Abstract
Soils from old Au-mine tailings (La Petite Faye, France) were investigated in relation to the natural vegetation cover to evaluate the risk of metals and metalloids (Pb, As, Sb) mobilizing and their potential transfer to native plants (Graminea, Betula pendula, Pteridium aquilinum, Equisetum telmateia). The soils are classified as Technosols with high contamination levels of As, Pb, and Sb. The single selective extractions tested to evaluate available fraction (CaCl2, acetic acid, A-Rhizo, and DTPA) showed low labile fractions (<5 % of bulk soil contents), but still significant levels were observed (up to 342.6 and 391.9 mg/kg for As and Pb, respectively) due to the high contamination levels of soils. Even at high soil contaminations (considered as phytotoxic levels for plants), translocation factors for native plants studied are very low resulting in low concentrations of As, Sb, and Pb in their aerial part tissues. This study demonstrates the important role of (1) native plant cover in terms of “stabilization” of these contaminants, and (2) the poor effectiveness of extraction procedures used for this type of soil assemblages, i.e., rich in specific mineral phases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
After the extraction of economic elements, abandoned mine sites contain large amounts of waste material characterized by high concentrations of metals and metalloids such as As, Sb, Pb, Ni, Cr, and Zn, increasing the abundance of these elements at the Earth crust (e.g., Rodríguez et al. 2009; Wang et al. 2008). The dispersal of these pollutants into the environment around mine tailings and smelters, through wind and water erosion, is an important type of anthropogenic contamination. Due to the potential hazards posed by water resource contamination, there is growing interest in pollution control, remediation, and revegetation of abandoned mine sites (Lei and Duan 2008; Maiz et al. 2000; Remon et al. 2005; Wang et al. 2008).
There is extensive literature devoted to studies of soils contaminated by metals and metalloids around mine sites (e.g., Boussen et al. 2013; Dudka and Adriano 1997; Yang et al. 2009), with studies focusing on methods for evaluating the factors controlling the vertical distribution, behavior, and environmental impact of these pollutants with respect to both vegetation and human health. However, there are few data regarding “naturally” developed soils on mine tailings, most likely due to the disparity between the amount of time needed for soil development and the time elapsed since the end of industrial activity in most mining areas. Due to the large extent of contaminated mining areas that may extent over several hectares, economic interests have appeared regarding the use of these soils for crops or forest land applications. Studies are needed to understand the reuse potential of these soils and their biogeochemical cycles (Yao et al. 2009).
Due to the special chemical and physical properties of contaminated soils (e.g., extreme pH (Conesa et al. 2006), low nutrient availability, and biological deficiency (Chiu et al. 2006; Vega et al. 2006; Wang et al. 2008), only plants adapted to harsh environments can colonize such sites (Lei and Duan 2008). Indeed, remediation of sites by revegetation with appropriate plants offers the most effective method for achieving sustainable restoration and visual improvement (Remon et al. 2005; Wang et al. 2008). Maintaining and restoring natural site vegetation can be considered to be the first step because existing vegetation consists of appropriate plant species (Lei and Duan 2008; Madejón et al. 2003; Wang et al. 2008). Indeed, natural vegetation plays a considerable role in reclaiming contaminated land by tolerating inorganic contamination through many mechanisms for growth and reproduction in harsh environments. However, recent studies with commercial species clearly show failure in land reclamation (Andres and Jorba 2000; Martinez-Ruiz et al. 2007), most likely due to their lack of adaptation to growth on contaminated soils (Wang et al. 2008) and potentially to growth in the prevailing climate for a large amount of metalliferous mine around the world (Singh et al. 2002; Li 2006).
This study was performed on old gold mine tailings in France, which presents an exceptionally high content in As, Sb, and Pb (several percent) compared to other studies. This mining site has been abandoned since 1964 and is colonized by native pioneer plants. The objectives of this work were to assess bioavailability of the inorganic pollutants (As, Sb, and Pb) through various single extraction (SE) procedures (CaCl2, acetic acid, A-Rhizo, and DTPA) and to compare natural vegetation cover. Moreover, the influence of soil mineralogy as well as the feasibility of site reclamation will be discussed.
