Abstract
Black carbon (BC) is an important class of geosorbents that control the fate and transport of organic pollutants in soil and sediment. We previously demonstrated a new role of BC as an electron transfer mediator in the abiotic reduction of nitroaromatic and nitramine compounds by Oh and Chiu (Environ Sci Technol 43:6983–6988, 2009). We proposed that BC can catalyze the reduction of nitro compounds because it contains microscopic graphitic (graphene) domains, which facilitate both sorption and electron transfer. In this study, we assessed the ability of different types of BC—graphite, activated carbon, and diesel soot—to mediate the reduction of 2,4-dinitrotoluene (DNT) and 2,4-dibromophenol (DBP) by H2S. All three types of BC enhanced DNT and DBP reduction. H2S supported BC-mediated reduction, as was observed previously with a thiol reductant. The results suggest that BC may influence the fate of organic pollutants in reducing subsurface environments through redox transformation in addition to sorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Black carbon (BC), such as soot, charcoal, coke, cenosphere, graphite, coal, kerogen, and activated carbon, is produced by incomplete combustion or pyrolysis of fossil fuels and biomass (Goldberg 1985; Schmidt and Noack 2000). BC is composed of polycyclic aromatic carbon (graphene) sheets, often arranged in a highly disordered manner resulting in a microporous structure (Goldberg 1985; Schmidt and Noack 2000). BC particles have variable morphologies ranging from spherical to irregular shapes and from smooth to rough surfaces (Brodowski et al. 2005). The elemental O/C ratio can be as high as 0.4, depending on the type of BC (Brodowski et al. 2005; Hopkins et al. 2007). BC represents, on the average, 9 and 4% of total organic carbon in sediments and soils, respectively (Cornelissen et al. 2005a). In some marine sediments, BC can make up 10–30% of the total organic carbon (Gustafsson and Gschwend 1998; Middelburg et al. 1999). The global annual production rate of BC is on the order of several million tons (Dusek et al. 2006).
In recent years, BC has received increasing attention because of its many environmental impacts. BC is a well-known global warming agent. Atmospheric BC can cause warming by trapping solar radiation and altering cloud formation (Jacobson 2001). Deposition of BC on snow and ice can greatly accelerate melting (Flanner et al. 2007). Because of the short lifetime of BC in the atmosphere, controlling BC emissions has been proposed as a fast-acting strategy to mitigate climate change (Molina et al. 2009). Moreover, BC plays a critical role in biogeochemical processes, such as formation of soil organic matter, global carbon cycle in which it acts as a sink, and a reservoir for nutrients for microorganisms (Goldberg 1985; Schmidt and Noack 2000).
In addition, BC is an important class of geosorbents that govern the fate and transport of organic contaminants in soil and sediment. Sorption of hydrophobic compounds to soil used to be attributed to soil organic matter and correlated solely to the organic carbon content (Eq. 1) (Schwarzenbach et al. 2002).
In Eq. 1, K d and K OC are the partitioning coefficients between soil and water and between organic carbon and water, respectively, and f OC is the mass fraction of organic carbon in soil. The discovery of BC’s role as a geosorbent changed this paradigm. In contrast to amorphous organic matter, which behaves like a solvent and accommodates organic molecules in a linear and noncompetitive manner, BC is hard and glassy and binds sorbate molecules through competitive, nonlinear adsorption onto its condensed surfaces and/or into the nanopores in its structures (Nguyen et al. 2004, 2007). Because of these sorption mechanisms, BC is a particularly important sorbent for molecules that have or can adopt a planar configuration, such as polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) (Cornelissen and Gustafsson 2005; Cornelissen et al. 2005b; Lohmann et al. 2005).
Chiu and Kile (1998) observed nonlinear sorption of organic compounds to soils and hypothesized that the presence of high-surface area carbonaceous material (HSACM), such as charcoal, may explain the increased sorption at low concentrations. Gustafsson et al. (1997) found that soot carbon affected the partitioning of PAHs such as naphthalene and phenanthrene in soil. These authors proposed an expanded organic sorption model that includes a BC fraction of soil (f BC), a BC-normalized partition coefficient (K BC), and a Freundlich exponent for sorption to BC (Nf) (Gustafsson et al. 1997; Bucheli and Gustafsson 2000; Cornelissen and Gustafsson 2004).
