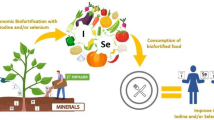
Abstract
Iodine, as one of the essential trace elements for human body, is very important for the proper function of thyroid gland. In some regions, people are still suffering from iodine deficiency disorder (IDD). How to provide an effective and cost-efficient iodine supplementation has been a public health issue for many countries. In this review, a novel iodine supplementation approach is introduced. Different from traditional iodine salt supplement, this approach innovatively uses cultivated iodine-rich phytogenic food as the supplement. These foods are cultivated using alga-based organic iodine fertilizer. The feasibility, mechanics of iodine absorption of plants from soil and the bioavailability of iodine-rich phytogenic food are further discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iodine is an essential element for the synthesis of thyroid hormone, which plays a key role in metabolism, including enhancing protein synthesis, promoting growth and maintaining normal brain function (Gerber et al. 1999). If iodine daily intake falls below certain levels, such as 40 μg for infants, 50 μg for 7–12 months, 70 μg for 1–7 years, 120 μg for 7–12 years, 150 μg for adults (above 13 years), 200 μg for pregnant and 290 μg for lactating women (Andersson et al. 2007), iodine deficiency disease (IDD) such as goiter, cretinism (clinical manifestations including impaired cognition, dwarf, deaf, dumb and paralysis), abortion, stillbirths, prenatal and infant mortality, and congenital malformation could occur (Melse-Boonstra and Jaiswal 2010; Rose et al. 2002; Zimmermann 2008). Iodine deficiency is the leading cause of preventable mental retardation. According to an epidemiological investigation (Hetzel 2005; WHO et al. 1999), iodine deficiency occurs in 130 countries and regions. About two billion people live in iodine deficiency areas, and 655 million have thyroid enlargement which accounts for 14 % of total population around the world. The prevalence of iodine deficiency is lowest in the Americas (13.7 %) and the highest in Europe (44.2 %). The Southeast Asia region accounts for 28.8 % of the global population with insufficient iodine intake (Andersson et al. 2012). As a major affected area of IDD, China has a population of 210 million who are under the threat of iodine deficiency (de Benoist et al. 2004). The potential of iodine deficiency for these people posed severe negative effects on the quality of life in these communities. Thus, eliminating iodine deficiency had been adopted as a public health policy for decades in many countries. Practically, many governments enforced to manufacture and sell iodine-enriched supplement, such as iodized salt (Horton and Miloff 2010; Sooch et al. 2001).
Seafood is a naturally iodine-enriched food. Unfortunately, seafood may be only consumed by population along costal area where IDD often is not an issue. For those people who live in inland, they usually do not have sufficient access to seafood due to its comparably high price. Another reason is that seafood is not in the preferred diet list for local resident. Therefore, seafood may not be an ideal source of iodine supplement for iodine deficiency population in general.
Iodized salt is currently the most prevalent food supplement to control IDD (Vejbjerg et al. 2007; WHO et al. 1999). However, inorganic iodine (KI or KIO3) from salt is considered less bioavailable than the iodine from plant (or animal) sources (Zheng et al. 2001). Furthermore, it is well known that iodine is readily to volatilize in the process of production, storage and transport of iodized salt (Biber et al. 2002; Longvah et al. 2012; Waszkowiak and Szymandera-Buszka 2008). Such volatilization is reinforced when it is under Chinese high-temperature cooking practice. To compensate the iodine loss in cooking practice, Chinese government originally set a high iodine standard in salt (60 mg/kg) to meet the World Health Organization (WHO) recommended level, which is 150–200 μg day L−1 in urinary. This practice was later found to result in excess iodine absorption for certain population because iodine intake pattern is not always the same for individuals, and led to the government to reset the iodine level in salt (30 mg/kg) (de Benoist et al. 2008). Overdose of iodine will also cause adverse effects such as hyperthyroidism and chronic lymphocytic thyroiditis (Feldt-Rasmussen 2001; Zahidi et al. 1999).
In a normal food chain, 75–85 % of iodine in human body comes from phytogenic food (DeLong et al. 1997; Welch and Graham 2005). Iodine in phytogenic food usually combines with amino acid, which can be readily assimilated after the reduction of iodide to iodine ion. The bioavailability of iodine in many foods can be as high as 99 % (Katamine et al. 1987). And the whole process is totally safe for human. Researches have shown that plant products, such as rice, spinach, and cereal, can be considered as potential source of iodine supply to mitigate iodine deficiencies (Dai et al. 2006; Hong et al. 2008a, b; Mackowiak and Grossl 1999; Weng et al. 2008a; Yu et al. 2011). Iodine in phytogenic food comes primarily from soil. Thus, the background value and bioavailability of iodine in soil determines whether or not the iodine in vegetables or fruits can meet the need of human body. It is the low background content of iodine in the ecosystem, especially in soil, that directly contributes IDD in a region (Korobova 2010). It is possible to achieve effective iodine supplementation through daily diet when the phytogenic food is produced from the iodine-rich soil. Thus, IDD may be well controlled or even eliminated. In this article, a sustainable strategy to control IDD by growing vegetables in iodine-enriched soil is proposed and its feasibility is reviewed.