Materials and methods
The study site
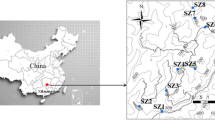
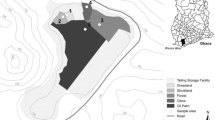
This study focuses on a former Au mine (La Petite Faye), located 60 km northeast (1°34′25.3″E, 46°08′37.0″N) of Limoges (French Massif central). The annual average rainfall and temperature are approximately 1,020 mm and 11.3 °C, respectively. Old mining activity has led to the accumulation of approximately 35,000 t of waste contaminated by heavy metals and metalloids. All wastes were stored in a settling basin (150 × 80 m) delimited by loamy dams on its sides. Since 45 years ago, mining activity has been discontinued, and vegetation has begun to slowly colonize the site and leads to the development of a soil from these wastes. Differences in parent material (pH, particle size distribution, metal content, and mineralogy, see previous study by Néel et al. 2003) have been clearly identified previously, corresponding to the delimitation of distinct zones (Fig. 1). In the paper of Néel and co-authors, the differentiation in 3 zones was conducted according to the vegetation cover of the site: no vegetation in the zone 1, zone 3 characterized by the presence of E. telmateia, the zone 2 being a transition zone. The present work started in 2008, since major changes were observed on the site: (1) the vegetation cover changed in terms of colonization since E. telmateia colonizes both zones 2 and 3 as described by Néel and co-authors; (2) vegetation (in the majority of Graminea) appears in the zone 1; and (3) soils are developed all over the site. Because of the evolution of the site since the precedent study, it was decided to divide the area in two zones, based on the vegetation cover, i.e., the both zones 2 and 3 become zone 2, whereas no change was observed in the zone 1. In more detail, the type of vegetation is quite different in both zones (Fig. 1): The zone 1 has very scarce vegetation cover with mainly grass (Graminea), jennets (Cytisus decumbens), ferns (Pteridium aquilinum), and some birch trees (Betula pendula) located at the periphery of the zone 1. On the contrary, the vegetation is very dense and vigorous in the second zone (zone 2) but with less diversity: giant horsetails (E. telmateia), birch trees (B. pendula), brambles (Rubus fructicosus), nettles (Urticae), and also some birch trees (B. pendula) near the zone 1 (periphery of zone 1 and zone 2).
Soil sampling and pedological parameters
Analyses were focused on two representative soil profiles located in both zones 1 and 2 (Fig. 1). To select the two representative profiles, 35 profiles (15 profiles in zone 1 and 20 in the zone 2) were carried out in order to make sure of the homogeneity and the representativeness of the sampling. The geochemical spatial approach from all sites allows to well separate the two zones: one highly contaminated, i.e., the zone 1; and another zone (zone 2), which is less contaminated (Joussein et al. 2012). Each horizon from the two representative profiles, as well as its parent material, was sampled, air-dried, and sieved at 2 mm before analysis. Color was determined using a Munsell chart, and pH was measured for a solid/liquid ratio of 2/5 with ultra pure water and 1 M KCl. The cation-exchange capacity (CEC) was determined by the 0.05 N cobalt hexamine method at the soil’s normal pH. This method was chosen as the actual soil pH values were lower than 6. Exchangeable cations were measured by atomic absorption spectroscopy, and the exchangeable acidity was measured by potentiometry. The organic C and N contents were determined by dry combustion with a C/H/N elemental analyzer (INRA Arras labs, France). Bulk densities of soil horizons were measured by the core cylinder method, with at least three replicates per horizon.
The mineralogy of bulk soil samples (<2 mm) was determined from crushed powder by X-ray diffraction (XRD) using a Siemens D 5000 diffractometer (CuKα, 40 kV, 30 mA) between 5 and 65°2θ at 0.12° 2θ/min. The chemical composition of each minerals phases, their morphology, and their organizations in the soil horizons were identified by scanning electron microscopy (SEM–EDS) using a Philips XL30 SEM at 20 kV (SerMiEL, Université de Limoges, France).
Soil and plant chemical analyses
The total chemical composition of each soil horizon (<2 mm; dried at room temperature) was performed by ACME Analytical Laboratories ltd (Vancouver, Canada). An aliquot of each sample was dried at 105 °C to determine relative humidity, and chemical analyses were corrected by the water content. Major elements were determined by ICP-AES after a lithium metaborate/tetraborate fusion and nitric acid digestion. Trace elements were measured by ICP-MS after an aqua regia digestion.
In this study, 4 representative plants species were sampled (i.e., Graminea and P. aquilinum for zone 1, E. telmateia for zone 2, and B. pendula, which are ubiquitous). For each representative plants species, 10 samples were collected (for B. pendula, 10 plants were sampled in each zones). All the plants tissues were carefully rinsed with ultrapure water and analyzed for their total metal contents. Plants were dried at 60 °C to a constant weight. Roots, shoots, and leaves were separated, and heavy metal contents were determined for each fraction after mineralization of 0.5 g of split sample (digestion by HNO3 and aqua regia) and analysis by ICP-MS (ACME Analytical Laboratories ltd; Vancouver, Canada). All the chemical analyses for plants are expressed in dry weight (dw). Each analysis was conducted in triplicate, and the data shown are the mean values of the three measurements.
Bioavailability assessment of chemical elements
The availability of metallic and metalloid elements was assessed through different selective extractions currently used in the literature: CaCl2, A-Rhizo, acetic acid, and DTPA (diethylene triamine pentaacetic acid) according to the protocols listed in Table 1. Extracted lead (Pb) was determined by flame atomic absorption spectrometry using a Varian SpectrAA 220 AAS equipped with a deuterium background correction system, and Arsenic (As) was determined by graphite furnace atomic absorption spectrometry using a Varian SpectrAA 880 Z GFAS equipped with a Zeeman background correction system. All measurements were performed in triplicate.