Cornelissen et al. (2004) showed that BC was particularly important for the sorption of planar compounds and that sorption data were in good agreement with the model that includes BC. For small planar compounds, BC can be 10 to 1,000 times stronger as a sorbent than natural organic matter on a per carbon atom basis (Cornelissen et al. 2005a). Thus, through sorption BC may strongly influence the bioavailability and ecotoxicity of organic compounds in soils and sediments (Gustafsson et al. 1997; Accardi-Dey and Gschwend 2002; Thorseon et al. 2004).
Zhu and Pignatello (2005) showed that the extent of sorption of aromatic compounds to BC was greater than predicted based on sorbate hydrophobicity and was related to the electron-accepting or donating tendency of the sorbate. These authors suggested that π–π electron donor–acceptor (EDA) interactions between sorbates and BC could explain the sorption behavior, and that electron-rich and poor regions of graphene moieties in BC might play a role in the sorption of π-acceptors (e.g., nitrobenzene, 2,4-dinitrotoluene (DNT) and 2,4,6-trinitrotoluene (TNT)) and π-donors (e.g., naphthalene and phenanthrene), respectively (Zhu and Pignatello 2005; Sander and Pignatello 2005). Pignatello’s group also showed that polar interactions involving the O-functions in BC were not important, since removal of O-functional groups actually enhanced sorption by decreasing adsorption of water molecules (Zhu et al. 2005).
Organic molecules sorbed to BC have been commonly assumed to be chemically and biologically inert. This assumption is the basis for a new cleanup approach where a sorbent such as granular activated carbon (GAC) is added to sediment to reduce the free concentration and bioavailability of PCBs or PAHs (Zimmerman et al. 2004; Cho et al. 2009). Around the same time, however, increasing evidence indicated that graphite, because of its ability to conduct electron and atomic hydrogen, could catalyzed the reduction of sorbed nitrogenous compounds (Oh et al. 2002, 2004, 2005; Ye and Chiu 2006). This suggests that the graphitic regions of BC may also be catalytically active, and that organic molecules sorbed to BC may be chemically reactive. This was subsequently demonstrated by us and other researchers (Kemper et al. 2008; Oh and Chiu 2009; Xu et al. 2010).
We showed that abiotic reduction of nitroaromatic compounds (NACs) such as DNT and heterocyclic nitramines such as hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by a thio reductant was greatly enhanced by soot or graphite (Oh and Chiu 2009). Kemper et al. (2008) also reported that RDX degradation by hydrogen sulfide could be accelerated in the presence of BC including activated carbon and graphite. Xu et al. (2010) subsequently showed that BC could also mediate the reduction of nitroglycerin by H2S.
With respect to the mechanism for BC-mediated reactions, two hypotheses have been put forth: one attributing the catalytic activities of BC to surface quinone functions and the other to graphene moieties in BC. van der Zee et al. (2003) observed enhanced reduction of an azo dye by H2S in the presence of activated carbon and suggested that quinone groups in activated carbon were involved. Kemper et al. (2008) observed activated carbon and other BC promoted RDX degradation by H2S. While these authors did not draw definitive conclusions, they suggested that quinone groups in BC might be responsible for the faster RDX degradation. In contrast, our results on the reduction of NACs and other nitrogenous compounds with soot or graphite (Oh and Chiu 2009) support the latter hypothesis; i.e., the catalytic property of BC arises from its graphene domains, where sorption of organic molecules and electron transfer to these molecules from an external reductant both occur, as illustrated in Fig. 1.
This study assessed different types of BC in terms of its ability to mediate the reductive transformation of NACs and halogenated aromatics. Batch experiments were performed using graphite, GAC, and diesel soot as BC materials and DNT and 2,4-dibromophenol (DBP) as model compounds. Hydrogen sulfide was chosen as a reductant in this study for its ubiquity in anaerobic soil and sediment (Schwarzenbach et al. 2002; Rickard and Luther 2007). Probable mechanisms for BC-mediated reduction reactions will be discussed based on data from this and other studies.
