Abstract
In the present study, an investigation of the mineralogy and morphology, the heavy metal content and the health risk of urban road dusts from the second largest city of Greece was conducted. For this reason road dust samples from selected sites within the city core area were collected. No differences were observed in the mineralogy of road dusts coming from different sampling sites, and they were mainly consisted of quartz and calcite, while an elevated amorphous content was detected. Morphologically road dusts presented Ca-rich, Fe-rich and silicates particles with various shapes and sizes. The mean concentrations of Cd, Cr, Cu, Mn, Ni, Pb and Zn in road dust were 1.76, 104.9, 662.3, 336.4, 89.43, 209 and 452.8 μg g−1, respectively. A series of spatial distribution patterns revealed that the hotspot areas were tended to associate with major road junctions and regions with high traffic. Combination of pollution indexes and statistical analyses (correlation analysis, cluster analysis and principal component analysis) revealed that road dusts have a severe influence by anthropogenic activities. In attempt to identify the source of metals through geostatistical and multivariate statistical analyses, it was concluded as follows: Cr, Cu, Fe and Zn mainly originated from tire/break wear and vehicle abrasions, while Cd, Mn and Pb were mainly related to fuel/oil leakage from automobiles along with oil lubricants and vehicle abrasion. Hazard quotient values for children based on total metal concentrations for the road dust ingestion route were lower than safe level (=1). However, the fact that the Hazard Index value for Pb (0.459) which is a particularly toxic metal, was close to safe level, renders essential further investigation in order to provide more reliable characterizations of potential health risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urban areas throughout the world are experiencing rapid change as a result of both urbanization and accelerated development of the social economy (Oh et al. 2011; Latif and Saleh 2012). As a consequence, there is a decline in the quality of urban environment (Ordonez et al. 2003; Brown and Peake 2006).
Road dust, an accumulation of solid particles in the form of organic and inorganic pollutants on outdoor ground surfaces, is a valuable medium for characterizing urban environmental quality (Godish 2005; Liu et al. 2014). The deposited particles that constitute urban road dust can originate from diverse sources including erosion of surrounding soil, atmospheric deposition and anthropogenic activities such as industrial, urban and traffic-related activities (Fergusson and Kim 1991; Amato et al. 2009, 2011; Kreider et al. 2010). As noted by Beckwith et al. (1986), these particles are subjected to complex mixing processes which occur during various on-road activities. Thus, their chemical composition can be continuously modified, a process which also takes place in the natural soil inputs found on road surfaces. Concerning the traffic-related particles, they comprise tire abrasion products, brake pad dust, combustion exhaust, road surface/paint wear, lubricating oil and pavement wear (Zhang and Wang 2009; Kreider et al. 2010; Amato et al. 2011). Frequent traffic activities have as a result the uniform combination of these traffic-related particles with the soil minerals, forming in that way the particles of urban road dust (Kreider et al. 2010). The evaluation of road deposited particles’ content is therefore required, in order to determine the contributing sources.
The contamination of urban road dusts has been of major concern in recent decades, mostly because they may act as a temporary sink of contaminants such as heavy metals (Amato et al. 2010; Gunawardana et al. 2012). These pollutants are emitted from various sources and they can have a direct influence on human health because they can be transferred into the human body by different pathways, e.g., inhalation, ingestion, and dermal absorption (USEPA 2001). Urban road dusts may also contribute to atmospheric pollution through resuspension (Amato et al. 2010; Moreno et al. 2013). Therefore, they might pose a health risk to the urban habitants, since half of the world’s population resides in urban areas (Vegter 2007). Thus, the composition of urban road dust has been intensively investigated by many researchers around the world (Charlesworth et al. 2003; Ordonez et al. 2003; de Miguel et al. 2007; Tanner et al. 2008; Lu et al. 2009; Fujiwara et al. 2011).
Thessaloniki, a metropolitan city in Greece, is one of the most densely populated cities in Greece accounting for approximately 16,000 inhabitants km−2 (Samara et al. 2003). Despite its significant size, it is not large enough to have developed a transport infrastructure involving metro, light railway or tram, but only public transport buses. As a consequence, many people use a car for their transportation resulting in abundant road traffic which is considered as the major cause of atmospheric contamination in the city center (Manoli et al. 2002; Samara et al. 2003). Furthermore, there is an already elevated number of private motor vehicles (5 % increase per year) contributing to a high level of road dust within Thessaloniki’s city center. The numerous residential suburbs that surround the city have the same effect, as well as the major point sources of heavy industrial contaminants that are located mainly on its northwestern part. Whereas sporadic studies reported the contents of urban road dusts in Thessaloniki, concerning especially heavy metals (Misaelides et al. 1989; Samara et al. 2003; Ewen et al. 2009), no investigation was focused on the mineralogical and morphological characteristics of the dust particles, on the chemical forms of heavy metals, or on a health risk assessment of the potentially toxic metals in road dusts.
Based on the aforementioned facts, the aims of the present study were: (1) the characterization of the road dust particles including particle size distribution, determination of their mineralogical composition and morphological characteristics, (2) the determination of heavy metals content and spatial distribution in order to identify their spatial patterns in the region, (3) the identification of the possible main sources of individual metals in the road dust using multivariate statistical analysis, and (4) the health risk assessment of potentially toxic metals in the road dusts.
Materials and methods
Site description
The city of Thessaloniki (40°62′E, 22°95′N) is located on the inner part of Thermaikos Gulf, while at its north, north-eastern part is surrounded by the Hortiatis mountain (1200 m height). According to Manoli et al. (2002), the study area exhibits a typical Mediterranean climate: mild and strongly influenced by the sea breeze. Mean monthly values of relative humidity vary between 47 and 80 % (mean annual rainfall is 490 mm), while temperature varies between 5.5 °C (in January) and 28 °C (in August). Prevailing wind directions are N/NW (25 %), S/SW (30 %) and calms (20 %).
Sample collection and preparation
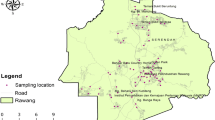
In November 2009, a total of 31 (RD1 to RD31) road dust samples (each weighting almost 100 g) were collected from different locations in the most congested area of the city of Thessaloniki, the city core (Fig. 1). The dust samples were mainly collected by gently sweeping at all sites a comparable area of one square meter (1 m2) from pavement edges. Clean plastic dustpan and brushes were used at each sampling site, while sampling was carried out carefully in order to reduce the disturbance of fine particles. This sampling procedure is similar to those used in previous studies reported in the literature (Al-Khashman 2007; Tanner et al. 2008; Lu et al. 2009). The efficiency of this sample collection has been reported to be equivalent to that of vacuum cleaning (Tanner et al. 2008). Samples were placed in self-sealed polyethylene bags and transported to the laboratory. Firstly, extraneous matter such as small pieces of brick, paving stone, leaves and other debris were removed. Then, the samples were dried in an oven at 35 °C for 3 days and mechanically sieved. Thus, each sample was divided into four grain size fractions: <63, 63–125, 125–500, >500 μm so as to determine the grain size distribution. Any loss of material during sieving was less than 2 % w/w. Until analysis, sub-samples were weighed and stored in polyethylene flasks in a cool and dry place. The fine-grained fraction (<63 μm) was used for further investigations. The reason for analyzing the <63 μm diameter fraction is the fact that these particles are easily transported in suspension, with the finest particles being capable of remaining airborne for considerable durations (Shilton et al. 2005). Furthermore, higher health risks are usually more associated with the fine particles than with the coarser ones (Kennedy and Hinds 2002; Charlesworth et al. 2011; Li and Zuo 2013).
Analytical procedures
Total heavy metal (Cd, Cr, Cu, Fe, Ni, Pb, Mn and Zn) concentrations were determined by atomic absorption spectroscopy using a PC controlled Perkin Elmer AAnalyst 400 spectrometer equipped with flame atomizer and a Perkin Elmer GF 900 graphite furnace (electrothermal atomizer). A pre-weighted amount (0.5 g) of fine-grained (<63 μm) dust fraction was subjected to chemical extraction using 10 mL of concentrated HNO3 and was subsequently microwave-heated using a CEM, Mars HP500 microwave extraction system. This digestion method was proven to extract the total quantities of native metals from road dust samples (USEPA 1994). After digestion, the solution was centrifuged (4000 rpm for 3 min), diluted to 50 mL with double-distilled water, and stored in plastic bottles. Accuracy of the analytical results was controlled using reagent blanks, matrix matched calibration curves and by calculating recoveries of spiked samples. All the analyses were carried out in triplicate to ensure the precision. Repeatability ranged 5–10 % rsd, while the recoveries for the studied metals were determined between 90 and 110 %.
