Abstract
The relationships between two exposure media, garden soil and house dust, were studied for Pb uptake in Stratoni village in northern Greece, an industrial area of mining and processing of sulphide ore. Lead data for the two media were assessed in terms of total and bioaccessible content, measurement and geochemical variability, and mineralogical composition. It was found that total Pb was enriched in house dust samples by a factor of 2 on average. Total Pb concentration in soil samples had a maximum of 2,040 mg/kg and reached a maximum of 7,000 mg/kg in house dust samples. The estimated variability due to measurement uncertainty was dominated by the sampling process, and the proportion of sampling variance was greater for soil samples, indicating a higher degree of Pb heterogeneity in soil on the given spatial scale of sampling strata. Although the same general spatial trend was observed for both sampling media with decreasing Pb concentration by increasing distance from the ore-processing plant, Pb in dust samples displayed the highest concentrations within a 300–600-m zone from the ore-processing facility. The significant differences which were observed in Pb speciation between the studied media were explained by differences in mineralogical composition of outdoor soil and indoor dust. Lead-enriched Fe and Mn oxides predominated in soil samples while fine galena grains (<10–20 μm diameter) were the major Pb-bearing phase in dust samples. The integrated exposure uptake biokinetic model was used to predict the risk of elevated blood lead levels in children of Stratoni. Model prediction indicated an average probability of 61 % for blood-Pb to exceed 10 μg/dl. The results underline the importance of house dust in risk assessment and highlight the effect of outdoor and indoor conditions on the fate of Pb in the particular environment of Stratoni.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead from mining and ore processing is recognised as one of the world’s worst toxic pollution problems and was number three on the top ten list of industrial sources of pollutants according to the Blacksmith Institute Report (2012). Ingestion and inhalation of solid phases containing Pb are major exposure routes to humans, resulting in adverse health effects especially on children (Wixson and Davies 1994; Calabrese et al. 1999; Plumlee et al. 2006). This holds especially in mining and ore-processing areas where effective redistribution of metals occurs in the surface environment as a result of natural weathering processes as well as human activities. It is also widely accepted that apart from the total concentration, it is the chemical and physical aspects of Pb speciation that define and control its reactivity including its solubility and uptake behaviour (Casteel et al. 2006; Plumlee et al. 2006; Romero et al. 2008; Demetriades et al. 2010). Thus, thorough geochemical and mineralogical characterisation of the solid material can provide valuable data on the mobility of this potentially harmful element (PHE) and constrain pathways of exposure.
Research has shown that the concepts of bioavailability and bioaccessibility are important for quantifying the risks associated with exposure to environmental pollutants (Reeder et al. 2006; Pelfrêne et al. 2011). Both soil and house dusts are heterogeneous mixtures of solid phases. Soil in mining, milling and smelting areas contains mineral phases of a range of stability under weathering conditions dictating the kinetics of Pb solubility (Davis et al. 1993; Bosso and Enzweiler 2008; Culson et al. 2009; Hayes et al. 2012). Casteel et al. (2006) estimated the relative bioavailability (RBA) of Pb for different types of soil test material and showed that certain types of Pb minerals such as cerussite and Mn/Pb oxide are better absorbed than others (e.g. galena, anglesite). Furthermore, Hogervorst et al. (2007), by exploring the association between biomarkers of the exposure to Cd and Pb, indicated that house dust is a more important route of exposure to heavy metals than consumption of vegetables in areas with contaminated soils. House dust is composed of finer particles, which are more mobile and metal enriched compared with external materials, and adhere to skin more effectively. Also, in areas with contaminated soil, indoor conditions of lower humidity limit the weathering of the grains and thus the dispersion-chemical degradation of contaminants (Hogervorst et al. 2007; Turner 2011).
The evaluation of bioavailability demands in vivo animal experiments; however, many researchers utilise more affordable and ethically acceptable in vitro tests in the form of sequential extractions or physiologically based extraction tests (PBET) for accessing PHEs bioaccessibility. Despite their operationally defined character, such in vitro tests have been widely used in recent years for identifying the carrier of PHEs in various environmental materials (Filgueiras et al. 2002; Hass and Fine 2010; Hayes et al. 2012) as well as estimating metal concentrations mobilised under simulated digestive conditions (Turner 2011 and references therein).
The study area
Kassandra mines in the Chalkidiki peninsula, Northern Greece, is the only example of active mining and processing of sulphide ore in Greece. One of the on-going projects of the operating mining company, the ‘Stratoni Operation’, includes mining of massive sulphide ore at the Mavres Petres mine, located about 100 km east of Thessaloniki, and ore processing to produce sphalerite and galena concentrates at the Stratoni flotation plant.
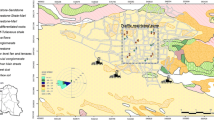
Stratoni village, with a population of 1,174 (2001 census data) lies by the sea on the north end of Ierissos Gulf, Aegean Sea (Fig. 1). The village is developed on a gentle sloping alluvial plain and has two parallel streams with seasonal run-off running through it, discharging into the Ierissos Gulf. Topography changes dramatically in the north of the village with rising steep slopes of the Stratonikon Mountain. The area is characterised by Mediterranean climate with rainy winters and dry, warm summers and prevailing winds are of NE–SW direction. Historically, the settlement of Stratoni has been developed as a residential area for miners, and most of its dwellings are one- or two-storey detached or semidetached houses; a few three-storey apartment buildings also exist. All buildings have yards and many also have vegetable gardens.
Geology and mineralisation
Geologically, the study area consists of Palaeozoic metamorphic rocks including biotite gneiss interlayered with marble horizons, biotite-muscovite gneiss, hornblende gneiss and amphibolite (Kockel et al. 1977). The metamorphic basement has been intruded during Tertiary by the post-orogenic Stratoni granodiorite (29 Ma) (Kalogeropoulos et al. 1989) outcropping in Stratonikon Mountain, north of Stratoni village. The granodiorite and several other Tertiary intrusive bodies are related to the formation of various ore deposits in the area that have been exploited since prehistoric times. The Madem Lakkos and Mavres Petres deposits that are currently exploited are classified as Pb–Zn (Ag–Au) carbonate-hosted replacement type and are structurally controlled by the Stratoni–Varvara fault (Nebel et al. 1991). The mineralisation has a relatively simple mineralogy, and the most abundant ore minerals are pyrite, sphalerite, galena, arsenopyrite and chalcopyrite. The dominant-exploited minerals historically have been sphalerite, pyrite and galena.