Materials and methods
Chemicals
2,4-Dinitrotoluene (DNT, 97%), 2,4-diaminotoluene (DAT, 98%), 4-amino-2-nitrotoluene (4A2NT, 97%), 2-amino-4-nitrotoluene (2A4NT, 99%), 2,4-dibromophenol (DBP, 97%), 2-bromophenol (2BP, 98%), 4-bromophenol (4BP, 99%), and Na2S (>98%) were purchased from Aldrich (Milwaukee, MI, USA). NaHCO3 (>99.0%) was obtained from Sigma (St. Louis, MO, USA). Phenol (>98.5%) and K2HPO4 were acquired from DC Chemical (Seoul, Korea). NaH2PO4·2H2O (>98%) was purchased from Junsei Chemical (Tokyo, Japan). All chemicals were used as received.
High-purity graphite powder (<20 μm, 99.9%) was acquired from Aldrich (Milwaukee, WI, USA). A charcoal-based GAC (DC Chemical, Seoul, Korea) was washed and rinsed with distilled water to remove fines and impurities, dried at 105°C overnight, and stored in a desiccator before use. Diesel soot was collected from a diesel engine (Hyundai Motor Co., Ulsan, Korea). Diesel soot particles were sampled carefully from an exhaust gas recirculation cooler in the diesel engine using a clean brush. The soot collected was rinsed with distilled water, dried at 60°C for 3 h, and stored in a desiccator for at least 10 days before characterization and experimental use. A scanning electron microscopy (SEM) image of the diesel soot shows that it has a lamellar structure consisting of agglomerates of spherical particles ~60 nm in diameter. An energy dispersive X-ray spectroscopy (EDX) analysis indicates that the carbon and oxygen contents of the soot surface were 83.8 and 16.2%, respectively. The surface areas of graphite, GAC, and diesel soot were 13.6 ± 0.3, 739 ± 1, and 9.2 ± 0.1 m2/g, respectively, as determined by the BET method with N2.
Batch reduction experiments
Batch experiments were conducted using 250-mL borosilicate amber bottles containing 200 mL of aqueous solution and BC: 0.1 g of graphite powder, 0.01 g of GAC, or 0.05 g of diesel soot. Prior to introduction of BC, solution in each bottle was deoxygenated by purging with N2 for 30 min to remove dissolved oxygen. For DNT, an initial concentration of 0.362 or 0.276 mM was used. By using 20 mM CO2/HCO3 − buffer, the pH of DNT solution was maintained at 6.3 during experiment. At this pH, 83% of the added sulfide was protonated (i.e., as H2S). For DBP, 20 mM of phosphate buffer was used to maintain the pH at 7.0, where approximately half of the added sulfide was HS−. The initial concentration of DBP was 0.395 mM. Three hundred twelve mg of Na2S (4 mmol) was added to the solution as a bulk reductant, corresponding to a total sulfide concentration of 20 mM. Mininert® valves (VICI Precision Sampling Inc, Baton Rouge, LA) and vinyl tape were used to seal the bottles and prevent air penetration. The bottles were placed in a horizontal position on a platform shaker rotating at 150 rpm. At selected times, 1 mL of aqueous sample was withdrawn using a glass syringe and immediately passed through a 0.025-μm cellulose membrane filter (Millipore, MA) for analytic determination of DNT, DBP, and their reduction products. For each experiment two controls were prepared under identical conditions, one without hydrogen sulfide and the other without BC.