Algal iodine fertilizer
It is well known that the low iodine content in soil results in low iodine phytogenic food, which causes IDD in those affected regions (Ren et al. 2008). How to improve the iodine content in soil in these areas? The most obvious way is to add exogenous iodine to soil and thus elevate the iodine content in phytogenic food. Kelp (Laminaria japonica Aresch) is an abundant marine species that can accumulate iodine as well as mineral substance such as potassium, magnesium and ferrum (Mišurcová et al. 2011). The resultant iodine concentration could be hundreds and thousands times higher than the environment where the kelp is grown. In addition, previous researchers indicated that the contents of total iodine in Laminaria japonica were highest, the iodine content could reach 734 mg kg−1 (wet basis), and most of which is water soluble (Hou et al. 1997; Teas et al. 2004). Therefore, kelp can be utilized as excellent exogenous iodine source. Weng et al. (2008a) found out that granulated algal iodine fertilizer manufactured by mixing grounded dry kelp with low-grade diatomite (passing through 100-mesh sieve) at a ratio of 1:1 could be an ideal fertilizer for elevating iodine level in soil and improving iodine uptake by plants. The porous structure in diatomite (as shown in Fig. 1) has strong absorption capability and can facilitate the plant to absorb more nutrients from the surrounding soil (UUSG 2008; Weng et al. 2008a; Wu et al. 2005). As a result, diatomite-containing algal iodine fertilizer can be an excellent soil amendment by creating a favorable environment for plant uptake of iodine.
Porous structure in diatomite provides strong adsorption capability to the host soil and facilitates plant growth. a Infrared spectra of diatomite. The strong absorption at 912, 798, 695, 537, 470 and 453 cm−1 indicates the coexistence of smectite and illite minerals in the diatomite. b Transmission electron microscopy (TEM) photograph of raw diatomite (×2,900). The floccules in the porous structures are smectite and illite minerals
To further study the residual iodine content after the soil was fertilized by algal iodine fertilizer, a series of measurements were taken 90 days after grounded kelp/kelp diatomite mixture was applied (Weng et al. 2008a). Figure 2 demonstrates the relationship between residual iodine content and the original iodine concentration in fertilized soil and iodine vertical distribution 90 days after fertilization (Weng et al. 2008a). As shown in Fig. 2a, when original fertilized soil has an iodine concentration lower than 40 mg/kg (Fig. 2a, black dots), the residual iodine concentration increases rapidly with increasing iodine fertilizes, and the residual iodine concentration in the soil is about 60 % of original iodine added (Fig. 2a, circles). However, if the exogenous iodine added in soil exceeds 40 mg/kg, this increment trend slows down. When the original iodine content in the soil is 100 mg/kg, the residual iodine is about 25 % of original amount. Even when the iodine content is increased to 300 mg/kg, only 13 % of original iodine is conserved. This illustrates that the ability of soil to conserve iodine is very limited. Besides those uptakes by plants and conserved in soil, the exogenous iodine added to soil can either enter atmosphere by volatilization or migrate to groundwater by leaching.
The iodine content in soil 90 days after iodine fertilizer application. a The residual iodine content and residual percentage 90 days after iodine fertilizer application at different strengths. b Depth profile of iodine content 90 days after iodine fertilizer application at 180 mg/kg application strength
Figure 2b shows iodine vertical distribution in soil 90 days after fertilization. Two set of fertilizers with same iodine concentration (180 mg/kg) were applied at 9 cm from the top of the soil. One is kelp diatomite mixture (1:1 kelp and diatomite), and the other is grounded kelp only. As shown in Fig. 2b, the iodine concentration of the soil has a symmetric decreasing distribution centered at the 9 cm from the top. For kelp–diatomite case, the remnant iodine concentration at 9 cm is significantly higher than that of kelp-only case. This difference suggests that the diatomite in algal iodine fertilizer plays a significant role in keeping iodine in soil by adsorption and further slowing down the release of iodine from the fertilizer. As a result, iodine loss through leaching or volatilization may be reduced and iodine concentration may be maintained for plant uptake. Previous researches had revealed that high exogenous iodine concentration may be harmful to crop growth (Shinonaga et al. 2001; Weng et al. 2003; Weng et al. 2008b; Hong et al. 2007). Once iodine concentration is lower than 25 mg/kg, there has no detectable influence on vegetable growth. As the concentration increases, the plants start to be affected. If the exogenous iodine concentration is higher than 50 mg/kg, the growth of plant is significantly affected such as yellow leaves, short plant and small fruits.
In addition, the algal iodine fertilizer is relatively easy to be stored and transported (Hong et al. 2009a, b). Long-term use of algal iodine fertilizer can significantly increase the iodine background value of soil and thus cultivate iodine-rich vegetables. After a long period of iodine fertilizer application, the iodine level of the whole food chain can be elevated gradually by biogeochemistry process. Furthermore, the iodine deficiency ecosystem may be rescued, and the regional IDD may be eliminated.
Characteristics and pathway of iodine uptake
Many works have studied the characteristics of plant uptake of exogenous iodine (Hong et al. 2009a, b; Shinonaga et al. 2001; Tsukada et al. 2008; Weng et al. 2013a; Zhu et al. 2003). Most researches conducted experiments using KI and KIO3 as iodine source. The iodine application methods include hydroculture, foliage spray and pot culture. These studies concluded that exogenous iodine was readily to be absorbed by vegetables. Weng et al. (2013b) cultured many types of vegetables in fields using algal iodine fertilizer as the iodine source. They found out that algal iodine in soil could be absorbed by vegetables under field conditions.