Mining activity and potential environmental considerations
The ore is transferred by heavy trucks to the concentrator plant located at the coastal village of Stratoni where it is dry crushed to <20 mm size and is conveyed to a fine ore bin. Ore is then wet ground to give a product sizing in the region of 80 % <212 μm in a conventional rod mill/ball mill circuit. A differential flotation scheme is operated to produce sequentially Pb and Zn concentrates which are conveyed to storage sheds awaiting shipment from Stratoni port to the smelters. According to reported data of the operating mining company, during 2009, the ore throughput was 226,784 dry metric tonnes. The average input grade is 5.6 % Pb, 8.6 % Zn and 145 g/t Ag, whereas the grade of the produced bulk lead/silver concentrate is typically 67 % Pb with 1,585 g/t Ag, and the zinc concentrate contains 49 % Zn (Forward et al. 2010).
Planned development of the Kassandra mines includes the operation of a new milling and metallurgical facility at Madem Lakkos, located a few kilometres west of Stratoni. However, Stratoni infrastructure will continue to be used and will serve in the future as the port for shipment of the total production from all mining operations in the area. The operating mining company was granted permissions and started the new mining developments after approval of the environmental impact assessment (EIA) by the Ministry of Environment in July 2011. Although the EIA report covers a wide range of environmental issues, a baseline health profile describing the current health status of the local population is lacking.
High concentrations of Pb, reaching 11,000 mg/kg, and other PHEs in surface soil of the wider area of Stratoni have been reported by Kelepertsis et al. (2006). Given the long mining history of the area and the lack of pre-mining data, their study indicated that the elevated concentrations in soil may be attributed to the combined effect of natural weathering of metalliferous rocks, mining activities and waste production processes. A contamination hot spot was located within Stratoni village, where high concentrations could be attributed to the operation of the ore-processing plant.
The aim of this paper is to explore the relation between two exposure media, garden soil and house dust, for Pb uptake within an area of mining and processing of sulphide ore in temperate—Mediterranean climate. The specific objectives were to assess and to compare the two media in terms of bulk sample total and bioaccessible Pb concentration, measurement and geochemical variability as well as mineralogical composition. In the light of planned future operations in the area, including the development and expansion of Stratoni port, this study also seeks to provide a geochemical baseline with respect to garden soil and household dust as exposure media for lead uptake in local population.
Materials and methods
Sampling procedures
Thirty-seven surface soil samples were collected from a total area of 0.7 km2 within the area of Stratoni mining village (Fig. 1). The total residential area was divided into strata based on building blocks following a stratified random-sampling design. One house was randomly selected inside each stratum, and one soil sample was collected from the garden. Thirty house dust samples were also collected from full vacuum cleaner bags wherever these were available from the house owner. Soil samples were also collected from communal, recreational areas within the settlement as well as from the primary school and the kindergarten playgrounds. The average distance between sampling sites was 150 m and the selected sampling depth was 0–5 cm in order to represent the public health layer and to avoid mixing of material from deeper soil horizons that might reflect different sources of metals. A hand auger was used to collect a fourfold composite sample from an area of 1 m2 at each sampling location after removing surface vegetation, making up a field sample of 500 g after mixing. Sampling was conducted in October, towards the end of the dry season.
Nine duplicate soil samples were collected at random locations, 5 m away from the original site for estimating sampling uncertainty of the measurements using the duplicate method (Ramsey and Ellison 2007). This distance represented the locational accuracy provided by the GPS instrument that was used. House dust duplicates were collected at seven locations by requesting full vacuum cleaner bags from adjacent houses. A second set of soil duplicates was collected accordingly in order to be able to compare variability at distances approximately equal to distances separating dust sample duplicates, i.e. ~10 m. A full balanced analysis of variance (ANOVA) design was subsequently followed by duplicate analysis of each sampling duplicate enabling the partition of the total variance into its components, i.e. geochemical, sampling and analytical variance. Robust ANOVA results were calculated by the ROBAN computer program (AMC 2012).
Analytical procedures
Soil samples were carried to the laboratory in sealed plastic bags and were prepared for chemical analysis by drying at a temperature of 40 °C, disaggregating, initially sieving to <2 mm and finally sieving to pass through a 100 μm plastic sieve, thus producing the laboratory sample. House dust samples were sieved to <75 μm prior to analysis. All utensils which were used were thoroughly cleaned between the samples in order to avoid cross-contamination.
Soil pH was measured after mixing soil with deionised water in a solid-to-liquid ratio of 1:2.5, and organic carbon content was determined by titration with K2Cr2O7 in an acid environment. Grain size distribution in the sand, silt and clay fractions was determined using the pipette sedimentation method. Concentrations of Pb, Zn, Cu, Cd, Mn and Fe in soil and dust samples were measured by Perkin Elmer 1100B flame AAS at the Laboratory of Economic Geology and Geochemistry, University of Athens, after total dissolution of 0.5 g soil with a mixture of HF, HClO4 and HNO3 acids. Arsenic concentrations in soil were measured by ICP-OES in the Quality Control Laboratory of Hellas Gold S.A. after a hot aqua regia attack. After determining the total content of metals in soil, a sequential extraction with five steps (Tessier et al. 1979; Li and Thornton 2001) was carried out on ten samples selected on a basis of total metal content and sample location. The method examines the possible ‘operationally defined’ geochemical phases of metals in overburden materials including metallurgical processing wastes and residual or alluvial contaminated soil. Extraction was carried out progressively on an initial weight of 1 g of test material.