Statistical data analysis
Microsoft Excel 2004 and SPSS 19.0 for windows were used for statistical analysis of data. Pearson’s correlation coefficients were calculated for the data (phytoavailability extractions, soils, plants tissues), and statistical significance was set at the p < 0.05 confidence level.
Results and discussion
Physicochemical properties and mineralogical assemblage of soils
The physicochemical properties of the soils are summarized in Table 2. Briefly, soils from all zones were characterized by a low pH (zone 1: 3.4–4; zone 2: 4.2–5.7) and were very thin, without any B horizon. However, high organic carbon and nitrogen contents were observed, especially in zone 2 (up to 426 g/kg organic carbon in the top horizon), most likely due to the heavy vegetation cover in this zone. This fact explains high CEC values observed in the top horizons (then attributed to the contribution of organic matter). Because the parent material has an anthropogenic origin, these soils were either classified as Technosols (“dominated by human-made materials”) as described in WRB (FAO 2006) or “Anthroposols Artificiels” in the French nomenclature (Referentiel Pédologique 2008). Remon et al. (2005) reported the same properties for Technosols developed from alkaline substrates, as well as the minimal profile development as a consequence of the soil’s young age (Scalenghe and Ferraris 2009).
As expected for industrial waste derived from acidic rock-mining activities, XRD and SEM–EDS data (Figs. 2, 3) indicated the presence of quartz and feldspars in each horizon studied, as well as phyllosilicates (clinochlore and muscovite). These silicate mineral phases essentially corresponded to primary minerals inherited from the known hydrothermal processes involved in gold ore genesis. Weathering and pedogenesis induce the formation of secondary mineral phases (clays and clay minerals; Figs. 2, 3): kaolinite, illite, and As- and Pb-bearing phases, such as beudantite (PbFe3(AsO4)SO4(OH)6), scorodite (FeAsO4 2H2O), and As-rich iron oxyhydroxides like symplesite (Fe3(AsO4)2 8H2O for theoretical formulae) can be identified based on their main reference peaks. These results are in accordance with the previous works of Néel et al. (2003) on waste materials and Entisol. The mineralogical phases observed are clearly associated with sulfide oxidation weathering processes, as already shown by numerous authors (e.g., Mihaljevič et al. 2009; Roussel et al. 2000). Basically, scorodite and beudantite are present in both profiles either in organic horizons or in parent material; however, their abundance increases with depth. From these results, it can be observed that soil properties (e.g., pH, CEC, and mineralogy) directly reflect those of the parent waste materials (i.e., acidic materials with the mineralogy in accordance with granitic raw material). Thus, the waste material seems to control the development of soil properties and, most likely, the behavior of chemical elements as well.
SEM photographs (backscattered electron mode) of the bulk soil fraction (<2 mm). a and b refer to the upper (2–5 cm) and lower (>11 cm) horizons from profile 1, while c and d refer to the upper (3–5 cm) and lower (>7 cm) horizons of profile 2, respectively. Musc, beu, sco, fds, and qtz refer to muscovite, beudantite, scorodite, feldspath, and quartz
Soil chemical concentrations
The mean chemical analyses of the studied soil profile are presented in Table 3. As expected, the most abundant elements of both studied horizons are Si, Al, and Fe, and the concentrations of these elements increase with depth, with Si being present in the highest concentration, reaching 27.7 and 24.2 % in zones 1 and 2, respectively. The amount of Al ranges from 3.8 % (zone 2) to 4.5 % (zone 1), and Fe is high up to 9.0 % (zone 2) and 12.2 % (zone 1). The chemical composition and mineralogical assemblages of the parent material (deeper horizons) match with those of the initial gold ores (Figs. 2, 3; see below).
The concentration of As, Sb, and Pb is very high in these soils (Table 3). Arsenic content varies between 32,200 and 119,900 mg/kg in zone 1 and between 5,465 and 68,100 mg/kg in zone 2. Antimony concentrations are higher in zone 1, ranging from 432 to 1,406 mg/kg, than in zone 2, in which they vary from 108 to 930 mg/kg. Lead concentrations increase with depth from 6,939 to 21,300 mg/kg in zone 1 and from 1,299 to 14,500 mg/kg in zone 2. As expected, the amount of elements increases with depth due to the pedogenesis leaching and soil evolution. These concentrations of As, Sb, and Pb are generally greater than (1) the “possible” toxicity values or maximum allowable concentrations of trace elements in agricultural soils (5–20, 150, and 30–300 mg/kg, respectively; Kabata-Pendias 2001) and (2) the normal background levels given by several authors (1–30 mg/kg for As, 9–50 mg/kg for Pb, 1–8.8 mg/kg for Sb; Baize and Sterckeman 2001; Wilson et al. 2010) for soils of diverse origins and lithologies throughout the world. Due to scarce literature on soils directly developed from highly polluted tailings, this study explores one of the most polluted young Technosols reported to date. In effect, the literature generally reports very high levels of inorganic pollutants in mine tailings but neglects soils developed on these tailings. For example, Saunders et al. (2010) described tailings with 42,000 mg/kg As, while Rodríguez et al. (2009) showed Pb concentrations reaching approximately 94,000 mg/kg in mine tailings. Moreover, other studies analyze adjacent soils affected by aerial contamination or by waste deposits mixed with the soils (Boussen et al. 2013). However, total chemical analysis of soils is not sufficient to predict the potential risk to contamination of water resource or accumulation in native plants.