Figure 3 shows the iodine content in edible part of 6 leaf vegetables and that of seven fruit vegetables (e.g., eggplant and tomato) under different intensity of iodine fertilizer application (Weng et al. 2008a). The leaf vegetables include cabbage, spinach, potherb mustard, Chinese cabbage, coriander and celery. And the fruit vegetables include tomato, cucumber, long cowpea, eggplant, hot pepper, young soybean and radish. There is significant increase in iodine concentration with increasing fertilizer application for both vegetables. The leaf vegetables absorb significantly more iodine than the fruit vegetables. Differences in iodine uptake exist between different types of vegetables. Among all leaf vegetables tested, Chinese cabbage has the highest iodine uptake, while young soybean and radish are the most iodine-absorbed vegetables in fruit vegetable category. The high iodine absorption of young soybean is due to its ability of continuous accumulation of exogenous iodine (Hong et al. 2008a, b). As for radish, it is absolutely different from other fruit vegetables. Radish, as the root of the plant, itself involves in iodine absorption process. It is obvious that uptake of exogenous iodine is not only influenced by the intensity of iodine fertilizer applied, but also by the types of vegetables.
The plant root can obtain element via two processes, passive and active uptake (Redjala et al. 2009). Passive uptake is dominant if the concentration of an element in external environment is much higher than that of normal circumstance (usually no fertilizer applied). In a hydroculture experiment, cabbage and celery were cultured in liquid culture supplied with different iodine concentration and different iodine forms, IO3 − or I−. As shown in Fig. 4, uptake of IO3 − by cabbage and celery follows Michaelis–Menten kinetic model if the exogenous iodine is in the range of 0.01–0.5 mg/L (Hong et al. 2009a, b). Under this situation, uptake of IO3 − by cabbage and celery is dominated by metabolism. For I− uptake, the pattern is different. When exogenous iodine concentration is much higher than 0.5 mg/L, uptake rate is positively related with iodine concentration (r ≥ 0.99), which shows characteristics of passive uptake. Obviously, under such high iodine concentration, the uptake is dominated by nonmetabolic factor.
As shown in Fig. 4, under low iodine concentration (< 0.5 mg/L), the two plants (cabbage and celery) tend to uptake more iodine in the form of IO3 − than I− (Hong et al. 2009a, b). When iodine concentration is higher than 0.5 mg/L, I− is the preferred form to be absorbed. This result reveals that the uptake of iodine is an active uptake process under low concentration, and IO3 − is a more favorable form to be absorbed. When iodine concentration of liquid culture is high, the iodine absorption is passive uptake. The smaller ionic radius of I− than IO3 − facilitates its entrance into plant plasma membrane.
As indicated previously, iodine absorbed by root can migrate to stalk and leaf. The analysis of 125I isotope tracing experiment shows that most (80.20 %) of iodine absorbed transfers to stalk, petiole and leaf in a descending order (Table 1) (Weng et al. 2009). In particular, 39.79 % of the 125I accumulates in the stem, 27.25 % in the petiole and 13.16 % in the leaf. The accumulation coefficient can be defined as
Table 1 shows the average accumulation coefficient of the cabbage is 2.63 (Weng et al. 2009). However, the accumulation coefficient of root and stalk of cabbage are 5.3 times and 2.7 times greater than the average value. The accumulation coefficient of petiole and leaf is lower than the average value. Figure 5 is the autoradiography of cabbage leaves fed with radioactive 125I (Weng et al. 2009). Figure 5a is the leaf containing 125I, while Fig. 5b is the leaf without 125I. It is obvious that petiole accumulates more 125I than leaf, which is indicated by darker shades. The autoradiographic data are consistent with the data of specific activity of 125I. Although the accumulation coefficients of petiole and leaf are smaller than those of root, most iodine uptake is stored in petiole and leaf because of their dominant mass fractions in cabbage.
The relationship between iodine in vegetables and iodine in soil
In order to investigate the fate and transport of iodine in a soil–plant system, a radioactive iodide (125I) tracer experiment was conducted in two different kinds of soils (Weng et al. 2009). Sandy loam means most of the soil particles are bigger than 2 mm in diameter, which gives good water drainage and has a low capability to hold nutrients. Paddy soil is a simple soil used for the cultivation of rice under a temporary-flooded condition, which cannot be considered as natural soil due to modifications such as levelling, cultivation, puddling, submerging and application of natural and artificial fertilizers. Figure 6 shows a dynamic relationship between 125I in vegetable (specifically Chinese cabbage) and 125I in soil (Weng et al. 2009). As the plant grows, more 125I in soil has been transferred to vegetable. A dual-box model can be used to describe this biogeochemistry relationship as indicated at the right corner of Fig. 6. The variation rate of total activity of 125I can be described as follows:
where q s and q v are total activity of 125I in soil and vegetable, respectively; k 12 is rate constant of iodine’s migration from soil to vegetable; k 21 is rate constant of iodine’s migration from vegetable to soil; k 10 and k 20 are the rate constants of iodine loss in vegetable and soil, respectively. The total activities are the product of the specific activity (C s or C v) and the mass (m s or m v): q s = C s m s, q v = C v m v. The initial specific activity of 125I namely C y (0) = 0. By defining E 1 = k 10 + k 20, E 2 = k 20 + k 21, C s and C v can be described as follows:
where
The specific activity of 125I in soil box model and vegetable box model can be expressed by biexponential equation. Taking k 20 as 0 due to negligible iodine volatilization, together with the initial specific activity value, k 10, k 12 and k 21 can be calculated. These rate constants are listed in Table 2 (Weng et al. 2009).
