Abstract
Radionuclide contamination in terrestrial ecosystems has reached a dangerous level. The major artificial radionuclide present in the environment is 137Cs, which is released as a result of weapon production related to atomic projects, accidental explosions of nuclear power plants and other sources, such as reactors, evaporation ponds, liquid storage tanks, and burial grounds. The release of potentially hazardous radionuclides (radiocesium) in recent years has provided the opportunity to conduct multidisciplinary studies on their fate and transport. Radiocesium’s high fission yield and ease of detection made it a prime candidate for early radio-ecological investigations. The facility setting provides a diverse background for the improved understanding of various factors that contribute toward the fate and transfer of radionuclides in the terrestrial ecosystem. In this review, we summarize the significant environmental radiocesium transfer factors to determine the damaging effects of radiocesium on terrestrial ecosystem. It has been found that 137Cs can trace the transport of other radionuclides that have a high affinity for binding to soil particles (silts and clays). Possible remedial methods are also discussed for contaminated terrestrial systems. This review will serve as a guideline for future studies of the fate and transport of 137Cs in terrestrial environments in the wake of the Fukushima Nuclear Power Plant disaster in 2011.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
137Cesium (a medium half-life approximately 30 years) is one of the most important and largely distributed radionuclides in the terrestrial environment as a result of anthropogenic sources, such as nuclear bomb tests, nuclear power plant accidents, leaching from waste disposal of radionuclides, and nuclear weapons race. (Zaborska et al. 2014; Steinhauser et al. 2013; Vallés et al. 2009). In recent years, considerable interest has been applied to the fate and transport of radionuclides in the terrestrial ecosystem, especially after the forest ecosystems of the Russian Federation and Ukraine which were badly affected by the Chernobyl accident (1986) and Fukushima Daiichi Nuclear Power Plant (FNPP) disaster on March 11, 2011. These accidents led to the release of large amounts of radionuclides into terrestrial and aquatic ecosystems (Al-Masri 2006; Szefer 2002; Ashraf et al. 2013); within a month after these accidents, estimates of the released hazardous materials were ~100 × 2015 Bq of 134Cs and ~13–50 × 1,015 Bq of 137Cs (Hinton et al. 2013; Hou et al. 2003; Zalewska and Lipska 2006). The 137Cs distribution and accumulation have also occurred in the history of the Baltic Sea sediments. The Baltic Sea is susceptible to pollution by hazardous substances as a result of its limited water exchange, shallowness and large catchment area (Zaborska et al. 2014). These anthropogenic sources have rapidly increased the radionuclide pollution, so their dispersion must be monitored to determine the resulting contamination levels and mitigate the human health and environmental risks. Therefore, this review paper has been conducted to analyze the fate and transfer of radiocesium in terrestrial environments. 137Cs in the environment originates from a variety of sources. In early 1990, the Institute of Nuclear Energy Research (INER, Taiwan) accidentally discharged radionuclide wastewater into an irrigation ditch and contaminated the agricultural ecosystem. Part of the agricultural soil contaminated with radionuclide was removed by INER in 1994 and 1995 (Chiang et al. 2010). The mobility and bioavailability of the radionuclides are determined by the ratio of radionuclide chemical forms in the fallout and site-specific environmental characteristics, which determines (a) the rates of leaching, (b) fixation/remobilization and (c) sorption–desorption of mobile fraction (its solid–liquid distribution). The total distribution coefficients for radionuclides can vary in a wide range (four orders of magnitude for radiostrontium and five orders of magnitude for radiocesium) as a function of the fallout characteristics and environmental conditions (Ritchie and McHenry 1990). The compartment model POSEIDON-R for the northwestern Pacific and adjacent seas estimates the fate and transfer of radioactivity for the period 1945–2010 and performs a radiological assessment of the release of radiocesium as a result of the Fukushima Daiichi accident for the period 2011–2014. A generic predictive dynamic food chain model has been used instead of a biological concentration factor (BCF) (Maderich et al. 2013). The spent nuclear fuel re-processing waste may introduce small amounts of contamination into the environment (Simpson and Law 2013). Industrial instruments containing 137Cs can be misplaced (Diaz Leon et al. 2011), so people who directly handle these tools may be affected. When this radionuclide enters the environment, it causes serious pollution (Mihaela et al. 2012). The largest deposition of cesium is in the forest soil ecosystem as a result of the Chernobyl-born fallout in the Russian Federation and Ukraine, which were badly affected by the Chernobyl accident (1986). In the soil profile, the intensity of 137Cs transport depends largely on the type of ecosystem and soil properties along with the forest litter structure and depth (Shcheglov et al. 2013). The solid liquid distribution coefficients (K d) of radiocesium for various soil types is based on the texture and organic matter content, which is derived from the geometric mean (GM) and use of soil cofactors governing the soil–radionuclide interaction (Nakanishi et al. 2014). The geographical trends in 137Cs fallout from the Chernobyl accident and leaching from natural surface soils in Norway have also been evaluated (Gjelsvik and Steinnes 2013). As a result of its chemical properties, 137Cs is readily transported through the environment and food chains. The abundance cesium is partly caused by its properties such as half-life (~30 years), which emits relatively high-energy beta particles, and its rather short-lived daughter 137mBa, which emits strong gamma rays and makes it a good contributor toward the terrestrial ecosystem (Chino et al. 2011). The Fukushima Daiichi nuclear power plant accident in Japan was triggered by a large earthquake, and the resulting tsunami on March 11, 2011, was the major source of radiocesium (137Cs and 134Cs) and contamination of soils in the terrestrial ecosystem. The vertical distributions of radiocesium and its physicochemical properties in soils (to 20 cm depth) at 15 locations under different land-use types (croplands, grasslands, and forests) within a 2 km × 2 km mesh area in Fukushima City were estimated (Nakanishi et al. 2014). Two Italian stations in the northern Apennines, Mt. Cimone (Modena) and Montecuccolino (Bologna), were used to investigate the Fukushima radioactive plume resulting from the tsunami on March 11, 2011. The analysis confirmed the arrival of radionuclides following atmospheric transport and processing. The Fukushima radioactivity data at the two stations were usually comparable and suggested a good vertical mixing of the plume; occasional discrepancies were found and attributed to the different occurrences of wet removal that are typically characterized by a scattered spatial pattern (Tositti et al. 2012). The total distribution coefficient for the radionuclides had a dynamic characteristic and was dependent on the transformation rates of the chemical forms. The high retention of radiocesium in soils was caused by two main processes: the selective reversible sorption on illitic clay minerals and fixation. Advanced methods have been proposed for determining the capacity of selective sorption sites (frayed-edge sites—FES) and exchangeable radiocesium interception potential (RIPex). Quantitative data were obtained for a wide range of soils and bottom sediments with respect to their FES capacities and RIPex (Wampler et al. 2012).

The effect of the accompanying anions (NO −13 , SO −24 , CO −23 and I−) on the retention and translocation of cesium experiments performed in a greenhouse by using droplets of stable cesium solution to the upper surface of four soybean trifoliate leaves showed that once cesium was dissolved and absorbed, it penetrated the cuticle rapidly (Yan et al. 2013). The uptake and translocation of radionuclide in tropical crops and ecosystems were dependent on the conditions of the tropical soil types and factors influencing the radionuclide biogeochemistry (Tagami et al. 2013). The soil-to-plant transfer of radionuclides and their effects on humans and the environment were also dependent on the change in global warming (Dowdall et al. 2008). The rate and capacity of the transformation and dynamics of Cs and Sr sorption by selected subtropical and tropical soils were also established under highly weathering intensities in Taiwan (Chiang et al. 2010). The effect of soil characteristics and fungal and plant transporters on radiocesium uptake and accumulation in plants was focused on radiocesium immobilization and plant accumulation in the mycorrhizal fungi. The fungi develop hyphae in the soil, which provides a transfer source of nutrients from the soil to plants (Dupré de Boulois et al. 2008). The movement of soil organic carbon (SOC) down the soil profile compared with the environmental tracer 137Cs in two neighboring field sites comprised of clay soils with different cracking characteristics (cracking black Vertisol and a red Luvisol) was observed (Wells and Hancock 2014). The sensitivity of rural and semi-natural environments toward radionuclide contamination by 137Cs, 90Sr and 131I after major nuclear accidents was also taken into account and indicated that the environmental sensitivity was highly radionuclide specific and time dependent, with 137Cs producing the highest doses for adults in terrestrial ecosystems and fresh water pathways than in coastal marine environments, where 131I was more noticeable during the first year. The sensitivity was dependent on the social and economic factors, such as the individual living habits, food consumption preferences and agricultural practices (Tracy et al. 2013). Water drawn from streams for irrigation resulted in the transfer of contaminated material from the stream to the land. However, runoff can also carry small soil particles containing 137Cs from the land to waterways (Yasunari et al. 2011). Compared with agricultural land, forests act as complex ecosystems because they involve miscellaneous plant species associations, several vegetative strata (overstory, shrubs, herbaceous and other annual plant layers) and multi-layered soil profiles (forest floor, hemi-organic and mineral layers) (Rochette et al. 2000). Large areas of forest in Sweden were analyzed for the deposition of radiocesium (137Cs) after the Chernobyl accident in 1986, and they showed large-scale accumulation. Twenty woody plants (12 evergreen and 8 deciduous species) grown in Abiko indicated radiocesium deposition after the Fukushima Power Plant accident, and they showed an average radiocesium activity 7.7 times higher than the activity in the leaves of deciduous species (Vinichuk et al. 2010; Okumura et al. 2014). The other factors that show a potential to release radiological contaminants to the environment include radionuclide activity concentrations in litter (i.e., vegetative debris) and duff (i.e., highly decomposed vegetative debris). A study was conducted by the United States’ Department of Energy at their Savannah River Site (SRS) in Aiken, South Carolina. The lemon tree (Citrus limon B) indicated the uptake and transfer from the soil of 137Cs to fruits in tropical plants during fruit growth. The maximum values of the transfer factors were reached in the initial phase of fruit growth and decreased as the fruit developed (Velasco et al. 2012). The Japanese population has placed emphasis on the estimation of radiation doses for radionuclide deposition from the inhalation of contaminated air and terrestrial and marine food contamination compared with other sources of ionizing radiation, such as anthropogenic sources (global fallout, Chernobyl accident, etc.), natural sources (radionuclides in food, cosmic radiation, etc.) and medical applications (X-ray tests, CT-tests, etc.) (Koizumi et al. 2012). Peat deposits in SW Spitsbergen are sources of man-made radionuclide activity. The maximum activity evident in the peats corresponds to the 1963/1964 global maximum fallout from the atmospheric testing of nuclear weapons; some of the activity profiles were altered after deposition by water infiltration (Lozano et al. 2011). The measurement of 137Cs in the Bratisalava stratospheric air showed a significant impact from 137Cs activity concentrations until the early 1980s, which was recognized as a typical spring or early summer maxim and winter minima (Povinec et al. 2012). As a result of similar chemical behaviors, potassium and phosphorous influence radiocesium transported by arbuscular mycorrhizal fungi (AMF). The arbuscular mycorrhizal plant (AMP) in in vitro culture systems associated with Medicagotruncatula plantlets with Glomusintraradices indicated the increasing concentrations of potassium and phosphorous on the transport of cesium (Gyuricza et al. 2010). The activity concentrations of radionuclides in the edible wild berries and mushrooms collected from Øvre Dividalen National Park in Northern Norway showed 210Po, 210Pb, 40K and 137Cs and indicated an effective ingestion dose to man based on high consumption rates. The activity concentrations of 137Cs in edible wild berries and mushrooms reflected lower levels of fallout of this radionuclide in Northern Norway compared with more central areas following the Chernobyl accident (Ben-Asher 2011). The seasonal variations in foliar 137Cs levels were examined in the Norway spruce (Piceaabies (L.) Karst.), and Scots pine (Pinussylvestris L.) in western Finland. The levels of total 137Cs in the three youngest needle age classes were compared with the levels of potassium and carbon. For the needle content and activity concentration of 137Cs in the time series phases of intensive growth, needle elongation and dormancy were apparent (Paasikallio et al. 1994). The Chernobyl accident in 1986 and recent Fukushima Dai-ichi disaster have produced many new studies in radioecology, primarily in Japan, the USA, the former Soviet Union and other European countries. Earlier work on 137Cs in the environment was motivated by scientific curiosity and concerns over the health and environmental impacts of global fallout from nuclear testing; subsequently, the studies were concerned with the safety of nuclear reactors in producing electricity. As a result of the rapid distribution of cesium in the environment and global climatic changes, the impacts of radiocesium are more hazardous. Most of the previous work on cesium distributions show its importance in the environment. This work adds to the significant body of literature from earlier studies.
