in the sampling areas. However, the sediment results in this study on the Langat River indicated that it was significantly contaminated by Pb. There were 12 sampling points with C f > 6 (very high C f). This could be due to the anthropogenic activities that occur along the river, particularly from industrial, domestic, and agricultural runoff (Juahir et al. 2011). Meanwhile, other stations such as SP01 show low C f (C f > 1) and considerable C f (3 ≤ C f < 6; at SP10 and SP11). This is because SP01 is located in the upstream area of the Langat River, which receives high water recharge from the Langat River catchment. Thus, low degrees of metal contamination are indicated in this particular area. In contrast, SP10 and SP11 are located in Banting and Kampung Bandar, which are highly populated areas with substantial industrial activities. Hence, a significantly higher pollution loading of Pb was recorded (3 ≤ C f < 6) which indicates considerable contamination with Pb.
Comparison with freshwater sediment quality guidelines
The concentrations of Pb and Zn found in this study were compared to several freshwater sediment quality guidelines (Fig. 4a, b). In general, the sediment quality guidelines are separated into two groups, namely the threshold effect concentrations (TECs) and the probable effect concentrations (PECs). Metal concentrations that are below the TECs (i.e., TEL—threshold effect level; LEL—lowest effect level; MET—minimal effect threshold; ERL—effect range-low; TEL-HA28—threshold effect level for Hyalella azteca in 28-day tests; and consensus-based TEC) are unlikely to cause harmful effects, while concentrations above the PECs (i.e., PEL—probable effect level; SEL—severe effect level; TET—toxic effect threshold; ERM—effect range median; PEL-HA28—probable effect level for H. azteca in 28-day tests; and consensus-based PEC) are most likely to cause harmful effects. Based on the results, at none of the sampling points was the recorded Zn concentration above all the TEC guidelines. Only SP06 (84.83 mg/kg) and SP09 (90.02 mg/kg) come close to the TEL-HA28 (98 mg/kg), which is the lowest of the TECs (Fig. 4b).
All of the sampling points had recorded Pb concentrations higher than all of the TEC guidelines with the exception of SP01, at which only 15.29 mg/kg of Pb was recorded. Twelve of the sampling points were found to have Pb concentrations higher than the ERM (110 mg/kg), and six of these stations (i.e., SP02, SP03, SP09, SP13, SP14, and SP15) even exceeded the consensus-based PEC (128 mg/kg) which is the highest Pb guideline value. These results are similar to those reported by Lim et al. (2013) who also found high Pb concentrations in the sediment of Langat River. The high concentration could be attributed to the anthropogenic activities occurring along the Langat River, which constitute point source pollution from industrial and domestic activities, and non-point source pollution, such as surface runoff from agriculture, roads, and urban areas.
Based on this result, it can be concluded that Pb concentration in the area has a high probability of causing harm to the environment. Therefore, identifying the sources of Pb and preventing further enrichment of Pb in the area should be given high priority.
Conclusions
The accumulation of metals in the sediments of urban rivers can have a negative impact on the environment. Therefore, in this study the sediment of Langat River was analyzed to assess the current state of Pb and Zn concentration in the area.
Based on the results obtained, the Langat River sediment is relatively free from Zn pollution because all the sampling points recorded concentrations below all the guideline values (23.70–90.02 mg/kg). This is further supported by the I geo and C f results, which indicated that most of the sampling points were practically uncontaminated and had low contamination factors for Zn. On the other hand, the concentrations of Pb were found to be higher than that of Zn and 40% (i.e., six sampling points) of the sampling points had Pb concentrations exceeding the consensus-based PEC guidelines (128 mg/kg), which is the highest guideline limit compared in this study. This indicates that harmful effects related to high Pb concentration are likely to be observed at these sampling points.
This result highlights the severity of the Pb contamination and the toxicity risk faced by the Langat River ecosystem. Therefore, necessary corrective and preventive action must be taken to avoid further deterioration of the environment. The relevant authorities, with the assistance of the affected communities, must make a concerted effort to reduce the pollution in the Langat River. If the condition is left unattended, the situation may worsen and jeopardize not only the health of the environment, but also the humans living along the river, especially the local populations that depend on the Langat River for their livelihood.