Chemical analysis
2,4-dinitrotoluene (DNT), 2,4-dibromophenol (DBP), and their reduction products were analyzed using a UltiMate™ 3,000 HPLC system (Dionex, Sunnyvale, CA) equipped with an Acclaim® 120 guard column (4.3 × 10 mm, Dionex, Sunnyvale, CA) and an Acclaim® 120 C18 column (4.6 × 250 mm, 5 μm, Dionex, Sunnyvale, CA). A methanol–water mixture (50/50, v/v) was used as the mobile phase at a flow rate of 1.0 mL/min for DNT, 4A2NT, and 2A4NT. The wavelength of the UV detector was set at 254 nm. The retention times for DNT, 4A2NT, and 2A4NT were 21.60, 10.12, and 11.26 min, respectively. For DAT analysis, an acetonitrile–phosphate buffer (20 mM, pH 7.0, 30/70, v/v) was used as eluent at 1.0 mL/min. The UV detector wavelength was 224 nm and the retention time was 4.73 min. For the quantification of DBP, 2BP, 4BP, and phenol, a methanol–water mixture (65/35, v/v) was used as the mobile phase at a flow rate of 1.0 mL/min. The wavelength of the UV detector was set at 224 nm. Retention times for DBP, 4BP, 2BP, and phenol were 18.22, 8.89, 7.25, and 4.83 min, respectively.
Results and discussion
BC-mediated reduction of DNT
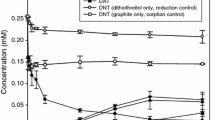
The reduction of DNT by hydrogen sulfide in homogeneous solution is shown in Fig. 2. Approximately, 90% of DNT was removed from solution over 168 h. As DNT disappeared, 4A2NT was formed as a major intermediate and its concentration reached 0.087 mM at 168 h, corresponding to 24.1% of the initial DNT mass. In contrast, 2A4NT concentration was below 0.01 mM and no DAT was detected in solution throughout the experiment. The mole ratio of aqueous 4A2NT to 2A4NT was approximately 8.5 at 168 h. This result indicates that a para nitro reduction pathway was favored in hydrogen sulfide solution, consistent with other electron transfer reactions in homogeneous systems containing H2S (88% para reduction) or H2S plus juglone (92% para reduction) (Barrows et al. 1996).
No DNT was reduced in the presence of graphite without hydrogen sulfide (Fig. 3). Approximately, 53% of dissolved DNT disappeared initially, presumably via sorption to graphite, and no reduction product was observed. In the presence of H2S, DNT removal was significantly enhanced due to a combination of sorption and reduction. DNT completely disappeared from solution within 72 h, and correspondingly the reduction intermediates (4A2NT, and to a lesser extent 2A4NT) and end product (DAT) were formed. At 120 h, the concentrations of 4A2NT and 2A4NT were 0.122 and 0.016 mM, respectively. The 4A2NT-to-2A4NT ratio was between 7 and 8 during the course of the experiment, lower than that (8.5) observed in homogeneous H2S solution. Note that this result is not necessarily inconsistent with the 1:1 ratio we previously observed for graphite-mediated DNT reduction by dithiothreitol (Oh and Chiu 2009). Graphite mediation with H2S may very well have shifted the 4A2NT-to-2A4NT ratio closer to 1, as in the case of dithiothreitol. However, due to the high reactivity of H2S, most DNT was transformed through direct reaction with H2S, which had a regio-selectivity of 8.5, while graphite-catalyzed reaction was secondary. At 168 h, 0.095 mM DAT was formed, accounting for 26.4% of the initial DNT, and the overall mass recovery was 61.0%. Based on the formation rates and yields of 4A2NT, 2A4NT, and DAT in Figs. 2 and 3, it is clear that graphite was able to catalyze the reduction of DNT with H2S as a bulk reductant.
The enhancement in DNT reduction was more marked with GAC (Fig. 4). Compared to Fig. 3, aqueous 4A2NT and 2A4NT masses peaked at earlier times and were removed almost completely after 168 h, while DAT concentration continued to increase during this time. The 4A2NT-to-2A4NT ratio was approximately 7, again smaller than that for the homogeneous reaction (8.5), suggesting that BC-mediated DNT reduction—by H2S or dithiothreitol—favored reduction of the ortho nitro group relative to the homogeneous reaction.
Result of DNT reduction by H2S in the presence of diesel soot shows similar trends, with 4A2NT being the predominant intermediate, which was further reduced to DAT (Fig. 5). Interestingly, though, 4A2NT and 2A4NT masses peaked at even earlier times, suggesting faster reduction of DNT than with graphite and GAC; however, further reduction of 4A2NT was rather slow and the final DAT yield was lower than with GAC and graphite. The reason why different BC materials selectively enhanced the reduction of DNT vs. 4A2NT/2A4NT is unclear and will require further investigation.