Solubility and extractability of metals and metalloids
Metal and metalloid soil phytoavailability were determined by four SEs, the results of which are reported in Fig. 4. As explained by Kabata-Pendias (2001), soluble, exchangeable, and chelated fractions are quite labile and are thus more bioavailable to plants and other organisms in the food chain. These SE procedures have been performed to study metal and metalloid mobility and, as a result, the potential bioavailability of these contaminants.
CaCl2 and acetic acid extracts reflect the exchangeable fraction of these contaminants, representing species that are weakly bound to particles in soils (mobile fraction; Novozamsky et al. 1993; Maiz et al. 2000). Both SE procedures should represent the “immediate” mobile and short-term available fractions in the soils. Neutral salt extraction with CaCl2 provides the most useful indication of metal phytoavailability and is more effective for estimating plant availability (Lebourg et al. 1996). To better evaluate the mobilizable fractions of these contaminants, DTPA and A-Rhizo methods were also used in this study. DTPA is a metal chelator currently used in the literature (Beckett 1989; Vázquez et al. 2008); it theoretically extracts metallic elements bounded to organic complexes and hydroxides. The A-Rhizo method extracts the metal and metalloid fractions supposedly mobilized in the rhizosphere by the action of roots exudates such as low-molecular-weight organic acids (LMWOAs) (Fang et al. 2007; Feng et al. 2005). The results for As and Pb are reported in Fig. 4 as extracted amounts and as percentages of the different fractions with respect to the total amount of soil extracted.
CaCl2-extractable As is present at concentrations between 3.6 and 42.9 mg/kg in zone 1 and between 2.7 and 55.2 mg/kg in the second zone. When DTPA was used, the extractable fraction of As was never greater than 2 mg/kg. The range of A-Rhizo-extractable As is from 24 to 269 mg/kg in zone 1 and from 76 to 578 mg/kg in zone 2. Overall, the behavior of As phytoavailability along profiles has the same trend in both zones: As phytoavailability decreases from the surface to deeper horizons. In zone 2, the trend is the same except in the case of A-Rhizo, for which higher phytoavailability is observed in deeper horizons.
Variations in Pb phytoavailability along profiles are clearly observed (Fig. 4). DTPA is the strongest extractant after acetic acid, followed by A-Rhizo and CaCl2. DTPA-extractable Pb is largely higher in zone 1 than in zone 2, reaching 391.9 mg/kg and 37.0 mg/kg, respectively. No more than 1.5 mg/kg of Pb are extracted by CaCl2 in both zones. Acetic acid extracts only 20.2 to 51.8 mg/kg of Pb in zone 1 and 0.4 to 17.0 mg/kg in zone 2. A-Rhizo extracts a maximum of 19.0 mg/kg of Pb in zone 1 and 15.5 mg/kg of Pb in zone 2.
As shown in Fig. 4, extracted fractions expressed as percentages of the total element concentration in the bulk soil could lead to the finding that inorganic pollutants are not very available to vegetation, as extractable concentration values were very low: generally less than 1 % for Pb that is extractable by CaCl2, A-Rhizo, or acetic acid but up to 5 % by DTPA. Extracted As was less than 4.4 % of total As concentration by the A-Rhizo method and even less than 0.001 %, on average, by the DTPA method. The labile fractions (mobile + mobilizable fractions corresponding to the largely available fraction by plants; Maiz et al. 2000) for both pollutants represented less than 5 % of the bulk soil contents. Marguí et al. (2007) and Remon et al. (2005) observed the same trend for heavy metals in contaminated environments near industrial activity or in mining contexts. Our results highlight the fact that the higher amount of contaminants are associated with the solid phases (e.g., the residue fraction) since the extraction pool (phytoavailable, adsorbed, and/or absorbed) is lower than 5 % of the total contaminants concentration in soils whatever the element (As or Pb). According to these results, the majority of elements are bound up in As- and Pb-bearing phases that seem to be thermodynamically stable (beudantite and scorodite). This fact results in the low As and Pb solubility in the studied soils, as already shown in other contexts (Frentiu et al. 2009; Paktunc and Bruggeman 2010). However, considering the highest levels of these pollutants in bulk soil, these values still represent considerable amounts of As and Pb (up to 342.6 mg/kg and 391.9 mg/kg for As and Pb, respectively) that are potentially available in the short- and medium term.