For the assessment of metal fractionation, a subset of eight road dust samples from sampling sites where big conjunctions or elevated traffic conditions exist, was selected (RD5, RD9, RD12, RD13, RD22, RD25, RD27, RD31). Fractionation was performed by applying a sequential extraction procedure presented in Fig. 2 (Tessier et al. 1979; Tokalioglu et al. 2003). More specifically, total heavy metal content was sequentially extracted and classified into 5 different fractions as following: (a) Exchangeable fraction corresponds to the most loosely sorbed fraction of metals, extracted with CH3COONa 1 M, (b) Bound to carbonates heavy metals in the form of carbonate compounds, were released using CH3COONa 1 M adjusted to pH 5.0 with CH3COOH 100 %, (c) Bound to Fe–Mn oxides is the fraction of metals sorbed onto Fe–Mn oxides or forming oxides, which is released with a redox change [NH2OH·HCl in 25 % (v/v) CH3COOH]. The extraction was performed at 96 °C for 6 h with occasional agitation. (d) Bound to organic matter fraction of metals was released with the oxidation of organic matter, using a mixture of HNO3 0.02 M and H2O2 30 % which was heated at 85 °C for 2 h with occasional agitation. After cooling, CH3COONa 1 M was added and the sample was diluted with deionized water and agitated continuously for 30 min. Centrifuge tubes of 50 mL were used to perform the extractions. Between each extraction step, separation was carried out by centrifuging at 4000 rpm for 5 min. The supernatant was removed with a micropipette in order to avoid any loss of solid residues. Afterward it was filtered, acidified with a drop of nitric acid (Merck) and stored at 4 °C for further analysis. Between each successive stage of extraction procedure, the remaining dust sample was washed with 8 mL of Milli Q water with vigorous shaking for 10 min. This second supernatant that resulted after the centrifugation was finally discarded. Ultrapure Milli-Q water was used for the preparation of heavy metal stock solutions and all dilutions. All the used solvents and chemicals were of analytical grade.
Mineralogical characterization of the dust samples as well as semi-quantitative mineral determination was performed by X-ray powder diffraction (XRPD) using a Philips PW1710 diffractometer. Ni-filtered copper Kα radiation was used energized to 35 kV and 25 mA. Randomly oriented road dust samples were scanned continuously from 3° to 63° 2θ angles at a scanning speed of 1.2° min−1. The characterization of the mineral phases was performed semi-quantitatively on the basis of the intensity (counts) of specific reflections, the density, and the mass absorption coefficient (CuKα) of the identified mineral phases. The percentage of total amorphous material contained in the road dust samples was determined using the methodology described by Kantiranis et al. (2004). The XRPD method is a very good, effective and useful tool for the determination of the percentage of amorphous material contained in a natural or synthetic sample (Kantiranis et al. 2004).
Finally, a scanning electron microscope-SEM (SEMTech Solutions, JEOL JSM-840) connected to an X-ray energy dispersion spectrometer-EDS (INCA 300) was employed for the morphological and semi-quantitative chemical characterization of the road dust particles. This technique allows the characterization of the particles with respect to their size and morphology as well as the identification of other properties such as particles association or aggregation (Buseck and Bradley 1982; Zou and Hooper 1997).
Estimation of pollution indicators
Enrichment factor (EF) was used to assess the degree of metal pollution and the probable contribution of anthropogenic sources (Christophoridis et al. 2009; Wei et al. 2010; Yuen et al. 2012). The calculation of this factor is carried out with reference to a normalization element (most commonly used Fe, Al, Ti, Mn etc.) in order to minimize the variations caused by heterogeneous samples. EF is calculated as follows:
where (C n /C ref)sample and (B n /B ref)background refer to the concentration ratios of a target metal and the reference element in the road dust samples and in the background material, respectively (Lu et al. 2010; Yuen et al. 2012). The reference element is selected so as to present minimum concentration variability or occur in abundant concentrations in the studied environment. Thus neither small concentration variations nor other synergistic or antagonistic effects toward the examined elements are significant (Christophoridis et al. 2009). In this study, EFs for all the studied metals, except Fe, were calculated selecting manganese (Mn) as normalization factor since it presented significantly lower concentrations than the background values and low concentration variability, while it has been also used as reference element in other studies (Yongming et al. 2006; Hu et al. 2013). Concentrations of elements in the upper crust as introduced by Rudnick and Gao (2003) were used as background. In general, EF values much higher than 10 are mainly considered as indicating anthropogenic sources of heavy metal pollution, while values lower than 10 predominantly originate in the background soil material (Liu et al. 2003). Moreover, EF also assists in determining the degree of metal contamination (overall pollution factor). Five contamination categories are recognized on the basis of the enrichment factor: EF < 2 states deficiency to minimal enrichment, EF = 2–5 moderate enrichment, EF = 5–20 significant enrichment, EF = 20–40 very high enrichment and EF > 40 extremely high enrichment (Yongming et al. 2006; Birch and Olmos 2008).
Furthermore, the contamination levels of heavy metals in urban dusts are assessed by using the Geoaccumulation Index (I geo), as introduced by Muller (1969). I geo is calculated by the following equation:
where C n is the measured concentration of the target metal and B n is the geochemical background concentration of the metal in the upper crust (Rudnick and Gao 2003). The constant 1.5 is the background matrix correction factor due to lithological variability. The I geo values are classified according to Muller (1969) in seven classes, as follows: unpolluted (I geo < 0); unpolluted to moderately polluted (0 < I geo < 1); moderately polluted (1 < I geo < 2); moderately to heavily polluted (2 < I geo < 3); heavily polluted (3 < I geo < 4); heavily to extremely polluted (4 < I geo < 5); and extremely polluted (I geo > 5).
Statistical analysis
Analysis of a large dataset with multiple variables requires analytical approaches capable of clustering similar data together while identifying relationships between variables. In these circumstances, the application of multivariate analytical techniques has been found to be the most appropriate statistical approach (Herngren et al. 2005; Settle et al. 2007). In the present study, Pearson correlation analysis, cluster analysis (CA) and principal component analysis (PCA) were applied to the analytical data obtained from road dust samples. The aim was to identify relationships among chemical elements and elucidate their origin. Basic statistical parameters for road dust data were established and the Shapiro–Wilk test was used for data normality assessment. The data obtained from Thessaloniki demonstrated that all parameters are non-normally distributed (significant level < 0.05) except for Cr, Ni and Zn (significant level > 0.05). Skewness values also indicate that only Cr, Ni and Zn approach a normal distribution, while the other elements are being positively skewed toward the lower concentrations. As a result, the data were log-transformed for performing the multivariate analysis. CA was performed according to the Ward’s method. The obtained data were standardized (z scores), and the squared Euclidean distance was used as similarity measurement, while the results are reported in the form of a dendrogram. PCA was performed with Varimax normalized rotation which facilitates the interpretation of the output results by minimizing the number of variables, leaving for consideration only factors with eigenvalues >1. The statistical analyses were performed using SPSS Statistics for Windows (version 22.0, SPSS Inc., USA). Contour maps demonstrating the distributions of metals concentrations in the road dust samples were depicted using OriginPro for Windows (version 8.5, OriginLab Corp., USA).
Health risk assessment model
The models that were used in this study to assess the health risk of the potentially toxic metals in the road dusts are based on those developed by the United States Environmental Protection Agency (USEPA 2001). Residential exposure can occur via three main pathways: (a) direct ingestion of substrate particles; (b) inhalation of re-suspended particles through mouth and nose; and (c) dermal absorption of trace elements in particles adhered to exposed skin (de Miguel et al. 2007; Chabukdhara and Nema 2013). Two major indices, i.e., chronic daily intake (CDI, mg kg−1 day−1) and hazard quotient (HQ), were calculated for each contaminant. The CDI values for human exposure to road dust particles indicate the quantity of chemical substance received through each of the three pathways over a specific time period and were calculated according to Eq. S1–S3 (Supplementary data) (USEPA 1989).