This review compiles and updates our knowledge of the transfer parameters in terrestrial ecosystems. Furthermore, from the radiocesium study in this review, it was found that the fate of other radionuclides in terrestrial ecosystems should be better documented. Because the information in this review indicates the fate and transfer of radiocesium, it should be shared so that radiocesium’s impact on terrestrial ecosystem can be minimized; this paper can also provide answers to questions that might arise after its distribution.
Chemistry of radiocesium
Cesium (atomic number 55) is ubiquitous and ranks forty-fifth in natural abundance; it constitutes approximately 3 parts per million in the earth’s crust. Cesium is found at relatively low concentrations. Granite contains cesium at approximately 1 ppm, and sedimentary rocks contain approximately 4 ppm. It is a white, soft, silvery gold metal with an atomic weight of 132.905; specific gravity of 1.88, melting point of 28.4 °C (83.1 °F), and boiling point of 641 °C (1,186 °F) (Lide 2004). Two German chemists, Robert Bunsen and Gustav Kirchhoff, discovered cesium in 1860. Since the 1990s, the largest applications for cesium have been as a format for drilling fluids. Although the metal is mildly toxic and hazardous as a metal, its radioactive isotopes present a high risk to the environment. Cesium exists in a +1 oxidation state. In the environment, it exists in salt forms (Hagan 1977), and its phosphates, acetate carbonates, halides, oxides, nitrates and sulfate salts are water soluble; however, double salts are often less soluble. Cesium hydroxide (CsOH) is hygroscopic and a very strong base that rapidly etches the surface of semiconductors such as silicon. Cesium has a total of 39 known isotopes in the range of their mass number from 112 to 151. The only stable isotope is 133Cs, which has a large spin (7/2+) and resonating frequency of 11.7 MHz. 135Cs has very long half-life of approximately 2.3 million years. The first beta decay mode that forms 137mBa accounts for approximately 95 % of the total intensity, and the second mode accounts for approximately 5 %. Radioactive 134Cs primarily decays to stable 134Ba by beta decay accompanied by gamma ray emission, and it less frequently decays to stable 134Xe by electron capture (EC) accompanied by a single gamma ray (Aoyama and Hirose 2008). Cesium-137, or radiocesium, is a radioactive isotope of cesium that is a common fission product by nuclear fission of uranium-235 and other fissionable isotopes in nuclear reactors nuclear weapons. It has a half-life of 30.17 years and is among the most problematic fission products, and it decays by β-emission (Fig. 1). One gram of cesium has an activity of 3.1325 terabecquerel (TBq) (Kitajima et al. 2014). The most common commercial source of cesium is pollucite, which contains between 5 and 32 % Cs2O. Cesium-137 with strontium-90 generate the largest sources of radioactivity in the area surrounding the Chernobyl disaster. Radioactive cesium comes from the original site of fission. With the commencement of nuclear weapons testing in 1945, cesium has been released into the atmosphere and returned to the earth’s surface as a component of radioactive fallout (Yablokov et al. 2010; Hohenemser and Renn 1988; Jargin 2010).
Current distribution of 137Cs in the environment
Cs-137 does not occur naturally on earth; it is exclusively anthropogenic in origin, such as through nuclear fission. The release of 137Cs associated with particles of different sizes and mineralogical composition to the aquatic ecosystems can considerably affect their transport and bioavailability (Dowdall et al. 2008; Dupré de Boulois et al. 2008). Cesium-137 contaminates bodies of water not only directly from deposition from the air and discharge as effluent, but also indirectly by washout from the catchment basin (Table 1). 137Cs contamination in large bodies of water is quickly redistributed and tends to accumulate in the bottom sediments, benthos, aquatic plants and fish (Wells and Hancock 2014). The main pathways of potential human exposure may be directly through contamination of drinking water or indirectly from the use of water for irrigation and consumption of contaminated fish (Tracy et al. 2013). Because contaminating radionuclides tend to disappear rapidly from water, human exposure is only likely in the initial fallout phase and late phase when the contamination washed out from the catchment area reaches drinking water supplies (Yasunari et al. 2011).
137Cs is produced primarily as a fission product in nuclear reactors and nuclear weapon detonations. There are many locations where 137Cs has been released to the environment (Rochette et al. 2000). These locations include nuclear weapons test areas, commercial or government fuel reprocessing sites, waste burial grounds, reactors, ocean disposal areas, and miscellaneous locations of release that result from theft or accident. Table 1 provides a summary of the major contributors to 137Cs in the global environment.
Radioactive contamination by 137Cs to the aquatic system has been caused by three main factors: global fallout, discharge from reprocessing plants and fallout after the Chernobyl accident in April 1986 and Fukushima nuclear incident in 2011. Currently, the average activity concentration of 137Cs in the surface water of the Pacific region has been estimated at approximately 60 Bq/m3, whereas the worldwide average concentration as a result of global fallout is approximately 2 Bq/m3 (Vinichuk et al. 2010). The infamous Chernobyl nuclear power plant accident in the Ukraine on April 26, 1986, was the second most significant large-scale fallout source of environmental radioactive contamination to the Arctic and has led to the addition of another significantly large-scale fallout source of environmental radioactive contamination. The accident resulted from uncontrolled fission in the reactor followed by a powerful explosion and fire. The released radioactive materials were carried away by air currents in the form of gases and dust particles (Okumura et al. 2014; Velasco et al. 2012; Koizumi et al. 2012). As a result, a total of 85 PBq of 137Cs was added to the atmosphere (Lozano et al. 2011). Furthermore, the tsunami in March 2011 caused the release of radionuclides from three of the boiling water reactor (BWR) units at the Fukushima Dai-ichi power station in Japan; this disaster is also considered to be a major contributor of artificial radionuclides. The main source of contamination was the direct discharge of contaminated water from the plant, which lasted until approximately April 8; to a lesser extent, oceanic contamination resulted from radionuclides discharged into the atmosphere between 12 and 22 March (Povinec et al. 2012). In the immediate vicinity of the plant, the concentrations in the seawater at the end of March and early April were up to several tens of thousands of Becquerels per liter (Bq/L) for 134 Cs and 137Cs, and the levels even exceeded 100,000 Bq/L for 131I, which is shown in Fig. 1 (Gyuricza et al. 2010). The radioactive release at sea was the largest one-time contribution of artificial radionuclides to the marine environment ever observed (Ben-Asher 2011). However, the location of the site of Fukushima made the dispersion of radionuclides exceptional because it is affected by the Kuroshio Current, one of the most important currents in the world, which distributed the contaminated water throughout the Pacific Ocean. Thus, the measurements obtained in the seawater and coastal sediments suggested that the consequences of the accident, in terms of radiation protection, would weaken pelagic species from the fall of 2011 (low concentrations in the seawater and limited storage in sediments) (Fig. 2).
Behavior of 137Cs in terrestrial ecosystems
The environmental transport of cesium is governed by many factors, most of which vary over space and time. The accumulation of cesium varies by orders of magnitude between different biological components within a single environment and also among different ecosystems. Much of this behavior can be understood from the chemical properties of cesium and its interactions in the soil.
Soil is particularly important because it is the primary reservoir of 137Cs in most ecosystems. The fraction of 137Cs in soil that is available for biological uptake and transport is determined by the strength of its binding to soil particles. This binding strength is mainly dependent on the clay mineral composition and abundance in the soil. Other chemical factors that modify its transport include the soil cation exchange capacity (CEC) and pH and potassium concentration of the soil water. For example, acidic conditions tend to enhance the biological availability of 137Cs in soil, whereas high concentrations of potassium in the soil water tend to depress cesium uptake by plants and subsequent transfers to higher trophic levels. Cesium exists in the environment in the +1 oxidation state, and there is little tendency for cesium to form aqueous complexes in the soil/water environment. Thus, the formation of inorganic complexes does not have a major influence on the chemical speciation of cesium, and the dominant aqueous species in most soil and groundwater systems is the uncomplexed Cs + ion. Unlike many other radionuclides, the sorption of cesium to sediments is highly dependent on the mineralogy of the sediment. Unweathered phyllosilicates such as micas can be transformed into illites, vermiculites or smectites depending on the extent to which they have been physically, chemically and biologically weathered. The extent of weathering increases in the order of mica < illite < vermiculite < smectite < kaolinite. Weathering has a profound effect on a number of physical and chemical properties that in turn have a direct effect on the mineral’s tendency to sorb cesium.
The transport of 137Cs through the environment involves a number of biogeochemical pathways. These include mainly physical processes that move contaminated particles and that are not specifically affected by the chemical nature of the contaminant of interest. For example, deposition from the atmosphere onto the soil or plant surfaces, soil erosion by wind or water, physical percolation of particles into the soil profile, weathering of particulate-bound material from plant surfaces, senescence of plant parts, and inhalation or ingestion rates of animals are generally not contaminant specific. However, there are processes that depend greatly on the specific contaminant and its physical and/or chemical form. Such contaminant-specific processes include foliar absorption, plant uptake from the soil, translocation within the plant, and assimilation, distribution and retention in animals. In the case of cesium, these contaminant-specific processes are affected by the concentrations of specific elements such as potassium, soil pH, and other site-specific conditions. With respect to cesium-specific processes, it has been observed that if cesium is in a soluble state, plant leaves will absorb a significant fraction that is on the order of 40–80 % of the surface deposit depending on the species of plant. Absorption can approach 100 % in lichens and mosses. Because of the greater absorption of cesium by lichens and the much longer retention after deposition, these organisms reach steady-state concentrations of 137Cs that can be over an order of magnitude higher than in adjacent herbaceous plant species.
The migration of initially soluble cesium in soil down through the soil profile by aqueous phase transport (leaching) is normally small because of cesium’s strong binding to clay minerals in the soil; however, the physical transport of material bound to small soil particles does occur. The transfer of 137Cs from the soil to plants occurs by different mechanisms, including uptake through the roots from the soil solution, resuspension from the soil surface, and rain splashes of contaminated soil particles. Uptake can be strongly affected by plant species and soil properties and influenced by fungi and other microbes.