Accumulation and translocation of metals in native plants tissues
The concentrations of inorganic pollutants (Pb, As, and Sb) in each part of the representative plants studied (Graminea, P. aquilinum, E. telmateia, and B. pendula) are shown in Table 4. Even if inorganic pollutant uptake by a plant depends on its speciation and the plant species in question, regardless of the element and the plants studied, metal concentrations in roots are higher than those in aerial plant parts (shoots and leaves). B. pendula is present in both zones, but without difference in their chemical analyses of their tissues (see Table 4). For example, the average obtained for As, Pb, and Sb is, respectively, about 1,141 (± 27), 306 (± 7), and 83.5 (± 2) mg/kg in the roots parts, 84 (± 4), 81 (± 4), and 1.7 (±0.1) mg/kg in the branches parts, and 48 (± 2), 15 (± 1.5), and 0.6 (± 0.1) mg/kg in the leaves parts. This fact may be explained by the following hypothesis: The B. pendula rhizospheric soils show no significantly differences in the concentrations of metals and metalloids between the both zones. This can be explained by the major localization of B. pendula at the transition between the both zones (zone 1 and zone 2). According to this, it is normal to find concentrations of each studied metals and metalloids (As, Pb, and Sb) substantially similar (no significant differences between chemical analyses of B. Pendula for the both zones) to each different organs of B. pendula, i.e., for example, the same concentration of As (1160 vs. 1,122 mg/kg) is effective in roots of B. Pendula sampled whatever the studied zone. Arsenic and lead were the elements that accumulated to the greatest extent in the roots: Up to 2,190 mg/kg As and 667 mg/kg Pb were found in Graminea (Table 4). These values are largely in excess of the maximum levels measured previously in plants grown in contaminated sites (Kabata-Pendias 2001). For example, Chang et al. (2009) reported As concentrations up to 17.6 mg/kg in the rhizome of P. aquilinum growing in contaminated abandoned mines in Korea. For Sb, B. pendula roots accumulate 83 mg/kg, while Graminea and P. aquilinum roots had similar Sb concentrations (approximately 40 mg/kg) (Table 4). These very high amounts of contaminants in roots may also be partly due to the potential presence of solid particles, which were not removed by ultrapure water washing. Despite the high Sb concentration in the soils, relatively low concentrations were observed in plants, as already shown by Hammel et al. (2000). In terms of aerial plant parts, As and Pb were the elements that accumulated to the greatest extent. Graminea concentrated the most As, Sb, and Pb (554, 9 and 155 mg/kg, respectively). These values are very high, particularly for As, compared to the concentrations in plants grown on uncontaminated soils, which vary from 0.009 to 1.5 mg/kg (Kabata-Pendias 2001) but are consistent with the levels of As found in plants grown in contaminated sites (e.g., Kabata-Pendias 2001). Several plant species are known to tolerate high levels of As in tissues. Arsenic toxicity has commonly been noted in plants growing on mine waste, on soils treated with arsenical pesticides, and on soils with As that has been added by the application of treated sewage sludge. Moreover, although Pb occurs naturally in all plants, it has not been shown to play any essential role in their metabolisms (Kabata-Pendias 2001). These authors reported that the levels of Pb in soils that are toxic to plants are not easy to evaluate, but several authors have given quite similar concentrations, ranging from 100 to 500 mg/kg (e.g., Davies 1980). Unterbrunner et al. (2007) describe Pb concentrations in B. pendula leaves that are in accordance with our results, between 74.9 and 263 mg/kg.
A hypothesis can be formulated based on the fact that Rao et al. (2008), referencing a study published by Basta et al. (2005), noted that for any given plant, rhizosphere biochemistry and plant physiology induce unique pollutant accumulation patterns in different organs: Two different crops growing on the same soil with the same level of contamination thus will not transfer inorganic pollutants in the same proportions. According to this, it is very difficult to compare results obtained from different plant species. However, the differences observed for the mobilizable fraction for each zone (see before) could also explain the nonrelationship between extractability of metals in soils and their concentration in plants. It is well known that exclusion and accumulation are the two basic tolerance strategies evolved by plants to cope with highly contaminated soils. Moreover, nonrelationship was clearly observed between the solubility of elements (from selective chemical methods; see before) in soils samples and the metals and metalloids accumulation in plants. In the literature, a correlation was observed for As or Pb in the case of contaminated soils by anthropogenic activities (e.g., industrial ponds inducing aerial input or soil near mine pounds) with a different plant species such as ryegrass (e.g., Karczewska et al. 2013 for As). In this study, the nonrelationship between solubility of As and Pb in soils and their accumulation in plants may be explained by the following: (1) the studied soils have directly developed on contaminated materials in which metals and metalloids are mainly in structural form located in the solid phases. As show before, the solid phase controls the mobility and the solubility of elements in soils. (2) The level of contamination is so elevated in soils inducing exclusion of these