Analar®—grade chemicals and deionised water were used throughout the analysis. All glass laboratory utensils were washed with a detergent, then soaked for 24 h in 10 % HNO3 acid solution and rinsed repeatedly with deionised water. Reference materials, duplicates and reagent blanks were distributed at random throughout the whole extraction procedures to make the most realistic assessments of data quality. Three internationally certified reference materials by NIST (SRM 2709, SRM 2710 and SRM 2711) were used for estimating analytical bias, and 18 analytical duplicates were used for estimating analytical precision.
Lead bioaccessibility from ten soil (<100 μm) and 10 dust (<75 μm) samples was examined by the relative bioavailability leaching procedure (RBALP) described by Drexler and Brattin (2007). This test uses a simple simulated gastric leach with hydrochloric acid and glycine. The ratio of solid mass to gastric fluid volume during the experiment was 1 g:100 ml. Samples were agitated for 1 h at 37 °C, and the solution pH was maintained at 1.5 during the leaching procedure. Two reagent blanks, ten analytical duplicates and two control soil samples (NIST Standard Reference Material SRM 2711—Montana soil) were included in the analytical run for quality control. After leaching, a 15 ml aliquot of fluid was removed from each sample and filtered into clean test tubes using a 0.45 μm cellulose acetate disc filter attached to a disposable syringe. Lead was immediately measured by flame AAS.
Mineralogical determination was performed on the high-density (specific gravity >2.96) fraction of selected soil and house dust samples, after gravity separation in tetrabromoethane. A Siemens’ D-500 instrument with Cu–Ka radiation was used for X-ray diffraction (XRD), scanning from 5° to 65° 2θ with a scan rate 2°/min; step interval = 0.02; voltage 40 = kV; current = 30 mA. Scanning electron microscopy (SEM) and energy dispersive spectra (EDS) analysis were carried out on resin-impregnated/carbon-coated samples, using a Jeol JSM 5600 SEM instrument, equipped with an Oxford ISIS 300 micro-analytical device. Examination in the backscattered electron (BSE) mode permitted the localisation of areas where heavy metals were concentrated. The mineralogical study was performed at the Laboratory of Economic Geology and Geochemistry, University of Athens.
Integrated exposure uptake biokinetic (IEUBK) model for lead in children application
The U.S. EPA’s integrated exposure uptake biokinetic (IEUBK) model estimates the probability that a child’s or a population of children’s blood lead (PbB) concentration will exceed a certain level of concern, e.g. 10 μg/dl (US-EPA 2007). The model utilises four interrelated modules (exposure, uptake, biokinetic and probability distribution) to estimate PbB levels in children exposed to Pb-contaminated media. In this study, the total Pb levels in house dust within the exposure module were changed according to collected data, and the default absorption value within the uptake module was modified based on the calculated bioaccessible Pb by the RBALP procedure. The age range was selected to be between 0 and 84 months.
Results and discussion
Physicochemical parameters of soil
The soil samples of Stratoni had a sandy–silty texture with 62 % sand, 4 % coarse silt, 10 % medium silt, 18 % fine silt and 6 % clay by mass on average. Soil pH ranged from 3.9 to 8.0 (average 7.0) and soil organic carbon from 0 to 9 % (average 5 %) with the highest concentrations in cultivated gardens. Grain size is a significant factor affecting the potential for metal transport, exposure and subsequent bioaccessibility. Studies on mine waste (chat) (Schaider et al. 2007) and smelter-impacted soils (Romero et al. 2008) have shown that the smallest particles (<38 μm) contain 15–22 times higher concentrations of Zn, Pb and Cd than those in bulk samples. This demonstrates the significance of selecting exposure-relevant particle sizes for analysis, i.e. <100 μm in this study. Although metal concentrations were not assessed in size-fractionated samples here, analysing the bulk 2-mm soil samples would probably lead to underestimation of metal exposure because of the great proportion of sand in soil.
Soil organic matter and organic acids also play a significant role in Pb sequestration as the latter may form sulfhydryl and carboxyl ligands on soil organic matter (Ruby et al. 1999; Steinnes 2013). Furthermore, bacteria and fungi present in organic rich soils accelerate the oxidation of Mn2+, facilitating the production of Mn4+ oxides which are very efficient scavengers of Pb (Villalobos et al. 2005a). In Stratoni, rhodochrosite (MnCO3) is one of the accessory minerals in the ore (Nebel et al. 1991; Forward et al. 2010) and a potential source of Mn2+. Rhodochrosite dissolution may be enhanced by fungal species (Tang et al. 2013) resulting in biologically mediated oxidation of Mn2+ and Mn4+ production.
Mineralogy of soil and house dust
Identified minerals with XRD analysis in soil samples comprise mainly (95 %) common soil inorganic phases, such as quartz, feldspar, mica and clay minerals. The absence of calcite is noted, reflecting the predominance of silicate rocks in the area. Sulphide minerals such as sphalerite and insoluble weathering products containing heavy metals such as alunite and tsumcorite were also recorded in the diffraction spectra. SEM–EDS observations and microanalysis results indicated that the studied material is rich in quartz > feldspar > Fe and Mn oxides > sulphide minerals.
Microanalysis of selected bright particles in backscattered mode indicated the presence of secondary minerals of very low solubility, enriched in heavy metals, particularly Pb (Fig. 2a). Specifically, the lead phosphate mineral pyromorphite (log Ksp = −84.4) has been identified in several grains. Also, the practically insoluble mineral corkite (log Ksp = −112.6), a Pb-rich member of the alunite supergroup, was identified (Argyraki et al. 2007). However, the most abundant Pb-bearing phase in soil found by SEM–EDS was Fe and Mn oxides and oxyhydroxides, enriched also in other heavy metals. Sulphide grains corresponding to the primary mineralisation of the wider area were also identified including galena, sphalerite, arsenopyrite and pyrite. The latter often appears oxidised, forming pseudomorph crystals. No weathering rinds as compared to altered pyrite were observed in galena particles. Finally, anthropogenic grains of weathered metallurgical slag were observed. The composition of such grains is characterised by a Si–Fe–Ca vitreous phase, enriched in heavy metals. In many instances, slag grains appear weathered with pyromorphite precipitates on their periphery.