The transfer of 137Cs from ingested plants and soil to animal tissues is also complex and depends on a host of conditions. One method of expressing the effectiveness of transfers from food to animal tissues is by the simple Cr. For example, the simple Cr in animal tissue/food ranges from less than one in herbivores to 20 in predators and reflects a general trophic-level increase in 137Cs. For animal products such as meat, milk and eggs, a preferred parameter is the feed transfer coefficient (C ft), which is the fraction of radiocesium that is ingested daily and transferred to 1 L of milk (d/L) or 1 kg of meat or eggs (d/kg) under steady-state conditions. Experimentally determined values have ranged from 0.004 to 0.012 d/L for milk, 0.003 to 0.06 d/kg for beef, and 0.3 to 3 d/kg for eggs. Animals consume soil, both inadvertently and purposefully. The absorption of 137Cs from ingested soil can be considerably less than absorption from ingested biological material depending on the degree of cesium binding to clay minerals. Unlike the vast majority of other radionuclides, the passage of radiocesium up through the animal food chain often increases from one trophic level to the next higher trophic level. For example, predatory animals tend to concentrate 137Cs in their soft tissues to a higher degree than do the animals upon which they feed. This trophic-level increase in cesium concentrations is frequently in the range of two- to fourfold higher for each step in the animal food chain. The physiological basis of this trophic-level effect is the chemical similarity of cesium and its nutrient analogue potassium and the roughly threefold increase in the excretion rate of potassium. Although potassium is homeostatically maintained within a certain range of concentrations in an animal’s soft tissues, the molar concentrations of 137Cs are far too low to be limited by physiological mechanisms. The assimilation fractions across the gut wall for cesium and potassium are generally similar. Because of these comparative behaviors, the ratios of intake to loss for body compartments (muscle tissue being the dominant compartment) are typically two to four times higher for cesium than for potassium.
The assimilation fractions (f a) from the gut to the blood or body fluids in animals vary with the physical/chemical form of cesium, species, potassium ingestion rate and other factors; however, the range of variation is relatively small. Most of the f a values fall in the range of ~0.6 to 0.9, but some can fall well outside of this range. Radiocesium bound 2:1 to clay minerals results in assimilation fractions that can be much smaller than the normal range. Considerable variation within a given species can occur depending on body mass and other factors. The body mass effect reflects the metabolic rates per unit mass that typically increase as body mass decreases. High metabolic rates are characterized by increased food intake per unit body mass and high nutrient turnovers in muscle and other soft tissues, and they are accompanied by rapid excretion rates of various elements, including cesium.
3-Distribution of 137Cs in terrestrial ecosystems
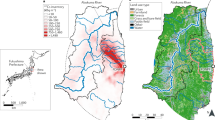
The Fukushima Daiichi Nuclear Power Plant (NPP) accident in Japan was triggered by an earthquake and caused by the resulting tsunami on March 11, 2011; it is considered to be one of the major contributors of cesium in the environment in terrestrial and aquatic ecosystems (Masamichi Chino et al. 2011). The atmospheric fallout from the Fukushima (NPP) accident released 137Cs, which is a source of great concern because of its potential impact to humans and ecosystems in the coming decades (Koarashi et al. 2012).
As an introduction to the transfer of radionuclides in the environment, a broad overview of some of the key processes influencing the behavior and fate of radionuclides is provided below and shown schematically in Fig. 3.
Processes affecting radionuclide behavior in ecosystems (Wicker and Schultz 1982)
Physical and chemical parameters
Once released into the air or water, radionuclides are influenced by physicochemical processes that lead to their dispersion in the environment. The physical and chemical form of the radionuclide and turbulence of the receiving medium play an important role in relation to the initial transport mechanisms.
Radionuclide interactions with solids
Radionuclides interact with all solid materials by numerous mechanisms, including electrostatic attraction and the formation of chemical bonds. In many cases, size alone can dictate the radionuclide activity per unit mass of solid because the surface area available for adsorption, per unit mass or volume, is greater for smaller objects.
Radionuclide interactions by vegetation
In the terrestrial environment, radionuclide interactions by vegetation occur by wet and dry deposition, and radionuclides may also be deposited on the ground directly (Pröhl 2009). Radioactive concentrations on vegetation may be reduced by a number of physical processes, including wash-off by rain or irrigation, surface abrasion, leaf bending from wind action, resuspension, tissue senescence, leaf fall, herbivore grazing, plant growth and evaporation. Various empirical formulas have been derived to model the retention of radionuclides on vegetation (Steinhauser et al. 2013).
Resuspension of soil
The resuspension of contaminated sediment or soil is an important process in both aquatic and terrestrial systems (Strand et al. 1999). Physical, chemical, and biological processes occurring in the soil and sediment can lead to the further redistribution of radionuclides within these environmental compartments. The radionuclides in soil can migrate to deeper soil depths by leaching, for example. Leaching rates are greatest under conditions of high rainfall and for soils containing a relatively high proportion of sand particles (Nimis 1996), and rainfall intensity also influences leaching rates.
What is an ecosystem?
An ecosystem is defined as an area of nature that includes living organisms and non-living substances interacting to produce an exchange of materials between living and non-living parts.
Components of an ecosystem
From a structural perspective, terrestrial ecosystems have two components: abiotic and biotic.
-
1.
Abiotic components include basic elements and compounds of the environment such as water, soil gases, such as oxygen and carbon dioxide, and minerals, such as carbonates and phosphates and variety of organic compounds.
-
2.
Biotic components include living components of an ecosystem in relation to its biodiversity, and they are divided into two main parts: producers, which are the major sustainers of life (McMichael 2013), and consumers.
Types of ecosystems
Ecosystems can be broadly divided into the following types (Mucina et al. 2013).
-
1.
Natural ecosystems are undisturbed ecosystems divided into aquatic and terrestrial ecosystems. (i) Aquatic ecosystems in open waters include marine ecosystems that consist of deep water bodies such as oceans, estuaries, and fresh water ecosystems that consist of lotic running fresh water such as streams or rivers, and lentic running water such as ponds and lakes. (ii) Terrestrial ecosystems pertain to forests, grassland, deserts, etc. These two ecosystems are considered as two extreme types of ecosystem. In five regions of the world, Mediterranean-type ecosystems (mtes) with mild wet winters and warm dry summers occur, and they are generally centered at latitudes of 30°–35° on the western margins of continental landmasses. Endangered ecosystems are found throughout the world, and although a numerous attempts have been made to classify the conservation status of ecosystems, a universal system does not yet exist (Peterson et al. 2014). Recent systematic assessments of the world’s major ecosystems have provided strong evidence of the nature and extent of threats to global biodiversity as a result of human activity (Gherardi and Padilla 2013). In recent decades, new threats to ecosystems such as climate change have emerged even as many of the past threats have continued or even escalated in magnitude (Brito et al. 2014; Ellis 2013).
Bioaccumulation and food chain transfer
What is bioaccumulation?
Bioaccumulation is the process through which chemicals or radionuclides accumulate and are stored more easily in living organisms than in the environment (Ashraf et al. 2013). The concentration of radionuclides increases more rapidly in living organisms as a result of storage and absorption rather than excretion and metabolism; therefore, it is important to understand the dynamic processes to protect human beings and living organisms from radionuclide exposure (Tateda et al. 2013).
Radionuclides can enter the lowest trophic level by numerous processes, such as adsorption, ingestion and absorption, and are often stored in detritus.
Adsorption
In terrestrial ecosystems, the direct adsorption of radionuclides to plant surfaces occurs by foliar uptake (Yan et al. 2013) and direct uptake via stomata and roots from the soil pore water. The transfer of radionuclides from soil to plant strongly depends on the physical and chemical characteristics of the soil, and there are phylogenetic influences on the transfer of radionuclides from soil to plants (Willey 2010). In terrestrial systems, fungi are known to play a key role in the mobilization, uptake, and translocation of nutrients, and they are likely to contribute substantially to the long-term retention of certain radionuclides in the organic horizons of forest soil (Steiner et al. 2002).
Ingestion
For radionuclides that are not readily taken up by plants, soil adhesion can represent the most important route of intake (Balonov et al. 2010). The transfer of radionuclides from terrestrial plants (and soil) to herbivores occurs by ingestion, and predation leads to the transfer of radionuclides to higher trophic levels. Radionuclides that are organically bound or present in ionic form within the plant itself may be assimilated by the herbivore to a greater degree than radionuclides that are adsorbed to soil matrices (Beresford et al. 2004).
Absorption
The absorption of radionuclides to all higher animals from the gastrointestinal tract depends on the physicochemical form of the radionuclide, composition of the source medium, and nutritional status of the animal; radionuclides are accumulated in particular organs or body structures, and for certain radionuclides, absorption is complete, whereas it is minimal for others.
Detritus
Detritus can serve as an important reservoir for radionuclides through which radionuclides can be recycled back into food chains. In terrestrial and aquatic ecosystems, dead plants and animals and their secretions and excretions contribute many radionuclides to detritus. With the passage of time, the release of solubilized radionuclides occurs through the decomposition of organic material by the action of detritivores and microbes. Deeper soil and sediment layers may act as permanent sinks for contaminants.
How does 137Cs bioaccumulate?
The way that the radionuclide 137Cs accumulates depends on its chemical behavior because it is a fission product with a radiological half-life of approximately 30 years. If radiocesium is released into the environment, it can concentrate at various steps in the food chain. The bioaccumulation of perfluorinated compounds from a food web in Taihu Lake in China was investigated (Yang et al. 2011). 137Cs has similar properties to potassium; because potassium is present in the blood, 137Cs can be mistaken by the body as potassium and absorbed as such. Therefore, cesium can be found in all parts of a body.
How does 137Cs behave in the food chain?
The transport of 137Cs in the environment is complex and depends on many conditions, including the climate, precipitation, plant and animal species and type of agriculture. Food web bioaccumulation models are scientific instruments that describe the relationship between chemical concentrations in abiotic media (such as water, sediments, soils, and air) and biological organisms in the food webs (De Hoop et al. 2013). For example, the same concentration of cesium-137 or strontium-90 in soil at two locations can lead to widely different concentrations in food grown in the soil depending on the environmental conditions. Scientists have described the behavior in computer models of different complexity. The root uptake of radionuclides during the growth of many food crops through acute soil deposition has been estimated, and the soil under the plants was contaminated to differentiate the foliar uptake from the root uptake subsequent to a radionuclide deposition during the vegetation period. The aggregated transfer factors specified for different time periods was used to quantify the soil-to-plant transfer of radionuclides from deposition until harvest (Niedrée 2013).
The behavior of monovalent inorganic cations in tropical plants in addition to the plant’s ability to store elements such as 137Cs, K and Na, especially in the fruit of lemon (Citrus limon B.) and coconut (Cocosnucifera L.) trees, was also estimated (Cid et al. 2013). The transport of cesium-137 in the environment is complex and depends on many conditions, including the climate, precipitation, plant and animal species and type of agriculture. For example, the same concentration of cesium-137 or strontium-90 in soil at two locations can lead to widely different concentrations in food grown in the soil depending on the environmental conditions. Scientists have described the behavior in computer models of different complexity, and an example is shown in Fig. 3.
There are numerous parameters that can be entered into such a computer model to describe the behavior of radionuclides in the environment and human body (Table 2), and they include the following:
-
1.
Transfer from the roots to plant tissues
-
2.
Ingestion rate of soil and plants by animals
-
3.
Loss during food preparation
-
4.
Uptake from the gut into the bloodstream
-
5.
Accumulation in the body organs
-
6.
Excretion from the body organs
-
7.
Dry deposition rate from the air to soil
-
8.
Wet deposition rate from the air to soil
-
9.
Resuspension from the soil into air
-
10.
Fraction that is initially deposited on the plant surfaces
-
11.
Removal rate from the plant surfaces
-
12.
Leach rate from the surface soil into the deeper soil layers
Radiation exposure of biota
The major pathways lead to radiation exposure of plants and animals in aquatic and terrestrial ecosystems multiple methods (Fig. 4), and they include the following:
-
Inhalation of (re)suspended contaminated particles or gaseous radionuclides (from air) is significant for terrestrial animals and aquatic birds, reptiles, amphibians, and mammals. The gaseous exchange of volatile and respired forms of radionuclides at the stomata of plants also contributes to exposure.