The difference between k 12 and k 21 represents iodine accumulation in vegetable. For paddy soil, the difference between these two rate constants is 0.0052, while this difference is 0.0008 for sandy loam. Apparently, vegetable cultivated in paddy soil absorbs more iodine than that in sandy loam. Clay contents of paddy soil and sandy loam are 36.86 and 2.5 %, respectively. For paddy soil, sandy particle content is 17.8 %, while for sandy loam, the sandy particle content is as high as 66.3 %. The content differences between paddy soil and sandy loam are correlated with the iodine uptake differences for plants. It is the soil texture that may influence the plant iodine uptake. For example, the porous structure of clay can serve as a reservoir to retain iodine and thus slowly release iodine for plant uptake. The clay minerals in diatomite also facilitate the plant uptake process.
Results of the previous studies indicate that the vegetable adsorption only counts for a small fraction of iodine loss in soil, iodine loss in soil, primarily through leaching and volatilization and is mainly caused by the physicochemical and biochemical properties of soil. Iodine loss was highly enhanced by the presence of plant, and a marked loss was found from rice plants cultivated under a flooded condition (Muramatsu and Yoshida 1995). It has been suggested that the soil sorption processes could affect iodine volatilization (Whitehead 1981), the rate of loss of iodine from the soil solution is dependent upon its speciation, while iodide is lost more rapidly (minutes–hours) than iodate (hours–days) especially in high organic matter soils (Sheppard and Hawkins 1995; Shetaya et al. 2012). What’s more, the soil bacteria plays an important role in the process of iodine volatilization; the emission of iodine was stimulated by the presence of yeast extract but was inhibited when soils were autoclaved (Amachi et al. 2003). In the leaching experiments conducted by Weng et al. (2009), they found out there was difference in 125I loss between paddy soil and sandy soil columns. In column experiments by Liu et al. (1998), they found out after passing the soil volume, about 5.26 % and 11.05 % of 125I was leached from the laterite and paddy soil. In addition, volatilization of plant iodine may be another process for soil iodine transportation to the atmospheric environment (Bostock et al. 2003; Muramatsu and Yoshida 1995).
Distribution of iodine in vegetables
To further understand iodine distribution in vegetables, different organs in two categories of vegetables, which include leaf vegetables and fruit vegetables, were systematic analyzed. For leaf vegetables, iodine concentration in root is higher than that in leaf. While for fruit vegetables, organ’s iodine concentration has a descending order of root> leaf> stalk> fruit (Weng et al. 2013b).
Figure 7 shows the relative concentration of iodine in subcell units such as cell wall, organelle and cytoplasm (soluble part of cell) (Weng et al. 2013b). Most iodine is accumulated in cytoplasm, while organelle has the least iodine accumulation. About 58 % (ranging from 40 to 77 %), 28 % (ranging from 14 to 40 %) and 14 % (ranging from 4 to 24 %) of the iodine are stored in cytoplasm, cell wall, and organelle, respectively.
AgI precipitate-based method was used to reveal the spatial distribution of iodine in vegetable cells (Weng et al. 2013b). Figure 8 is a transmission electron microscopy image of cells of iodine-rich cabbage and iodine-free cabbage (Weng et al. 2013b). No dark AgI precipitate can be found in root cell of iodine-free cabbage (8a), whereas it is abundant in root cell of iodine-rich cabbage (8b). The accumulation of iodine in fibrous tissue reveals that large amount of iodine absorbed is retained by fiber-rich root instead of transferred upward.
TEM micrographs of various cell types from cabbage indicating that iodine absorbed from root can be transferred with water to many important organelles. a Root cell (control group, ×8,000). b Root cell (experiment group, ×60,000). c Stalk cell(control group, ×12,000). d Stalk cell (experiment group, ×20,000). e Leaf cell (control group, ×12,000). f Leaf cell (experiment group, ×20,000). Chl chloroplast, Ft fibrous tissue, I intercellular space, Ig AgI, M mitochondria, N nucleus, Nm nuclear membrane, V vacuole, W cytoderm
Figure 8c is an image of cabbage stalk cell without AgI. In contract, black AgI precipitate appears in stalk cell of the iodine-rich cabbage (Fig. 8d). Lots of AgI dots can be observed in chloroplast, which is the place for photosynthesis. Iodine absorbed by root is transferring to the indispensable organelle involving important functions such as photosynthesis.
Chloroplast (Chl), cell wall (W), vacuole (V) and starch grain can be easily found in leaf cell of iodine-free cabbage, as shown in Fig. 8e. However, there is no AgI precipitate. Again, AgI can be found in chloroplast of leaf cell of iodine-rich cabbage, as shown in Fig. 8f. All the results indicate that iodine absorbed from root can be transferred with water to many important organelles.
Bioavailability of iodine in vegetables
We have known that consumption of more inorganic iodine in salt would result in iodine deficiency disorders (IDD). But what will happen if we ingest too much iodine from iodine-rich vegetables? Researches carried out by Chi (2002) gave us the answer. In his researches, he compared the effect on supplementing iodine of inorganic iodine with that of organic in kelp and found out if getting excessive inorganic iodine, the mice appeared the drop of weight and goiter. If a mouse was given beyond 40 μg iodine/day, obvious goiter appeared. Contrastingly, the dose of organic iodine in kelp even exceeded 200 μg iodine/mouse day, but goiter did not appear. Even long-term consumption of organic iodine in kelp (200 μg/mouse), iodine toxicity did not occur (Gu et al. 2003). When 10,000 mg/kg bw organic iodine was given at once, the mouse did not appear iodine poisoning and death (Zhuang et al. 2003). So iodine in vegetables is much more effective and safe than inorganic iodine in salt.