The majority of metal-bearing dust grains were metallic minerals that exist in the ore, e.g. galena (PbS), anglesite (PbSO4) and cerussite (PbCO3). Lead in the house dust samples also occurs to a lesser degree in association with precipitating species of Fe and Mn oxides and phosphates or encapsulated inside grains of inert mineralogical phases and slag, i.e. phases also identified in the soil samples (Fig. 2b). Fine-grained (<10–20 μm) euhedral galena was by far the most abundant Pb carrier in house dust samples, indicating that the ore mill facility is the primary source of the metal in house dust. Further evidence on this is provided by a mineralogical study of dust filters from the air sampler operating for environmental monitoring purposes near the ore-processing facility (Papastamatiou et al. 2010). That study did not demonstrate variation in the type of metallic minerals captured on the filters. Galena, sphalerite and pyrite dominated, whereas bournotite, boulanzerite, chalcopyrite, cerussite, barite, gypsum, goethite and tennantite were identified as subordinate mineral phases. Furthermore, Pb concentration on air filters showed positive correlation with NE wind direction, suggesting that dust grains originate from the ore milling and ore-processing facilities.
Total concentrations of Pb and other PHE in soil and dust
Quality control on measurements of total concentrations yielded acceptable results for most studied heavy metals. Analytical bias was estimated within ±10 % of the certified value, and analytical precision was better than ±5 % of mean value for a 2 s interval. In contrast to the above, analytical precision for Fe reached ±14 %, probably due to volumetric errors, and precision of Cd was ±30 %, probably due to low-concentration readings near the detection limit of the method.
The descriptive statistics of total concentrations of Pb, Zn, Cu, Cd, As, Fe and Mn in soil and house dust samples are presented in Table 1.
High total levels of the studied elements were found in both soil and dust samples with averages well above European reference values for soil (Salminen et al. 2005) and house dust (Seifert et al. 2000). Average concentrations were also higher than the regional soil mean values reported by Kelepertsis et al. (2006) for all elements except Fe. Total Pb concentration in soil samples had a maximum of 2,040 mg/kg and reached a maximum of 7,000 mg/kg in house dust. The statistical distributions of Pb, Zn, Cd and Cu in soil were normal while distributions of the same elements in dust were positively skewed. Total Pb was enriched in house dust samples by a factor of 1.5 on average, and there was no correlation between the two sampling media with respect to Pb. Even higher enrichment factors were observed for Zn, Cd and Cu while dust samples were depleted in Mn and Fe compared to soil (Table 1). The above relationships indicate that the ore milling and ore-processing facility in Stratoni presents a distinct point source for the ore-related elements.
Multivariate cluster analysis was applied to the normal scores of soil and house dust data in order to examine the classification of element groups and recognise relationships among them. The distance measure used in cluster analysis was the Pearson correlation coefficient at the 95 % confidence level. The results of the analysis are presented as a dendrogram in Fig. 3.
The similarity axis represents the degree of association between the variables, the greater the value the more significant the association. Three distinct clusters can be identified in the data: cluster I containing Pb, Mn, Zn, Cd in soil and Mn in dust, cluster II containing all parameters in dust except Mn and cluster III containing Cu and Fe in soil. The strongest observed associations (similarity >85 %) were between Zn and Cd in soil, reflecting the chemical composition of sphalerite; Pb, Cd and Zn in dust, indicating the produced ore concentrates as their common source; and between Pb and Mn in soil, probably due to the significant incorporation of Pb into the structure of soil Mn oxides. The above analysis indicates that Pb in the outdoor environment is sequestered into secondary weathering products and Mn oxides. The strong similarity between Pb, Zn and Cd in house dust as well as the elevated levels of these elements in dust compared with soil is attributed to preferential transport of fine-grained, airborne particles of metallic primary ore minerals indoors.
Spatial distribution of total Pb in soil and house dust
The highest concentrations of Pb were spatially located near the ore mill plant, and the lowest further away. However, the spatial trend was different for soil and house dust samples (Fig. 4).
Lead in soil samples showed a smooth decrease with distance while Pb concentration in dust samples reached its maximum within a zone of 300–600 m from the ore mill and decreased abruptly at greater distances. While the general spatial pattern is indicative of the ore mill facility being the source of Pb in soil samples, the observed dust Pb enrichment within the particular spatial zone may be influenced by several factors. It is possible that it is related to the potential for air transport of fine galena grains of the ore mill concentrate. Lead is enriched in dust compared with soil at distances <600 m from the ore mill, while the relationship is reversed at greater distances, probably due to insufficient air transport. Another influencing factor may be related to the inhabitants’ occupation. Many of the residents of Stratoni work in the mines or the ore mill plant and potentially transfer ground galena indoors with their clothes and shoes. Such a hypothesis could not be verified as no relevant data were collected during the sampling survey.
Information on the degree of spatial heterogeneity of Pb in soil was provided by the application of the duplicate method. Robust ANOVA results revealed that the analytical repeatability was low in all instances, i.e. soil sample duplicates separated by 5 and 10 m, and house dust duplicates separated by 10 m, contributing a minimal percentage to the total variance (Fig. 5). Thus, the measurement variance was always dominated by sampling variance. Sampling variance of soil sample duplicates at 5 m (collected within the same house yard) contributed 4 % to the total variance, indicating that soil Pb heterogeneity on this scale is significantly lower than the variance between locations within the total sampled area.