BC-mediated reduction of DBP
The reduction of DBP by hydrogen sulfide was shown in Fig. 6. After 24 h, 88.6% of DBP was transformed by hydrogen sulfide. Different from DNT reduction by H2S (Fig. 2), DBP was more rapidly removed and complete removal was observed in 72 h. As DBP disappeared, 4BP was formed as a major intermediate, showing 0.115 mM at 72 h. In contrast, 2BP was less than 0.01 mM and no phenol was detected in solution throughout the experiments. These results indicated that debromination of DBP by H2S was more favorable at the ortho position.
Compared to the results of sorption and reduction control experiments, the presence of graphite enhanced the reduction of DBP by hydrogen sulfide (Fig. 7). With graphite, complete removal of DBP was observed in 12 h. 4BP was produced as a debromination product, with a concentration of 0.115 mM, or 29.0% of the initial DBP mass, at 240 h. Similar to the homogeneous system, yield of 2BP was minimal, below 0.05 mM throughout the experiment. At 240 h, 0.023 mM of phenol was produced as the end product of DBP debromination, accounting for 5.8% of initial DBP. Unexplained mass balance may be attributed to sorption of DBP and its debrominated daughter products to graphite surface. However, considering the extent of DBP sorption to graphite (Fig. 7), sorption does not seem to be significant. Other reduction products may plausibly explain the incomplete mass recovery. As previously reported for chlorinated aliphatic compounds (Barbash and Reinhard 1989; Perlinger et al. 1996), an adduct or complex between DBP and hydrogen sulfide may be formed. LC chromatogram indicated that an unidentified broad peak developed during the reduction DBP at 17.1 min (data not shown), suggesting that a possible reactant-reductant adduct(s) might be formed. Further studies are needed to completely elucidate the mechanisms of reductive debromination of DBP.
Similar to graphite, the reduction of DBP by hydrogen sulfide was enhanced in the presence of GAC and diesel soot (Figs. 8, 9). Control experiments indicated that sorption of DBP to GAC was not significant; only 15% of DBP was sorbed to GAC in 240 h. In the presence of GAC and hydrogen sulfide, DBP was completely removed from solution in 12 h. Concentrations of 4BP and phenol were 0.127 and 0.032 mM in 240 h, explaining 40.2% of mass recovery (Fig. 8). Similarly, in the presence of diesel soot, DBP was rapidly removed from solution, giving 88.4 and 97.0% removal in 12 and 24 h, respectively (Fig. 9). 4BP was produced as a dominant intermediate and phenol was a reduction end product. Mass recovery as 4BP and phenol in 240 h was 19.2%. Unidentified reduction intermediates (H2S-DBP adducts) may be responsible for the incomplete mass recovery. These results indicated that diesel soot also played a role of electron transfer mediator in the reduction of DBP by hydrogen sulfide.
Potential mechanisms and environmental implications
Our results illustrate that BC—graphite, GAC, or diesel soot—was able to mediate the reductive transformation of NACs and brominated phenols by hydrogen sulfide. Therefore, BC may influence the fate and transport of these and related compounds in sulfidic environments such as anaerobic soils and sediments. Our results also have implications for site remediation. On the one hand, degradation of NACs and halogenated compounds may be accelerated in situ by adding BC as a catalyst (which is also a sorbent to reduce bioavailability) and/or by adding a reducing agent if one is not already present on site. On the other hand, sorbents such as GAC that has been applied for remediation purposes may have a beneficial (catalytic) effect that was previously unintended/unanticipated. In that regard, it remains to be determined whether BC can mediate the reductive dechlorination of chlorinated aromatics such as PCBs, at what rate and to what extent.
