Abstract
The Salí River Basin in north-west Argentina (7,000 km2) is composed of a sequence of Tertiary and Quaternary loess deposits, which have been substantially reworked by fluvial and aeolian processes. As with other areas of the Chaco-Pampean Plain, groundwater in the basin suffers a range of chemical quality problems, including arsenic (concentrations in the range of 12.2–1,660 μg L−1), fluoride (50–8,740 μg L−1), boron (34.0–9,550 μg L−1), vanadium (30.7–300 μg L−1) and uranium (0.03–125 μg L−1). Shallow groundwater (depths up to 15 m) has particularly high concentrations of these elements. Exceedances above WHO (2011) guideline values are 100% for As, 35% for B, 21% for U and 17% for F. Concentrations in deep (>200 m) and artesian groundwater in the basin are also often high, though less extreme than at shallow depths. The waters are oxidizing, with often high bicarbonate concentrations (50.0–1,260 mg L−1) and pH (6.28–9.24). The ultimate sources of these trace elements are the volcanic components of the loess deposits, although sorption reactions involving secondary Al and Fe oxides also regulate the distribution and mobility of trace elements in the aquifers. In addition, concentrations of chromium lie in range of 79.4–232 μg L−1 in shallow groundwater, 129–250 μg L−1 in deep groundwater and 110–218 μg L−1 in artesian groundwater. All exceed the WHO guideline value of 50 μg L−1. Their origin is likely to be predominantly geogenic, present as chromate in the ambient oxic and alkaline aquifer conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Chaco-Pampean Plain (about one million km2) is the largest and most populated geographical region in Argentina. The plain lies east of the Pampean Hills, extending from the Paraguay border in the north to the Patagonian Plateau in the south. Its central part is the site of the country’s most important cities. One of the greatest obstacles for the socioeconomic regional development is the quality of the water resources available for the rural population. Saline or hard groundwater is the only water resource in wide areas, and this condition limits its use for human consumption. In addition, high trace-element concentrations (especially arsenic) often render the groundwater toxic for potable supply, cattle consumption and irrigation.
A number of water-related health problems are linked to high arsenic concentrations in drinking water in the Chaco-Pampean region. From the early XIX century to 1913, the pathology was known as “Bell-Ville disease” because this was the city where the majority of recognized cases were found. That year, Dr. Mario Goyenechea proved by chemical analysis that arsenic caused the disease, a fact mentioned by Círculo Médico del Rosario (1917) and described by Ayerza (1917a, b, 1918). Skin-pigmentation disorders and increase in neoplasms, particularly of the skin (squamous-cell carcinoma), bladder and digestive tract, were reported in central Argentina (Besuschio et al. 1980). The relation between As in drinking water and skin cancer was reported by Astolfi et al. (1981, 1982). These health problems are regionally known as HACRE (the Spanish acronym for Hidro Arsenicismo Crónico Regional Endémico, Endemic Regional Chronic Hydro Arsenicism (Tello 1951)). Bladder cancer mortality associated with arsenic in drinking water was reported in Córdoba, central Argentina (Hopenhayn-Rich et al. 1996). Other studies concerned skin cancer (Cabrera and Gómez 2003) and bladder cancer in relation to direct arsenic exposure (Bates et al. 2004). In addition, high fluoride concentrations in these waters are associated with dental fluorosis and sometimes bone fluorosis (de la Sota et al. 1997). Arsenic- and fluoride-related disease affects, potentially, four million inhabitants in the region as a whole.
Moreover, the high salinity and the presence of excessive concentrations of As and F in irrigation waters are a strong limitation for agricultural production. Highly saline waters with high concentrations of potentially toxic elements (As > 200 μg L−1; F > 2,000 μg L−1) could lead to a risk for animals and may affect meat and milk quality. The health consequences due to other trace elements present in high concentrations and exceeding WHO guideline values assigned on health grounds is not known.
The aim of the present research was to characterize the water chemistry with respect to As and associated trace elements and to determine the principal sources and controlling geochemical factors. The main water-related health problems of the region are also described.
Regional setting
In the sedimentary basin of Tucumán Eastern Plain (10,000 km2), it is possible to differentiate two hydrogeological basins (Tineo 1998): the Burruyacú Basin in the north-eastern part and the Salí River Basin (the study area) in the south-west and south-east of the province. The Salí River Basin (Fig. 1), the most important basin in Tucumán Province, is located between the eastern borders of the del Aconquija Ranges, the Calchaquíes Summits and the de San Javier Ranges. The area covers almost 7,000 km2, extending as far as the structural lineament of the “Tacanas Ridge” and the Guasayán Ranges. The whole basin is covered by Quaternary sediments, which, according to geophysical studies and drilling data, may reach as much as 400 m thick.
Two climatic areas are defined. The first, in the piedmont area, exhibits a moderate rainy temperate climate with rainfall reaching 2,000 mm/year. On the nearby plain, which extends from the piedmont to the eastern boundary with Santiago del Estero Province, rainfall gradually decreases until it reaches 600 mm. Winters are dry and summers, warm and humid.