A substantial increase in the metal’s heterogeneity was noticed when the distance separating the sampling duplicates was increased to 10 m, i.e. for sample duplicates collected from adjacent house yards, with sampling variance (58 % of total) exceeding the geochemical variance (42 % of total). Such significant changes in spatial contaminant heterogeneity with scale have previously been reported in contaminated land studies (Thomas et al. 2008; Ramsey et al. 2013). In the present study, the change may be attributed to different property-scale management activities as some of the dwellings had cultivated yards with vegetables, others had lawns and flower beds and others just bare soil. The surface overburden was totally covered by imported, uncontaminated soil in the instance of the kindergarten playground, purposely for reducing exposure of young children to contamination. House dust duplicates collected from adjacent houses which were semidetached in majority also displayed a relatively high proportion of sampling variance (22 % of total), probably explained by cleaning practices at individual households as well as mine-related occupation of family members. However, compared with soil, dust Pb heterogeneity appeared substantially lower at the 10 m sample-duplicate separation distance, supporting the hypothesis of airborne-ore mill-associated Pb in dust rather than indoor tracking of Pb-enriched soil grains.
Overall, the results of total analyses suggest a common origin but different fate of Pb in soil and house dust. The ore mill facility is the source of primary ore fine-grained galena which is prone to air transport and deposition in the soil and house dust within the residential area. Based on the observed predominance of Pb-enriched Mn-oxide phases in soil, it is proposed that once deposited, humid, organic rich, oxidising conditions of surface soil facilitate dissolution of sulphide minerals and scavenging of Pb by Mn oxides. Villalobos et al. (2005b) have experimentally explored the kinetics of Pb2+ sequestration by layer-type, biogenic and synthetic manganese oxides. They reported relatively fast sorption, with maximum saturating concentrations reached within 1 day and extremely high sorption capacities, suggesting Pb incorporation into the structure of the oxides. Maximum sorption capacity was observed for δ-MnO2 at a pH of 6.5, which is close to the pH measured in the soil samples of Stratoni. Furthermore, Pb in soil is also captured by other secondary, weathering phases identified by SEM–EDS including anglesite, pyromorphite and corkite, all contributing to a dilution effect decreasing its concentration in the samples. Chemical degradation of primary Pb–sulphide grains is prevented in the indoor environment where Pb retains its high galena concentration.
Geochemically and physiologically based extraction tests results
Analytical precision of the sequential extraction procedure applied in soil samples was between 2 and 20 % for all elements and all extraction steps. Analytical bias was found to be within acceptable limits (<10 %) except for Fe. Proportions of metals in each one of the five operationally defined phases are presented in Fig. 6. These phases differ in respect with potential availability of elements into the environment and include in the order of decreasing availability (Tessier et al. 1979): (a) the exchangeable fraction, i.e. metals adsorbed in exchange sites on surfaces of clays and colloids; (b) the metal fraction associated with carbonate minerals and metals specifically adsorbed on surfaces of amorphous precipitates of Fe, Al, Mn oxides/hydroxides and phosphates; (c) the reducible phase comprising metals associated with Mn and Fe oxides through co-precipitation and binding within the oxide structure; (d) the oxidisable phase, i.e. the fraction of metals bound to soil organic matter and metals incorporated in the structure of sulphide minerals and (e) the residual phase including elements incorporated in the crystal lattice of silicate minerals and vitreous metallurgical phases such as slag.
The results of sequential extraction soil analysis in this study showed that the fractionation of studied heavy metals varies. This observation has significant implications on the potential bioavailability of metals. Specifically, Pb was fractioned mainly (53 %) in the residual phase of relatively insoluble soil components as well as the reducible phase of Fe–Mn oxides (40 %) in agreement with the mineralogical analysis of soil samples. On the contrary, Zn, Cd and Mn appeared in the residual phase in lower concentrations, fractioned by 25, 23 and 5 %, respectively. These elements had noticeable share in the first two extraction steps (5–25 %). Manganese was extracted by 90 % in the reducible phase, reflecting the abundance of Mn-oxide phases in soil, while Cu appeared in high proportion (40 %) in the oxidisable phase, probably indicating the significant role of organic matter as a bounding factor for this element. Iron was fractioned mainly in the reducible phase (45 %) and in the oxidisable phase of sulphides and organic matter (30 %).
Calculation of correlation coefficients between metals and organic carbon in the sequential extraction fractions provided evidence for the most significant factors affecting speciation of metals in soil. Organic carbon correlated significantly (p < 0.05) with Fe > Cu > Cd > Zn in the oxidisable phase (correlation coefficients 0.79, 0.74, 0.58, 0.55, respectively). Zinc and Cd were highly correlated in all extraction stages (correlation coefficients 0.86–0.96) indicating sphalerite as the source of these elements. Significant correlation between Zn, Cd and Mn in fractions I, II and III (coefficients 0.52–0.96) indicates the association of these elements with easily extractable phases, suggesting increased mobility in the environment. Lead was correlated with Fe in the residual phase, possibly indicating the coexistence of the two elements in metallurgical slag grains in soil. The significant correlation coefficient of 0.93 between the oxidisable soil fraction, and total dust Pb is also worth noting, indicating that galena is the dominant Pb-bearing phase in house dust in agreement with SEM–EDS observations.
Results of the RBALP extractions on soil and dust samples are presented in Table 2. Quality control of the test indicated that results for Pb were within acceptable limits with analytical precision of 5 % and method detection limit of 0.1 mg/l. The concentration of Pb in the two SRM 2711 samples that underwent the extraction was 9.26 and 9.51 mg/l (927 and 949 mg/kg), which is within the range reported in similar studies (MOE 2002). The bioaccessibility estimates in soil ranged from 12 to 58 % and between 22 and 74 % across all dust samples; average calculated bioaccessibilities for soil and dust were 33 and 38 %, respectively. A marginal statistically significant correlation coefficient of 0.57 (p = 0.085) was observed between soil and dust RBALP-extracted concentrations. Correlation coefficients were also calculated between Pb extracted by this test and Pb in the different sequential extraction soil fractions. Coefficients were decreasing in the order oxidisable phase (0.83, p = 0.003) > reducible phase (0.73, p = 0.016) > specifically adsorbed phase (0.69, p = 0.027). These data indicate that Pb sulphide followed by the Fe/Mn oxides are the predominant soil phases contributing to bioaccessible concentrations of the metal.