-
Contamination of the fur, feathers, skin, and vegetation surfaces contributes to the external exposure component (i.e., beta- and gamma-emitting radionuclides on or near the epidermis that cause irradiation of the underlying living cells) and internal exposure component (i.e., contaminants that are ingested and incorporated into the body of the animal).
-
Ingestion of plants and animals leads to direct irradiation of the digestive tract and internal exposure to the radionuclide within the animal’s body. For some faunal types, ingestion of detritus and sediment is included.
-
Direct uptake from the water column may lead to the direct irradiation of the gills or respiratory system and internal exposure if the radionuclide becomes assimilated and distributed within the animal’s body.
-
Ingestion from water leads to radiation exposure to animals. For plants, the corresponding pathway relates to the root uptake of water (Table 3).
Table 3 Cancer risk as a result of ingestion of cesium-137 -
Habitat exposure occurs mostly from gamma irradiation and to lesser extent by beta irradiation that originates from radionuclides present in the organism’s habitat. The configuration of the source relative to the target clearly depends on the organism’s ecological characteristics and habitat. A benthic-dwelling adult fish will be exposed to radiation from radionuclides present in the water column and deposited sediments, whereas a pelagic fish may only be exposed to the former, although its eggs may be laid on or in the sediment.
Approaches used to transfer radionuclides in the environment
A number of approaches have been proposed to estimate the transfer of radionuclides to biota when measurements of the activity concentrations are not available (Beresford et al. 2013; Wood et al. 2013; Beresford and Vives i Batlle 2013). The exposure pathways have been explained in details in Fig. 5. These approaches range from tabulated transfer parameters to integrated approaches that employ spreadsheets that incorporate transfer data and more highly parameterized food chain models (Brown et al. 2008).
Concentration ratios
There are many methods of expressing concentration ratios.
For terrestrial biota, CR = A biota/A soil, where A biota is the activity concentration of radionuclide r in the whole-body biota b (Bq/kg fresh weight) and A soil is the activity concentration of radionuclide r in the soil (Bq/kg dry weight).
The initial relative distribution of released 137Cs between terrestrial and aquatic ecosystems depends primarily on the source of the radioisotope. Additionally, cesium has a relatively low
Bq/L of approximately 3 × 103 mL/g for sediments/waters (Kinoshita et al. 2011).
Which pathways are typically important?
The environmental exposure pathway that is most relevant for humans or animals depends on the amount and type of food that is ingested. Much of the 137Cs in the environment surrounding LANL is from fallout of atmospheric nuclear weapons tests, but there is an additional contribution from the historical and current releases from LANL. Only a limited number of environmental media are sampled at LANL, and the predominant exposure pathway for the communities in northern New Mexico is likely the consumption of produce because the typical individual consumes more produce than meat or fish. The following table provides information regarding the 137Cs concentrations that were found in the regional environment surrounding LANL. The values correspond to the regional statistical reference levels (mean plus two standard deviations) based on data from 1994 to 1998.
What dangers are presented by 137Cs?
The danger from ingesting 137Cs depends on a person’s age and metabolism. A risk level of one in one million (1 in 1,000,000) is usually considered negligible, whereas a risk of greater than one in ten thousand (1 in 10,000) is typically deemed to be unacceptable. The EPA uses a simplified model to provide risk estimates for a typical adult who breathes contaminated air or ingests radionuclides with food or water (Eckerman et al. 1988). The values for ingestion of food or water are shown in the table below.
Effective measures to reduce the risk of 137Cs in terrestrial ecosystems
137Cs is radionuclide that generally drives human and ecological risks in the ecosystem; therefore, it is the radioactive contaminant targeted during remediation operations. Aquatic ecosystems such as lakes, rivers, groundwater and seas have characteristic and site-specific behavior governed by hydrological and morphological parameters of their reservoirs and its drainage area. Remediation of such reservoirs following the contamination of 137Cs is, therefore, largely dependent on site-specific parameters. Additionally, remediation plans for contaminated waters may be expensive and include significant engineering costs. The effects of such remediation efforts must be based on a cost–benefit basis, selected according to the well-known ALARA principles, and compared with the risks from other toxic substances present in the water (Brenner et al. 2003). The remediation of contaminated ecosystems involves physical, chemical and biological procedures that are described below.
A number of remediation techniques are available to remove or immobilize hazardous substances in environmental systems. Radiological risks associated with radiocesium in soils and sediments are not likely to be eliminated by current remediation technologies; however, the levels or mobility of radiocesium can sometimes be reduced by application of an appropriate technique. Because of the strong and often irreversible binding of the majority of cesium in soils and sediments, the complete elimination of the radionuclide is likely to require the physical removal of the soils or sediments and transport of the material to another site. Because this process is extremely expensive and may entail significant ecological damage, the so-called no-action alternative is usually considered (Zheng et al. 2012). It is generally believed by experienced scientists that the majority of cesium-contaminated areas may not warrant remedial action based on the actual human health or ecological risk posed by the contamination. However, it can be a significant challenge to convince the public, regulators and decision makers that action is not warranted.
Remediation techniques
Many techniques are effective for organic compounds and certain metals, but they are not necessarily effective for radiocesium. Such techniques are organized into the general categories of biological, chemical and physical remediation. In utilizing agricultural countermeasures for the remediation of any hazardous substance, the most desirable of conditions would be one in which plant uptake is enhanced and contaminant mobility is minimized (Endo et al. 2012). The physical means of decontamination generally result in negative impacts to the soil matrix and may be less desirable than agricultural processes. Compaction by large earth-moving vehicles has significant effects on the physical, chemical and microbial properties of soil, which may ultimately affect plant growth. Similarly, certain chemical treatments may remove specific contaminants while potentially removing soil nutrients as well (Fukushima Prefecture 2012). Alternatively, the selective addition of certain organic material, chelating agents, or fertilizers can enhance the uptake by vegetation while decreasing contaminant mobility in the soil.
Biological remediation
Biological remediation addresses the utilization of microorganisms and higher plants to alter the distribution or mobility of radiocesium in soils or sediments. Microorganisms can alter the mobility of radiocesium in some cases, but cesium cannot be directly removed or degraded by these organisms as certain other organic compounds can (Hirose et al. 2008). Phytoremediation includes both phytoextraction (the concentration of contaminants into harvestable portions of plant biomass) and phytostabilization (the use of plants to minimize the off-site losses of contaminants through erosion and leaching losses). The potential for successful application of phytoextraction and/or phytostabilization techniques to contaminated soils depends strongly on the site-specific conditions. Factors such as cleanup goals, level of radionuclide contamination, depth of contamination, soil properties, the presence of other toxic materials, disposal of harvested biomass and climatic conditions all influence the likelihood of success (International Atomic Energy Agency 2001a).
Microbiological effects
Biological techniques of soil remediation often involve the use of microorganisms to facilitate the breakdown of toxic materials to something less hazardous. The targets of biological remediation are generally organic compounds (International Atomic Energy Agency 2011). Such approaches would not be effective for reducing radioactivity except possibly to alter their availability or mobility. Have shown that some metals become more mobile under the influence of active microorganisms depending on the properties of the soil and metal (Ministry of Land Infrastructure Transport and Tourism 2011). They also found that cysteine is an effective agent for enhancing the release of some metals from soil. A soil bacterium (Pseudomonas putida) has been used to determine the adsorption of cesium and other elements and its influence on the mobility of metals in soils (Bossew et al. 2007). The investigators examined adsorption as a function of pH and ionic strength for low metal concentrations. Cesium was shown to exhibit adsorption by the bacteria (a K d of ~102 to 103); however, adsorption was much higher for other metals (e.g., the K d for mercury adsorption was on the order of 106) (International Atomic Energy Agency 2000).
Phytoremediation
Phytoremediation has been proposed as a promising eco-friendly, cost effective, in situ alternative treatment technology that relies on the capacity of plants and their associated microorganisms to stabilize or extract contaminants from aquatic ecosystems (International Atomic Energy Agency 2001b). The potential of C. roseus plants has also been studied for the uptake of 137Cs at three different activity concentrations and has been found to remediate 73, 59.3 and 51.3 % of 137Cs within 15 days of the experiment (Valentin 2003). The research findings showed that 137Cs and 90Sr are bioaccumulated by C. roseus, which could be an ideal hyperaccumulator with the potential to remove radionuclides. After remediation, this weed can be harvested, burned to ash and disposed of as waste in a safe environment. The aquatic plant Lemna gibba was utilized to biosorb 137Cs from simulated aqueous radioactive waste (Brechignac et al. 2003). In batch-wise laboratory-scale experiments, the study discusses the parameters (e.g., contact time, pH value and the initial activity content of the simulated waste, light effect, biomass used) that may affect the efficiency of Lemna gibba in bioremoving and bioaccumulating two radionuclides (Jasiulionis et al. 2006). The uptake values, biosorption efficiency percentages, rate constant and isotherm factors were evaluated for the process. The uptakes values for Co-60 and Cs-137 recorded values of 1,213 and 872 Bq/g, respectively, from the simulated waste solution containing 6,100 Bq at pH = 6.9 after 24 h of contact time (Jasiulionis and Rozkov 2007). The results exhibited the potential of the widely distributed aquatic plant Lemna gibba to be used as a biological sorber for 137Cs because it successfully and efficiently biosorbed low and intermediate level aqueous radioactive waste streams.
Wetlands
Aquatic plants can also be used for wetland development. The most important role of plants in wetlands is to increase the residence time of water and thereby increase the sedimentation of particles and associated pollutants (Medici 2001). Thus, they are indirectly involved in water cleaning. Plants also add oxygen to the roots and generate favorable conditions for microbes and bioremediation. For the efficient removal of pollutants, a high biomass per volume of water for the submerged plants is required (Japanese Ministry of Education Culture Sports Science and Technology 2011f). The uptake of metals in emergent plants only accounts for 5 % or less of the total removal capacity in wetlands.
Biological dilution
To reduce the amount of bioavailable 137Cs in lakes, biological dilution can be used whereby the 137Cs already taken up in the nutrient web can be removed by reducing the fish stock (intensive fishing) in the lake and influencing the predation pressure so to alter the nutrient web to contain relatively more plankton, which could result in lower concentrations of 137Cs in the biota (Morino et al. 2011). Biological dilution can also decrease the concentration of 137Cs in each individual fish through the treatment of the lakes with different types of fertilizer (discharges from aqua culture, commercial fertilizer and P-enriched lime) (Butler 2011). The intention is to increase the lake biomass and thereby disperse the given amount of 137Cs (Lee et al. 2008). The biological dilution method is based on theories involving biological buffering.
Phytoextraction and phytostabilization
The partial removal of radiocesium from soils can be accomplished by relying on the natural uptake of metals by plants, which is termed phytoextraction. The radioactive vegetation is then collected and disposed of separately as a hazard, which reduces the biologically available contaminant concentrations in the soil (Real et al. 2002). This technique has been applied to radiocesium remediation of contaminated soil (Japanese Ministry of Education Culture Sports Science and Technology 2011b); however, several potential constraints may limit the use of phytoextraction at DOE sites. Phytostabilization can greatly reduce the migration of radionuclides such as 137Cs that tend to sorb strongly to soils by preventing or reducing soil erosion. Recent studies at Oak Ridge National Laboratory suggest that forest vegetation can be important to the retention of radionuclides at contaminated sites (Masamichi Chino et al. 2011). The removal of vegetation reduces evapotranspiration and potentially increases the export of water, nutrients and radionuclides from contaminated sites. Such losses can be critical to environmental protection when the water quality in streams draining contaminated areas approaches the standards set to protect the public health (Stohl et al. 2011). If methods of planting and amending soil increase the availability of 137Cs for phytoextraction, then phytostabilization buffer zones might be incorporated into field designs to minimize losses by erosion and leach of 137Cs following plant harvest. Thus, phytostabilization can significantly contribute to environmental management at contaminated sites by helping to minimize the off-site migration of particle-reactive radionuclides through processes of wind and water erosion (Japanese Ministry of Education Culture Sports Science and Technology 2011d). In situations where contaminants can be leached from surface soils into the groundwater, plants can significantly reduce the flux of infiltrating water, thus reducing the driving force for leaching.