For the non-carcinogenic effects, the CDIs calculated for each element and exposure pathway are subsequently divided by the corresponding Reference Dose (RfD) in order to yield a HQ (Eq. S4a, Supplementary data). Moreover, in order to calculate the level of cancer risk, the dose is multiplied by the corresponding slope factor (SF) (Eq. S4b, Supplementary data). The reference doses are defined as the maximum allowable level of metals that will not pose any deleterious effects on human health. In this study, RfDing (mg kg−1 day−1) and RfDinh (mg m−3) for road dust ingestion and inhalation, respectively, were taken into account. The reference toxicity values for dermal absorption were calculated by multiplying the road dust oral reference doses by a gastrointestinal absorption factor as suggested by USEPA IRIS (U.S. Environmental Protection Agency’s Integrated Risk Information System) (2011) and implemented by Ferreira-Baptista and de Miguel (2005) and Luo et al. (2012). The magnitude of adverse effect is accumulative for each element, thus the Hazard Index (HI) (Eq. S5, Supplementary data) is presented as the sum of HQ for each exposure pathway. Non-carcinogenic risk is low when the HI value is <1 and as HI increases, the magnitude of risk increases, too (USEPA 2001). Carcinogenic risk is the probability of an individual developing any type of cancer from lifetime exposure to carcinogenic hazards. The acceptable or tolerable risk for regulatory purposes is in the range of 10−6–10−4 (Ferreira-Baptista and de Miguel 2005).
Results and discussion
Grain size distribution
The grain size distribution of the road dust samples (Fig. 3) revealed that the predominant grain size fraction was of 125–500 μm with a variation between 29.3 and 57.1 %. The second most dominant grain size fraction was of 63–125 μm ranging between 14.8 and 35.2 %. The dust particles which could easily be entrained and suspended in the wind stream were those which had the smallest particle size (<63 μm). The mass fraction of these suspendable dust particles was ranging between 8.0 and 41.3 %, exhibiting greater variability than the other grain size fractions. This variation seems to be independent of the location of the sample. Even at short distances, the <63 μm fraction was significantly different between sampling locations. Assuming that the source of the road dust does not vary, especially in adjacent sampling sites, this variation was attributed to other factors. These factors involve the action of wind (stronger winds transport the lightest dust fractions and leave the coarser in shorter distances) and rainwater flow in combination with the topography (flat places with smoother inclination are places where the <63 μm fraction is deposited).
Mineralogy
Road dust composition can be affected by various factors including geographical location, land use, traffic characteristics and precedent dry period (Amato et al. 2011; Gunawardana et al. 2012). Road dusts were dominated by quartz and calcite, while lower abundances of plagioclases (mostly albite) and dolomite occured in almost all the studied samples (Bourliva et al. 2010, 2012). The minor phases detected in some samples include orthoclase, tremolite, muscovite and chlorite. The high content of quartz in road dust samples is likely to be a road pavement-related signature, given the abundance of granite stones in all pavements of sampling roads (Amato et al. 2011), while the fact that calcite content formed the second most common mineral phase after quartz indicated probably the presence of construction wear materials (Varrica et al. 2003). Additionally, the presence of albite in road dusts was also strongly related to road pavement eroded particles (Kupiainen et al. 2003; Kupiainen and Tervahattu 2004; Raisanen et al. 2005; Tervahattu et al. 2006). Furthermore, a significant proportion of amorphous content was detected (Bourliva et al. 2010, 2012), which could probably involve non-crystalline mineral phases, amorphous silicates like non-crystalline quartz and also organic matter, as reported by Bish and Post (1989). According to Gunawardana et al. (2012), the elevated amorphous content is strongly correlated with heavy metals. This is an implication that traffic-related material would be the main source of amorphous content, since Zn, Cu, Ni, Cr and Cd originate from sources such as tire wear, brake pad wear and asphalt. Finally, minerals identified in road dust samples were similar to those found in soil samples (Xie et al. 2000) clearly indicating that there might be inputs from surrounding soil to the road surfaces.
Morphology of road dust particles
SEM observations of representative road dust samples were employed in order to distinguish particles of anthropogenic origin, and selected images are presented in Fig. 4. As shown in Fig. 4, dust particles were present in a wide range of sizes and shapes. Depending on the origin of dust particles, they mostly consisted of subhedral to anhedral crystalline grains, plate-like and near-spherical particles, and finally irregular agglomerates that comprised various sizes and amount of particles. EDS results revealed that the composition of dusts was dominated by the presence of Ca-rich or Fe-rich particles and silicates. The samples were predominately composed of angular Si-rich particles (Fig. 4a) consisting of Si, Al, Fe and Mg as shown by the elemental analysis, a composition which is characteristic of natural sources. Additionally, Ca-dominant particles (Fig. 4b) mainly composed of calcite (CaCO3) and agglomerates of Ca-rich particles composed of Ca with traces of Si, Al, Mg, K and S were identified. Agglomerates of Ca-aluminosilicates were also determined. Furthermore, Fe-rich particles (Fig. 4c) either spherule-shaped or angular with complex chemical compositions were also recognized. The spherical Fe-rich particles could be fly ash originating from asphalt and coal combustion (Goodarzi 2006). SEM analysis was mainly focused on the particles originating from anthropogenic activities. It is possible to detect the presence of particles derived from traffic-related activities or anthropogenic sources by their detailed morphology and elemental composition (Adachi and Tainosho 2004). Road dust contained a high proportion of particles with rough surface texture (Fig. 4d) suggesting that they were released from vehicles via exhaust emission and from the abrasion or corrosion of the vehicle engine and body work (Hoffman et al. 1999). Elemental analysis indicated that these particles exhibit high Fe content and heavy metal elements such as Cr and Mn. Heavy metals were observed in the form of flakes attached either on the surface or within the road dust particles (Fig. 4e).
Total heavy metal concentrations
Descriptive statistics of heavy metal concentrations determined in road dusts in Thessaloniki, as well as background values, are presented in Table 1. The mean concentration of heavy metals in road dust samples followed the order Fe ≫ Cu > Zn > Mn > Pb > Cr > Ni > Cd, which is consistent with the study by Han et al. (2014) in road dusts from Kuala Lumpur city center. In the present study, mean concentrations of all metals except Mn were higher than the background values in world soils and the upper crust, suggesting that the presence of these metals in road dust samples from Thessaloniki city core is highly influenced by anthropogenic sources.
In order to assess the possible sources of metal enrichment and to identify hot-spot areas with elevated metal concentrations, spatial distribution patterns of the concentrations of the studied heavy metals were plotted (Shi et al. 2008; Wei et al. 2009). The resulting contour maps for the metal concentration levels in road dust samples from Thessaloniki city core are illustrated in Fig. 5. Similar patterns of spatial distribution were observed for Cr, Cu and partly Zn. These elements presented relatively high spatial variability. Their hot-spot areas were mainly associated with main roads that reveal high traffic density (Tsimiski str. and streets surrounding Aristotelous Square). The elevated concentrations could be attributed to traffic characterized by low speeds and long periods of vehicle idling (Thorpe and Harrison 2008). Cadmium and manganese showed similar spatial distributions coinciding with the arterial coastal road, while hot-spot area was associated with the Port entrance area, revealing an increased but narrow spreaded traffic flow across the sampling sites. The aforementioned suggest that these two metals may be derived from the same sources, although it is demonstrated by several studies that elevated Mn concentrations in road dust mainly attribute to natural sources (Sezgin et al. 2003; Wang and Qin 2007). These findings are in good agreement with Ewen et al. (2009), according to which roadside dust samples highly contaminated in Cd and Mn occurred along the coastal road, within the Thessaloniki area. Concerning Pb and Ni, their spatial distributions were very different as compared with those of the rest metals. Spatial distribution of Pb revealed hot-spot areas associated with large conjunctions due to the substantial increase in traffic volume and the stopping and idling while waiting at the traffic lights. Nickel displayed a different distribution pattern presenting low variability, while the hotspot area located close to a tourist attraction area (White Tower) probably implying the existence of a different source.
Heavy metal enrichment factors were calculated for each road dust sample, and their values are presented in Table 1 and Fig. 6a. Mean EF values are arranged in the following decreasing order: Cu > Cd > Pb > Zn > Ni > Cr. The highest EF mean value that was recorded was the one for Cu (70.78) followed by 55.91 for Cd. EF mean value for Pb was 37.08, while the corresponding one for Zn was 19.60. The fact that Cu, Cd, Pb and Zn reveal mean EF values >10 suggested that the elevated concentrations of these heavy metals were attributed to anthropogenic activities. On the contrary, Ni and Cr with mean EF values of 5.54 and 3.50, respectively, are considered to have minimal anthropogenic influences.