elements by the plant which must adapt to this specific site. Therefore, there is not necessarily correlation between the solubility of metals and metalloids elements in the soil and the concentration in the plant organs since a part will be blocked. If this is the case, the translocation factors (TFs) will potentially be lower. This is not necessarily the case in soils having much lower contamination then less toxic. Despite very high As, Sb, and Pb concentrations in the roots, translocation to aerial parts, which is evaluated, using a TF, was calculated for each species and element as the ratio of the contaminant concentration in the plant’s aerial part (taking into account organ weight) to the concentration in the plant’s roots. Regardless of the plant samples and organs studied, the TFs were found to be very low <1 (Table 4) in accordance with the literature. However, the lowest TFs values can be due to the presence of solid particles, which were not potentially removed by ultrapure water washing. Then, in this case, the TFs may be underestimated but still very low. Some authors define plants with TFs < 1 as excluders (Baker and Whiting 2002). Briefly, angiosperms accumulate more contaminants overall than pteridophytes (namely P. aquilinum and E. telmateia). Multiple studies confirm this behavior, e.g., Müller et al. (2009), who reported accumulation of Sb and As by Pteris vittata, the well-known As hyperaccumulator. These results indicate that plants are considered to play an important “stabilization” role (Mendez and Maier 2008), even at pollutant concentrations over phytotoxic levels (Pb: 30–300 mg/kg; As: 15–50 mg/kg; and Sb: 5-10 mg/kg; Kabata-Pendias 2001). To assess the potential for plants to spread these contaminants to higher trophic levels, bioconcentration factors (BCFs) reflecting plants’ abilities to accumulate metals and metalloids from contaminated soils have been determined (expressed as metal concentration in aboveground parts divided by total metal concentration in the soil, data not shown). The BCF values of each studied species for As, Sb, and Pb were very low, ranging from 0.001 to 0.18. In other words, these low BCF values are among the features ascribing a “stabilization” role to all of the studied species due to their tendency to prevent metals from entering the ecosystem through the food chain. Overall, native plants growing at La Petite Faye were able to tolerate As, Sb, and Pb contamination with reduced transfer to their aerial parts, leading to the phytostabilization of elements and preventing transfer to groundwater as well as eolian dispersion.
Conclusion
The studied soils located in Limousin (France) have developed rapidly over 40 years on highly contaminated waste piles with significant natural vegetation cover. This work highlighted the following:
-
(1)
The short-term development of soils (over the course of up to 40 years) under acidic conditions, with pedogenesis being strongly related to (i) the parent material, which is heavily contaminated by As, Sb, and Pb controlling the development of soil properties, and (ii) differences in endemic vegetation cover. These soils were either classified as Technosols or Anthroposols artificiels;
-
(2)
It was observed a natural colonization of various vegetation species (e.g., Graminea, B. pendula, E. telmateia, and P. aquilinum) even in the presence of highly contents of metals and metalloids in the soils. The translocation and/or the bioaccumulation factors for the studied plants were very low (TF and BCF << 1);
-
(3)
Labile fractions of As and Pb obtained by SEs were low (<5% of bulk soil content). This finding may be explained by (i) the role of roots to concentrate contaminants in the roots zone (excluder plants) and (ii) the potential presence of solid phases inducing the increase of contaminant amount in roots zone;
-
(4)
No relationship is evident between phytoavailability and concentration in plants. A possible explanation may be the poor effectiveness of extraction procedures used for this type of soil assemblages, i.e., rich in specific mineral phases (beudantite and scorodite);
-
(5)
The natural vegetation cover played an important role in terms of “stabilization,” even at contaminant concentrations over phytotoxic levels, as a result of its adaptability to these contaminated soils.
References
Andres, P., & Jorba, M. (2000). Mitigation strategies in some motorway embankments. Restoration Ecology, 8, 268–275.
Baize, D., & Sterckeman, T. (2001). Of the necessity of knowledge of the natural pedo-geochemical background content in the evaluation of the contamination of soils by trace elements. The Science of The Total Environment, 264, 127–139.
Baker and Whiting. (2002). In search of holy grail—a further step in understanding metal hyperaccumulation? New Phytologist, 155, 1–4.
Basta, N. T., Ryan, J. A., & Chaney, R. L. (2005). Trace element chemistry in residual-treated soil: Key concepts and metal bioavailability. Journal of Environmental Quality, 34, 49–63.
Beckett, P. H. T. (1989). The use of extractants in studies on trace metals in soils, sewage sludges, and sludge-treated soils. Advances in soils sciences, 9, 143–176.
Boussen, S., Soubrand, M., Bril, H., Ouerfelli, K., & Abdeljaouad, S. (2013). Transfer of lead, zinc and cadmium from mine tailings to wheat (Triticum aestivum) in carbonated Mediterranean (Northern Tunisia) soils. Geoderma, 192, 227–236.
Chang, J. S., Yoon, I. H., & Kim, K. W. (2009). Heavy metal and arsenic accumulating fern species as potential ecological indicators in As-contaminated abandoned mines. Ecological Indicators, 9, 1275–1279.