In the mountain area (5,000–500 m a.s.l.), steep slopes are about 10%. These facilitate runoff, particularly during the spring-summer rain period. The piedmont (500–350 m a.s.l.) has a slope decreasing to 2% and is characterized by modern deposits, alluvial fans and terrace levels with smaller grain-size towards the plain. Permeability is high, favouring water seepage for the recharge of basin aquifers. The plain (350–250 m a.s.l.) has a sharp change in slope (approximately 0.2%). This area consists of modern fluvial and aeolian deposits with intercalations of gravel and loess.
Hydrogeology
The western edge of the Salí River hydrogeological basin is characterized by outcrops of the metamorphic basement, with scarce outcrops of Cretaceous-Tertiary sedimentary rocks and a Quaternary cover hosting the main aquifer. The upper part of this (3–30 m) consists of silty-sandy soils and silty-loessic deposits. The artesian aquifers, located in the basal part of the Quaternary, consist of gravel and sands intercalated with clayey-silt sediments. A series of alluvial fans extend from the western edge of the basin, the most prominent being the Salí River and the Lules River alluvial fans (Fig. 1). Coalescing fans occur further south in the basin. The western marginal sediments form the recharge zone for the aquifers in the basin, and groundwater flow is predominantly eastwards.
The Salí River alluvial fan (Tineo et al. 1995), which extends from San Miguel de Tucumán city towards the south-east, covers an area of approximately 2,000 km2. It is one of the most important groundwater reservoirs in the province. In the apical zone, near the city, thick gravels and conglomerates intercalated with more scarce clayey silts of variable thickness extend to depths between 70 and 150 m. These aquifers have good yields. In the intermediate zone, horizons more than 300 m thick consist of layers of coarse sand and gravel intercalated with clayey-silt layers. The latter produce confined conditions. Throughout the whole of the intermediate zone, below 80 m depth, aquifers with natural artesian pressure occur. In the distal zone, sediments are of finer grain-size and the aquifers with natural artesian pressure have lower flows.
The eastern edge consists of de San Javier Ranges, a series of well-developed alluvial fans, with the coarse sediments and sandy-silt or clayey-silt intercalations (some 200 m thick).
The sedimentary column is formed by an igneous-metamorphic basement (Upper Precambrian to Lower Cambrian) covered by conglomerates and pelitic sediments of Cretaceous age, superposed unconformably by very fine-grained Cretaceous sediments and tuffs (middle to upper Miocene age). Very thick sedimentary layers of silty-clay sediments with sands belong to the Pliocene. The Quaternary section closer to the surface is formed by silty sands and silty-loess sediments modified by pedogenesis (4–30 m thickness) and buried by conglomerates. The Pliocene-Holocene cover hosts the main aquifers of the area; the artesian aquifers are developed at the base of Quaternary sediments. According to geological observations together with geophysical studies and drilling data, three hydraulic systems occur:
-
A lower hydraulic system: aquifer in fine- to medium-quartzose sands of Tertiary age between 200 and >400 m depth; the lowest limit is unknown. This is well developed in the plain area, with natural artesian pressure and groundwater temperatures up to 45.1°C. This system has a high groundwater yield and is recharged in the piedmont areas. The aquifer is used for public water supply, irrigation and cattle.
-
A middle hydraulic system: confined aquifers in coarse sands and gravels (between 30 and 150 m depth) with natural artesian pressure in the distal part of alluvial fans. These reservoirs have a high yield.
-
An upper hydraulic system: unconfined or semi-confined aquifers between 3 and 15 m depth, hosted in Quaternary silty-loess sediments with low permeability. They have very low yields and produce small supplies used by the rural population.
Sampling and analytical methods
Groundwater samples from shallow aquifers (3–15 m depth; 42 samples), deep aquifers (>200 m depth; 26 samples) and artesian aquifers (variable depths, >30 m; 17 samples) have been collected from public water-supply boreholes and private boreholes or hand-dug wells. In addition, 13 surface-water samples were collected.
In situ analysis included water temperature, pH, redox potential (Eh), dissolved oxygen (DO), specific electrical conductance (SEC, 25°C), turbidity and alkalinity (as HCO3). Samples for major- and trace-element analysis were filtered (0.45 μm pore) in situ into polyethylene bottles. Those collected for cation and sulphate analysis were acidified to 1% HNO3 (v/v). Samples for anion analysis were not acidified and those for total arsenic and other trace elements were acidified to 1% HCl (v/v). Unacidified aliquots were prepared with the addition of boric acid for nitrate analysis, mercuryII chloride for nitrite analysis and sulphuric acid for chemical oxygen demand (COD). Ca and Mg were analysed by AAS, Na and K by emission flame photometry, and sulphate by turbidimetry or ion chromatography. Chloride, fluoride and nitrate were determined by ion-selective electrode (ISE), nitrite and SiO2 by spectrophotometry, and COD by permanganometric titration. Arsenic and other trace elements were analysed by ICP-MS and ICP-OES. Speciation modelling of the groundwater was carried out using PHREEQC (Parkhurst and Appelo 1999).