Similar to enrichment factor, the mean values of I geo decreased in the order of Cu > Cd > Pb > Zn > Ni > Cr > Mn (Table 1; Fig. 6b). The data indicated that the majority of the road dusts could be characterized as “unpolluted” for Cr and Mn. On the contrary the contamination levels of Cu, Cd and Pb in road dusts were high and the majority of dust samples were characterized as “heavily polluted” concerning those metals. The highest I geo values for Cd and Pb were recorded to road dust samples associated with heavy traffic due to big conjunction sites (Vardaris Square, Dikastiria, Limani). Overall, EF and I geo values show similar trends, indicating the hot-spots of anthropogenic pollution.
The comparison of heavy metals mean concentrations in road dusts collected from different urban environments is a common practice, despite the fact that there are no widely accepted sampling and analytical procedures for geochemical studies of urban deposits. Table 2 presents the mean concentrations of heavy metals that were determined in road dust samples from Thessaloniki’s city center, in comparison with the corresponding ones from other cities around the world. As shown, there is a considerable variation of heavy metals concentrations in the road dust particles among various cities probably depending on population, traffic density, industrial activities in the area as well as local weather conditions and wind patterns. Cadmium and chromium concentrations in Thessaloniki were comparable to those found in most of the past studies, showing a slight decrease in relation to a past study carried out in Thessaloniki (sampling dates 2008). The mean concentration of Cu in Thessaloniki’s road dusts (this study) were higher than the corresponding ones determined in other cities, except of those recorded by Joshi et al. (2009) in the industrial area of Singapore, although in this study the majority of the detected metals exhibited extremely high concentrations. According to a past study carried out in Thessaloniki (Ewen et al. 2009), Cu content was significantly lower in samples originating from almost adjacent sampling locations. It is quite remarkable that Ni concentration in Thessaloniki’s road dusts is comparable with that of Shanghai, an urban area with a population of more than 17 million, while the majority of the other cities present lower concentrations. Manganese in Thessaloniki was also detected in levels higher than those determined in road dust samples from other highly populated cities (Kuala-Lumpur, Toronto, Nanjing). On the other hand, Pb and Zn mean concentrations in Thessaloniki’s road dusts were comparable to the corresponding ones reported in an earlier study in the same region (Ewen et al. 2009) and almost similar to those of Xi’an, China (Yongming et al. 2006). Moreover, they were lower than those reported by Soltani et al. (2015) and Shi et al. (2008) for Isfahan, Iran and Shanghai, China, respectively. In general, each city has its own characteristic combination of elemental compositions and the observed similarities as well as variations may not reflect the actual natural and anthropogenic diversities among different urban settings. The chemical mixture of traffic-related metals varies significantly among different studies (Thorpe and Harrison 2008; Gietl et al. 2010; Gunawardana et al. 2012). This is probably due to the variance of the metal content in brake lining and tire materials and/or to the differences in traffic volume density and driving behavior (Thorpe and Harrison 2008; Apeagyei et al. 2011; Duong and Lee 2011).
Source identification of selected pollutants
In order to establish inter-element relations in the road dust samples, Pearson correlation coefficients were employed for all the studied metals and the obtained correlation matrix is presented in Table 3. There was a positive correlation among all the heavy metals, except for the pair Ni and Pb (−0.196). Strong correlations (p < 0.01) were evident for the pairs Cr and Cu (0.872), Cr and Zn (0.583), Cu and Ni (0.548) and Cu and Zn (0.568) indicating that there is one group of elements i.e. Cr–Cu–Ni–Zn which probably obtain similar characteristics and share common origins of emission. According to Amato et al. (2011) Zn, Ni, Cu and Cr can be derived from tire and brake wear. Furthermore, a significant positive correlation was also identified among a second group of elements comprising Mn and Cd (0.729) suggesting similar characteristics, fate, and common origin. Significant correlations (p < 0.05) were also observed for the pairs Cr and Fe (0.432), Cu and Fe (0.439), Ni and Cr (0.428), Cd and Zn (0.443), Fe and Zn (0.444).
Cluster analysis was applied to the z scores of road dust data in order to examine the classification of element groups and to recognize relationships among them. The results of the analysis are presented as a dendrogram in Fig. 7. The similarity axis represents the degree of association between the variables, the greater the value the more significant the association. The cluster analysis presents two distinct larger subgroups: the first contains Cu, Cr, Zn, Fe, Ni and the second includes Cd, Mn and Pb. The strongest observed association (similarity > 95 %) was between Cu and Cr which form another cluster with Zn joining following the other elements in the subgroup 1. Furthermore, the long distance between Ni and the rest elements in subgroup 1 probably suggests a different source (Yongming et al. 2006). Strong association was observed also for Cd and Mn in subgroup 2 which form another cluster with Pb.
PCA was performed for the dataset of urban road dusts so as to assist in identifying the sources of the contaminants. Table 4 displays PCA results, including factor loadings with Varimax rotation as well as eigenvalues, while a 3D plot of PCA loadings is presented in Fig. 8 and the relationships among the studied metals are readily seen. As indicated, three main components were obtained accounting for 78.02 % of the total variance and the eigenvalues of the extracted factors were >1. The first component is dominated by Cr, Cu, Fe, Ni and Zn, accounting for 37.60 % of the total variance. The source of this component may be tire and brake wear and vehicle abrasion. The high EFs (>10) for Cu and Zn observed in road dusts (Fig. 6a) are denoting major contribution of tire and brake abrasion. The possible sources of copper include wearing of machinery parts and brake pads. Brake dust is already recognized as a significant carrier of Cu in aerosol composition since Cu is generally used in brakes to control heat transfer (Sternbeck et al. 2002; Adachi and Tainosho 2004; Huang et al. 2009). Copper and zinc in road dust can also derive from tire wear and the level of Cu present in diesel (Contini et al. 2012). Elevated Zn contents may have originated from wear and tear of vulcanized vehicle tires as well as from the corrosion of galvanized automobile parts. According to the study of Apeagyei et al. (2011), Zn is the most abundant metal in tires, with an average of 17,720 mg kg−1 in 115 measured tires. Zn may have also derived from both the mechanical abrasion of vehicles (Jiries et al. 2001) and the lubricating oils and tires of motor vehicles (Akhter and Madany 1993; Arslan 2001). Furthermore, according to Adachi and Tainosho (2004) and Apeagyei et al. (2011), considerable amount of heavy metals such as Fe and Zn may originate from serious wear and tear of tires and from the brake lining. The source of Cr in road dusts is believed to be chrome plating of some motor vehicle parts (Al-Shayep and Seaward 2001), while the source of Ni in street dust is believed to be corrosion of cars (Fergusson and Kim 1991; Akhter and Madany 1993). Finally, according to Al-Khashman (2007) and Han et al. (2014), Fe usually originates from geogenic sources like soil and the weathering of earth crust. Moreover, Fe in road dust can also originate from the use of vehicle brake pads which are constantly utilized to reduce vehicle acceleration in heavy traffic along with industrial activity (Apeagyei et al. 2011; Han et al. 2014). Component 2, which includes Cd and Mn, accounts for 24.70 % of the total variance. Based on the significantly high EF (>10) for Cd, which indicate that component 2 is significantly influenced by anthropogenic sources, we could consider Mn as a traffic-related element taking into account its clustering with Cd. However, the low EF for Mn is characteristic of crustal origin, while further studies are essential in order to explain if it is also influenced by anthropogenic activities. The source of Cd in component 2 most likely is vehicle abrasion (Akhter and Madany 1993).According to a study undertaken by Tamrakar and Shakya (2011), metal plating and metal-enforced tires are considered the most likely and common anthropogenic sources of Cd in road dust through the burning of tires and bad roads. Additionally, Cd is typically used as plating in brake pads to prevent corrosion (McKenzie et al. 2009). On the other hand, Mn, apart from its crustal origin, could also be present in both, automotive parts as a component of aluminum-based alloys and like Pb be used as a vehicle fuel additive. Component 3 is strongly correlated with Pb, which has a high loading value (0.873) and explains 15.72 % of the total variance. The main source of Pb in component 3 is fuel/oil leakage from automobiles along with oil lubricants. Even though most vehicles now use unleaded fuel, a large amount of Pb has been still identified in road dust or street dust samples (Sezgin et al. 2003; Christoforidis and Stamatis 2009; Faiz et al. 2009). Lead (Pb) compounds are also used as anti-wear agents in lubricant oils for engines (Mang and Dresel 2007). The results are in good agreement with the findings of the cluster analysis (Fig. 7).