With respect to the mechanism(s) for BC-mediated redox reactions, our previous work (Oh and Chiu 2009) and results from Mitch’s group (Xu et al. 2010) appear to be consistent with the graphene hypothesis (i.e., graphene moieties of BC can serve as adsorbent as well as electron shuttle to promote the reduction of sorbed organic molecules), but not the quinone hypothesis. First, given that sorption to BC is prerequisite to reduction, NACs have been proposed to sorb to the electron-rich graphitic regions in BC through π–π EDA interactions (Zhu and Pignatello 2005) rather than to polar, oxygen-containing quinone groups (Zhu et al. 2005). Second, our previous work (Oh et al. 2002, 2004, 2005) shows that NACs, nitroglycerin, and RDX sorbed to high-purity graphite could all be reduced without involving quinones or other functional groups. Third, in this and earlier studies (Oh and Chiu 2009; Kemper et al. 2008; Xu et al. 2010), graphite (99.9% C), soot (83–90% C), and GAC (84–96% C) could all mediate redox reactions despite their very different oxygen contents. Fourth, Xu et al. (2010) confirmed the graphene hypothesis by demonstrating that, in an electrochemical system where nitroglycerin and H2S were physically separated, electrons were transferred from H2S to nitroglycerin through graphite electrodes. Moreover, Xu et al. (2010) observed that the carbon-normalized catalytic activity was related to the conductivity of BC, which also agree strongly with the graphene hypothesis. A similar correlation between catalytic activity and conductivity of single-walled nanotubes—a class of synthetic BC—was also observed and was attributed to the cytotoxicity of nanotubes (Vecitis et al. 2010). Finally, the para-to-ortho selectivity in DNT reduction was unmistakably altered in this and our previous study (Oh and Chiu 2009), whereas quinone-mediated reduction of DNT would exhibit a similar regio-selectivity as with H2S alone (Barrows et al. 1996). To date, the only piece of evidence that appears ostensibly inconsistent with the graphene hypothesis is that, RDX transformation by H2S was enhanced in the presence of BC but, unlike nitroglycerin, RDX was not transformed when it was physically apart from H2S (Xu et al. 2010). However, it is yet unclear whether the transformation of RDX by H2S, with and without BC, was reductive or not, since H2S is also a strong nucleophile and RDX is known to undergo non-redox reactions (e.g., hydrolysis) to yield the same products such as formaldehyde and nitrite. Further studies are necessary to fully elucidate the mechanism for BC-mediated reduction of RDX.
As a final remark, comparison of results with H2S and dithiothreitol (Oh and Chiu 2009) suggests that reductants, such as H2S, may be more than merely electron donors for BC-mediated reduction reactions but can actually induce side reactions with organic reactants and possibly with functional groups in BC. These confounding reactions need to be further investigated in order to better understand and quantify BC-catalyzed reactions.
References
Accardi-Dey, A., & Gschwend, P. M. (2002). Assessing the combined roles of natural organic matter and black carbon as sorbents in sediments. Environmental Science and Technology, 36, 21–29.
Barbash, J. E., & Reinhard, M. (1989). Abiotic dehalogenation of 1, 2-dichloroethane and 1, 2-dibromoethane in aqueous solution containing hydrogen sulfide. Environmental Science and Technology, 23, 1349–1358.
Barrows, S. E., Cramer, C. J., Truhlar, D. G., Evolitz, M. S., Weber, E. J., et al. (1996). Factors controlling regioselectivity in the reduction of polynitroaromatics in aqueous solution. Environmental Science and Technology, 30, 3028–3038.
Brodowski, S., Amelung, W., Haumaier, L., Abetz, C., Zech, W., et al. (2005). Morphological and chemical properties of black carbon in physical soil fractions as revealed by scanning electron microscopy and energy-dispersive X-ray spectroscopy. Geoderma, 128, 116–129.
Bucheli, T. D., & Gustafsson, Ö. (2000). Quantification of the soot-water distribution coefficient of PAHs provides mechanistic basis for enhanced sorption observations. Environmental Science and Technology, 34, 5144–5151.
Chiu, C. T., & Kile, D. E. (1998). Deviations from sorption linearity on soils of polar and nonpoloar organic compounds at low relative concentrations. Environmental Science and Technology, 32, 338–343.