Sediment samples were also collected from selected sites in the basin and at Los Pereyra site (see Fig. 1), where the highest arsenic concentrations in groundwater were found. Sediment from a hand-dug well at Los Pereyra was sampled from ground level down to the water table (−10.20 m).
Sediment samples were prepared by lithium metaborate/tetraborate fusion and analysed by ICP-MS or ICP-OES for major constituents and trace elements (As, V, U, Se, Sb, Mo, Cr, Al, Fe, Mn). Fluoride was determined by ISE and B by PGNAA. Loss on ignition (LOI) was also measured at 925°C. Selected samples of volcanic glass separated from loess sediments have been analysed by the same procedure. In the 0.5–0.05 mm fraction, volcanic glass was concentrated using an isodynamic separator and bromoform-acetone mixtures. A final washing was performed using a sodium-hexametaphosphate solution as a dispersant agent in an ultrasonic vibrator; a clean glass was thus obtained.
Hydrochemistry
Table 1 shows summary data for the shallow (unconfined) groundwater, deep groundwater and waters from flowing artesian wells, considered separately. Groundwater compositions are highly variable. Groundwater temperatures vary in the range of 19.0–45.1°C. Geothermal waters have been identified in the region (Tineo et al. 1989) and have been linked to the generation of high-arsenic groundwater (García et al. 2009). However, the significance of geothermal inputs in the study area is unclear as the ranges of temperature observed in the groundwater can be explained simply as a function of the geothermal gradient. Samples with temperatures above 40°C correspond to deep and artesian wells, in some cases more than 400 m deep.
Salinity is highly variable. SEC values vary from 112 to 7, 110 μS cm−1 and TDS values from 146 to 9, 240 mg L−1. The highest solute concentrations occur mainly in the shallow groundwater. The maximum SEC value for shallow levels is 7,110 μS cm−1 and that for deep groundwater is 1,570 μS cm−1; the TDS maximum value for shallow groundwater is 9,240 mg L−1 and that for deep groundwater is 1,250 mg L−1.
Most groundwater is hard or very hard. The hardness (as CaCO3) reaches up to 1,370 mg L−1 in shallow groundwater; concentrations are lower in deep groundwater and artesian aquifers. The pH values are mostly alkaline, ranging from 6.28 to 9.24. As shown in a Piper diagram (Fig. 2), sodium is in most cases the dominant cation (maximum 2,270 mg L−1) and bicarbonate the dominant anion (maximum 1,260 mg L−1). Sulphate and chloride are also important anions in the more saline groundwater. The most saline samples (e.g. high Na, Cl; Fig. 3) are from shallow depths and support the conclusion that the salinity is caused by the evaporation in the shallow horizons of the aquifer.
Major ion compositions are also controlled by silicate hydrolysis and ion exchange (Smedley et al. 2002). In the case of sodium, the release by reaction of sodic plagioclase is an important process. However, the trend of much groundwater towards Na-HCO3-dominated compositions (Fig. 2) is consistent with evolution via ion-exchange reactions. Bicarbonate concentrations exhibit a wide range: 50.0–1,260 mg L−1. The differences in concentrations between shallow and deep groundwater are significant.
Potassium concentrations are often high, largely as a result of hydrolysis of K-bearing minerals and evaporation; ion-exchange reactions may also be involved.
Since carbonate minerals are important components of sediments in the basin, groundwater chemistry is strongly controlled by carbonate reactions. Most groundwater is supersaturated with respect to dolomite, calcite, aragonite and rhodochrosite.
Concentrations of SiO2 are usually very high (28.2–138 mg L−1). The wide range reflects the high-temperature groundwater (up to 45.1°C) and, in loess sediments, abundance of fine-grained silicate minerals. These contain a very high proportion of feldspars and volcanic glass, which can be weathered easily. A key process responsible for the generation of high-pH groundwater is the reaction of albite to form kaolinite that also accounts for the origin of high sodium concentrations in groundwater.
The groundwater is oxidizing with DO concentrations between 1.8 and 6.1 mg L−1 and redox potentials up to 445 mV. Nitrate concentrations are highly variable. High concentrations are generally found in the shallow groundwater and have likely been increased by evaporation, although surface pollution is a possible additional factor. Mostly low nitrite concentrations (<5–712 μg L−1) are consistent with the oxic groundwater conditions observed.
Groundwater arsenic concentrations lie in the range of 11.4–1,660 μg L−1. Of the 85 groundwater samples collected, all exceed the WHO (2011) provisional guideline value for arsenic of 10 μg L−1. No definite regional trend has been observed in As distribution, but significant local variation is apparent. The highest arsenic concentrations are found in the shallow groundwater (Fig. 4). In these, concentrations reach up to 1,660 μg L−1, i.e. more than 160 times the WHO guideline value.