Heavy metal fractionation
Sequential fractionation of the total metal content enables the classification of the heavy metals that are present in the samples, according to the type of bonding on the surface of the particles, thus permitting the assessment of heavy metal mobility from road dust to water or air. The bioavailable fractions of each heavy metal for any given sample, excluding the non-available residual fraction (located in the silicate lattice of the sample particles), are illustrated in Fig. 9. In general, the exchangeable fraction, which represents the heavy metal content easily dissociated and released through water dissolution or ion exchange, accounted for the lowest amount of metals. Fractions with the highest content were usually the carbonate, redox and organic fraction, where heavy metals are bound in various forms.
In the case of Mn, the exchangeable fraction was minimal, showing that a simple release of this metal in the rain water, or air humidity was not likely to occur. Mn was mainly found in the redox fraction (52–90 %). This was expected since reductive/oxidative changes in the surface of the particles (dissolution in water at different redox potential) may greatly affect the oxidation state of this transition metal, releasing large part of its content to the surrounding environment. The largest quantities of oxide-bound Mn were found in samples RD9, RD25, RD27 and RD31. Second most abundant fraction was the carbonate-bound fraction (8–31 %), at the sampling points RD9, RD12, RD13 and RD22. The release of Mn from this fraction occurred through carbonate dissolution in water with low pH (such as acidic rainwater), since this metal is usually bound on the surface of other carbonate salts (Tokalioglu et al. 2003). The immobilized residual fragment accounted for 21–32 % of the total heavy metal content.
Cu on the other hand was mainly observed in the organic-bound fraction (51–84 %), especially at sampling points RD12, RD22, RD27 and RD31. This part is not easily released in the surrounding environment, unless excessively oxidative conditions occur. The second largest fraction was the redox-bound (12–45 %), and its maximum values were determined at sampling points RD9, RD13 and RD25. Changes in the redox potential of humidity and rainwater could release part of this fraction. The exchangeable part was minimal, while the immobilized residual part varied between 4 and 27 %.
Nickel and chromium exhibited similar fractionation pattern, with the highest fraction being the redox-bound (52–71 and 58–76 % for Ni and Cr, respectively), followed by the organic fraction. The release of these transition metals to the surrounding environment increases as the redox potential varies and their oxidative state changes. A large part of Cr and Ni was bound to the organic fraction, which can be released through strong oxidation or gradual degradation of the sorptive substances. The amount of Cr and Ni in the exchangeable fraction was significantly higher compared to the corresponding of other heavy metals, rendering their presence in rain water more than likely.
Lead was also found in higher abundance in the redox-bound fraction, followed by the carbonate-bound fraction, which was not expected since it usually forms insoluble carbonate compounds. Reductive processes and changes in the acidity of the sample’s environment could lead to dissolution of Fe/Mn oxides found in the sample, which could consequently release sorbed quantities of heavy metals, including Pb in this fraction (Luo and Christie 1998; Alvarez et al. 2001; Ure and Davidson 2002). The part of Pb which was bound to the organic fraction ranged between 27 and 45 %. Slightly higher amount of exchangeable Pb was found in locations RD13, RD22 and RD25.
Cadmium, an extremely toxic heavy metal, originating mainly from anthropogenic processes, presented a diverse fractionation pattern, since it occurred in almost every fraction of the extracted particles, partly due to its different sources of pollution. Moreover, slight variations and transport from one fraction to another, may considerably have affected the overall fractionation pattern of Cd, since its total concentration was significantly lower compared to other heavy metals.
In the case of Zn, most of its content was bound to the redox and carbonate fraction, with higher values at sampling points RD25 and RD27.
The wt% amount of every analyzed heavy metal in each fraction of the total metal content is presented in Fig. 10. The exchangeable fraction, accounting for the lower available heavy metal quantity, mainly consisted of Cu Mn and Pb. At sampling point RD9, Mn and Cu were more easily released. The same occurred at sampling point RD13 for Cr and Cu, while Mn, Cu and Zn were more easily released at RD25. The carbonate-bound fraction mainly comprised Mn, Pb and Zn, metals that are considered as easily bound to carbonate structures. Mn, Cu and Pb were mainly sorbed on the Fe/Mn oxide-bound fraction which has been reported to dissolve under reductive conditions, possibly resulting in the release of Mn, Cu and Zn in the surrounding environment (Luo and Christie 1998; Alvarez et al. 2001; Tokalioglu et al. 2003).
Health risk assessment
The results of non-carcinogenic and carcinogenic health risks due to metal exposure in road dusts, for both children and adults concerning the population in the studied area are presented in Table 5. Generally, the non-carcinogenic health risk for children is always higher compared to adults. Regarding the exposure pathway, ingestion of dust particles appeared to be the main risk followed by dermal and inhalation routes; contribution of inhalation and dermal contact to the overall HI can be stated that is almost negligible. The results obtained were in agreement with previously published research data (Ferreira-Baptista and de Miguel 2005; Zheng et al. 2010; Liu et al. 2014).
Exposure of children via dust ingestion resulted in a health risk in the order of Pb > Cr > Cu > Ni > Cd > Zn. All the HQ values for the studied elements were lower than the safe level (=1); however, Pb (HQing = 0.451), Cr (HQing = 0.255) and Cu (HQing = 0.134) exhibited values close to that safe level. Additionally, HIs for Pb, Cr and Cu with reference to children were close to the safe level signifying that children exposure to the road dusts can potentially trigger adverse but non-cancer health effects. Specifically, though HI for Pb (0.459) is below the safe level (1), Pb is a cumulative toxic metal which interferes with the development of the central nervous system and it is therefore particularly toxic to children, causing potentially permanent learning and behavior disorders (Sarkar 2002). Hence, Pb exposure through the road dust cannot be overlooked and its ecological and health implications need further detailed investigations. Young children are the most sensitive subpopulation because of their hand-to-mouth activity, whereby contaminated dust can be readily ingested (Meza-Figueroa et al. 2007). In addition, considering the lower body weight of children, the metals intake (mg/kg-bodyweight/day) of a child is believed to be greater than that of an adult (Wang et al. 2011). Thus, the hazard health risk for children exposed to road dust metals is thought to be greater than that of adults.
Compared to children, adult health risk due to heavy metal exposure from road dust was lower. The ingestion of dust particles appeared to be the route of exposure to street dust. Both HQ and HI values for all studied elements were far below the safe level, indicating no non-carcinogenic risks from these elements for adults. However, the possible health risk due to exposure to road dust in hot-spot areas cannot be overlooked for the outdoor workers, such as street sweepers, in view of the potential increase in airborne particle concentrations during the manual sweeping (Gertler et al. 2006; Amato et al. 2010). Taxi drivers may also be at health risk due to the long-term exposure to the road dust.
Regarding cancer risk, only the carcinogen risk due to inhalation exposure was considered in the model and the cancer risk of Cd, Cr and Ni was extremely low.
Conclusions
Mineralogical characterization of road dusts revealed that the dominant mineral phases were quartz and calcite, strongly indicating toward road and pavement-related signature. The amorphous content detected in road dust samples is probably due to partially weathered minerals, which are strongly related to the presence of heavy metals. Concerning the morphology of dust particles, SEM observations revealed subhedral to anhedral crystalline grains, plate-like and near-spherical particles, or irregular agglomerates. Road dust contained a large amount of particles with rough surface texture which could suggest that they originate from vehicle exhaust emission as well as from the abrasion or corrosion of engines and vehicle parts. Elemental analysis (EDS) showed that these particles comprise high Fe content and heavy metals such as Cr and Mn (mainly in the form of flakes attached on the surface of road dust particles).