Relevant studies in mining and smelting areas from the Copperbelt, Zambia (Ettler et al. 2012), report comparable average values (40 %) of bioaccessible Pb in mining-affected soils, noting that the simple gastric conditions simulation of the RBALP test may overestimate the total bioaccessibility of metals but provides a robust tool for human risk assessment in areas with high levels of metals in soils. The high correlations between the RBALP test and sequential extraction results for Pb in soil as well as the predominance of galena grains in house dust indicate that the influence of Pb sulphide dissolution on the estimated bioaccessibility is of particular significance in the Stratoni environment. Although Pb present as metal sulphide is extracted by HCl (Schaider et al. 2007), galena is considered a mineral of intermediate solubility, and its dissolution rate controlling process has been explained in the literature by a combination of surface-reaction-controlled kinetics and transport-controlled kinetics (Ruby et al. 1999). The first process affects primarily less soluble Pb minerals while the second drives dissolution of more soluble Pb phases, e.g. Pb oxides. Particle size of a metal species is another important factor in the mobilisation of the metal as solubility increases with increased surface area. The relatively high percentages of bioaccessible Pb in samples calculated in the present study were attributed to the small size (<10 μm) of airborne galena grains.
IEUBK model results and health implications
The IEUBK model was applied to predict the risk of elevated PbB levels in children of Stratoni using multiple source analysis and the ‘sum of individual risks’ approach (US-EPA 2007). The model was run in a single-simulation mode for each sampled location after inserting the site-specific total concentrations of Pb in soil and dust. The output included the log-normal probability density function of blood-Pb levels for a single-exposure scenario predicting geometric mean blood-Pb as well as the portion of the upper tail of the probability distribution exceeding 10 μg/dl blood-Pb. In this way, it provided an estimate of the risk of blood-Pb exceeding that level. Results from individual homes were combined to form an estimate of community risk. Model prediction based on the 30 sampling locations indicated an average geometric mean of 12.6 ± 5.2 μg/dl and an average probability of 61 % of blood-Pb to exceed 10 μg/dl.
The IEUBK model was also used to predict three levels of blood-Pb concentrations for the ten houses with available data on in vitro bioaccessibility (IVBA) calculated by the RBALP test: one using only total soil’s Pb data, a second using total soil and dust Pb and a third using the bioaccessibility data obtained for soil and dust (Table 2). In the third run, in vivo RBA was approximated by the empirical equation (US-EPA 2007):
The average probabilities of blood-Pb exceeding 10 μg/dl calculated by the three runs of the model were 53, 48 and 55 %. Although taking into account the IVBA data gave a slightly higher probability compared with the other two runs, the between-run variability in the model’s output was not statistically significantly different at 95 % confidence interval from the within-run variability when comparing output values by one-way ANOVA. These results are presented as side by side box plots in Fig. 7 and indicate that there was no significant effect on the model’s output by changing the default value based on the calculated in vitro Pb bioaccessibility. However, the small sample number in this instance might have had an influence on the results.
Predictions of the IEUBK model are usually validated in comparison with measured values of blood-Pb concentration (e.g. Tristan et al. 2000; Yu et al. 2006). In the absence of relevant PbB data, we must reserve judgment on the validity of IEUBK prediction and potential health threats for Stratoni population. Nevertheless, the data of this research call for a health impact assessment study involving biomonitoring of individuals, and, if possible, a PbB survey in the village in order to provide knowledge about the health baseline of the local population before further mining development activities takes place. Such knowledge would provide a clearer answer to the question of exposure and its effects and aid the understanding of any source-pathway-receptor linkages. There is a growing body of evidence that causal connections between environment and health are better addressed within the frame of interdisciplinary collaborations, enabling more effective research strategies (Stewart and Ramsey (2009) and references therein). In the instance of Stratoni, researchers and practitioners from different disciplines including geochemists, medics and social scientists should work together and in collaboration with the operating mining company in order to ensure that the health of the local population is not compromised by mining development.
Finally, it should be noted that the in vitro test used in this study assesses Pb bioaccessibility through the gastric exposure path; however, the identification of fine-grained sulphide as the predominant Pb-bearing phase raises concern about exposure through inhalation. It is known that because of differential toxicity, various minerals have a different impact on human health when inhaled (Schaider et al. 2007). According to Plumlee et al. (2006), Pb as a constituent of cerussite (a highly acid-soluble lead carbonate), Pb oxides, and Pb sorbed onto atmospheric aerosols generated by lead–zinc smelting is substantially more bioaccessible than Pb as a major constituent of galena and various lead phosphate minerals. Consequently, the dominant presence of galena in the dust may signify limited bioavailability via this exposure pathway. More appropriate methodologies could verify if this hypothesis holds for Pb exposure in Stratoni. Another potential hazard, not examined here, is the consumption of home-grown vegetables since many of the houses have cultivated yards. This could also be a significant source of Pb intake, considering the high proportion of fresh vegetables in the typical Mediterranean diet. Future studies have the opportunity to utilise information provided by this research for optimising sampling protocols with respect to this exposure medium. Specifically, data of in situ heterogeneity and airborne transfer of contaminants would have to be taken into account as they might affect both root and foliar uptake of PHE by plants and vegetables grown in the gardens.
Conclusions
Lead in soil and in house dust was for the first time assessed in terms of total and bioaccessible concentrations, measurement and geochemical variability, and mineralogical speciation in the industrial area of Stratoni. The results underline the importance of house dust in risk assessment and highlight the effect of outdoor and indoor conditions on the fate of Pb in the particular environment of Stratoni. Overall, this study provides appropriate guidance for further exploring risk assessment issues in the study area. It also contributes to the existing database of hazard characterisation in sulphide-ore-processing areas and provides initial information for health impact assessment by defining the role of soil and house dust as exposure media and identifying research gaps. All of the above could be utilised by public health authorities and decision makers in Stratoni and other areas of similar geoenvironmental conditions.
