Abstract
The aim of this work is to investigate the application of fly ash adsorbent for removal of arsenite ions from dilute solution (100–1,000 ppm). Experiments were carried out using material from the “Turów” (Poland) brown-coal-burning power plant, which was wetted, then mixed and tumbled in a granulator to form spherical agglomerates. Measurements of arsenic adsorption from aqueous solution were carried out at room temperature and natural pH of fly ash agglomerates, in either a shaken flask or circulating column, to compare two different methods of contacting solution with adsorbent. Adsorption isotherms of arsenic were determined for agglomerated material using the Freundlich equation. Kinetic studies indicated that sorption follows a pseudo-second-order model. Preferable method to carry out the process is continuous circulation of arsenite solution through a column.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many efforts have been made to remove arsenic from water and wastewater using various adsorbents (Mohan and Pittman 2007; Sari and Tuzen 2009). Cement, hydrated lime and other pozzolanic materials, e.g. fly ash, have been widely and successfully applied for such purpose (Singh and Pant 2006; Wang et al. 2008; Fan et al. 2008).
Our interest has been focussed on fly ash, a common by-product of coal incineration. A relatively small percentage of this material finds application as an ingredient of cement and other construction materials. However, more than half of this waste material is collected in dumps or ponds (Reijnders 2005).
The contents of particular components in fly ash samples differ depending on combustion conditions and the nature of fuel, i.e. samples from brown coal contain more unburned coal and much more calcium compared with those from black coal, because of the lime method used for flue gas desulphurisation (Vassilev and Vassileva 2005). Fly ash consists mainly of silica and alumina particles, and is strongly alkaline (pH 10–13) when added to water, hence one can expect that metal ions can be removed from aqueous solutions by precipitation or electrostatic adsorption.
Arsenic can be chemically fixed into cementation environment of solidified/stabilized matrices by three important immobilization mechanisms: (1) sorption onto C–S–H phase (calcium silicate hydrate), (2) replacing SO 2−4 ions of ettringite and (3) reaction with cement components to form calcium-arsenic compounds (Phenrat et al. 2005). The effectiveness of both As(III) and As(V) immobilization in lime-treated slurries increases with increasing Ca-to-As molar ratio (Moon et al. 2004). Vandecasteele et al. (2002) demonstrated that formation of Ca3(AsO4)2 and CaHAsO3 precipitates in the presence of Ca(OH)2 controls immobilization of As in fly ash.
In many different branches of industry, fine material of this type is converted into more convenient compressed form of granules, pellets or briquettes. Agglomeration of fly ash from various origins and with different kinds of additives has been investigated for application in the construction sector, for instance in lightweight aggregates (Baykal and Döven 2000; Hycnar 2006). Granulated fly ash has also been used as a sorbent for heavy metals such as arsenic (Polowczyk et al. 2006, 2007) and copper or cadmium (Papandreou et al. 2007).
Adsorption of arsenic on fly ash was found to conform to Freundlich’s isotherm (Cho et al. 2005), and adsorption efficiency was comparable to that of activated carbon. Some authors reported better fit with Freundlich’s than Langmuir isotherm for arsenic adsorption onto activated carbon (Lorenzen et al. 1995), reused sanding waste (Lim et al. 2009) or ferric hydroxide (Deliyanni et al. 2006). Aguilar-Corrillo and co-workers (2006) applied Langmuir isotherms for sorption of As, Cd and Tl onto fly ash.
Determination of kinetics parameters and explanation of the mechanism in heterogeneous systems are often complex. Kinetic models, including the pseudo-first-order (PFO) model of Lagergren and the pseudo-second-order (PSO) model of Ritchie, have been widely tested for simulation of experimental results of adsorption of heavy metals (Deliyanni et al. 2006). The rate constants for adsorption of metal ions (zinc, lead, cadmium and copper) on fly ash were determined using the pseudo-first-order model of Lagergren (Cho et al. 2005). The PSO equation has been used in the case of arsenic, cadmium and thallium adsorption kinetics onto fly ash, followed by the parabolic diffusion equation (Aguilar-Carrillo et al. 2006).
The purpose of this study is to investigate the possibility of utilization of coal fly ash as a low-cost and effective adsorbent for arsenic removal.