References
Ahmad, A. K., Mushrifah, I., & Shuhaimi-Othman, M. (2009). Water quality and heavy metal concentrations in sediment of Sungai Kelantan, Kelantan, Malaysia: A baseline study. Sains Malaysiana, 34(4), 435–442.
APHA. (2005). Standard method for the examination of water and wastewater (21st ed.). Washington, DC: America Water Works Association Water Environment Federation.
Appelo, C. A. J., & Postma, D. (2005). Geochemistry, groundwater and pollution (2nd ed.). Rotterdam, Balkema: CRC Press.
Aris, A. Z., Abdullah, M. H., Praveena, S. M., Yusoff, M. K., & Juahir, H. (2010). Extenuation of saline solutes in shallow aquifer of a small tropical Island: A case study of Manukan Island, North Borneo. Environment Asia, 3, 84–92.
Balogh, Z., Harangi, S., Kundrát, J. T., Gyulai, I., Tóthmérész, B., & Simon, E. (2016). Effects of anthropogenic activities on the elemental concentration in surface sediment of oxbows. Water, Air, and Soil pollution, 227(1), 13. doi:10.1007/s11270-015-2714-x.
Çevik, F., Göksu, M. Z. L., Derici, O. B., & Fındık, Ö. (2009). An assessment of metal pollution in surface sediments of Seyhan dam by using enrichment factor, geoaccumulation index and statistical analyses. Environmental Monitoring and Assessment, 152(1), 309–317. doi:10.1007/s10661-008-0317-3.
EC & MENVIQ. (1992). Interim criteria for quality assessment of St. Lawrence River sediment. Ottawa: Environment Canada and Ministere de l’Envionnement du Quebec.
Fatima, M., Usmani, N., & Hossain, M. M. (2014). Heavy metal in aquatic ecosystem emphasizing its effect on tissue bioaccumulation and histopathology: A Review. Journal of Environmental Science andTechnology, 7(1), 1–15. doi:10.3923/jest.2014.1.15.
Haris, H., & Aris, A. (2013). The geoaccumulation index and enrichment factor of mercury in mangrove sediment of Port Klang, Selangor, Malaysia. Arabian Journal of Geosciences, 6(11), 4119–4128. doi:10.1007/s12517-012-0674-7.
Haris, H., & Aris, A. (2015). Distribution of metals and quality of intertidal surface sediment near commercial ports and estuaries of urbanized rivers in Port Klang, Malaysia. Environmental Earth Sciences, 73(11), 7205–7218. doi:10.1007/s12665-014-3900-7.
Haris, H., Aris, A. Z., & Mokhtar, M. B. (2017). Mercury and methylmercury distribution in the intertidal surface sediment of a heavily anthrophogenically impacted saltwater-mangrove-sediment interplay zone. Chemosphere, 166, 323–333. doi:10.1016/j.chemosphere.2016.09.045.
Idriss, A. A., & Ahmad, A. K. (2013). Heavy metals nickel and chromiumin sediments in the Juru River, Penang, Malaysia. Journal of Environmental Protection, 4(11), 1245–1250. doi:10.4236/jep.2013.411144.
Islam, M. S., Ahmed, M. K., & Habibullah-Al-Mamun, M. (2015). Determination of heavy metals in fish and vegetables in bangladesh and health implications. Human and Ecological Risk Assessment: An International Journal, 21(4), 986–1006. doi:10.1080/10807039.2014.950172.
Jamil, T., Lias, K., Hanif, H. F., Norsila, D., Aeisyah, A., & Kamaruzzaman, B. Y. (2014). The spatial variability of heavy metals concentrations and sedimentary organic matter in estuary sediment of Sungai Perlis, Perlis, Malaysia. Science Postprint, 1(1), e00016. doi:10.14340/spp.2014.02A0003.
Juahir, H., Zain, S. M., Yusoff, M. K., Hanidza, T. I. T., Armi, A. S. M., Toriman, M. E., et al. (2011). Spatial water quality assessment of Langat River Basin (Malaysia) using environmetric techniques. Environmental Monitoring and Assessment, 173(1), 625–641. doi:10.1007/s10661-010-1411-x.