Bioavailability is a term used to describe the way chemicals are absorbed by humans and other animals. It can be defined as the proportion of the total mineral in a food utilized for normal body functions (Fairweather-Tait 1992). The bioavailability of iodine in vegetables is very important when evaluating the effectiveness of the vegetable-based iodine. In order to estimate the bioavailability of iodine in foods in human nutrition, an in-vivo study using two seaweeds (laminaria hyperborean and gracilaria verrucosa) was carried out by Aquaron et al. (2002). The research shows iodine bioavailability is from 61.5 to 85 %. However, when Romarís Hortas et al. (2011) used an in-vitro digestion method based on element dialyzability using a gastric and intestinal digestion with pepsin and pancreatin with bile salts, respectively, they found out that the concentration of bioavailable iodine is just from 2.0 to 18 % within the tested nine different types of edible seaweed (Dulse, Nori, Kombu, Wakame, Sea Spaghetti, Sea Lettuce, Spirulina, Agar–agar, NIES-09). The difference may come from the different research methods (in-vivo study and in-vitro procedure). Iodine dialyzability was found to be independent on the total iodine content. What’s more, it is not easy to found correlations between the type of seaweed and the dialyzability. The bioavailability of a substance can be influenced by the major nutrients released from the foodstuff during the simulated in vitro process (Romaris-Hortas et al. 2012).
The stability of iodine in vegetables is an important issue in evaluating the effectiveness of the vegetable-based iodine supplement approach to reduce IDD. Research by Weng et al. (2012) performed a series of release studies and cooking tests to evaluate the stability of iodine in iodine-rich celery. Figure 9 shows the release of iodine from iodine-rich celery with soaking time (Weng et al. 2012). In this experiment, 1 g stalk of iodine-rich celery was soaked in 10 ml deionized water at room temperature for 36 h. The release profile of iodine can be classified into three stages. The first 8-h release is a low release stage. By the end of eighth hour, only about 4.08 mg/kg iodine is released, which counts for 10.5 % of the original iodine. The second stage spans from 8th to 24th hour, which is the major release stage. By the end of 24th hour, the iodine released reaches 12.89 mg/kg, which is 33.3 % of the original iodine. The third stage is a stabilized stage during which almost no more iodine was released. The release study indicates that iodine-rich celery has the good ability to retain iodine in aqueous environment. Even after 24-h soaking, only 1/3 of the iodine is lost.
In many cultures, cooking is a necessary step to make vegetables more tasty (Longvah et al. 2012; Weng et al. 2012). It is important to address the possibility of iodine loss during cooking process. To compare the iodine loss of iodized salt and iodine-rich celery in cooking process, two sets of celery/salt mixture were tested under the same cooking condition (Fig. 10) (Weng et al. 2012). These two combinations include (1) common celery with iodized salt and (2) iodine-rich celery with iodine-free salt. After the mixture was boiled to 100 °C, at predetermined time point, the celery and soup samples were taken and the iodine concentrations were measured. Figure 10a shows iodine concentration change in iodine-rich celery and soup while cooking. The iodine preserved is the summation of iodine in celery and soup. Obviously, after 30-min cooking, only about 15 % of iodine in celery is lost. It is important to notice that about 60 % of original iodine remains in celery; iodine in soup only counts small percentile in total iodine preserved. In many cases, the soup of the vegetable is discarded after cooking. Since most iodine is reserved in celery which is ready to be consumed, it is easy to keep track of the amount of iodine taken. When iodized salt cooked with celery, after 2-min cooking, about 50 % total iodine is lost and the iodine absorption by celery is negligible, as shown in Fig. 10b. After 5 min, the loss of iodine reached 69 %, whereas the iodine absorbed by celery is only 1.6 %. As comparison, for iodine-rich celery case, the celery retains 93 and 86 % of iodine after cooking for 2 and 5 min, respectively. These results demonstrate that the iodine in iodine-rich celery is more stable than that in the iodized salt during cooking processes, and iodine-rich celery has better bioavailability.
The average iodine content of edible part of cabbage, spinach, potherb mustard, Chinese cabbage, coriander and celery is 9.1, 1.8, 5.8, 4.2, 19.3 and 9.4 mg/kg, respectively, when the application intensity of algal iodine fertilizer reaches 12 mg/m2. Assuming the moisture content of fresh vegetables as 75 %, then the iodine content of edible part of fresh vegetables is 2.28, 0.45, 1.45, 1.05, 4.83 and 2.35 mg/kg (fresh mass), respectively. The daily iodine intake, recommended by WHO, is 150 μg for adults (above 12 years) and 200 μg for pregnant and lactating women. Assuming the bioavailability of iodine in vegetables as 73.25 %, therefore, 45–455 g iodine-rich leaf vegetables are sufficient to meet the recommended dose for adult. For fruit vegetables, if the application intensity of algal iodine fertilizer reaches 75 mg/m2, the average iodine content of eggplant, hot pepper, cucumber, tomato and long cowpea is 1.23, 0.88, 0.91, 1.12 and 0.92 mg/kg, respectively, and 15.56, 21.30, 10.48, 7.74 and 8.42 mg/kg, respectively, for the leaf and stalk part. When application intensity of algal iodine fertilizer is 50 and 35 mg/m2, average iodine content of young soybean and radish can reach 1.82 and 1.74 mg/kg, respectively. Since fruit iodine content is comparable to that of leaf vegetables, iodine-rich fruit can be an alternative to iodine-rich leaf vegetables. Instead of discard the stalks and leaves of fruit vegetables, they can be grounded as feedstock for poultry and livestock. By this way, iodine background of food chain can be elevated without extra effort is made and eventually benefit the iodine deficiency ecosystem.