Total heavy metal content in the road samples was dominated by Fe, while the concentrations of the other metals followed the decreasing order: Cu > Zn > Mn > Pb > Cr > Ni > Cd. Since the mean concentrations of all the studied metals, except Mn, were higher than the background reference values in upper crust, it is very likely that their presence is significantly influenced by anthropogenic sources. Spatial distribution of the metals was evaluated using contour maps of the metal concentration levels in road dust samples from Thessaloniki. Similar patterns of spatial distribution were observed for Cr, Cu and partly Zn, while the areas of their highest abundance were associated with main roads and high traffic density. Their presence in such abundance was attributed to low vehicle speeds and long periods of vehicle idling. Pb was mainly determined at large conjunctions, due to the substantial increase in the traffic volume and the stopping and idling because of the traffic lights. Metal enrichment factors displayed the following decreasing trend: Cu > Cd > Pb > Zn > Ni > Cr. Similar to enrichment factors, the mean values of I geo decrease in the order of Cu > Cd > Pb > Zn > Ni > Cr > Mn. The obtained data indicated that the road dusts were slightly contaminated by Cr and Mn, while the contamination levels of Cu, Cd and Pb were elevated.
Two distinct larger subgroups are revealed by cluster analysis: the first contains Cu, Cr, Zn, Fe, Ni and the second includes Cd, Mn and Pb. In the first subgroup, the strongest association was observed between Cu and Cr, while in the second among Cd and Mn. The first component of PCA is dominated by Cr, Cu, Fe, Ni and Zn, with a possible source of this component to be tire and brake wear, vehicle abrasion, tire and brake abrasion and also lubricating oils. Component 2, which includes Cd and Mn, is significantly influenced by anthropogenic sources. The possible metal source is most likely to be vehicle abrasion. Metal plating and metal-enforced tires are considered the most likely and common anthropogenic sources of Cd in road dust through the burning of tires and bad roads, while Mn could be a component of aluminium-based alloys. Component 3 is strongly correlated with Pb, with the major source being fuel/oil leakage from automobiles along with oil lubricants.
Heavy metal fractionation suggested that Mn, Ni and Cr are mainly bound to the redox fraction and associated with Mn and Fe-oxides. The carbonate-bound fraction mainly consists of Zn, Pb and Mn, likely to be released under increased acidity conditions. Oxidative or gradual degradation of the organic fraction could result in the release of Cu, Mn and Zn in the surrounding environment. The exchangeable fraction, which can easily be released in the surrounding environment through water dissolution or ion exchange, accounts only for a very small portion of the total heavy metal content, comprising mainly Cu Mn and Pb.
Exposure of children via dust ingestion resulted in a health risk in the order of Pb > Cr > Cu > Ni > Cd > Zn. The HQ values for the studied elements were all lower than the safe level (=1); however, Pb (HQing = 0.451), Cr (HQing = 0.255) and Cu (HQing = 0.134) exhibited values closer to that safe level. HI for Pb (0.459) is close but below the safe level (1); nevertheless, Pb is a cumulative toxic metal particularly toxic to children. In Thessaloniki city center, cancer risk of Cd, Cr and Ni due to road dust exposure was calculated to be extremely low.
References
Adachi, K., & Tainosho, Y. (2004). Characterization of heavy metal particles embedded in tire dust. Environment International, 30, 1009–1017.
Akhter, M. S., & Madany, I. M. (1993). Heavy metals in street and house dust in Bahrain. Water, Air, and Soil Pollution, 66, 111–119.
Al-Khashman, O. A. (2007). Determination of metal accumulation in deposited street dusts in Amman, Jordan. Environmental Geochemistry and Health, 29, 1–10.
Al-Shayep, S. M., & Seaward, M. R. D. (2001). Heavy metal content of roadside soils along ring road in Riyadh (Saudi Arabia). Asian Journal of Chemistry, 13, 407–423.
Alvarez, M. B., Malla, M. E., & Batistoni, D. A. (2001). Comparative assessment of two sequential chemical extraction schemes for the fractionation of cadmium, chromium, lead and zinc in surface coastal sediments. Fresenius Journal of Analytical Chemistry, 369, 81–90.
Amato, F., Pandolfi, M., Escrig, A., Querol, X., Alastuey, A., Pey, J., et al. (2009). Quantifying road dust resuspension in urban environment by multilinear engine: A comparison with PM2. Atmospheric Environment, 43, 2770–2780.
Amato, F., Pandolfi, M., Moreno, T., Furger, M., Pey, J., Alastuey, A., et al. (2011). Sources and variability of inhalable road dust particles in three European cities. Atmospheric Environment, 45, 6777–6787.
Amato, F., Querol, X., Johansson, C., Nagl, C., & Alastuey, A. (2010). A review on the effectiveness of street sweeping, washing and dust suppressants as urban PM control methods. Science of the Total Environment, 408, 3070–3084.
Apeagyei, E., Bank, M. S., & Spengler, J. D. (2011). Distribution of heavy metals in road dust along an urban–rural gradient in Massachusetts. Atmospheric Environment, 45, 2310–2323.
Arslan, H. (2001). Heavy metals in street dust in Bursa, Turkey. Journal of Trace and Microprobe Techniques, 19, 439–445.
Beckwith, P. R., Ellis, J. B., & Revitt, D. M. (1986). Heavy metal and magnetic relationships for urban source sediments. Physics of the Earth and Planetary Interiors, 42, 67–75.
Birch, G. F., & Olmos, M. A. (2008). Sediment-bound heavy metals as indicators of human influence and biological risk in coastal water bodies. ICES Journal of Marine Science, 65, 1407–1413.
Bish, D. L., & Post, J. E. (1989). Modern powder diffraction (Vol. 20). Chelsea, MI: Geological Society of America.
Bourliva A., Kantiranis N., Papadopoulou L., Christoforidis C., & Kollias P. (2012). Heavy metals in road dusts from the center of the city of Thessaloniki, Greece: A mineralogical and chemical assessment. In Proceedings of 11th International Conference of Protection and Restoration of the Environment Conference (PRE XI), Thessaloniki, Greece, e-Proceedings, (pp. 1040–1049).
Bourliva, A., Papadopoulos, A., Giouri, A., Papadopoulou, L., & Kantiranis, N. (2010). On the mineralogy, physical characteristics and the main elemental content of urban road dust particles from the historic center of the city of Thessaloniki, Northern Greece. Scientific Annals, 100, 31–38.
Brown, J. N., & Peake, B. M. (2006). Sources of heavy metals and polycyclic aromatic hydrocarbons in urban stormwater runoff. Science of the Total Environment, 359, 145–155.
Buseck P. R., & Bradley J. P. (1982). Electron beam studies of individual natural and anthropogenic microparticles: Compositions, structures and surface reactions. In D. R. Schryer (Ed.), Heterogeneous atmospheric chemistry (pp. 57–76). Washington, DC: American Geophysical Union.
Chabukdhara, M., & Nema, A. K. (2013). Heavy metals assessment in urban soils around industrial clusters in Ghaziabad, India: Probabilistic health risk approach. Ecotoxicology and Environmental Safety, 87, 57–64.
Charlesworth, S., De Miguel, E., & Ordóñez, A. (2011). A review of the distribution of particulate trace elements in urban terrestrial environments and its application to considerations of risk. Environmental Geochemistry and Health, 33, 103–123.
Charlesworth, S., Everett, M., McCarthy, R., Ordónẽz, A., & de Miguel, E. (2003). A comparative study of heavy metal concentration and distribution in deposited street dusts in a large and a small urban area: Birmingham and Coventry, West Midlands, UK. Environment International, 29, 563–573.
Christoforidis, A., & Stamatis, N. (2009). Heavy metal contamination in street dust and roadside soil along the major national road in Kavala’s region, Greece. Geoderma, 151, 257–263.
Christophoridis, C., Dedepsidis, D., & Fytianos, K. (2009). Occurrence and distribution of selected heavy metals in the surface sediments of Thermaikos Gulf, N. Greece. Assessment using pollution indicators. Journal of Hazardous Materials, 168, 1082–1091.
Contini, D., Belosi, F., Gambaro, A., Cesari, D., Stortini, A. M., & Bove, M. C. (2012). Comparison of PM10 concentrations and metal content in three different sites of the Venice Lagoon: An analysis of possible aerosol sources. Journal of Environmental Sciences, 24, 1954–1965.
de Miguel, E., Iribarren, I., Chacón, E., Ordoñez, A., & Charlesworth, S. (2007). Risk-based evaluation of the exposure of children to trace elements in playgrounds in Madrid (Spain). Chemosphere, 66, 505–513.