Materials and methods
Fly ash from the “Elektrownia Turów” power plant (Poland), from brown coal (lignite) burned in fluidal boiler (unit 4), was used in these investigations.
Particle analysis was carried out using a Mastersizer 2000 laser diffractometer (Malvern), equipped with a HydroMu dispersion unit (Malvern). Surface area was measured by the Brunauer–Emmett–Teller (BET) method for helium/nitrogen mixture using a FlowSorbII (Micromeritics). Density of fly ash was determined using a pycnometer.
Powder diffraction measurements were carried out by using a D8 Advance instrument (Bruker) with measured angle (2θ) from 7° to 100° and scanning step of 0.016°.
Tumble agglomeration experiments were conducted in a laboratory granulator (Polowczyk et al. 2007). The agglomeration process was performed in a 6-L, 185-mm-diameter plastic drum placed horizontally, driven by a motor at 60 rpm. Water (90 mL) was used as the binder liquid. In each experiment, 200 g fly ash samples were taken, and the agglomeration process was carried out for 1 h. After this time, green agglomerates were formed. Wet product was cured in a curing chamber at room temperature for 1 week under cover to achieve hydration of cementitious components. Mean size of agglomerates was about 15 mm.
Mechanical strength of agglomerates was investigated by monoaxial compression method, using an MTS insight electromechanical testing system. The destructive force was determined for ten pairs of agglomerates, stacked together, which were placed between two plates and crushed. The mean destructive force was calculated.
Removal of arsenic by fly ash agglomerates from solution was determined as follows. Sorbent (2.5 or 5 g) was added to 10 mL acidic As(III) stock solution (1–1,000 ppm), and the resulting mixtures were shaken for 1 week to achieve equilibrium. After this time, 0.5 mL aliquot was taken and analysed spectrophotometrically (UV–Vis Helios Gamma; ThermoFisher) by means of the molybdenum blue method, according to the standard procedure. Adsorption experiments were carried out in native pH, which varied from 11 to 12, as imposed by fly ash agglomerates.
Batch adsorption of arsenic was carried out as follows: 50 or 100 g fly ash agglomerates with 200 mL arsenic solution [1,000 and 100 ppm As(III)] in glass Erlenmeyer flask was kept in laboratory shaker, and shaken at 60 cpm for a few days.
In the continuous-flow adsorption experiments, 50 or 100 g adsorbent with 200 mL arsenic solution was placed in a glass column with the ceramic sinter at the bottom. The diameter of the column was 45 mm, and the height was 250 mm. The packing density in the column was 0.53 ± 0.04 g/cm3. Circulation of arsenic solution through the bed was performed at 5 mL/min using a peristaltic pomp. All experiments were conducted at room temperature of 25°C.
Static uptake of arsenic by fly ash agglomerates (mg/g of adsorbent) was determined for the following adsorbent/arsenic ratios: 0.1, 0.15, 0.2, 0.25 and 0.5 g/mL of 1,000 ppm As solution.
After adsorption experiments, the adsorbent was separated on a sieve and dried on air, then mixed with 200 mL distilled water. The content of As(III) in leachate was analysed after 72 h using the method described above.
Results and discussion
X-ray diffraction analysis of fly ash showed the presence of the following minerals: anhydrite (desulphurisation product), calcite (unreacted sorbent for desulphurisation), quartz, haematite, illite and calcium oxide (reactive). After granulation and hardening of agglomerates, as a result of pozzolanic reaction, whole calcium oxide disappeared, whereas new phases appeared: ettringite (3CaO·Al2O3·3CaSO4·32H2O) and C–S–H phase (calcium silicate hydrate).
Particle size analysis showed volume median diameter (d50) of about 27.7 μm, while d10 and d90 were 4.3 and 112.6 μm, respectively. Density of fly ash was found as 2.70 g/cm3. Surface area was 6.5 m2/g for the fly ash powder. After granulation in the presence of water, the surface area of agglomerates increased to 8.5 m2/g, as a consequence of C–S–H phase creation (Giergiczny 2006).