Summary
The iodine-rich phytogenic food could be a more effective iodine supplement compared with traditional iodized salt, due to its high bioavailability and low risk of overdose. The process to produce iodine-rich phytogenic food is environmental-friendly and sustainable. As the main component of algal iodine fertilizer, kelp (Laminaria japonica Aresch) is an excellent species for large-scale cultivation in coast area with high productivity. Besides iodine, kelp also can absorb other nutritious elements such as nitrogen, phosphorus, potassium and magnesium. The algal iodine fertilizer provides an organic source of iodine for vegetables or crops; meanwhile, it can also improve the physical and chemical properties of soil, providing necessary macro- and micro-nutrients, which cannot be found in other iodine enrichment approach such as adding inorganic iodine in drip irrigation. People in underdeveloped regions not only benefit from iodine supplement from iodine-rich phytogenic food, but also take the advantage of sustainable improvement of the soil quality locally. Therefore, the iodine-rich phytogenic food could be a promising method for the prevention and control of iodine deficiency. Heavy metal contaminant of algal could be an issue in some regions, and the related monitoring should be a part of quality control of the algal iodine fertilizer production. On the other hand, although the transport cost of algal iodine fertilizer could be increased for the inland region, the development of modern efficient transportation system could well reduce the cost to a degree that for most areas, the transport cost would be acceptable considering the overall benefits obtained from the organic fertilizer.
References
Amachi, S., Kasahara, M., Hanada, S., Kamagata, Y., Shinoyama, H., Fujii, T., et al. (2003). Microbial participation in iodine volatilization from soils. Environmental Science Technology, 37(17), 3885–3890.
Andersson, M., de Benoist, B., Darnton-Hill, I., & Delange, F. (2007). Iodine deficiency in Europe: A continuing public health problem. France, Geneva: World Health Organization.
Andersson, M., Karumbunathan, V., & Zimmermann, M. B. (2012). Global iodine status in 2011 and trends over the past decade. Journal of Nutrition, 142(4), 744–750. doi:10.3945/jn.111.149393.
Aquaron, R., Delange, F., Marchal, P., Lognon E, V., & Ninane, L. (2002). Bioavailability of seaweed iodine in human beings. Cellular and Molecular Biology (Noisy-le-Grand, France), 48(5), 563–569.
Biber, F. Z., Unak, P., & Yurt, F. (2002). Stability of iodine content in iodized salt. Isotopes Environ Health Stud, 38(2), 87–93. doi:10.1080/10256010208033316.
Bostock, A. C., Shaw, G., & Bell, J. N. (2003). The volatilisation and sorption of 129I in coniferous forest, grassland and frozen soils. Journal of Environmental Radioactivity, 70(1–2), 29–42. doi:10.1016/S0265-931X(03)00120-6.
Chi, Y. S. (2002). The evaluation of organic iodine in kelp supplementing iodine on animal. Journal of Chinese Institute of Food Science and Technology, 2(3), 37–42.
Dai, J. L., Zhu, Y. G., Huang, Y. Z., Zhang, M., & Song, J. L. (2006). Availability of iodide and iodate to spinach (Spinacia oleracea L.) in relation to total iodine in soil solution. Plant and Soil, 289(1–2), 301–308.
de Benoist, B., Andersson, M., Egli, I. M., El Bahi, T., & Allen, H. (2004). Iodine status worldwide: WHO global database on iodine deficiency. France, Geneva: World Health Organization.
de Benoist, B., McLean, E., Andersson, M., & Rogers, L. (2008). Iodine deficiency in 2007: Global progress since 2003. Food and Nutrition Bulletin, 29(3), 195–202.
DeLong, G. R., Leslie, P. W., Wang, S. H., Jiang, X. M., Zhang, M. L., Rakeman, M., et al. (1997). Effect on infant mortality of iodination of irrigation water in a severely iodine-deficient area of China. Lancet, 350(9080), 771–773.
Fairweather-Tait, S. J. (1992). Bioavailability of trace elements. Food Chemistry, 43(3), 213–217. doi:10.1016/0308-8146(92)90176-3.
Feldt-Rasmussen, U. (2001). Iodine and cancer. Thyroid, 11(5), 483–486.
Gerber, H., Peter, H. J., Burgi, E., Bigler, S., Kaempf, J., & Zbaeren, J. (1999). Colloidal aggregates of insoluble inclusions in human goiters. Biochimie, 81(5), 441–445.
Gu, J., Sun, P., & Zhuang, G. D. (2003). Study on the chronic accumulating of biological organic iodine in kelp. Food Research and Development, 24(4), 48–49.
Hetzel, B. S. (2005). Towards the global elimination of brain damage due to iodine deficiency—The role of the International Council for Control of Iodine Deficiency Disorders. International Journal of Epidemiology, 34(4), 762–764. doi:10.1093/ije/dyi073.
Hong, C. L., Weng, H. X., Qin, Y. C., Yan, A. L., & Xie, L. L. (2008a). Transfer of iodine from soil to vegetables by applying exogenous iodine. Agronomy for Sustainable Development, 28(4), 575–583. doi:10.1051/agro:2008033.
Hong, C. L., Weng, H. X., Yan, A. L., & Islam, E. U. (2009a). The fate of exogenous iodine in pot soil cultivated with vegetables. Environmental Geochemistry and Health, 31(1), 99–108. doi:10.1007/s10653-008-9169-6.