Cho, Y. M., Ghosh, U., Kennedy, A. J., Grossman, A., Ray, G., Tomaszewski, J. E., et al. (2009). Field application of activated carbon amendment for in situ stabilization of polychlorinated biphenyls in marine sediment. Environmental Science and Technology, 43, 3815–3823.
Cornelissen, G., Elmquist, M., Groth, I., Gustafsson, Ö., et al. (2004). Effect of sorbate planarity on environmental black carbon sorption. Environmental Science and Technology, 38, 3574–3580.
Cornelissen, G., & Gustafsson, Ö. (2004). Sorption of phenanthrene to environmental black carbon in sediment with and without organic matter and native sorbates. Environmental Science and Technology, 38, 148–155.
Cornelissen, G., & Gustafsson, Ö. (2005). Importance of unburned coal carbon, black carbon, and amorphous organic carbon to phenanthrene sorption in sediments. Environmental Science and Technology, 39, 764–769.
Cornelissen, G., Gustafsson, Ö., Bucheli, T. D., Jonker, M. T. O., Koelmans, A. A., van Noort, P. C. M., et al. (2005a). Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environmental Science and Technology, 39, 6881–6895.
Cornelissen, G., Haftka, J., Parsons, J., Gustafsson, Ö., et al. (2005b). Sorption to black carbon of organic compounds with varying polarity and planarity. Environmental Science and Technology, 39, 3688–3694.
Dusek, U., Reischl, G. P., Hitzenberger, R., et al. (2006). CCN activation of pure and coated carbon black particles. Environmental Science and Technology, 40, 1223–1230.
Flanner, M. G., Zender, C. S., Randerson, J. T., Rasch, P. J., et al. (2007). Present-day climate forcing and response from black carbon in snow. Journal of Geophysical Research, 112, D11202.
Goldberg, E. D. (1985). Black carbon in the environment. New York: Wiley.
Gustafsson, Ö., & Gschwend, P. M. (1998). The flux of black carbon to surface sediments on the New England continental shelf. Geochimica et Cosmochimica Acta, 62, 465–472.
Gustafsson, Ö., Haghseta, F., Chan, C., MacFarlane, J., Gschwend, P. M., et al. (1997). Quantification of the dilute sedimentary soot phase: Implications for PAH speciation and bioavailability. Environmental Science and Technology, 31, 203–209.
Hopkins, R. J., Tivanski, A. V., Marten, B. D., Gilles, M. K., et al. (2007). Chemical bonding and structure of black carbon reference materials and individual carbonaceous atmospheric aerosols. Journal of Aerosol Science, 38, 573–591.
Jacobson, M. Z. (2001). Strong radiative heating due to the mixing state of black carbon in atmospheric aerosols. Nature, 409, 695–697.
Kemper, J. M., Ammar, E., Mitch, W. A., et al. (2008). Abiotic degradation of hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine in the presence of hydrogen sulfide and black carbon. Environmental Science and Technology, 42, 2118–2123.
Lohmann, R., MacFarlane, J. K., Gschwend, P. M., et al. (2005). Importance of black carbon to sorption of native PAHs, PCBs, and PCDDs in Boston and New York harbor sediments. Environmental Science and Technology, 39, 141–148.
Middelburg, J. J., Nieuwenhuize, J., Van Breugel, P., et al. (1999). Black carbon in marine sediments. Marine Chemistry, 65, 245–252.
Molina, M., Zaelke, D., Sarma, K. M., Anderson, S. O., Ramanathan, V., et al. (2009). Reducing abrupt climate change risk using the Montreol Protocol and other regulatory actions to complement cuts in CO2 emissions. Proceedings of the National Academy of Sciences, 106, 20616–20621.
Nguyen, T. H., Cho, H.-H., Poster, D. L., Ball, W. P., et al. (2007). Evidence for a pore-filling mechanism in the adsorption of aromatic hydrocarbons to a natural wood char. Environmental Science and Technology, 41, 1212–1217.
Nguyen, T. H., Sabbah, I., Ball, W. P., et al. (2004). Sorption nonlinearity for organic contaminants with diesel soot: Method development and isotherm interpretation. Environmental Science and Technology, 38, 3595–3603.