The shallow high-arsenic groundwater is also among the most saline samples observed (e.g. high Cl; Fig. 4). This suggests that at least some of the arsenic accumulation is related to near-surface evaporation. Deeper and artesian groundwater (including those with temperatures >40°C) has comparatively low arsenic concentrations.
Arsenic also shows a general positive correlation with HCO3 and a range of associated anion and oxyanion trace elements (F, V, B, U; Fig. 5, as well as Se and Mo). Such associations have been reported elsewhere in the Quaternary sediments of the Chaco-Pampean Plain (Smedley et al. 2002; Bundschuh et al. 2004; Florentino et al. 2007; Nicolli et al. 2008). Arsenic shows a poor correlation with dissolved Al, Fe and Mn.
Of the other anion and oxyanion trace elements, many also have very high concentrations. Fluoride (F−) shows a wide range of concentrations (50–7,340 μg L−1). Exceedances with respect to the WHO guideline value (1,500 μg L−1) are 17% for shallow groundwater. Boron concentrations lie in the range of 34–9,550 μg L−1 in shallow groundwater but are lower in the deeper and artesian groundwater. The WHO (2011) guideline value for boron is 2,400 μg L−1, and exceedances are about 35% in shallow aquifers.
Vanadium varies in concentration between 30.7 and 300 μg L−1 in shallow groundwater, and significantly lower values were found in deep groundwater and artesian aquifers. The health consequences of vanadium ingestion are poorly known and so no WHO guideline value exists for this element.
Molybdenum concentration varies between 0.2 and 727 μg L−1 in unconfined groundwater. Deep and artesian aquifers have lower values.
Uranium varies in concentration between 0.032 and 125 μg L−1 in shallow groundwater. The exceedances with respect to the WHO provisional guideline value (30 μg L−1) are 21%. The U concentrations in deep groundwater and artesian aquifers are significantly lower. A close positive correlation is observed between U and As and between U and HCO3.
Antimony concentrations vary between 0.04 and 0.46 μg L−1 in shallow groundwater, in which the highest concentrations have been found. The concentrations are low, and none of the groundwater samples has a concentration exceeding 20 μg L−1, the WHO guideline value.
The Se concentrations range between 0.2 and 9.0 μg L−1, and the highest values have been found in shallow groundwater. All are below the WHO guideline value of 40 μg L−1 (WHO 2011).
Concentrations of chromium lie in the range of 79–250 μg L−1 in the Salí River Basin groundwater. All exceed the WHO provisional guideline value for Cr of 50 μg L−1. Deep groundwater has the highest Cr concentrations. Shallow groundwater exhibits a maximum of 232 μg L−1, and the artesian aquifer Cr concentration reaches up to 218 μg L−1. Due to the acidic or intermediate composition of loess sediments (rhyolitic, dacitic, andesitic or trachyandesitic), the Chaco-Pampean aquifers usually have low dissolved chromium concentrations (up to 20 μg L−1: Smedley et al. 2002). However, high chromium concentrations (up to 230 μg L−1) were reported in some groundwater from the central Chaco-Pampean Plain by Farías et al. (2003). The fact that high concentrations occur in deep groundwater and are not of localized extent argues against contamination from industrial sources. The chromium is likely to be largely of natural origin, mobilized as hexavalent chromium under the oxic and alkaline groundwater conditions in the aquifer.
Loess sediments
The main sources of the above-mentioned trace elements are the Quaternary loess sediments, which have been reworked by aeolian and fluvial processes and cover the whole basin. Textural analyses show that the dominant sediment is a clayey-silt and, in some places, a silty-clay (Nicolli et al. 2000, 2005).
Polarizing microscopy was used for the mineralogical study of sand and coarse-silt fractions, and X-ray diffraction for those of fine silt and clay. Among light minerals (greater than 10 μm size), the main components are feldspars (mainly plagioclase but also K-feldspar) and volcanic glass. Feldspars usually range between 45 and 70% and volcanic glass shards between 25 and 50% (exceptionally up to 63%). Smaller amounts of quartz, muscovite, calcite and lithic fragments (some of them with Al-, Mn- and Fe-oxide coatings) are also represented. Opal and chalcedony are present in smaller proportions with an irregular distribution. In the fraction corresponding to the 0.5–0.05 mm size, the most abundant heavy minerals (usually <5%) are pyroxene and amphibole (hypersthene, enstatite, hornblende, lamprobolite). Biotite, epidote and Fe- and Mn oxides are less abundant, and garnet, tourmaline, apatite, zircon, chlorite and rutile are scarce.
The X-ray diffraction analysis demonstrated the presence of low-crystallinity minerals, illite prevailing over smectite. These minerals include interstratified illite-montmorillonite, as well as rare pure montmorillonite, kaolinite and interstratified chlorite-montmorillonite. Usually, fine grain-size fractions show variable amounts of amorphous material (volcanic glass shards).