References
AMC. (2012). Software, Roban, program for robust analysis of variance. http://www.rsc.org/Membership/Networking/InterestGroups/Analytical/AMC/Software/ROBAN.asp. Accessed 22 July 2013.
Argyraki, A., Plakaki, A., & Godelitsas, A. (2007). Characterization of garden soil pollution in the mining village of Stratoni. In N. Greece (Ed.), Bulletin of the Geological Society of Greece vol. XXXX, Proceedings of the 11th International Congress, Athens 24–26 May, 1331–1342. http://geolib.geo.auth.gr/digeo/index.php/article/download/4691/4503. Accessed 22 July 2013.
Blacksmith Institute Report. (2012). The World’s worst pollution problems: Assessing health risks at hazardous waste sites. The Blacksmith Institute. www.worstpolluted.org/files/WWPP_2012.pdf. Accessed 22 July 2013.
Bosso, S. T., & Enzweiler, J. (2008). Bioaccessible lead in soils, slag, and mine wastes from an abandoned mining district in Brazil. Environmental Geochemistry and Health, 30, 219–229.
Calabrese, E. J., Stanek, E. J., James, R. C., & Roberts, S. M. (1999). Soil ingestion: A concern for acute toxicity in children. Journal of Environmental Health, 61, 1354–1358.
Casteel, S. W., Weis, C. P., Henningsen, G. M., & Brattin, W. J. (2006). Estimation of relative bioavailability of lead in soil and soil-like materials using young swine. Environmental Health Perspectives, 114, 1162–1171.
Culson, B., Korsch, M., Matisons, M., Douglas, C., Gillam, L., & McLaughlin, V. (2009). Windblown lead carbonate as the main source of lead in blood of children from a seaside community: An example of local birds as “canaries in the mine”. Environmental Health Perspectives, 117, 148–154.
Davis, A., Drexler, J. W., Ruby, M. V., & Nicholson, A. (1993). Micromineralogy of mine waste in relation to lead bioavailability, Butte, Montana. Environmental Science and Technology, 27, 1415–1425.
Demetriades, A., Li, X.-D., Ramsey, M. H., & Thornton, I. (2010). Chemical speciation and bioaccessibility of lead in surface soil and house dust, Lavrion urban area, Attiki, Hellas. Environmental Geochemistry and Health, 32, 529–552.
Drexler, J. W., & Brattin, W. J. (2007). An in vitro procedure for estimation of lead bioavailability: With validation. Human and Ecological Risk Assessment, 13, 383–401.
Ettler, V., Kribek, B., Majer, V., Knesl, I., & Mihaljevic, M. (2012). Differences in the bioaccessibility of metals/metalloids in soils from mining and smelting areas (Copperbelt, Zambia). Journal of Geochemical Exploration, 113, 68–75.
Filgueiras, A. V., Lavilla, I., & Bendicho, C. (2002). Chemical sequential extraction for metal partitioning in environmental solid samples. Journal of Environmental Monitoring, 4, 823–857.
Forward, P., Francis, A. & Liddell, N. (2010). Technical report on the stratoni project: Pb, Zn, Ag deposit, northern Greece. European Goldfields Limited, Yukon. http://www.eldoradogold.com/pdf/StratoniTechnicalReportSept2010.pdf. Accessed 22 July 2013.
Hass, A., & Fine, P. (2010). Sequential selective extraction procedures for the study of heavy metals in soils, sediments and waste materials—a critical review. Critical Reviews in Environmental Science and Technology, 40, 365–399.
Hayes, S. M., Webb, S. M., Bargar, J. R., O’Day, P. A., Maier, R. M., & Chorover, J. (2012). Geochemical weathering increases lead bioaccessibility in semi-arid mine tailings. Environmental Science and Technology, 46, 5834–5841.
Hogervorst, J., Plusquin, M., Vangronsveld, J., Nawrot, T., van Cuypers, A., Hecke, E., et al. (2007). House dust as possible route of environmental exposure to cadmium and lead in the adult general population. Environmental Research, 103, 30–37.
Kalogeropoulos, S. I., Kilias, S. P., Bitzios, D. C., Nicolaou, M., & Both, R. A. (1989). Genesis of the Olympias carbonate-hosted Pb–Zn(Au, Ag) sulfide ore deposit, eastern Chalkidiki Peninsula, northern Greece. Economic Geology, 84, 1210–1234.
Kelepertsis, A., Argyraki, A., & Alexakis, D. (2006). Multivariate statistics and spatial interpretation of geochemical data for assessing soil contamination by potentially toxic elements in the mining area of Stratoni, north Greece. Geochemistry: Exploration, Environment, Analysis, 6, 349–355.
Kockel, F., Mollat, H., & Walther, H.W. (1977). Erlauterungen zur geologischen Karte der Chalkidiki und angrenzender Gebiete 1:100000 (Nord-Griechenland). Hannover: Bundesanstalt fur Geowissenschaften und Rohstoffe, p. 119.
Li, X., & Thornton, I. (2001). Chemical partitioning of trace and major elements in soils contami-nated by mining and smelting activities. Applied Geochemistry, 16, 1693–1706.
MOE (Ontario Ministry of Environment). (2002). Soil investigation and human health risk assessment for the Rodeny Street Community, Port Colbourne.
Nebel, M., Hutchinson, R. W., & Zartman, R. E. (1991). Metamorphism and polygenesis of the Madem Lakkos polymetallic sulphide deposit, Chalkidiki, Greece. Economic Geology, 86, 81–105.
Papastamatiou, D., Skarpelis, N., & Argyraki, A. (2010). Air quality in mining areas: The case of Stratoni, Chalkidiki, Greece. Bulletin of the Geological Society of Greece, Proceedings of the 12th International Congress, Patras May 2010, XLIII, 5, 2510–2519. http://geolib.geo.auth.gr/digeo/index.php/article/viewFile/6972/6730. Accessed 22 July 2013.
Pelfrêne, A., Waterlot, C., Mazzuca, M., Nisse, C., Bidar, G., & Douay, F. (2011). Assessing Cd, Pb, Zn human bioaccessibility in smelter-contaminated agricultural topsoils (northern France). Environmental Geochemistry and Health, 33, 477–493.