Removal by continuous cropping
A frequently proposed concept for reducing the 137Cs inventory in soil is to repeatedly grow and dispose of successive crops of plants that show a high uptake of the element. The consideration of plant growth factors on atolls demonstrates the limited application of the concept (United Nations Scientific Committee on the Effects of Atomic Radiation 2000). The source of fresh water is rainfall that occurs mostly from June through November, which limits natural vegetation growth to that six-month interval (Japanese Ministry of Education Culture Sports Science and Technology 2011c). The average annual mass of vegetation that can be produced is approximately 1 kg/m or perhaps even less with continued cropping. The maximum uptake of 137Cs in vegetation expressed as a concentration ratio (Bq/g in plants (wet weight)/Bq/g in soil (dry weight) is approximately 3. A square meter of soil that is 40 cm deep weighs approximately 440 kg, so the loss of 137Cs would be:
Additionally, whole coconut trees have been analyzed to determine the 137Cs concentration in each component, and their litter fall (fronds and coconuts) has been quantified to determine the annual loss of 137Cs in a coconut grove if all of the litter were collected and disposed of during the year (Dlugosz-Lisiecka and Bem 2012).
Chemical remediation
Currently there are a few chemical remediation techniques available for 137Cs. Dodge and Francis (1994) developed a process to recover 137Cs from soils using citric acid and visible light photodegradation (Tyler et al. 2001). Their early studies have shown that the uranyl ion is photochemically active in the presence of organic acids, and on exposure to visible light, a uranyl citrate complex undergoes photochemical oxidation/reduction reactions (Sanzharova et al. 2009). The mixture of citric acid and contaminated soil is first treated with bacteria that will degrade free of the complexed citric acid to carbon dioxide and water. The supernatant containing the uranium–citrate complex is then separated and subjected to photodegradation for uranium recovery (International Atomic Energy Agency 2009). It is doubtful, however, if such approaches would aid 137Cs extraction from contaminated sediments.
Fertilizers
Chemical amendments in the form of fertilization practices have been shown to have dramatic effects on plant accumulation of 137Cs and the effectiveness of phytoextraction. The most important nutrients in aquatic ecosystems are phosphorus and nitrogen (Shaw 2007). Total phosphorus has long been recognized as the nutrient most likely to limit the primary productivity in lakes. The most important focus of the remediation of aquatic ecosystem is the adaptation of potassium (K) and nitrogen (N) fertilization techniques; therefore, land use/management and agricultural water management practices may be the best options to minimize radioactive 137Cs in the local food chain (Japanese Ministry of Education Culture Sports Science and Technology 2012). In particular, information on the K status of the ecosystem is essential for predicting the efficiency of K fertilizer application in reducing the transfer of 137Cs (Japanese Ministry of Education Culture Sports Science and Technology 2011e). Several useful and ecologically relevant methods exist to remediate lakes contaminated by 137Cs, and they include liming, potash treatment and fertilization of low-production lakes (Kinoshita et al. 2011). Nitrogen fertilization results in an increased uptake of 137Cs as a result of increased plant growth rates, whereas fertilization with large amounts of potassium results in a substantial reduction in 137Cs uptake (Hirose 2012). Ammonium sulfate fertilizers can also be used for growth stimulation and to displace the exchangeable fraction of 137Cs in the soil matrix with the ammonium ion, thus increasing the uptake availability of 137Cs. Fertilizers containing competing elements (e.g., potassium) have significant effects on the behavior of 137Cs and plant-root uptake (Cyranoski and Brumfiel 2011). Potassium fertilization has been shown to be a successful counter measure for ryegrass until the potassium loading in soil reaches ~5 % of the CEC. Above that amount, the soil-to-plant transfer may increase as the potassium supply increases because the K d decreases and the concentration ratio may remain essentially constant (Taira and Hatoyama 2011). In sandy soils, especially those with generally low CEC values and low K d, only a small range of potassium fertilization can be used as a counter measure for ryegrass. In some instances, such as with spinach, the correlation between the soil-to-plant transfer and K d does not produce the desired countermeasure reaction when using potassium fertilization (Moller et al. 2012).
Potash treatment
Blocking 137Cs and/or it substituting it with potassium is another method of reducing the proportion of 137Cs taken up by fish. For this reason, potash treatments have been carried out to increase the potassium concentration of lake waters. Potassium and cesium are taken up in fish in a similar manner (Moller et al. 2013). Other ions may also participate in different blocking processes (e.g., Ca, Mg and Na), which implies that different liming measures that produce a general increase in the ionic strength of water may also produce a similar positive effect (Japanese Ministry of Education Culture Sports Science and Technology 2011a). However, pH can be increased without changing the concentration of potassium in a lake by adding primary rock lime with no potassium; however, it is not possible to increase the K concentration without also increasing the lake pH.
Liming
To increase the proportion of 137Cs in the sediments of a lake bottom, which prevents or delays 137Cs biouptake, lake liming has been performed based on the hypothesis that the flocculation tendency of 137Cs-carrying particles is increased by increases in the pH, alkalinity and hardness of the lake water (Tsukada et al. 2003). Naturally low pH occurs in many oligotrophic lakes with catchments dominated by acidic rocks and mires, and numerous natural processes and properties in the catchment influence the lake pH. Therefore, the natural, preindustrial or precivilization values of the lake pH vary from lake to lake and cannot be determined by current methods; however, there are predictive methods to determine the original conditions of a lake (Ministry of Agriculture Forestry and Fisheries 2011). In Sweden, a crude ‘rule-of-the-thumb’ system is applied by the National Environmental Protection Agency and regional authorities whereby lakes are generally limed to approximately 6.4–6.5 (Bowyer et al. 2011). The ‘natural’ range in mean annual pH in small glacial lakes varies from approximately 5.5 to approximately 7.2, so 6.4 is only occasionally correct.
Ammonium addition
Recent studies have shown that the addition of ammonium increases the availability of 137Cs to plants growing in aged contaminated soils (Brandt et al. 2002). Field trials associated with this research indicate that plants that have both high uptake and high biomass production could be used to remediate a 137Cs-contaminated site (ZAMG 2011). Historical research has also shown that the addition of ammonium-based fertilizers increases 137Cs availability. Thus, the application of ammonium fertilizers has the potential to enhance the plant uptake of soil 137Cs (Chino et al. 1993). Changes in the soil chemistry that are caused by rhizosphere development may subsequently promote or inhibit plant uptake of radionuclides; however, these changes are not fully understood.
Physical remediation
There are a number of different physical methods for stabilizing, immobilizing or removing contaminants in aquatic ecosystems. Physical processes generally apply to a wide variety of contaminants in various chemical forms (Chino et al. 2011), and physical remediation of radioactive constituents does not alter the level of radioactivity unless the contaminated soil or sediment is physically removed. However, physical remediation may reduce the potential for exposure or movement through the soil or sediments.
Capping and in situ grouting
137Cs binds tightly to clays, but methods that prevent water intrusion and solution phase migration of contaminants (e.g., capping and in situ grouting) are generally less cost effective than other remediation methods (Davoine and Bocquet 2007). Sulfur polymer cement, saltstone, and solidification are methods for containing wastes in cementitious forms. Electrokinetic techniques require the dissolution of the contaminant in soil water to facilitate its movement to a collecting electrode (Devell et al. 1996). A large fraction of 137Cs, however, will likely be contained in the soil structure and not in the soil solution. The properties of 2:1 lattice clays such as micas, vermiculites, illites and smectites lead to an almost totally irreversible adsorption of 137Cs into the lattice structures and effectively immobilizes a large fraction of 137Cs if sufficient time is allowed for equilibration. This concept led to the addition of illite to a 137Cs-contaminated wetland to reduce concentrations in the biota (Galmarini et al. 2011). Replicated 3 m diameter limnocorrals were used to field test the method at different application rates of illite. Spreading the illite on the water surface so that ~0.25 cm settled and covered the contaminated sediments resulted in a 25- to 30-fold reduction in 137Cs concentrations in the water. Concomitant reductions in 137Cs concentrations in plants were two- to five-fold and two- to threefold in fish (Antonio et al. 1997). This simple method reduced the biological availability of 137Cs and preserved the integrity and function of the wetland at a cost that was 5–20 times less than sediment removal.
Addition of micaceous minerals
High 137Cs mobility in aquatic systems results from the low retention capacity of the kaolinitic-dominated sediments; however, the addition of micaceous minerals to contaminated water bodies is an effective in situ remediation technique that sequesters 137Cs and reduces its bioavailability (Haritonidis and Malea 1995). The use of micaceous amendments such as illites to sequester contaminants, including 137Cs, is not new. Many of the previous methods, however, physically mixed the amendment within the contaminated soil and then tested the effectiveness using chemical adsorptive and/or desorptive tests.
Soil washing
Soil washing, or the separation of soils by particle size, may be an appropriate removal technique because 137Cs is known to preferentially attach to small particles, especially clays (Sokolov et al. 2001). This method, however, is generally only effective for soils that contain less than ~20 % of fine particles. Other sorting technique that rely on the radioactive properties of 137Cs can be used to physically separate soils that contain activity above a given threshold. This sorting involves soil passing by radiation detectors on a conveyor belt; when high activities are detected, that segment of soil is mechanically removed, whereas the low-activity soil continues on and is discarded as clean (Manolopoulou et al. 2011). As with many methods, this approach is likely to be scale-limited.
Removal of contaminated surface soil
The disposal of contaminated soil is not a trivial issue; however, it is beyond the scope of this paper. This method is definitely an effective way to reduce the radionuclide inventory on an island. However, the environmental consequences of excavation are significant and long-lasting (Norman et al. 2011). First, all of the vegetation must be removed. On Bikini Island, this would include 25,000 producing coconut palms, breadfruit trees, Pandanus, papaya and other miscellaneous food crops as well as native vegetation (Bowyer et al. 2011). Scraping off the surface 40 cm would remove the radionuclides; however, it would also remove the organic layer that has developed over centuries. The organic layer provides for nutrient exchange, greatly increases the rainfall retention, stabilizes the surface against wind and water erosion, and renders the soil friable for root development (Sinclair et al. 2011). The cost of these elements in a fertilizer bag would be several thousand dollars per hectare. Some of our studies have demonstrated how the barren scraped surface could be revegetated. Restoring and maintaining productivity, however, requires a decades-long commitment of effort and expertise that is far from assured. The long-term costs of restoration are above and beyond the estimated $100 million dollars required to excavate only the one square mile of surface of Bikini Island (Pittauerova et al. 2011). Because of the severe environmental impacts of the excavation option, we have examined other remedial possibilities that might reduce 137Cs in the terrestrial food chain (Lozano et al. 2011).
Leaching 137Cs from the soil
A large-scale field irrigation experiment was designed to determine whether a significant fraction of the 137Cs inventory in the soil could be removed by leaching (Bolsunovsky and Dementyev 2011). Atolls simply do not have the large stores of fresh water required for single or multiple leaching operations (Stohl et al. 2005). However, the supply of seawater is boundless, and the contained cations might be expected to dislodge any cesium by simple exchange forces (Aoyama et al. 2011). The adverse consequences of such treatments are the destruction of the freshwater lens and salinization of the soil itself. However, the latter turned is a short-lived effect, whereas the restoration of the freshwater lens is a much longer process.