Duong, T., & Lee, B. K. (2011). Determining contamination level of heavy metals in road dust from busy traffic areas with different characteristics. Journal of Environmental Management, 92, 554–562.
Ewen, C., Anagnostopoulou, M. A., & Ward, N. I. (2009). Monitoring of heavy metal levels in roadside dusts of Thessaloniki, Greece in relation to motor vehicle traffic density and flow. Environmental Monitoring and Assessment, 157, 483–498.
Faiz, Y., Tufail, M., Javed, M. T., Chaudhry, M. M., & Siddique, N. (2009). Road dust pollution of Cd, Cu, Ni, Pb and Zn along Islamabad Expressway, Pakistan. Microchemical Journal, 92, 186–192.
Fergusson, J. E., & Kim, N. (1991). Trace elements in street and house dusts: Source and speciation. Science of the Total Environment, 100, 125–150.
Ferreira-Baptista, L., & de Miguel, E. (2005). Geochemistry and risk assessment of street dust in Luanda, Angola: A tropical urban environment. Atmospheric Environment, 39, 4501–4512.
Fujiwara, F., Rebagliati, R. J., Dawidowski, L., Gómez, D., Polla, G., Pereyra, V., & Smichowski, P. (2011). Spatial and chemical patterns of size fractionated road dust collected in a megacity. Atmospheric Environment, 45, 1497–1505.
Gertler, A., Kuhns, H., Abu-Allaban, M., Damm, C., Gillies, J., & Etyemezian, V. (2006). A case study of the impact of winter road sand/salt and street sweeping on road dust re-entrainment. Atmospheric Environment, 40, 5976–5985.
Gietl, J. K., Lawrence, R., Thorpe, A. J., & Harrison, R. M. (2010). Identification of brake wear particles and derivation of a quantitative tracer for brake dust at a major road. Atmospheric Environment, 44, 141–146.
Godish, T. (2005). Air quality (4th ed.). Boca Raton: CRC Press Company.
Goodarzi, F. (2006). Morphology and chemistry of fine particles emitted from a Canadian coal-fired power plant. Fuel, 85, 273–280.
Gunawardana, C., Goonetilleke, A., Egodawatta, P., Dawes, L., & Kokot, S. (2012). Source characterisation of road dust based on chemical and mineralogical composition. Chemosphere, 87, 163–170.
Han, N. M. M., Latif, M. T., Othman, M., Dominick, D., Mohamad, N., Juahir, H., & Tahir, N. M. (2014). Composition of selected heavy metals in road dust from Kuala Lumpur city centre. Environmental Earth Science, 72, 849–859.
Herngren, L., Goonetilleke, A., & Ayoko, G. A. (2005). Understanding heavy metal and suspended solids relationships in urban stormwater using simulated rainfall. Journal of Environmental Management, 76, 149–158.
Hoffman, V., Knab, M., & Appel, E. (1999). Magnetic susceptibility mapping of roadside pollution. Journal of Geochemical Exploration, 66, 313–326.
Hu, Y., Liu, X., Bai, J., Shih, K., Zeng, E. Y., & Cheng, H. (2013). Assessing heavy metal pollution in the surface soils of a region that had undergone three decades of intense industrialization and urbanization. Environmental Science and Pollution Research, 20, 6150–6159.
Huang, S., Tu, J., Liu, H., Hua, M., Liao, Q., Feng, J., et al. (2009). Multivariate analysis of trace element concentrations in atmospheric deposition in the Yangtze River Delta, East China. Atmospheric Environment, 43, 5781–5790.
Jiries, A., Hussein, H. H., & Halaseh, Z. (2001). The quality of water and sediments of street runoff in Amman, Jordan. Hydrological Processes, 15, 815–824.
Joshi, U. M., Vijayaraghavan, K., & Balasubramanian, R. (2009). Elemental composition of urban street dusts and their dissolution characteristics in various aqueous media. Chemosphere, 77, 526–533.
Kabata-Pendias, A., & Pendias, H. (2001). Trace elements in soils and plants (3rd ed.). Boca Raton, FL: CRC Press.
Kantiranis, N., Stergiou, A., Filippidis, A., & Drakoulis, A. (2004). Calculation of the percentage of amorphous material using PXRD patterns. Bulletin of Geological Society of Greece, 36, 446.
Kennedy, N. J., & Hinds, W. C. (2002). Inhalability of large solid particles. Journal of Aerosol Science, 33, 237–255.
Kreider, M. L., Panko, J. M., McAtee, B. L., Sweet, L. I., & Finley, B. L. (2010). Physical and chemical characterization of tire-related particles: Comparison of particles generated using different methodologies. Science of the Total Environment, 408, 652–659.
Kupiainen, K., & Tervahattu, H. (2004). The effect of traction sanding on urban suspended particles in Finland. Environmental Monitoring and Assessment, 93, 287–300.
Kupiainen, K., Tervahattu, H., & Raisane, M. (2003). Experimental studies about the impact of traction sand on urban road dust composition. Science of the Total Environment, 308, 175–184.
Latif, A. N. M., & Saleh, I. A. (2012). Heavy metals contamination in road site dust along major roads and correlation with urbanization activities in Cairo, Egypt. The Journal of American Science, 8, 379–389.
Li, R., Cai, G., Wang, J., Ouyang, W., Cheng, H., & Lin, C. (2014). Contents and chemical forms of heavy metals in school and roadside topsoils and road-surface dust of Beijing. Journal of Soils and Sediments, 14, 1806–1817.
Li, H., & Zuo, X. J. (2013). Speciation and size distribution of copper and zinc in urban road runoff. Bulletin of Environmental Contamination and Toxicology, 90, 471–476.
Liu, Q. T., Diamond, M. E., Gingrich, S. E., Ondov, J. M., Maciejczyk, P., & Sterm, G. A. (2003). Accumulation of metals, trace elements and semivolatile organic compounds on exterior windows surfaces in Baltimore. Environmental Pollution, 122, 51–61.
Liu, E., Yan, T., Birch, G., & Zhu, Y. (2014). Pollution and health risk of potentially toxic metals in urban road dust in Nanjing, a mega-city of China. Science of the Total Environment, 476–477, 522–531.
Lu, X., Wang, L., Lei, K., Huang, J., & Zhai, Y. (2009). Contamination assessment of copper, lead, zinc, manganese and nickel in street dust of Baoji, NW China. Journal of Geochemical Exploration, 161, 1058–1062.
Lu, X., Wang, L., Li, L. Y., Lei, K., Huang, L., & Kang, D. (2010). Multivariate statistical analysis of heavy metals in street dust of Baoji NW China. Journal of Hazardous Materials, 173, 744–749.
Luo, Y. M., & Christie, P. (1998). Choice of extraction technique for soil reducible trace metals determines the subsequent oxidisable metal fraction in sequential extraction schemes. International Journal of Environmental Analytical Chemistry, 72, 59–75.
Luo, X. S., Ding, J., Xu, B., Wang, Y. J., Li, H. B., & Yu, S. (2012). Incorporating bioaccessibility into human health risk assessments of heavy metals in urban park soils. Science of the Total Environment, 424, 88–96.
Mang, T., & Dresel, W. (2007). Lubricants and lubrication (2nd ed.). Weinheim: Wiley-VCH.
Manoli, E., Voutsa, D., & Samara, C. (2002). Chemical characterization and source identification/apportionment of fine and coarse air particles in Thessaloniki, Greece. Atmospheric Environment, 36, 949–961.
McKenzie, E. R., Money, J. E., Green, P. G., & Young, T. M. (2009). Metals associated with stormwater-relevant brake and tire samples. Science of the Total Environment, 407, 5855–5860.
Meza-Figueroa, D., De la O-Villanueva, M., & De la Parra, M. L. (2007). Heavy metal distribution in dust from elementary schools in Hermosillo, Sonora, México. Atmospheric Environment, 41, 276–288.
Misaelides, P., Samara, C., Georgopoulos, Μ., Kouimtzis, Th, & Synetos, S. (1989). Toxic elements in the environment of Thessaloniki, Greece. Part 1: Roadside dust analysis by I.N.A.A. and A.A.S. Toxicological and Environmental Chemistry, 24, 191–198.
Moreno, T., Karanasiou, A., Amato, F., Lucarelli, F., Nava, S., Calzolai, G., et al. (2013). Daily and hourly sourcing of metallic and mineral dust in urban air contaminated by traffic and coal-burning emissions. Atmospheric Environment, 68, 33–44.