Lim, W. Y., Aris, A. Z., & Tengku Ismail, T. H. (2013). Spatial geochemical distribution and sources of heavy metals in the sediment of Langat River, Western Peninsular Malaysia. Environmental Forensics, 14(2), 133–145. doi:10.1080/15275922.2013.781078.
Lim, W. Y., Aris, A. Z., & Zakaria, M. P. (2012). Spatial variability of metals in surface water and sediment in the Langat River and geochemical factors that influence their water–sediment interactions. The Scientific World Journal, 2012, 1–14. doi:10.1100/2012/652150.
Long, E. R., & Morgan, L. G. (1991). The potential for biological effects of sediment-sorbed contaminants tested in the National Status and Trends Program. NOAA Technical Memorandum NOS OMA 52 (pp. 175). Seattle, WA: National Oceanic and Atmospheric Administration.
Looi, L. J., Aris, A. Z., Wan Johari, W. L., Yusoff, F. M., & Hashim, Z. (2013). Baseline metals pollution profile of tropical estuaries and coastal waters of the Straits of Malacca. Marine Pollution Bulletin, 74, 471–476.
Looi, L. J., Aris, A. Z., & Yusoff, F. M. (2014). Metals concentrations (Co, Ni, Pb, Zn) in the estuarine and coastal waters from western part of Johor Strait. Malayan Nature Journal, 66, 86–96.
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39(1), 20–31. doi:10.1007/s002440010075.
Mohd Zahari, A., Veronica, C. L., & Mohd Tahir, A. (2015). Metal pollution and ecological risk assessment of Balok River Sediment, Pahang, Malaysia. American Journal of Environmental Engineering, 5(3A), 1–7. doi:10.5923/c.ajee.201501.01.
Mokhtar, M., Aris, A. Z., Munusamy, V., & Praveena, S. M. (2009). Assessment level of heavy metals in Penaeus monodon and Oreochromis spp. in selected aquaculture ponds of high densities development area. European Journal of Scientific Research, 30(3), 348–360.
Mokhtar, N. F., Aris, A. Z., & Praveena, S. M. (2015). Preliminary study of heavy metal (Zn, Pb, Cr, Ni) contaminations in Langat River Estuary, Selangor. Procedia Environmental Sciences, 30, 285–290. doi:10.1016/j.proenv.2015.10.051.
Müller, G. (1979). Schwermetalle in den sedimenten des Rheins, Veranderungen Seit 1971. Umschau, 79(24), 778–783.
Naji, A., Ismail, A., & Ismail, A. R. (2010). Chemical speciation and contamination assessment of Zn and Cd by sequential extraction in surface sediment of Klang River, Malaysia. Microchemical Journal, 95(2), 285–292. doi:10.1016/j.microc.2009.12.015.
Ong, M. C., Fok, F. M., Sultan, K., & Joseph, B. (2016). Distribution of heavy metals and rare earth elements in the surface sediments of Penang River Estuary, Malaysia. Open Journal of Marine Science, 6, 79–92. doi:10.4236/ojms.2016.61008.
Persaud, D., Jaagumagi, R., & Hayton, A. (1993). Guidelines for the protection and management of aquatic sediment quality in Ontario (p. 27). Ontario: Ministry of Environment and Energy.
Pillay, S., Naidoo, K., Bissessur, A., Agjee, N., Pillay, K., Purves, B., et al. (2014). Sand mining impacts on heavy metal concentrations in two important river systems of northern Kwazulu-Natal, South Africa. Journal of Human Ecology, 47(2), 155–162.
Praveena, S. M., Ismail, S. N. S., & Aris, A. Z. (2015). Health risk assessment of heavy metal exposure in urban soil from Seri Kembangan (Malaysia). Arabian Journal of Geosciences, 8(11), 9753–9761. doi:10.1007/s12517-015-1895-3.
Radojević, M., & Bashkin, V. N. (2006). Practical environment analysis (2nd ed.). Cambridge: The Royal Society of Chemistry.
Ramsie, S. A., Zulkifli, S. Z., Mohamat-Yusuf, F., & Ismail, A. (2014). Geochemical fractionations of heavy metals in sediments of Sepang Besar River, Malaysia. Acta Biologica Malaysiana, 3(1), 1–9.