The average chemical composition of the sediments usually corresponds to that of a dacite, andesite or trachyandesite, with SiO2 ranging between 60.5 and 66.8%. The As contents vary between 7 and 14 mg kg−1; F contents between 534 and 862 mg kg−1; V between 53 and 99 mg kg−1; U between 3.62 and 12.4 mg kg−1; and B between 42 and 72 mg kg−1.
The chemical composition of volcanic glass is typically rhyolitic with SiO2 ranging between 72.2 and 78.6%. Arsenic contents in glass are relatively low, ranging between <5 and 8 mg kg−1; F varies between 109 and 1,020 mg kg−1; V between <5–17 mg kg−1; U between 4.3 and 27.7 mg kg−1; and B between 35 and 101 mg kg−1.
Discussion
Arsenic and associated trace elements in groundwater from the Salí River Basin show a wide range of variation, although all groundwater samples investigated were found to be seriously contaminated with arsenic. The range in As concentration (11.4–1,660 μg L−1) indicates that 100% are above the WHO provisional guideline value (10 μg L−1); 14.1% exceed 100 μg L−1, 3.0% exceed 500 μg L−1 and 1.5% exceed 1,000 μg L−1. In shallow groundwater, the exceedances in concentration above the WHO guideline values are also 35% for B, 21% for U and 17% for F. All groundwater samples exceed the WHO provisional guideline value for Cr.
No distinct regional trend in trace-element distribution has been found. Thus, local-scale phenomena, such as small slope variations that influence groundwater flow velocities or spatial variations in grain-size and texture of sediments, play significant roles in the trace-element distributions.
The positive correlations observed between As and other anions and oxyanions (F, V, B, U, Mo) suggest that these trace elements have the same mineral source, although the generally alkaline and oxic groundwater conditions present are also likely to lead to correlation of these variables. The dissolution/flow velocity relationship controls their concentrations in the groundwater (Nicolli et al. 2001 , 2004, 2008). A high degree of spatial variability in groundwater chemistry and trace-element concentrations over short distances indicates restricted groundwater flow as a result of low permeability in loess sediments, especially at shallow levels.
The Quaternary loess sediments are the source of these trace elements. The average chemical composition of loess is similar to that of a dacite and arsenic concentration ranges between 7 and 14 mg kg−1. The composition of volcanic glass is, on the other hand, similar to that of a rhyolite, and the arsenic content ranges between <5 and 8 mg kg−1. Significant uranium concentrations (4.3–27.7 mg kg−1) are also present. Trace-element contents of volcanic glass from the Salí River Basin are similar to those in glass separated from loess sediments in the south-eastern part of the plain, in Córdoba Province, central Argentina (Nicolli et al. 1989).
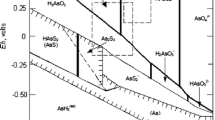
The high pH values in the basin (up to 8.72 in shallow groundwater) favour the volcanic glass dissolution and leaching of volcanic-origin material in loess and loess-like sediments. In the oxidizing conditions, arsenic occurs dominantly as AsV (arsenate). The presence of Mn oxides as a coating on lithic fragments could contribute to maintaining the arsenic mainly in the oxidized form (Oscarson et al. 1981a, b). Speciation modelling suggests that the other dominant anion and oxyanion species are HVO4 2−, H2VO4 −, UO2(CO3) 2−2 , UO2(CO3) 4−3 , F− and other F-complexes with B, FeIII and Al. The groundwater with high concentrations of these elements is mostly of bicarbonate type with high pH values.
Under neutral pH conditions, concentrations of solute As and associated trace elements would be expected to be low due to their strong sorption onto surfaces of Fe- and Al oxides and oxyhydroxides (Dzombak and Morel 1990). The high pH and oxic conditions, characteristic of the Salí River Basin aquifers, render sorption of AsV and associated elements less favourable and hence contribute to the observed high concentrations.
Taking into account the saturation indices (SI) for shallow groundwater (with high As concentrations), many (usually 15–25) saturated or supersaturated mineral phases occur. Some of them are Fe- and Al oxides and hydroxides, and clay minerals with high sorption capacities, carbonate species (calcite, anorthite, aragonite, siderite, rhodochrosite), chalcedony and gypsum. Significant differences are shown in deep groundwater (with lower As concentration), where fewer (usually <10) supersaturated mineral phases occur and absolute values are lower (Nicolli et al. 2004, 2008). All groundwater is unsaturated with respect to arsenic minerals.
The shallow groundwater has relatively high salinity, which is related to a strong evaporation process near the surface in this semi-arid area. The evaporative concentration of solutes near the surface in areas with limited recharge means that water-rock reactions can proceed without significant dilution (Smedley et al. 2002). These features, that produce a high solute concentration, are similar to those that may be found in other arid or semi-arid areas of the Chaco-Pampean Plain (Nicolli et al. 1989, 1997, 2008; Smedley et al. 2002, 2005, 2008; Bundschuh et al. 2004, 2006, 2008; Bhattacharya et al. 2006), in northern Mexico (del Razo et al. 1990), in southwestern USA, at Tulares Basin, San Joaquin Valley, California (Fujii and Swain 1995) and in other world regions.