Hong, C. L., Weng, H. X., Yan, A. L., & Xie, L. L. (2007). Characteristics of iodine uptake and accumulation by vegetables. Chinese Journal of Applied Ecology, 18(10), 2313–2318.
Hong, C. L., Weng, H. X., Yan, A. L., & Xie, L. L. (2008b). Study on characteristics of iodine absorption and accumulation of vegetable soybean. Chinese journal of oil crop sciences, 30(1), 95–99.
Hong, C. L., Weng, H. X., Yan, A. L., & Xie, L. L. (2009b). Dynamic characterization of iodine uptake in vegetable plants. Acta Ecologica Sinica, 29(3), 1438–1447.
Horton, S., & Miloff, A. (2010). Iodine status and availability of iodized salt: an across-country analysis. Food and Nutrition Bulletin, 31(2), 214–220.
Hou, X. L., Chai, C. F., Qian, Q. F., Yan, X. J., & Fan, X. (1997). Determination of chemical species of iodine in some seaweeds (I). Science of the Total Environment, 204(3), 215–221.
Katamine, S., Mamiya, Y., Sekimoto, K., Hoshino, N., Totsuka, K., & Suzuki, M. (1987). Differences in bioavailability of iodine among iodine-rich foods and food colors. Nutrition Reports International, 35(2), 289–297.
Korobova, E. (2010). Soil and landscape geochemical factors which contribute to iodine spatial distribution in the main environmental components and food chain in the central Russian plain. Journal of Geochemical Exploration, 107(2), 180–192.
Liu, X. H., Liu, Q. Y., Kuang, Y. H., Chai, H., & Yu, H. H. (1998). The leaching and moving of 125I in subtroptics soils of south China. Acta Agriculturae Nucleatae Sinica, 12(3), 44–47.
Longvah, T., Toteja, G. S., Bulliyya, G., Raghuvanshi, R. S., Jain, S., Rao, V., et al. (2012). Stability of added iodine in different Indian cooking processes. Food Chemistry, 130(4), 953–959.
Mackowiak, C. L., & Grossl, P. R. (1999). Iodate and iodide effects on iodine uptake and partitioning in rice (Oryza sativa L.) grown in solution culture. Plant and Soil, 212(2), 135–143.
Melse-Boonstra, A., & Jaiswal, N. (2010). Iodine deficiency in pregnancy, infancy and childhood and its consequences for brain development. Best Practice and Research Clinical Endocrinology and Metabolism, 24(1), 29–38. doi:10.1016/j.beem.2009.09.002.
Mišurcová, L., Machů, L., & Orsavová, J. (2011). Seaweed minerals as nutraceuticals. Advances in Food and Nutrition Research, 64, 371–390. doi:10.1016/B978-0-12-387669-0.00029-6.
Muramatsu, Y., & Yoshida, S. (1995). Volatilization of methyl iodide from the soil-plant system. Atmospheric Environment, 29(1), 21–25.
Redjala, T., Sterckeman, T., & Morel, J. L. (2009). Cadmium uptake by roots: Contribution of apoplast and of high- and low-affinity membrane transport systems. Environmental and Experimental Botany, 67(1), 235–242. doi:10.1016/j.envexpbot.2009.05.012.
Ren, Q., Fan, J., Zhang, Z., Zheng, X., & DeLong, G. R. (2008). An environmental approach to correcting iodine deficiency: Supplementing iodine in soil by iodination of irrigation water in remote areas. Journal of Trace Elements in Medicine and Biology, 22(1), 1–8.
Romarís Hortas, V., García-Sartal, C., Barciela-Alonso, M. D. C., Domínguez-González, R., Moreda-Piñeiro, A., & Bermejo-Barrera, P. (2011). Bioavailability study using an in vitro method of iodine and bromine in edible seaweed. Food Chemistry, 124(4), 1747–1752. doi:10.1016/j.foodchem.2010.07.117.
Romaris-Hortas, V., Bermejo-Barrera, P., Moreda-Pineiro, J., & Moreda-Pineiro, A. (2012). Speciation of the bio-available iodine and bromine forms in edible seaweed by high performance liquid chromatography hyphenated with inductively coupled plasma-mass spectrometry. Analytica Chimica Acta, 745, 24–32. doi:10.1016/j.aca.2012.07.035.
Rose, N. R., Bonita, R., & Burek, C. L. (2002). Iodine: an environmental trigger of thyroiditis. Autoimmunity Reviews, 1(1–2), 97–103.
Sheppard, M. I., & Hawkins, J. L. (1995). Iodine and microbial interactions in an organic soil. Journal of Environmental Radioactivity, 29(2), 91–109.
Shetaya, W. H., Young, S. D., Watts, M. J., Ander, E. L., & Bailey, E. H. (2012). Iodine dynamics in soils. Geochimica et Cosmochimica Acta, 77, 457–473.
Shinonaga, T., Gerzabek, M. H., Strebl, F., & Muramatsu, Y. (2001). Transfer of iodine from soil to cereal grains in agricultural areas of Austria. Science of the Total Environment, 267(1–3), 33–40.
Sooch, S. S., Deo, M. G., Karmarkar, M. G., Kochupillai, N., Ramachandran, K., & Ramalingaswami, V. (2001). Prevention of endemic goitre with iodized salt. National Medical Journal of India, 14(3), 185–188.
Teas, J., Pino, S., Critchley, A., & Braverman, L. E. (2004). Variability of iodine content in common commercially available edible seaweeds. Thyroid, 14(10), 836–841.