Future work
The 2011 Fukushima nuclear accident in Japan was the worst nuclear disaster after the 1986 Chernobyl accident. The Fukushima nuclear accident occurred in the wake of the double disaster of the 9.0 magnitude Tohoku earthquake and tsunami on March 11, 2011. The earthquake triggered the shutdown of the three active reactors at the Fukushima Daiichi nuclear power station, and the tsunami stopped the station’s backup diesel generators, which caused a station blackout. The subsequent lack of cooling led to a series of explosions and complete meltdowns of three active reactor cores at the Fukushima facility and additional problems at all six reactor units and the central spent fuel pool. At the time of the accidents, air transport in the mid-latitudes was dominated by prevailing westerly winds, which can circle around the globe in 2–3 weeks. Therefore, if Fukushima-derived radionuclides were introduced into and above the planetary boundary layer, their dispersal at both regional and global scales would be inevitable. Indeed, the fission products released from the accidents have been spread not only across the entire Northern Hemisphere but also into the Southern Hemisphere (data available from the CTBTO Preparatory Commission). In approximately 2 weeks, the fission products of 15 radionuclides released from the Fukushima plant site had been detected around the globe.
To date, areas contaminated by radionuclides discharged from the Fukushima Daiichi nuclear power plant accident have been mapped in detail. However, the fate and transport of 137Cs in ecosystems have not yet been revealed, and this information is critical for the removal of 137Cs from the environment. Therefore, this review provides the basic information on the fate and transport of 137Cs in aquatic ecosystems and will serve as a starting point for future work plans. Figure 6 illustrates the flow sheet diagram for future required research work.
This research has a significant impact as a demonstrable contribution to society and the economy. Below is a brief introduction of the potential range of impacts that can be generated from the present research.
Academic impact
The present research will provide scientific and technical information required by the governments and related organizations for evaluations and future planning, especially after the events of the Tsunami in March 2011, which resulted in the release of radionuclides from the Fukushima Daiichi Nuclear Power Plant. This research is a worldwide academic advancement to address global issues caused by radionuclides. The fate and transport of 137Cs in the aquatic environment is not well known. Therefore, this research provides a significant contribution to determining the mechanism of radionuclide transport in aquatic ecosystems. The development and utilization of new and innovative methodologies, equipment and cross-disciplinary approaches are major contributions toward a vibrant academic discipline. Furthermore, this research can add to the development and growth and training of highly skilled researchers.
Economic and societal impact
This research also contributes toward evidence-based policy-making, has the potential to influence public policies and legislation at a local, regional, national and international level, and can enhancing cultural enrichment, quality of life, health and well-being. In addition, it contributes toward environmental sustainability, protection and impact reduction and also increases the public awareness and understanding of science and economic and societal issues.
Conclusions
The evidence discussed in the present review article indicates that 137Cs may persist in a biologically available form for many years following its release into the environment. The high solubility of cesium and its almost exclusive existence as 137Cs in the ecosystem indicate a high degree of mobility and bioavailability. Moreover, 137Cs displays very similar chemical properties to other alkali metal cations with a similar ionic radius and charge, particularly the biologically essential K+. Thus, Cs+ may be taken up via transport systems that would normally catalyze the intracellular accumulation of K+, so the toxic effects of 137Cs may result from a perturbation of K+-mediated processes. Despite these properties, the mobility of 137Cs is strongly influenced by a number of parameters, which is especially apparent in terrestrial ecosystems. One factor that is critical in determining cesium mobility in the environment is its tendency to become strongly complexed with the inorganic components of soils. This behavior contrasts markedly with its weak coordination with organic ligands. Complexation of 137Cs coincides with a dramatic reduction in its bioavailability. However, binding 137Cs to minerals is not necessarily irreversible, which is especially true in terrestrial ecosystems where the recycling of bound 137Cs may result from changes in the redox conditions of the water column. Remediation of terrestrial systems following contamination by 137Cs is largely dependent on the site-specific parameters. Therefore, the effects of such remediation efforts must consider a cost-benefit analysis according to the environmental conditions. The remediation of contaminated ecosystems involves physical, chemical and biological procedures that have their own advantages and disadvantages. This review provides basic information on the fate and transport of 137Cs in terrestrial ecosystems and serves as a starting point for future work plans in the wake of the Fukushima Nuclear Power Plant disaster of 2011.
References
Al-Masri, M. S. (2006). Vertical distribution and inventories of 137Cs in the Syrian soils of the eastern Mediterranean region. Journal of Environmental Radioactivity, 86(2), 187–198.
Antonio, M. R., Dietz, M. L., Jensen, M. P., Soderholm, L., & Horwitz, E. P. (1997). EXAFS studies of cesium complexation by dibenzo-crown ethers in tri-n-butyl phosphate. Inorganica Chimica Acta, 255, 13–20.
Aoyama, M., Fukasawa, M., Hirose, K., Hamajima, Y., Kawano, T., Povinec, P. P., et al. (2011). Cross equator transport of 137Cs from North Pacific Ocean to South Pacific Ocean (BEAGLE2003 cruises). Progress in Oceanography, 89, 7–16.
Aoyama, M., & Hirose, K. (2008). Radiometric determination of anthropogenic radionuclides in seawater. In P. Povinec (Ed.), Radioactivity in the environment (pp. 137–162). Amsterdam: Elsevier.
Ashraf, M. A., Rehman, M. A., Maah, M. J., & Yusoff, I. (2013). Cesium-137: Radio-chemistry, fate and transport, remediation and future concerns. Critical Reviews in Environmental Science and Technology. doi:10.1080/10643389.2013.790753.
Balonov, M., Barnett, C., Belli, M., Beresford, N., Berkovsky, V., Bossew, P., et al. (2010). Handbook of parameter values for the prediction of radionuclide transfer in terrestrial and freshwater environment. Vienna: IAEA.
Ben-Asher, J. (2011). Regulating in a radioactive world: The 1593 FDA and radionuclide contamination. Harvard student paper. http://nrs.harvard.edu/urn-3:HUL.InstRepos:8789611.
Beresford, N. A., Broadley, M. R., Howard, B. J., Barnett, C. L., & White, P. J. (2004). Estimating radionuclide transfer to wild species—Data requirements and availability for terrestrial ecosystems. Journal of Radiological Protection, 24(4A), A89.
Beresford, N. A., & Vives i Batlle, J. (2013). Estimating the biological half-life for radionuclides in homoeothermic vertebrates: A simplified allometric approach. Radiation and Environmental Biophysics, 52(4), 505–511.
Beresford, N. A., Yankovich, T. L., Wood, M. D., Fesenko, S., Andersson, P., Muikku, M., et al. (2013). A new approach to predicting environmental transfer of radionuclides to wildlife: A demonstration for freshwater fish and caesium. Science of the Total Environment, 463–464, 284–292.
Bolsunovsky, A., & Dementyev, D. (2011). Evidence of the radioactive fallout in the center of Asia (Russia) following the Fukushima Nuclear Accident. Journal of Environmental Radioactivity, 102(11), 1062–1064.
Bossew, P., Lettner, H., Hubmer, A., Erlinger, C., & Gastberger, M. (2007). Activity ratios of 137Cs, 90Sr and 239 + 240Pu in environmental samples. Journal of Environmental Radioactivity, 97(1), 5–19.
Bowyer, T. W., Biegalski, S. R., Cooper, M., Eslinger, P. W., Haas, D., Hayes, J. C., et al. (2011). Elevated radioxenon detected remotely following the Fukushima nuclear accident. Journal of Environmental Radioactivity, 102(7), 681–687.
Brandt, J., Christensen, J. H., & Frohn, L. M. (2002). Modelling transport and deposition of caesium and iodine from Chernobyl accident using the DREAM model. Atmospheric Chemistry and Physics, 2, 397–417.
Brechignac, F., Polikarpov, G., Oughton, D. H., Hunter, G., Alexakhin, R., Zhu, Y. G., et al. (2003). Protection of the environment in the 21st century: Radiation protection of the biosphere including humankind. Statement of the International Union of Radioecology. Journal of Environmental Radioactivity, 70(3), 155–159.
Brenner, D. J., Doll, R., Goodhead, D. T., Hall, E. J., Land, C. E., Little, J. B., et al. (2003). Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proceedings of the National Academy of Sciences of the United States of America, 100(24), 13761–13766.
Brito, J. C., Godinho, R., Martinez-Freiria, F., Pleguezuelos, J. M., Rebelo, H., Santos, X., et al. (2014). Unravelling biodiversity, evolution and threats to conservation in the Sahara-Sahel. Biological Reviews of the Cambridge Philosophical Society, 89(1), 215–231.
Brown, J. E., Alfonso, B., Avila, R., Beresford, N. A., Copplestone, D., Pröhl, G., et al. (2008). The ERICA tool. Journal of Environmental Radioactivity, 99(9), 1371–1383.
Butler, D. (2011). First estimates of total radioactive cesium and iodine emissions from Fukushima Plant. Nature Newsblog. http://blogs.nature.com/news/2011/03/firstestimatesofradioactive.html.
Chiang, P. N., Wang, M. K., Huang, P. M., Wang, J. J., & Chiu, C. Y. (2010). Cesium and strontium sorption by selected tropical and subtropical soils around nuclear facilities. Journal of Environmental Radioactivity, 101(6), 472–481.
Chino, M., Ishikawa, H., & Yamazawa, H. (1993). SPEEDI and WSPEEDI: Japanese emergency response systems to predict radiological impacts in local and workplace areas due to a nuclear accident. Radiation Protection Dosimetry, 50, 145–152.
Chino, M., Nakayama, H., Nagai, H., Terada, H., Katata, G., & Yamazawa, H. (2011). Preliminary estimation of release amounts of 131I and 137Cs accidentally discharged from the Fukushima Daiichi nuclear power plant into the atmosphere. Journal of Nuclear Science and Technology, 48(7), 1129–1134.
Cid, A. S., Anjos, R. M., Zamboni, C. B., Velasco, H., Macario, K., Rizzotto, M., et al. (2013). Temporal evolution of (1)(3)(7)Cs(+), K(+) and Na(+) in fruits of South American tropical species. The Science of the Total Environment, 444, 115–120.
Cyranoski, D., & Brumfiel, G. (2011). Fukushima impact is still hazy. Nature, 477(7363), 139–140.
Davoine, X., & Bocquet, M. (2007). Inverse modelling-based reconstruction of the Chernobyl source term available for long-range transport. Atmospheric Chemistry and Physics, 7, 1549–1564.
De Hoop, L., Huijbregts, M. A. J., Schipper, A. M., Veltman, K., De Laender, F., Viaene, K. P. J., et al. (2013). Modelling bioaccumulation of oil constituents in aquatic species. Marine Pollution Bulletin, 76(1–2), 178–186.
Devell, L., Guntay, S., & Powers, D. A. (1996). The Chernobyl reactor accident source term: Development of a consensus view. Paris: OECD Nuclear Energy Agency.
Diaz Leon, J., Jaffe, D. A., Kaspar, J., Knecht, A., Miller, M. L., Robertson, R. G. H., et al. (2011). Arrival time and magnitude of airborne fission products from the Fukushima, Japan, reactor incident as measured in Seattle, WA, USA. Journal of Environmental Radioactivity, 102(11), 1032–1038.
Długosz-Lisiecka, M., & Bem, H. (2012). Determination of the mean aerosol residence times in the atmosphere and additional 210po input on the base of simultaneous determination of 7be, 22na, 210pb, 210bi and 210po in urban air. Journal of Radioanalytical and Nuclear Chemistry, 293, 135–140.