Chiu, K. K., Ye, Z. H., & Wong, M. H. (2006). Growth of Vetiveria zizanioides and Phragamities australis on Pb/Zn and Cu mine tailings amended with manure compost and sewage sludge: A greenhouse study. Bioresource Technology, 97, 158–170.
Conesa, H. M., Faz, Á., & Arnaldos, R. (2006). Heavy metal accumulation and tolerance in plants from mine tailings of the semiarid Cartagena-La Union mining district (SE Spain). Science of the Total Environment, 36, 1–11.
Davies, B. E., 1980. Applied Soil Trace Elements, John Wiley & Sons, New York, 1980, 482 pp.
Dudka, S., & Adriano, D. C. (1997). Environmental impacts of metal ore mining and processing: A review. Journal of Environmental Quality, 26, 590–602.
Fang, J., Wen, B., Shan, X.-Q., Lin, J.-M., & Owens, G. (2007). Is an adjusted rhizosphere-based method valid for field assessment of metal phytoavailability. Application to non-contaminated soils. Environmental Pollution, 150, 209–217.
FAO, 2006. World Reference Base for Soil Researches. World Soil Resources Report. Vol. 103. FAO, Rome.
Feng, M.-H., Shan, X.-Q., Zhang, S., & Wen, B. (2005). A comparison of the rhizosphere-based method with DTPA, EDTA, CaCl2, and NaNO3 extraction methods for prediction of bioavailbility of metals in soil to barley. Environmental Pollution, 137, 231–240.
Frentiu, T., Ponta, M., Levei, E., & Cordos, E. A. (2009). Study of partitioning and dynamics of metals in contaminated soil using modified four-step BCR sequential extraction procedure. Chemical Paper, 63, 239–248.
Hammel, W., Debus, R., & Steubing, L. (2000). Mobility of antimony in soil and its availability to plants. Chemosphere, 41, 1791–1798.
Joussein, E., Soubrand, M., Lévèque, F., Mathé, V., Lenain, J-F., Salvador-Blanes, S. et al. (2012). XRF portable et magnétisme environnemental sur des Anthroposols à contamination polymétallique : relation physique, chimique et minéralogique. 11èmes Journées d’Etude des Sols, 19–23 mars 2012, Versailles, pp 350–351, available online.
Kabata-Pendias, A. (2001). Trace elements in soils and plants (3rd ed.). NY: CRC Press.
Karczewska, A., Lewińska, K., Gałka, B. (2013). Arsenic extractability and uptake by velvetgrass Holcus lanatus and ryegrass Lolium perenne in variously treated soils polluted by tailing spills. Journal of Hazardous Materials,, 262, 1014–1021
Lebourg, A., Sterckeman, T., Ciesielski, H., & Proix, N. (1996). Intérêt des différents réactifs d’extraction chimique pour l’évaluation de la biodisponibilité des métaux en traces du sol. Agronomie, 16, 201–215.
Lei, D., & Duan, C. (2008). Restoration potential of pioneer plants growing on lead-zinc mine tailings in Lamping, southwest China. Journal of Environmental Sciences, 20, 1102–1109.
Li, M. S. (2006). Ecological restoration of mineland with particular reference to the metalliferous mine wasteland in China: A review of research and practice. The Science of the Total Environment, 357, 38–53.
Lindsay, W. L., & Norvell, W. A. (1978). Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Science Society of America Journal, 42, 421–428.
Madejón, P., Murillo, J. M., Marañón, T., Cabrera, F., & Soriano, M. A. (2003). Trace element and nutrient accumulation in sunflower plants two years after the Aznalcóllar mine spill. The Science of the Total Environment, 307, 239–257.
Maiz, I., Arambarri, I., Garcia, R., & Millán, E. (2000). Evaluation of heavy metal availability in polluted soils by two sequential extraction procedures using factor analysis. Environmental Pollution, 110, 3–9.
Marguí, E., Queralt, I., Carvalho, M. L., & Hidalgo, M. (2007). Assessment of metal availability to vegetation (Betula pendula) in Pb–Zn ore concentrate residues with different features. Environmental Pollution, 145, 179–184.
Martinez-Ruiz, C., Fernandez-Santos, B., Putwain, P. D., & Fernandez-Gomez, M. J. (2007). Natural and man-induced revegetation on mining wastes: Changes in the floristic composition during early succession. Ecological Engineering, 30, 286–294.
Mendez, M. O., & Maier, R. M. (2008). Phytostabilization of mine tailings in arid and semiarid environments—An emerging remediation technology. Environmental Health Perspective, 116, 278–283.
Mihaljevič, M., Ettler, V., Sisr, L., Šebek, O., Strnad, L., & Vonásková, V. (2009). Effect of low concentrations of phosphate ions on extraction of arsenic from naturally contaminated soil. Bulletin of Environmental Contamination and Toxicology, 83, 422–427.