Plumlee, G. S., Morman, S. A., & Ziegler, T. L. (2006). The toxicological geochemistry of earth materials: An overview of the processes and the interdisciplinary methods used to understand them. Reviews in Mineralogy and Geochemistry, 64, 5–57.
Ramsey M.H., & Ellison S. L. R. (eds.) (2007). Eurachem/EUROLAB/CITAC/Nordtest/AMC Guide: Measurement uncertainty arising from sampling: A guide to methods and approaches Eurachem. ISBN 978 0 948926 26 6. http://www.eurachem.org/guides/pdf/UfS_2007.pdf. Accessed 22 July 2013.
Ramsey, M. H., Solomon-Wisdom, G., & Argyraki, A. (2013). Evaluation of in situ heterogeneity of elements in solids: Implications for analytical geochemistry. Geostandards Geoanalytical Research,. doi:10.1111/j.1751-908X.2013.00236.x.
Reeder, R. J., Schoonen, M. A. A., & Lanzirotti, A. (2006). Metal speciation and its role in bioaccessibility and bioavailability. Reviews in Mineralogy and Geochemistry, 64, 59–113.
Romero, F. M., Villalobos, M., Aquirre, R., & Gutierrez, M. E. (2008). Solid-phase control on lead bioaccessibility in smelter-impacted soils. Archives of Environmental Contamination and Toxicology, 55, 566–575.
Ruby, M. V., Schoof, R., Brattin, W., Goldade, M., et al. (1999). Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environmental Science and Technology, 33, 3697–3705.
Salminen, R., Batista, M. J., Bidovec, M. & Demetriades, A. et al. (2005). Geochemical Atlas of Europe. Part 1: Background information, methodology and maps. http://weppi.gtk.fi/publ/foregsatlas. Accessed 22 July 2013.
Schaider, L. A., Senn, D. B., McCarthy, K. D., & Shine, J. P. (2007). Characterization of zinc, lead, and cadmium in mine waste: Implications for transport, exposure and bioavailability. Environmental Science and Technology, 41, 4164–4171.
Seifert, B., Becker, K., Helm, D., Krause, C., Schulz, C., & Seiwert, M. (2000). The German Environmental Survey 1990/1992 (GerES II): Reference concentrations of selected environmental pollutants in blood, urine, hair, house dust, drinking water and indoor air. Journal of Exposure Analysis and Environmental Epidemiology, 10, 552–565.
Steinnes, E. (2013). Lead. In B. J. Alloway (ed.), Heavy metals in soils: Trace metals and metalloids in soils and their bioavailability, Environmental Pollution 22, doi:10.1007/978-94-007-4470-7-14.
Stewart, A. G., & Ramsey, M. H. (2009). Multiple links towards integrating teams for understanding of disease and environment (MULTITUDE). Environmental Geochemistry and Health, 31, 161–163.
Tang, Y., Zeiner, C. A., Santelli, C. M., & Hansel, C. M. (2013). Fungal oxidative dissolution of the Mn(II)-bearing mineral rhodochrosite and the role of metabolites in manganese oxide formation. Environmental Microbiology, 15, 1063–1077.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for speciation of particulate trace metals. Analytical Chemistry, 51, 844–851.
Thomas, J. Y., Ramsey, M. H., John, E. A., & Barnes, R. (2008). Quantification of in situ heterogeneity of contaminants in soil: A fundamental prerequisite to understanding processes controlling plant uptake. Proceedings of ConSoil 2008 (10th International UFZ-Deltares/TNO Conference on Soil-Water Systems), Milan, Italy, 3–6 June 2008 (ISBN: 978-3-00-024598-5). Theme C, 101–106.
Tristan, E., Demetriades, A., Ramsey, M. H., Rosenbaum, M. S., Stavrakis, P., et al. (2000). Spatially resolved hazard and exposure assessments: An example of lead in soil at Lavrion, Greece. Environmental Research Section A, 82, 33–45.
Turner, A. (2011). Oral bioaccessibility of trace metals in household dust: A review. Environmental Geochemistry and Health, 33, 331–341.
US Environmental Protection Agency. (2007). User’s guide for the integrated exposure uptake biokinetic model for lead in children (IEUBK) windows ® version 1.1, EPA 540-K-01-005, North Syracuse, NY.
Villalobos, M., Bargar, J., & Sposito, G. (2005a). Trace metal retention on biogenic manganese oxide nanoparticles. Elements, 1, 223–226.
Villalobos, M., Bargar, J., & Sposito, G. (2005b). Mechanisms of Pb(II) sorption on a biogenic manganese oxide. Environmental Science and Technology, 39, 569–576.
Wixson, B. G., & Davies, B. E. (1994). Guidelines for lead in soil. Proposal of the society for environmental geochemistry and health. Environmental Science Technology, 28, 26A–31A.
Yu, C. H., Yiin, L. M., & Lioy, P. J. (2006). The bioaccessibility of lead (Pb) from vacuumed house dust on carpets in urban residences. Risk Analysis, 26, 125–134.
Acknowledgments
This work was partially funded by the Special Account for Research Grants, National and Kapodistrian University of Athens, Project No. 70/4/7624. The postgraduate students A. Plakaki and S. Nicolaou are greatly acknowledged for their involvement in sampling and laboratory analyses. V. Gazea, Director of the Environmental Monitoring Programme of Hellas Gold S.A., is thanked for the useful discussions during the research and arrangements for analysis of part of the samples in the Laboratory of Quality Control in Stratoni. G. Zagorakis of the Municipality of Stratoni is also thanked for his invaluable help during field sampling. Zafar Iqbal and Terpsi Kamtsiou are thanked for improving the English grammar and style of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Argyraki, A. Garden soil and house dust as exposure media for lead uptake in the mining village of Stratoni, Greece. Environ Geochem Health 36, 677–692 (2014). https://doi.org/10.1007/s10653-013-9589-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-013-9589-9