Dodge, C. J. X., & Francis, A. J. (1994). Photodegradation of uranium citrate complex with uranium recovery. Environmental Science and Technology, 28(2), 1300–1306.
Dowdall, M., Standring, W., Shaw, G., & Strand, P. (2008). Will global warming affect soil-to-plant transfer of radionuclides? Journal of Environmental Radioactivity, 99(11), 1736–1745.
Dupré de Boulois, H., Joner, E. J., Leyval, C., Jakobsen, I., Chen, B. D., Roos, P., et al. (2008). Role and influence of mycorrhizal fungi on radiocesium accumulation by plants. Journal of Environmental Radioactivity, 99(5), 785–800.
Eckerman, K. F., Wolbarst, A. B., & Richardson, A. C. B. (1988). Limiting values of radionuclide intake and air concentration and dose conversion factors for inhalation, submersion, and ingestion: Federal guidance report No. 11. Washington, DC: EPA.
Ellis, E. C. (2013). Sustaining biodiversity and people in the world’s anthropogenic biomes. Current Opinion in Environmental Sustainability, 5(3), 368–372.
Endo, S., Kimura, S., Takatsuji, T., Nanasawa, K., Imanaka, T., & Shizuma, K. (2012). Measurement of soil contamination by radionuclides due to the Fukushima Dai-ichi Nuclear Power Plant accident and associated estimated cumulative external dose estimation. Journal of Environmental Radioactivity, 111, 18–27.
Fukushima Prefecture. (2012). http://www.pref.fukushima.jp/j/koukyouyousuiiki0217.pdf.
Galmarini, S., Stohl, A., & Wotawa, G. (2011). Fund experiments on atmospheric hazards. Nature, 473(7347), 285.
Gherardi, F., & Padilla, D. K. (2013). Climate-induced changes in human behavior and range expansion of freshwater species. Ethology Ecology & Evolution, 26(1), 86–90.
Gjelsvik, R., & Steinnes, E. (2013). Geographical trends in 137Cs fallout from the Chernobyl accident and leaching from natural surface soil in Norway. Journal of Environmental Radioactivity, 126, 99–103.
Gyuricza, V., Dupré de Boulois, H., & Declerck, S. (2010). Effect of potassium and phosphorus on the transport of radiocesium by arbuscular mycorrhizal fungi. Journal of Environmental Radioactivity, 101(6), 482–487.
Hagan, L. (1977). Bibliography on atomic energy levels and spectra, July 1971 through June 1975 (Vol. 363). Washington, DC: US Dept. of Commerce, National Bureau of Standards.
Haritonidis, S., & Malea, P. (1995). Seasonal and local variation of Cr, Ni and Co concentrations in Ulva rigida C. Agardh and Enteromorpha linza (Linnaeus) from Thermaikos Gulf, Greece. Environmental Pollution, 89(3), 319–327.
Hinton, T. G., Garnier-Laplace, J., Vandenhove, H., Dowdall, M., Adam-Guillermin, C., Alonzo, F., et al. (2013). An invitation to contribute to a strategic research agenda in radioecology. Journal of Environmental Radioactivity, 115, 73–82.
Hirose, K. (2012). 2011 Fukushima Dai-ichi nuclear power plant accident: Summary of regional radioactive deposition monitoring results. Journal of Environmental Radioactivity, 111, 13–17.
Hirose, K., Igarashi, Y., & Aoyama, M. (2008). Analysis of the 50-year records of the atmospheric deposition of long-lived radionuclides in Japan. Applied Radiation and Isotopes, 66(11), 1675–1678.
Hohenemser, C., & Renn, O. (1988). Shifting public perceptions of nuclear risk: Chernobyl’s other legacy. Environment: Science and Policy for Sustainable Development, 30(3), 4–45.
Hou, X. L., Fogh, C. L., Kucera, J., Andersson, K. G., Dahlgaard, H., & Nielsen, S. P. (2003). Iodine-129 and Caesium-137 in Chernobyl contaminated soil and their chemical fractionation. Science of the Total Environment, 308(1–3), 97–109.
International Atomic Energy Agency. (2000). Report of the specialists’ meeting on environmental protection from the effects of ionizing radiation: International perspectives. Ref. 723-J9-SP-1114.2. Vienna, Austria: IAEA.
International Atomic Energy Agency. (2001a). Present and future environmental impact of the Chernobyl accident. Vienna: IAEA.
International Atomic Energy Agency. (2001b). Report of the Second FAO, “The classification of soil systems on the basis of transfer factors of radionuclide from soil to reference plants”. Vienna: IAEA.
International Atomic Energy Agency. (2009). Quantification of radionuclide transfer in terrestrial and freshwater environments for radiological assessments. Vienna: IAEA.
International Atomic Energy Agency. (2011). Please add title. Vienna: Ministry of Education, Culture, Sports, Science and Technology.
Japanese Ministry of Education Culture Sports Science and Technology. (2011a). Environmental radiation database. Tokyo: MEXT.
Japanese Ministry of Education Culture Sports Science and Technology. (2011b). Reading of radioactivity level in fallout by prefecture. Tokyo: MEXT.
Japanese Ministry of Education Culture Sports Science and Technology. (2011c). Readings of environmental radioactivity level by prefecture. Tokyo: MEXT.
Japanese Ministry of Education Culture Sports Science and Technology. (2011d). Readings of sea area monitoring. Tokyo: MEXT.
Japanese Ministry of Education Culture Sports Science and Technology. (2011e). Results of airborne monitoring by MEXT and the U.S. Department of Energy. Tokyo: MEXT.
Japanese Ministry of Education Culture Sports Science and Technology. (2011f). Results of the 2nd airborne monitoring by the MEXT and U.S. Department of Energy. Tokyo: MEXT.
Japanese Ministry of Education Culture Sports Science and Technology. (2012). Add the title. MEXT: In. Tokyo.
Jargin, S. V. (2010). Overestimation of Chernobyl consequences: Poorly substantiated information published. Radiation and Environmental Biophysics, 49(4), 743–745.
Jasiulionis, R., & Rozkov, A. (2007). 137Cs activity concentration in the ground-level air in the Ignalina NPP region. Lithuanian Journal of Physics, 47(2), 195–202.
Jasiulionis, R., Rozkov, A., & Vycinas, L. (2006). Radionuclides in the ground-level air and deposition in the Ignalina NPP region during 2002–2005. Lithuanian Journal of Physics, 46, 101–108.
Kinoshita, N., Sueki, K., Sasa, K., Kitagawa, J.-I., Ikarashi, S., Nishimura, T., et al. (2011). Assessment of individual radionuclide distributions from the Fukushima nuclear accident covering central-east Japan. Proceedings of the National Academy of Sciences, 108(49), 19526–19529.
Kitajima, A., Ogawa, H., Kobayashi, T., Kawasaki, T., Kawatsu, Y., Kawamoto, T., et al. (2014). Monitoring low-radioactivity caesium in Fukushima waters. Environmental Science: Processes & Impacts, 16(1), 28–32.
Koarashi, J., Atarashi-Andoh, M., Matsunaga, T., Sato, T., Nagao, S., & Nagai, H. (2012). Factors affecting vertical distribution of Fukushima accident-derived radiocesium in soil under different land-use conditions. Science of the Total Environment, 431, 392–401.
Koizumi, A., Harada, K., Niisoe, T., Adachi, A., Fujii, Y., Hitomi, T., et al. (2012). Preliminary assessment of ecological exposure of adult residents in Fukushima Prefecture to radioactive cesium through ingestion and inhalation. Environmental Health and Preventive Medicine, 17(4), 292–298.
Lee, C. P., Kuo, Y. M., Tsai, S. C., Wei, Y. Y., Teng, S. P., & Hsu, C. N. (2008). Numerical analysis for characterizing the sorption/desorption of cesium in crushed granite. Journal of Radioanalytical and Nuclear Chemistry, 275(2), 343–349.
Lide, D. R. (2004). CRC handbook of chemistry and physics 2004–2005: A ready-reference book of chemical and physical data. Boca Raton, FL: CRC Press.
Lozano, R. L., Hernández-Ceballos, M. A., Adame, J. A., Casas-Ruíz, M., Sorribas, M., Miguel, E. G. S., et al. (2011). Radioactive impact of Fukushima accident on the Iberian Peninsula: Evolution and plume previous pathway. Environment International, 37(7), 1259–1264.
Maderich, V., Bezhenar, R., Heling, R., de With, G., Jung, K. T., Myoung, J. G., et al. (2013). Regional long-term model of radioactivity dispersion and fate in the Northwestern Pacific and adjacent seas: Application to the Fukushima Dai-ichi accident. Journal of Environmental Radioactivity, 131(1), 4–18.
Manolopoulou, M., Vagena, E., Stoulos, S., Ioannidou, A., & Papastefanou, C. (2011). Radioiodine and radiocesium in Thessaloniki, Northern Greece due to the Fukushima nuclear accident. Journal of Environmental Radioactivity, 102(8), 796–797.
McMichael, P. (2013). Food Sovereignty: A critical dialogue. International Institute of Social Studies (ISS),Hague.
Medici, F. (2001). The IMS radionuclide network of the CTBT. Radiation Physics and Chemistry, 61, 689–690.
Mihaela, T., Otto, K., & Ovidiu, T. (2012). Naturally occurring 137Cs, 90Sr and 226Ra radionuclides in raw milk in the Sibiu province of Romania. International Journal of Dairy Technology, 65(4), 511–515.
Ministry of Agriculture Forestry and Fisheries. (2011). A point of view on planting rice plant. Tokyo: MAFF.
Ministry of Land Infrastructure Transport and Tourism. (2011). Soil map of Japan. http://tochi.mlit.go.jp/tockok/tochimizu/F3/ZOOMA/0719/index.html.
Moller, A. P., Hagiwara, A., Matsui, S., Kasahara, S., Kawatsu, K., Nishiumi, I., et al. (2012). Abundance of birds in Fukushima as judged from Chernobyl. Environmental Pollution, 164, 36–39.
Moller, A. P., Nishiumi, I., Suzuki, H., Ueda, K., & Mousseau, T. A. (2013). Differences in effects of radiation on abundance of animals in Fukushima and Chernobyl. Ecological Indicators, 24, 75–81.
Morino, Y., Ohara, T., & Nishizawa, M. (2011). Atmospheric behavior, deposition, and budget of radioactive materials from the Fukushima Daiichi nuclear power plant in March 2011. Geophysical Research Letters, 38(7), L00G11.
Mucina, L., Daniel, G., Stephenson, G., Boonzaier, I., van Niekerk, A., Barret, M., et al. (2013). Floristic-ecological mapping in the Northern Kimberley. Western Australia: Field survey methods and mapping protocols.
Nakanishi, T., Matsunaga, T., Koarashi, J., & Atarashi-Andoh, M. (2014). 137Cs vertical migration in a deciduous forest soil following the Fukushima Dai-ichi Nuclear Power Plant accident. Journal of Environmental Radioactivity, 128, 9–14.
Niedrée, B. (2013). Effects of 137Cs and 90Sr on structure and functional aspects of the microflora in agricultural used soils. Julich: Forschungszentrum Jülich.
Nimis, P. L. (1996). Radiocesium in plants of forest ecosystems. Studia Geobotanica, 15(3–49), 3–38.
Norman, E. B., Angell, C. T., & Chodash, P. A. (2011). Observations of fallout from the Fukushima reactor accident in San Francisco Bay area rainwater. PLoS ONE, 6(9), e24330.