Conclusions
-
Groundwater from the Salí River Basin, Tucumán Province, Argentina, has a wide range of trace-element quality problems. Highest concentrations of arsenic, boron, molybdenum, uranium, fluoride and vanadium derive from the shallow Quaternary loess aquifer. Lower concentrations occur in deep groundwater (>200 m) and groundwater from artesian boreholes.
-
High concentrations of chromium have been found in all the waters of the Salí River Basin. Deep groundwater appears to be the most contaminated (Cr concentrations: 129–250 μg L−1; WHO provisional guideline value: 50 μg L−1). The wide extent of the high concentrations and the fact that high concentrations occur in deep groundwater (>200 m) as well as shallow ones suggests that the Cr is mainly naturally derived.
-
Arsenic concentrations are in the range of 11.4–1,660 μg L−1, and 100% of the investigated groundwater samples exceed the provisional WHO guideline value for arsenic in drinking water of 10 μg L−1. The median value is 3.5 times higher than the guideline value, and the maximum value is 160 times the guideline value. Exceedances above WHO guideline values also occur for boron (maximum value up to 4 times the WHO guideline value), uranium (up to 8 times the guideline value) and fluoride (up to 5 times the guideline value). No WHO guideline value currently exists for vanadium in drinking water.
-
The dominant loess sediment is a clayey-silt and in some places a silty-clay. Its main components are feldspars (45–70%), volcanic glass (typically 25–50%; up to 63%) and smaller amounts of quartz, muscovite, calcite and lithic fragments. Chemical composition is similar to that of a dacite, andesite or trachyandesite (SiO2: 60.5–66.8%), with As contents between 7 and 14 mg kg−1 and high associated trace-element concentrations.
-
The groundwater is universally oxidizing with high dissolved oxygen concentrations and high redox potential values and with arsenic concentrations likely occurring mainly as AsV. Groundwater has neutral to strongly alkaline pH (values up to 9.24); many groundwaters with high alkalinity values (HCO3). These alkaline conditions are concluded to be generated by silicate hydrolysis reactions (especially involving sodic plagioclase), together with carbonate reaction under closed conditions and associated cation exchange. Evaporation, as evidenced by high salinity (e.g. TDS values up to 9,240 mg L−1; Cl concentrations up to 1,100 mg L−1), has been an important process in the shallow loess aquifer of this semi-arid/arid region. This may have contributed to a further increase in the concentrations of the trace elements described.
-
Strong positive correlations have been found between arsenic and bicarbonate, as well as with fluorine, vanadium, boron and uranium. The presence of high concentrations of anion and oxyanion species results from the dissolution of primary minerals, particularly volcanogenic minerals (including volcanic glass), in the loess aquifer. Under the oxic and alkaline conditions, sorption of As and other oxyanions to secondary oxide-mineral surfaces (Fe-, Al- and Mn oxides) is limited.
-
No clear regional trend has been observed in the trace-element distributions. Short-range variability is likely to be controlled by local groundwater flow cells and residence time and by local variability in sediment texture and lithology.
-
Long-term consumption of groundwater in the Salí River Basin has been associated with a number of health problems in exposed populations (some four million inhabitants in the Chaco-Pampean Plain). The main observed symptoms are hydroarsenicism (skin and internal cancers) and teeth/bone fluorosis.
References
Astolfi, E., Besuschio, S. C., García, J. C., Guerra, C., & Maccagno, A. (1982). Hidroarsenicismo Crónico Regional Endémico (p. 144). Buenos Aires, Argentina: Edit. Coop. General Belgrano.
Astolfi, E. A. N., Maccagno, A., García Fernández, J. C., Vaccaro, R., & Stimola, R. (1981). Relation between arsenic in drinking water and skin cancer. Biological Trace Element Research, 3, 133–143.
Ayerza, A. (1917a). Arsenicismo regional endémico (keratodermia y melanodermia combinadas). Bol. Acad. Medicina 2–3 (pp. 11–24). Buenos Aires, Argentina.
Ayerza, A. (1917b). Arsenicismo regional endémico (keratodermia y melanodermia combinadas) (continuación). Bol. Acad. Medicina 2–3 (pp. 41–55). Buenos Aires, Argentina.
Ayerza, A. (1918). Arsenicismo regional endémico (keratodermia y melanodermia combinadas). Bol. Acad. Nac. Medicina I (pp. 11–41). Buenos Aires, Argentina.
Bates, M. N., Rey, O. A., Biggs, M. L., Hoppenhayn, C., Moore, L. E., Kalman, D., et al. (2004). Case–control study of bladder cancer and exposure to arsenic in Argentina. American Journal of Epidemiology, 159, 381–389.
Besuschio, S. C., Desanzo, A. C., Pérez, A., & Croci, M. (1980). Epidemiological associations between arsenic and cancer in Argentina. Biological Trace Element Research, 2, 41–55.