Muller, G. (1969). Index of geo-accumulation in sediments of the Rhine River. Geochemical Journal, 2, 108–118.
Nazzal, Y., Ghrefat, H., & Rosen, M. A. (2014). Application of multivariate geostatistics in the investigation of heavy metal contamination of roadside dusts from selected highways of the Greater Toronto Area, Canada. Environmental Earth Science, 71, 1409–1419.
Oh, M. S., Lee, T. J., & Kim, D. S. (2011). Quantitative source apportionment of size-segregated particulate matter at urbanized local site in Korea. Aerosol and Air Quality Research, 11, 247–264.
Ordonez, A., Loredo, J., De Miguel, E., & Charlesworth, S. (2003). Distribution of heavy metals in the street dusts and soils of an industrial city in northern Spain. Archives of Environmental Contamination and Toxicology, 44, 160–170.
Raisanen, M., Kupiainen, K., & Tervahattu, H. (2005). The effect of mineralogy, texture and mechanical properties of anti-skid and asphalt aggregates on urban dust, stages II and III. Bulletin of Engineering Geology and the Environment, 64, 247–256.
Rudnick, R. L., & Gao, S. (2003). Composition of the continental crust. In R. L. Rudnick (Ed.), The Crust (pp. 1–64). Amsterdam: Elsevier.
Samara, C., Kouimtzis, Th, Tsitouridou, R., Kanias, G., & Simeonov, V. (2003). Chemical mass balance source apportionment of PM10 in an industrialized urban area of Northern Greece. Atmospheric Environment, 37, 41–54.
Sarkar, B. (2002). Heavy metals in the environment. NY: CRC Press.
Settle, S., Goonetilleke, A., & Ayoko, G. A. (2007). Determination of surrogate indicators for phosphorus and solids in urban stormwater: Application of multivariate data analysis techniques. Water, Air, and Soil Pollution, 182, 149–161.
Sezgin, N., Ozcan, H. K., Demir, G., Nemlioglu, S., & Bayat, C. (2003). Determination of heavy metal concentrations in street dusts in Istanbul E-5 highway. Environment International, 29, 979–985.
Shi, G. T., Chen, Z. L., Xu, S. Y., Zhang, J., Wang, L., & Bi, C. J. (2008). Potentially toxic metal contamination of urban soils and roadside dust in Shanghai, China. Environmental Pollution, 156, 251–260.
Shilton, V. F., Booth, C. A., Smith, J. P., Giess, P., Mitchell, D. J., & Williams, C. D. (2005). Magnetic properties of urban street dust and their relationship with organic matter content in the West Midlands, UK. Atmospheric Environment, 39, 3651–3659.
Singh, A. K. (2011). Elemental chemistry and geochemical partitioning of heavy metals in road dust from Dhanbad and Bokaro regions, India. Environmental Earth Science, 62, 1447–1459.
Soltani, N., Keshavarzi, B., Moore, F., Tavakol, T., Lahijanzadeh, A. R., Jaafarzadeh, N., & Kermani, M. (2015). Ecological and human health hazards of heavy metals and polycyclic aromatic hydrocarbons (PAHs) in road dust of Isfahan metropolis, Iran. Science of the Total Environment, 505, 712–723.
Sternbeck, J., Sjödin, A., & Andréasson, K. (2002). Metal emissions from road traffic and the influence of resuspension-results from two tunnel studies. Atmospheric Environment, 36, 4735–4744.
Tamrakar, C. S., & Shakya, P. R. (2011). Assessment of heavy metals in street dust in Kathmandu Metropolitan City and their possible impacts on the environment. Pakistan Journal of Analytical and Environmental Chemistry, 12, 31–42.
Tanner, P. A., Ma, H. L., & Yu, P. K. N. (2008). Fingerprinting metals in urban street dust of Beijing, Shanghai, and Hong Kong. Environmental Science and Technology, 42, 7111–7117.
Tervahattu, H., Kupiainen, K. J., Raisanen, M., Makela, T., & Hillamo, R. (2006). Generation of urban road dust from anti-skid and asphalt concrete aggregates. Journal of Hazardous Materials, 132, 39–46.
Tessier, A., Campbell, P. G. C., & Blsson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51, 844–851.
Thorpe, A., & Harrison, R. M. (2008). Sources and properties of non-exhaust particulate matter from road traffic: a review. Science of the Total Environment, 400, 270–282.
Tokalioglu, S., Kartal, S., & Birol, G. (2003). Comparison of three sequential extraction procedures for partitioning of heavy metals in car park dusts. Journal of Environmental Monitoring, 5, 468–476.
Ure A. M., & Davidson, C. M. (Eds.). (2002). Chemical speciation in soils and related materials by selective chemical extraction. In Chemical speciation in the environment (pp. 265–298). London: Blackwell Science Ltd.
USEPA. (1989). Risk assessment guidance for superfund. Volume 1: Human health evaluation manual (part A). Washington: Office of Emergency and Remedial Response.
USEPA. (1994). Microwave assisted acid digestion of sediments, sludges, soils, and oils. SW-846, test methods for evaluating solid waste. Washington, DC: U.S. Environmental Protection Agency.
USEPA. (2001). Supplemental guidance for developing soil screening levels for superfund sites. Washington: U.S. Environmental Protection Agency, Office of Emergency and Remedial Response.
USEPA. (2011). Integrated Risk Information System (IRIS). U.S. Environmental Protection Agency.
Varrica, D., Dongarrà, G., Sabatino, G., & Monna, F. (2003). Inorganic geochemistry of roadway dust from the metropolitan area of Palermo, Italy. Environmental Geology, 44, 222–230.
Vegter, J. (2007). Urban soils—An emerging problem? Journal of Soils and Sediments, 7, 63.
Wang, W., Huang, M. J., Cheung, K. C., & Wong, M. H. (2011). Polycyclic aromatic hydrocarbons (PAHs) in urban surface dust of Guangzhou, China: Status, sources and human health risk assessment. Science of the Total Environment, 409, 4519–4527.
Wang, X., & Qin, Y. (2007). Some characteristics of the distribution of heavy metals in urban topsoil of Xuzhou, China. Environmental Geochemistry and Health, 29, 11–19.
Wei, B., Jiang, F., Li, X., & Mu, S. (2009). Spatial distribution and contamination assessment of heavy metals in urban road dusts from Urumqi, NW China. Microchemical Journal, 93, 147–152.
Wei, B., Jiang, F., Li, X., & Mu, S. (2010). Heavy metal induced ecological risk in the city of Urumqi, NW China. Environmental Monitoring and Assessment, 160, 33–45.
Xie, S., Dearing, J. A., & Bloemendal, J. (2000). The organic matter content of street dust in Liverpool, UK, and its association with dust magnetic properties. Atmospheric Environment, 34, 269–275.
Yongming, H., Peixuan, D., Junji, C., & Posmentier, E. S. (2006). Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Science of the Total Environment, 355, 176–186.
Yuen, J. Q., Olin, P. H., Lim, H. S., Benner, S. G., Sutherland, R. A., & Ziegler, A. D. (2012). Accumulation of potentially toxic elements in road deposited sediments in residential and light industrial neighborhoods of Singapore. Journal of Environmental Management, 101, 151–163.
Zhang, M., & Wang, H. (2009). Concentrations and chemical forms of potentially toxic metals in road-deposited sediments from different zones of Hangzhou, China. Journal of Environmental Sciences, 21, 625–631.
Zheng, N., Liu, J., Wang, Q., & Liang, Z. (2010). Health risk assessment of heavy metal exposure to street dust in the zinc smelting district, Northeast of China. Science of the Total Environment, 408, 726–733.
Zou, L. Y., & Hooper, M. A. (1997). Size resolved airborne particles and their morphology in central Jakarta. Atmospheric Environment, 31, 1167–1172.
Acknowledgments
The authors gratefully acknowledge Assist. Prof. N. Kantiranis for his help in XRD analysis and the determination of the amorphous content.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bourliva, A., Christophoridis, C., Papadopoulou, L. et al. Characterization, heavy metal content and health risk assessment of urban road dusts from the historic center of the city of Thessaloniki, Greece. Environ Geochem Health 39, 611–634 (2017). https://doi.org/10.1007/s10653-016-9836-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-016-9836-y