Oh, S. Y., Cha, D. K., Chiu, P. C., et al. (2002). Graphite-mediated reduction of 2, 4-dinitrotoluene with elemental iron. Environmental Science and Technology, 36, 2178–2184.
Oh, S. Y., Cha, D. K., Kim, B. J., Chiu, P. C., et al. (2005). Transformation of hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine (RDX), octahydro-1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine (HMX) and methylene-dinitramine (MDNA) with elemental iron. Environmental Toxicology and Chemistry, 24, 2812–2819.
Oh, S. Y., & Chiu, P. C. (2009). Graphite- and soot-mediated reduction of 2,4-dinitrotoluene and hexahydro-1,3,5-trinitro-1,3,5-triazine. Environmental Science and Technology, 43, 6983–6988.
Oh, S.-Y., Cha, D. K., & Chiu, P. C. (2004) Reduction of nitroglycerin with cast iron: Pathway, kinetics, and mechanisms. Environmental Science & Technology, 38(13), 3723–3730.
Perlinger, J. A., Angst, W., Schwarzenbach, R. P., et al. (1996). Kinetics of the reduction of hexachloroethane by juglone in solutions containing hydrogen sulfide. Environmental Science and Technology, 30, 3408–3417.
Rickard, D., & Luther, G. W. (2007). Chemistry of iron sulfides. Chemical Reviews, 107, 514–562.
Sander, M., & Pignatello, J. J. (2005). Characterization of charcoal adsorption sites for aromatic compounds: Insights drawn from single-solute and bi-solute competitive experiments. Environmental Science and Technology, 39, 1606–1615.
Schmidt, M. W. I., & Noack, A. G. (2000). Black carbon in soils and sediments: Analysis, distribution, implications, and current challenges. Global Biogeochemical Cycles, 14, 777–793.
Schwarzenbach, R. P., Gschwend, P. M., Imboden, D. M., et al. (2002). Environmental organic chemistry (2nd ed.). New York: Wiley.
Thorseon, W. A., Cope, W. G., Shea, D., et al. (2004). Bioavailability of PAHs: Effects of soot carbon and PAH source. Environmental Science and Technology, 38, 2029–2037.
van der Zee, F. P., Bisschops, I. A. E., Lettinga, G., Field, J. A., et al. (2003). Activated carbon as an electron acceptor and redox mediator during the anaerobic biotransformation of azo dyes. Environmental Science and Technology, 37, 402–408.
Vecitis, C. D., Zodrow, K. R., Kang, S., Elimelech, M., et al. (2010). Electronic structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano, 4, 5471–5479.
Xu, W., Dana, K. E., Mitch, W. A., et al. (2010). Black carbon-mediated destruction of nitroglycerin and RDX by hydrogen sulfide. Environmental Science and Technology, 44, 6409–6415.
Ye, J., & Chiu, P. C. (2006). Transport of atomic hydrogen through graphite and its reaction with azoaromatic compounds. Environmental Science and Technology, 40, 3959–3964.
Zhu, D., Kwon, S., Pignatello, J. J., et al. (2005). Adsorption of single-ring organic compounds to wood charcoals prepared under different thermochemical conditions. Environmental Science and Technology, 39, 3990–3998.
Zhu, D., & Pignatello, J. J. (2005). Characterization of aromatic compound sorptive interactions with black caron (charcoal) assisted by graphite as a model. Environmental Science and Technology, 39, 2033–2041.
Zimmerman, J. R., Ghosh, U., Millward, R. N., Bridges, T. S., Luthy, R. G., et al. (2004). Addition of carbon sorbents to reduce PCB and PAH bioavailability in marine sediments: Physicochemical tests. Environmental Science and Technology, 38, 5458–5464.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0064688).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh, SY., Son, JG., Lim, OT. et al. The role of black carbon as a catalyst for environmental redox transformation. Environ Geochem Health 34 (Suppl 1), 105–113 (2012). https://doi.org/10.1007/s10653-011-9416-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-011-9416-0