Bhattacharya, P., Claesson, M., Bundschuh, J., Sracek, O., Fagerberg, J., Jacks, G., et al. (2006). Distribution and mobility of arsenic in the Río Dulce alluvial aquifers in Santiago del Estero Province, Argentina. Science of the Total Environment, 358, 97–120.
Bundschuh, J., Farias, B., Martin, R., Storniolo, A., Bahattacharya, P., Cortes, J., et al. (2004). Groundwater arsenic in the Chaco-Pampean Plain, Argentina: case-study from Robles County, Santiago del Estero Province. Applied Geochemistry, 19(2), 231–243.
Bundschuh, J., García, M. E., & Bhattacharya, P. (2006). Arsenic in groundwater of Latin America—a challenge of the 21st century. Geological Society of America Annual Meeting, Philadelphia, 22–25 Oct. 2006, Geological Society of America Abstracts with Programs, 38:7 (320 pp.).
Bundschuh, J., Perez Carrera, A., & Litter, M. (Eds.). (2008). Distribución del arsénico en las regiones Ibérica e Iberoamericana (223 pp.). Buenos Aires, Argentina: Editorial Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo. Available at: http://www.cnea.gov.ar/xxi/ambiental/iberoarsen/.
Cabrera, H. N., & Gómez, M. L. (2003). Skin cancer induced by arsenic in the water. Journal of Cutaneous Medicine and Surgery, 7(2), 106–111.
Círculo Médico del Rosario. (1917). Sobre la nueva enfermedad descubierta en Bell-Ville. Revista Médica del Rosario, VII (pp. 485). Rosario, Argentina.
de la Sota, M., Puche, R., Rigalli, A., Fernández, M. L., Benassati, S., & Boland, R. (1997). Modificaciones de la masa ósea y en la homeóstasis de la glucosa en residentes de la zona de Bahía Blanca con alta ingesta de flúor. Medicina, 57, 417–420. Buenos Aires, Argentina.
del Razo, L. M., Arellano, M. A., & Cebrián, M. E. (1990). The oxidation states of arsenic in well-water from a chronic arsenicism area of northern Mexico. Pollution, 64, 143–153.
Dzombak, D. A., & Morel, F. M. M. (1990). Surface complexation modeling: Hydrous ferric oxide (p. 393). New York: Wiley.
Farías, S. S., Casa, V. A., Vazquez, C., Ferpozzi, L., Pucci, G. N., & Cohen, I. M. (2003). Natural contamination with arsenic and other trace elements in ground waters of Argentine Pampean Plain. Science of the Total Environment, 309, 187–199.
Florentino, C. E., Paoloni, J. D., Sequerra, M. E., & Arosteguy, P. (2007). The presence of vanadium in groundwater of southeastern extreme the Pampean region Argentina: Relationship with other chemical elements. Journal of Contaminant Hydrology, 93, 122–129.
Fujii, R., & Swain, W. C. (1995). Areal distribution of selected trace elements, salinity, and major ions in shallow ground water, Tulares Basin, southern San Joaquin Valley, California (67 pp.). US geological survey water-resources investigations report, 95-4048. Denver, USA: US Geological Survey.
García, M. G., Moreno, C., Galindo, M. C., Hidalgo, M. del V., Fernández, & Sracek, O. (2009). Intermediate to high levels of arsenic and fluoride in deep geothermal aquifers from thr northwestern Chaco-Pampean plain, Argentina. CRC Press/Balkema. In J. Bundschuh, M. A. Armienta, P. Birkle, P. Bhattacharya, J. Matschullat, & A. B. Mukherjee (Eds.), Natural arsenic in groundwater of Latin America—occurrence, health impact and remediation (pp. 69–79). Balkema, Boca Raton: CRC Press.
Hopenhayn-Rich, C., Biggs, M. L., Fuchs, A., Bergoglio, R., Tello, E., Nicolli, H., et al. (1996). Bladder cancer mortality associated with arsenic in drinking water in Córdoba, Argentina. Epidemiology, 7, 117–124.
Nicolli, H. B., Suriano, J. M., Gómez Peral, M. A., Ferpozzi, L. H., & Baleani, O. M. (1989). Groundwater contamination with arsenic and other trace elements in an area of the Pampa, Province of Córdoba, Argentina. Environmental Geology and Water Sciences, 14, 3–16.
Nicolli, H. B., Tineo, A., Falcón, C. M., & García, J. W. (2005). Distribución del arsénico y otros elementos asociados en aguas subterráneas de la región de Los Pereyra, provincia de Tucumán, Argentina. In G. Galindo, J. L. Fernández Turiel, M. A. Parada, & D. Gimeno Torrente (Eds.), Arsénico en aguas: origen, movilidad y tratamiento (pp. 83–92). Río Cuarto, Argentina: IV Cong. Argentino de Hidrogeología.