Müller, K., Daus, B., Mattusch, J., Stärk, H.-J., & Wennrich, R. (2009). Simultaneous determination of inorganic and organic antimony species by using anion exchange phases for HPLC-ICP-MS and their application to plant extracts of Pteris vittata. Talanta, 78, 820–826.
Néel, C., Bril, H., Courtin-Nomade, A., & Dutreuil, J.-P. (2003). Factors affecting natural development of soil on 35-year-old sulphide-rich mine tailings. Geoderma, 111, 1–20.
Novozamsky, I., Lexmond, T. H. M., & Houba, V. J. G. (1993). A single extraction procedure of soil for evaluation of uptake of some heavy metals by plants. International Journal Environmental Analytical Chemistry, 51, 47–58.
Paktunc, D., & Bruggeman, K. (2010). Solubility of nanocrystalline scorodite and amorphous ferric arsenate: Implications for stabilization of arsenic in mine wastes. Applied Geochemistry, 25, 674–683.
Pérez-Cid, B., Lavilla, I., & Bendicho, C. (1998). Speeding up of a three-stage sequential extraction method for metal speciation using focused ultrasound. Analytica Chimica Acta, 360, 35–41.
Rao, C. R. M., Sahuquillo, A., Sanchez, J., & Lopez, F. (2008). A review of the different methods applied in environmental geochemistry for single and sequential extraction of trace elements in soils and relate materials. Water Air Soil Pollution, 189, 291–333.
Referentiel Pédologique, 2008. Association Française pour l’Etude du Sol, Ed Quæ.
Remon, E., Bouchardon, J.-L., Cornier, B., Guy, B., Leclerc, J.-C., & Faure, O. (2005). Soil characteristics, heavy metal availability and vegetation recovery at a former metallurgical landfill: Implications in risk assessment and site restoration. Environmental Pollution, 137, 316–323.
Rodríguez, L., Ruiz, E., Alonso-Azcárate, J., & Rincón, J. (2009). Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. Journal of Environmental Management, 90, 1106–1116.
Roussel, C., Néel, C., & Bril, H. (2000). Minerals controlling arsenic and lead solubility in an abandonned gold mine tailings. The Science of the Environment, 263, 209–219.
Saunders, J. R., Knopper, L. D., Koch, I., & Reimer, K. J. (2010). Arsenic transformations and biomarkers in meadow voles (Microtus pennsylvanicus) living on an abandoned gold mine site in Montague, Nova Sciota, Canada. Science of the Total Environment, 408, 829–835.
Scalenghe, R., & Ferraris, S. (2009). The first forty years of a Technosol. Pedosphere, 19, 40–52.
Singh, A. N., Raghubanshi, A. S., & Singh, J. S. (2002). Plantations as a tool for mine spoil restoration. Current Science, 82, 1436–1441.
Unterbrunner, R., Puschenreiter, M., Sommer, P., Wieshammer, G., Tlustoš, P., Zupan, M., et al. (2007). Heavy metal accumulation in trees growing on contaminated sites in Central Europe. Environmental Pollution, 148, 107–114.
Vázquez, S., Moreno, E., & Carpena, R. O. (2008). Bioavailability of metals and As from acidified multicontaminated soils: use of white lupin to validate several extraction methods. Environmental Geochemistry and Health, 30, 193–198.
Vega, F. A., Covelo, E. F., & Andrade, M. L. (2006). Competitive sorption and desorption of heavy metals in mine soil: Influence of mine soil characteristics. Journal of Colloid and Interface Science, 298, 582–592.
Wang, X., Liu, Y., Zeng, G., Chai, L., Xiao, X., Song, X., et al. (2008). Pedological characteristics of Mn mine tailings and metal accumulation by native plants. Chemosphere, 72, 1260–1266.
Wilson, S. C., Lockwood, P. V., Ashley, P. M., & Tighe, M. (2010). The chemistry and behaviour of antimony in the soil environment with comparison to arsenic: A critical review. Environmental Pollution, 158, 1169–1181.
Yang, J. S., Lee, J. Y., Baek, K., Kwon, T. S., & Choi, J. (2009). Extraction behavior of As, Pb, and Zn from mine tailings with acid and base solutions. Journal of Hazardous Materials, 171, 443–451.
Yao, F. X., Macías, F., Virgel, S., Blanco, F., Jiang, X., & Camps Arbestain, M. (2009). Chemical changes in heavy metals in the leachates from Technosols. Chemosphere, 77, 29–35.
Acknowledgments
This work was supported by the “Contrat de plan Etat - Région Limousin,” the “Conseil Regional du Limousin,” the C2R Research Program, and finally the GOLDorak program. The authors thank the owner of the site for allowing access, as well as the seven anonymous reviewers for their helpful and constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wanat, N., Joussein, E., Soubrand, M. et al. Arsenic (As), antimony (Sb), and lead (Pb) availability from Au-mine Technosols: a case study of transfer to natural vegetation cover in temperate climates. Environ Geochem Health 36, 783–795 (2014). https://doi.org/10.1007/s10653-014-9596-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-014-9596-5