Okumura, T., Tamura, K., Fujii, E., Yamada, H., & Kogure, T. (2014). Direct observation of cesium at the interlayer region in phlogopite mica. Microscopy, 63(1), 65–72.
Paasikallio, A., Rantavaara, A., & Sippola, J. (1994). The transfer of cesium-137 and strontium-90 from soil to food crops after the Chernobyl accident. Science of the Total Environment, 155(2), 109–124.
Peterson, D., Wolken, J., Hollingsworth, T., Giardina, C., Littell, J., Joyce, L., et al. (2014). Regional highlights of climate change. In D. L. Peterson, J. M. Vose, & T. Patel-Weynand (Eds.), Climate change and United States forests (Vol. 57, pp. 113–148)., Advances in global change research Dordrecht: Springer.
Pittauerova, D., Hettwig, B., & Fischer, H. W. (2011). Fukushima fallout in Northwest German environmental media. Journal of Environmental Radioactivity, 102(9), 877–880.
Povinec, P. P., Sýkora, I., Holý, K., Gera, M., Kováčik, A., & Brest’áková, L. (2012). Aerosol radioactivity record in Bratislava/Slovakia following the Fukushima accident—A comparison with global fallout and the Chernobyl accident. Journal of Environmental Radioactivity, 114, 81–88.
Pröhl, G. (2009). Interception of dry and wet deposited radionuclides by vegetation. Journal of Environmental Radioactivity, 100(9), 675–682.
Real, J., Persin, F., & Camarasa-Claret, C. (2002). Mechanisms of desorption of 134Cs and 85Sr aerosols deposited on urban surfaces. Journal of Environmental Radioactivity, 62(1), 1–15.
Ritchie, J. C., & McHenry, J. R. (1990). Application of radioactive fallout cesium-137 for measuring soil erosion and sediment accumulation rates and patterns: a review. Journal of Environment Quality, 19(2), 215.
Rochette, P., van Bochove, E., Prévost, D., Angers, D. A., Côté, D., & Bertrand, N. (2000). Soil carbon and nitrogen dynamics following application of pig slurry for the 19th consecutive year. Soil Science Society of America Journal, 64(4), 1396.
Sanzharova, N., Shubina, O., Vandenhove, H., Olyslaegers, G., Fesenko, S., Shang, Z. R., et al. (2009). Root uptake: Temperate environment. Quantification of radionuclide transfer in terrestrial and freshwater environments for radiological assessments (pp. 139–206). Vienna: IAEA.
Shaw, G. (2007). Radionuclides in forest ecosystems. In G. Shaw (Ed.), Radioactivity in the enviornment (pp. 127–155). Amsterdam: Elsevier.
Shcheglov, A. I., Tsvetnova, O. B., & Klyashtorin, A. (2013). The fate of Cs-137 in forest soils of Russian Federation and Ukraine contaminated due to the Chernobyl accident. Journal of Geochemical Exploration.
Simpson, M. F., & Law, J. D. (2013). Reprocessing of nuclear fuel. In N. Tsoulfanidis (Ed.), Nuclear energy (pp. 153–173). New York: Springer.
Sinclair, L. E., Seywerd, H. C., Fortin, R., Carson, J. M., Saull, P. R., Coyle, M. J., et al. (2011). Aerial measurement of radioxenon concentration off the west coast of Vancouver Island following the Fukushima reactor accident. Journal of Environmental Radioactivity, 102(11), 1018–1023.
Sokolov, N. V., Grodsinsky, D. M., & Sorochinsky, B. V. (2001). How does low does chronic irradiation under the condition of 10 km Chernobyl exclusion zone influence on processes of seed aging? In Fifteen years after the Chernobyl accident: Lessons learned (p. 117). Kiev.
Steiner, M., Linkov, I., & Yoshida, S. (2002). The role of fungi in the transfer and cycling of radionuclides in forest ecosystems. Journal of Environmental Radioactivity, 58(2–3), 217–241.
Steinhauser, G., Merz, S., Hainz, D., & Sterba, J. (2013). Artificial radioactivity in environmental media (air, rainwater, soil, vegetation) in Austria after the Fukushima nuclear accident. Environmental Science and Pollution Research, 20(4), 2527–2534.
Stohl, A., Forster, C., Frank, A., Seibert, P., & Wotawa, G. (2005). Technical note: The Lagrangian particle dispersion model FLEXPART version 6.2. Atmospheric Chemistry and Physics, 5, 2461–2474.
Stohl, A., Seibert, P., Wotawa, G., Arnold, D., Burkhart, J. F., Eckhardt, S., et al. (2011). Xenon-133 and caesium-137 releases into the atmosphere from Fukushima Dai-ichi nuclear power plant: Determination of the source term, atmospheric dispersion, and deposition. Atmospheric Chemistry and Physics, 11, 28319–28394.
Strand, P., Brown, J. E., Drozhko, E., Mokrov, Y., Salbu, B., Oughton, D., et al. (1999). Biogeochemical behaviour of 137Cs and 90Sr in the artificial reservoirs of Mayak PA, Russia. Science of the Total Environment, 241(1–3), 107–116.
Szefer, P. (2002). Metal pollutants and radionuclides in the 1982 Baltic Sea-an overview. Oceanologia, 44(2), 129–178.
Tagami, K., Uchida, S., Ishii, N., & Zheng, J. (2013). Estimation of Te-132 distribution in Fukushima Prefecture at the early stage of the Fukushima Daiichi nuclear power plant reactor failures. Environmental Science and Technology, 47(10), 5007–5012.
Taira, T., & Hatoyama, Y. (2011). Nuclear energy: Nationalize the Fukushima Daiichi atomic plant. Nature, 480(7377), 313–314.
Tateda, Y., Tsumune, D., & Tsubono, T. (2013). Simulation of radioactive cesium transfer in the southern Fukushima coastal biota using a dynamic food chain transfer model. Journal of Environmental Radioactivity, 124, 1–12.
Tositti, L., Brattich, E., Cinelli, G., Previti, A., & Mostacci, D. (2012). Comparison of radioactivity data measured in PM10 aerosol samples at two elevated stations in northern Italy during the Fukushima event. Journal of Environmental Radioactivity, 114, 105–112.
Tracy, B. L., Carini, F., Barabash, S., Berkovskyy, V., Brittain, J. E., Chouhan, S., et al. (2013). The sensitivity of different environments to radioactive contamination. Journal of Environmental Radioactivity, 122, 1–8.
Tsukada, H., Hisamatsu, S., & Inaba, J. (2003). Transfer of 137Cs and stable Cs in soil-grass-milk pathway in Aomori, Japan. Journal of Radioanalytical and Nuclear Chemistry, 255, 455–458.
Tyler, A. N., Cartier, S., Davidson, D. A., Long, D. J., & Tipping, R. (2001). The extent and significance of bioturbation on Cs-137 distribution in upland soils. Catena, 43, 81–99.
United Nations Scientific Committee on the Effects of Atomic Radiation. (2000). Sources and effects of ionizing radiation. New York: United Nations.
Valentin, J. (2003). A framework for assessing the impact of ionising radiation on non-human species. ICRP Publication 91. Annals of the ICRP, 33(3), 207–266.
Vallés, I., Camacho, A., Ortega, X., Serrano, I., Blázquez, S., & Pérez, S. (2009). Natural and anthropogenic radionuclides in airborne particulate samples collected in Barcelona (Spain). Journal of Environmental Radioactivity, 100(2), 102–107.
Velasco, H., Cid, A. S., Anjos, R. M., Zamboni, C. B., Rizzotto, M., Valladares, D. L., et al. (2012). Variability of 137Cs and 40K soil-to-fruit transfer factor in tropical lemon trees during the fruit development period. Journal of Environmental Radioactivity, 104, 64–70.
Vinichuk, M., Taylor, A. F. S., Rosén, K., & Johanson, K. J. (2010). Accumulation of potassium, rubidium and caesium (133Cs and 137Cs) in various fractions of soil and fungi in a Swedish forest. Science of the Total Environment, 408(12), 2543–2548.
Wampler, J. M., Krogstad, E. J., Elliott, W. C., Kahn, B., & Kaplan, D. I. (2012). Long-term selective retention of natural Cs and Rb by highly weathered coastal plain soils. Environmental Science and Technology, 46(7), 3837–3843.
Wells, T., & Hancock, G. (2014). Comparison of vertical transport of 137Cs and organic carbon in agricultural cracking soils. Geoderma, 214–215, 228–238.
Wicker, W. F., & Schultz, V. (1982). Radioecology: Nuclear energy and environment (Vol. 1, pp. 153–156). Boca Raton, FL: CRC Press.
Willey, N. (2010). Phylogeny can be used to make useful predictions of soil-to-plant transfer factors for radionuclides. Radiation and Environmental Biophysics, 49(4), 613–623.
Wood, M. D., Beresford, N. A., Howard, B. J., & Copplestone, D. (2013). Evaluating summarised radionuclide concentration ratio datasets for wildlife. Journal of Environmental Radioactivity, 126, 314–325.
Yablokov, A. V., Nesterenko, V. B., Nesterenko, A. V., & Sherman-Nevinger, J. D. (2010). Chernobyl: Consequences of the catastrophe for people and the environment. Hoboken, NJ: Wiley.
Yan, D., Zhao, Y., Lu, A., Wang, S., Xu, D., & Zhang, P. (2013). Effects of accompanying anions on cesium retention and translocation via droplets on soybean leaves. Journal of Environmental Radioactivity, 126, 232–238.
Yang, L., Zhu, L., & Liu, Z. (2011). Occurrence and partition of perfluorinated compounds in water and sediment from Liao River and Taihu Lake, China. Chemosphere, 83(6), 806–814.
Yasunari, T. J., Stohl, A., Hayano, R. S., Burkhart, J. F., Eckhardt, S., & Yasunari, T. (2011). Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Proceedings of the National Academy of Sciences, 108(49), 19530–19534.
Zaborska, A., Winogradow, A., & Pempkowiak, J. (2014). Caesium-137 distribution, inventories and accumulation history in the Baltic Sea sediments. Journal of Environmental Radioactivity, 127, 11–25.
Zalewska, T., & Lipska, J. (2006). Contamination of the southern Baltic Sea with 137Cs and 90Sr over the period 2000–2004. Journal of Environmental Radioactivity, 91(1–2), 1–14.
ZAMG. (2011). Accident in the Japanese NPP Fukushima: Spread of radioactivity/first source estimates from CTBTO data show large source terms at the beginning of the accident/weather currently not favourable/low level radioactivity meanwhile observed over U.S. East Coast and Hawaii. Wien: ZAMG.
Zheng, J., Tagami, K., Watanabe, Y., Uchida, S., Aono, T., Ishii, N., et al. (2012). Isotopic evidence of plutonium release into the environment from the Fukushima DNPP accident. Scientific Reports, 2, 304.
Acknowledgments
This research is supported by High Impact Research MoE Grant UM.C/625/1/HIR/MoE/SC/04 from the Ministry of Education Malaysia. Thanks also for the support by UMRG (RG257-13AFR) and FRGS (FP038-2013B).
Conflict of interest
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article has been retracted by the Editor-in-Chief. After a thorough investigation carried out according to the Committee on Publication Ethics guidelines, we have strong reason to believe that the peer review process was compromised.
An erratum to this article can be found online at http://dx.doi.org/10.1007/s10653-016-9811-7.
About this article
Cite this article
Ashraf, M.A., Khan, A.M., Ahmad, M. et al. RETRACTED ARTICLE: Release, deposition and elimination of radiocesium (137Cs) in the terrestrial environment. Environ Geochem Health 36, 1165–1190 (2014). https://doi.org/10.1007/s10653-014-9620-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-014-9620-9