Rodríguez Martín, J. A., De Arana, C., Ramos-Miras, J. J., Gil, C., & Boluda, R. (2015). Impact of 70 years urban growth associated with heavy metal pollution. Environmental Pollution, 196, 156–163. doi:10.1016/j.envpol.2014.10.014.
Sarmani, S. B. (1989). The determination of heavy metals in water, suspended materials and sediments from Langat River, Malaysia. Hydrobiologia, 176(1), 233–238. doi:10.1007/bf00026558.
Shafie, N. A., Aris, A. Z., & Haris, H. (2014). Geoaccumulation and distribution of heavy metals in the urban river sediment. International Journal of Sediment Research, 29(3), 368–377. doi:10.1016/S1001-6279(14)60051-2.
Shafie, N. A., Aris, A. Z., & Puad, N. H. A. (2013a). Influential factors on the levels of cation exchange capacity in sediment at Langat River. Arabian Journal of Geosciences, 6(8), 3049–3058. doi:10.1007/s12517-012-0563-0.
Shafie, N. A., Aris, A. Z., Zakaria, M. P., Haris, H., Lim, W. Y., & Isa, N. M. (2013b). Application of geoaccumulation index and enrichment factors on the assessment of heavy metal pollution in the sediments. Journal of Environmental Science and Health, Part A, 48(2), 182–190. doi:10.1080/10934529.2012.717810.
Shaw, D. M., Dostal, J., & Keays, R. R. (1976). Additional estimates of continental surface Precambrian shield composition in Canada. Geochimica et Cosmochimica Acta, 40, 73–83.
Sherene, T. (2010). Mobility and transport of heavy metals in polluted soil environment. Biological Forum: An International Journal, 2(2), 112–121.
Shuhaimi-Othman, M., Ahmad, A. K., Nadzifah, Y., & Azmah, M. (2012). Metal concentrations in Sungai Sedili Kecil, Johor, Peninsular Malaysia. Journal of Tropical Marine Ecosystem, 1, 15–23.
Smith, S. L., MacDonald, D. D., Keenleyside, K. A., Ingersoll, C. G., & Jay Field, L. (1996). A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. Journal of Great Lakes Research, 22(3), 624–638. doi:10.1016/S0380-1330(96)70985-1.
Sorme, L., & Lagerkvist, R. (2002). Sources of heavy metals in urban wastewater in Stockholm. Science of Total Environment, 298, 131–145.
Thomasi, S. S., Fernandes, R. B. A., Fontes, R. L. F., & Jordão, C. P. (2015). Sequential extraction of copper, nickel, zinc, lead and cadmium from Brazilian Oxysols: Metal leaching and metal distribution in soil fractions. International Journal of Environmental Studies, 72(1), 41–55. doi:10.1080/00207233.2014.983331.
US EPA. (1996). Calculation and evaluation of sediment effect concentrations for the amphipod Hyalella azteca and the midge Chironomus riparius. Chicago, IL: Great Lakes National Program Office, Region V.
Wedepohl, K. H. (1995). The composition of the continental crust. Geochimica et Cosmochimica Acta, 59(7), 1217–1232. doi:10.1016/0016-7037(95)00038-2.
Wei, B., & Yang, L. (2010). A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchemical Journal, 94(2), 99–107. doi:10.1016/j.microc.2009.09.014.
Yin Chi, C. (2013). An investigation of the bioavailability of heavy metals in tailing run-off sediment at Devon Great Consols. The Plymouth Student Scientist, 6(2), 211–309.
Zeitoun, M. M., & Mehana, E.-S. E. (2014). Impact of water pollution with heavy metals on fish health: Overview and updates. Global Veterinaria, 12(2), 219–231. doi:10.5829/idosi.gv.2014.12.02.82219.
Acknowledgements
The author would like to acknowledge the Exploratory Research Grant Scheme (ERGS, Project No. ERGS/1/2013/STWN01/UPM/02/2) from the Department of Higher Education Malaysia and the Research University Grant Scheme (RUGS, Project No. UPM/700-2/1/GP-IPB/2013) from Universiti Putra Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haris, H., Looi, L.J., Aris, A.Z. et al. Geo-accumulation index and contamination factors of heavy metals (Zn and Pb) in urban river sediment. Environ Geochem Health 39, 1259–1271 (2017). https://doi.org/10.1007/s10653-017-9971-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-017-9971-0