Nicolli, H. B., Tineo, A., Falcón, C. M., García, J. W., Merino, M. H., Etchichury, M. C., Alonso, M. S., & Tofalo, O. R. (2008). Hydrogeochemistry of arsenic in groundwaters from Burruyacú basin, Tucumán Province, Argentina. In J. Bundschuh, M. A. Armienta, P. Bhattacharya, J. Matschullat, & A. B. Mukherjee (Eds.), Natural arsenic in groundwaters of Latin America—Occurrence, health impact and remediation. In J. Bundschuh, & P. Bhattacharya (series Eds.), Arsenic in the environment (Vol. 1, pp. 47–59). Leiden, The Netherlands: CRC Press, Balkema.
Nicolli, H. B., Tineo, A., Falcón, C. M., & Merino, M. H. (2001). Movilidad del arsénico y de otros oligoelementos asociados en aguas subterráneas de la cuenca de Burruyacú, provincia de Tucumán, República Argentina. In A. Medina, J. Carrera, & L. Vives (Eds.), Congreso Las Caras del Agua Subterránea I (pp. 27–33). Madrid: Instituto Geológico y Minero de España.
Nicolli, H. B., Tineo, A., & García, J. W. (2000). Estudio hidrogeológico y de calidad del agua en la cuenca del río Salí, provincia de Tucumán. Revista Association Argentina Geología Aplicada a la Ingeniería y al Ambiente, 15, 82–100. Buenos Aires, Argentina.
Nicolli, H. B., Tineo, A., García, J. W., Falcón, C. M., Merino, M. H., Etchichury, M. C., et al. (2004). The role of loess in groundwater pollution at Salí River Basin, Argentina. In R. B. Wanty & R. R. Seals II (Eds.), Water–rock interaction (2nd ed., pp. 1591–1595). Leiden, The Netherlands: Balkema.
Oscarson, D. W., Huang, P. M., Defosse, D., & Herbillion, A. (1981a). Oxidative power of Mn(IV) and Fe(III) oxides with respect to As(III) in terrestrial and aquatic environments. Nature, 291, 50–51.
Oscarson, D. W., Huang, P. M., & Liaw, W. K. (1981b). Role of manganese in the oxidation of arsenite by freshwater lake sediments. Clays & Clay Minerals, 29, 219–225.
Parkhurst, D. L., & Appelo, C. (1999). User’s guide to PHREEQC (Version2)—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. U.S. Geol. Survey Water-Resources Investigations, Report 99–4259. Denver, USA: US Geological Survey.
Smedley, P. L., Kinniburgh, D. G., Macdonald, D. J. M., Nicolli, H. B., Barros, A. J., Tullio, J. O., et al. (2005). Arsenic associations in sediments from the loess aquifer of La Pampa, Argentina. Applied Geochemistry, 20(5), 989–1016.
Smedley, P. L., Nicolli, H. B., Macdonald, D. M. J., Barros, A. J., & Tullio, J. O. (2002). Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Applied Geochemistry, 17(3), 259–284.
Smedley, P. L., Nicolli, H. B., Macdonald, D. M. J., & Kinniburgh, D. G. (2008). Arsenic in groundwater and sediments from La Pampa province, Argentina. In J. Bundschuh, M. A. Armienta, P. Birkle, P. Bhattacharya, J. Matschullat, & A. B. Mukherjee (Eds.), Natural arsenic in groundwater of Latin America—occurrence, health impact and remediation (pp. 35–45). Boca Raton: CRC Press, Balkema.
Tello, E. (1951). Hidroarsenicismo Crónico Regional Endémico (HACRE), sus manifestaciones clínicas (p. 165). Córdoba, Argentina: Ed. Univ. Nac. de Córdoba.
Tineo, A. (1998). Los acuíferos del cono aluvial del río Salí, provincia de Tucumán, República Argentina. IV Cong. Latinoamericano de Hidrología Subterránea I (pp. 14–24). Uruguay: Montevideo.
Tineo, A., García, J., Falcón, C., D’Urso, C., & Rodríguez, G. (1995). Hidrogeología del cono aluvial del río Salí, provincia de Tucumán, Argentina. IX° Cong. Latinoamericano de Geología II (pp. 515–524). Venezuela: Caracas.
Tineo, A., Iglesias, E., Durán, M., Verma, M., García, J., Falcón, C., et al. (1989). Geochemical survey of the Llanura Tucumana geothermal area, Argentina. Geothermal Resources Council Transactions, XIII, 165–171.
WHO—World Health Organization. (2011). Guidelines for drinking-water quality. Fourth Edition. On line: http://www.who.int/publications/2011/9789241548151_eng.pdf.
Acknowledgments
The authors wish to acknowledge CONICET, Consejo Nacional de Investigaciones Científicas y Técnicas (Argentine National Council for Scientific and Technical Research) for providing additional funding from PIP N° 5775 as support for the research. Arturo J. Barros assisted in chemical analysis of waters, sediments and volcanic glasses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nicolli, H.B., García, J.W., Falcón, C.M. et al. Mobilization of arsenic and other trace elements of health concern in groundwater from the Salí River Basin, Tucumán Province, Argentina. Environ Geochem Health 34, 251–262 (2012). https://doi.org/10.1007/s10653-011-9429-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-011-9429-8