Tsukada, H., Takeda, A., Tagami, K., & Uchida, S. (2008). Uptake and distribution of iodine in rice plants. Journal of Environmental Quality, 37(6), 2243–2247.
UUSG. (2008). USGS minerals information—Diatomite statistics and information. http://minerals.usgs.gov/minerals/pubs/commodity/diatomite/#pubs.
Vejbjerg, P., Knudsen, N., Perrild, H., Carle, A., Laurberg, P., Pedersen, I. B., et al. (2007). Effect of a mandatory iodization program on thyroid gland volume based on individuals’ age, gender, and preceding severity of dietary iodine deficiency: A prospective, population-based study. Journal of Clinical Endocrinology and Metabolism, 92(4), 1397–1401. doi:10.1210/jc.2006-2580.
Waszkowiak, K., & Szymandera-Buszka, K. (2008). Effect of storage conditions on potassium iodide stability in iodised table salt and collagen preparations. International Journal of Food Science & Technology, 43(5), 895–899.
Welch, R. M., & Graham, R. D. (2005). Agriculture: The real nexus for enhancing bioavailable micronutrients in food crops. Journal of Trace Elements in Medicine and Biology, 18(4), 299–307. doi:10.1016/j.jtemb.2005.03.001.
Weng, H. X., Hong, C. L., Xia, T. H., Bao, L. T., Liu, H. P., & Li, D. W. (2013a). Iodine biofortification of vegetable plants—An innovative method for iodine supplementation. Chinese Science Bulletin,. doi:10.1007/s11434-013-5709-2.
Weng, H. X., Hong, C. L., & Yan, A. L. (2013b). Biogeochemical transport of iodine and its quantitative model,. doi:10.1007/s11430-013-4594-5.
Weng, H. X., Weng, J. K., Yan, A. L., Hong, C. L., Yong, W. B., & Qin, Y. C. (2008a). Increment of iodine content in vegetable plants by applying iodized fertilizer and the residual characteristics of iodine in soil. Biological Trace Element Research, 123(1–3), 218–228. doi:10.1007/s12011-008-8094-y.
Weng, H. X., Weng, J. K., Yong, W. B., Sun, X. W., & Zhong, H. (2003). Capacity and degree of iodine absorbed and enriched by vegetable from soil. Journal of Environmental Science (China), 15(1), 107–111.
Weng, H. X., Yan, A. L., Hong, C. L., Qin, Y. C., Pan, L., & Xie, L. L. (2009). Biogeochemical transfer and dynamics of iodine in a soil-plant system. Environmental Geochemistry and Health, 31(3), 401–411. doi:10.1007/s10653-008-9193-6.
Weng, H. X., Yan, A. L., Hong, C. L., Xia, T. H., & Liu, H. P. (2012). The absorption of iodide and iodate by celery and their bioavailability. Geochimica (Beijing), 41(4), 393–400.
Weng, H. X., Yan, A. L., Hong, C. L., Xie, L. L., Qin, Y. C., & Cheng, C. Q. (2008b). Uptake of different species of iodine by water spinach and its effect to growth. Biological Trace Element Research, 124(2), 184–194. doi:10.1007/s12011-008-8137-4.
Whitehead, D. C. (1981). The volatilisation, from soils and mixtures of soil components, of iodine as potassium iodide. Journal of Soil Science, 32(1), 97–102.
WHO, ICCIDD, & UNICEF. (1999). Progress towards the elimination of iodine deficiency disorders. India, New Delhi: WHO publication.
Wu, J., Yang, Y. S., & Lin, J. (2005). Advanced tertiary treatment of municipal wastewater using raw and modified diatomite. Journal of Hazardous Materials, 127(1–3), 196–203. doi:10.1016/j.jhazmat.2005.07.016.
Yu, W. J., Yao, Y., Wei, H. M., Long, M. H., & Tang, X. F. (2011). Absorption of exogenous iodine in rhizosphere and its effects on physiological parameters of cherry tomato plants. Guihaia, 4, 20.
Zahidi, A., Hababa, L., Idrissi, M. O., & Taoufik, J. (1999). Use of iodized salt and the risk of iodine overload. Therapie, 54(5), 549–552.
Zheng, B. S., Wang, B. B., Zhu, G. W., & Yu, X. Y. (2001). Environmental Geochemistry of iodine in atmosphere and plant—review and a hypothesis. Earth Science Frontiers, 8(2), 359–365.
Zhu, Y. G., Huang, Y. Z., Hu, Y., & Liu, Y. X. (2003). Iodine uptake by spinach (Spinacia oleracea L.) plants grown in solution culture: Effects of iodine species and solution concentrations. Environment International, 29(1), 33–37. doi:10.1016/S0160-4120(02)00129-0.
Zhuang, G. D., Gu, J., & Chi, Y. S. (2003). Study on the toxicology of biological organic iodine in kelp. Science and Technology of Food Industry, 24(12), 87–88.
Zimmermann, M. B. (2008). Iodine requirements and the risks and benefits of correcting iodine deficiency in populations. Journal of Trace Elements in Medicine and Biology, 22(2), 81–92. doi:10.1016/j.jtemb.2008.03.001.
Acknowledgments
This work was supported by the National Science Foundation of China (40873058 and 40373043).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weng, HX., Liu, HP., Li, DW. et al. An innovative approach for iodine supplementation using iodine-rich phytogenic food. Environ Geochem Health 36, 815–828 (2014). https://doi.org/10.1007/s10653-014-9597-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-014-9597-4