There are many tests to determine the structural strength of pellets or granules. One of these is compression testing between two plates. The experimental data indicate that agglomerates without any additives, such as blast-furnace (slag) or Portland cement, withstood compression to approximately 160 N. Hycnar (2006) investigated maximum compressive force for 8- to 20-mm pellets of fly ash from fluidized bed and pulverized coal-fired bed with addition of ground bottom ash from fluidized-bed coal combustion. The maximum compressive force varied from 0 N for hard coal-burning fly ash to 275 N per granule for Ca-rich brown-coal-combustion fly ash.
C–S–H phase originates from hydration of tri- and di-calcium silicates. This phase not only has a direct influence on the compressive strength of the solidified matrices, but also plays the main role in waste immobilization (Phenrat et al. 2005). In the presence of Ca2+, charge reversal of C–S–H is possible, and in alkaline condition the apparent positive charges are supposed to be compensated by adsorption of arsenic oxyanions (Jösson et al. 2004).
The scope of this study was not to determine thermodynamic conditions, but to show the potential utility and robustness of aggregated material as a cheap, irreversible adsorbent for large quantities of As(III). Due to this, all experiments were carried out at room temperature and in native pH conditions. This corresponds well to the parameters employed during regeneration of commercial sorbents, such as ArsenXnp or Lewatit FO 36, i.e. 2% NaOH solution, containing a few percent NaCl (pH ~12). Native pH of agglomerate solution ranged between 11 and 12, thus adjustment with acid did not need to be applied.
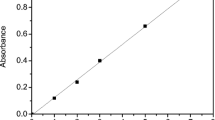
In this study, equilibrium adsorption data of As(III) for fly ash agglomerates did not obey the Langmuir equation, perhaps due to changes on the sorbent surface. The best fit was achieved using a Freundlich isotherm given by the equation:
where q eq is the amount of metal ions adsorbed at equilibrium [mg/gsolid], c eq is the concentration of metal ions in solution at equilibrium [ppm], and K F [mg/g] and n [–] are constants related to adsorption capacity and adsorption intensity. Experimental data and model curves are shown in Fig. 1. For two solid/liquid ratios (2.5 and 5.0 g/10 mL) K F was 0.026 and 0.057 and 1/n was 0.77 and 0.64, respectively. In both cases the R 2 coefficient was about 0.999. Unpublished authors’ data for arsenic adsorption on non-agglomerated fly ash can be fitted using a Langmuir model. This suggests that adsorption sites have equal activity but contradicts the heterogeneity of the fly ash surface. After agglomeration and hardening of the material, significant changes occurred in morphology, as a result of hydration reaction. This is reflected in X-ray diffraction results as well as in specific surface area increase.
In order to investigate the mechanisms of sorption and potential rate-controlling step, such as mass transport and chemical reaction processes, kinetic models were used to test experimental data. The sorption process can be described by four consecutive steps: (1) transport in the bulk of the solution, (2) diffusion across the film surrounding the sorbent particles, (3) particle diffusion in the liquid contained in the pores and in the sorbate along the pores walls and (4) sorption and desorption within the particle and on the external surface (Ho et al. 2000). Previous research (Polowczyk et al. 2007) revealed that adsorption of arsenic on brown- and black-coal-burning fly ash agglomerates follows a pseudo-first-order model. Addition of blast-furnace cement (ca. 10–20%) as a binder and hardener in the granulation process can affect the kinetics, due to the change in adsorption properties of fly ash. The current kinetic studies indicated that sorption of arsenic onto fly ash agglomerates follows a PSO chemisorption model. The linear form of this model is as follows:
where k is the adsorption rate constant [g/mg·min], q m is the amount of adsorbate at equilibrium [mg/gsolid], q t is the amount of adsorbate at any time, and kq 2 m = h denotes the initial adsorption rate in mg/g·min. Plots of t/q t versus t showed very good linearity in all cases of adsorbent amount in relation to arsenic concentration in solution. The calculated values of the model parameters are shown in Table 1. The parameter q m is the amount of arsenic at equilibrium estimated from the model, while q eq was obtained from experiment. The values of the correlation coefficient, R 2, confirm that the kinetics of arsenic adsorption onto fly ash agglomerates follows this model. The initial adsorption rate, h, as well as the equilibrium adsorption capacity, q m , increases when the concentration of arsenic in solution and per unit mass of adsorbent increases. As expected, both h and k parameters are higher (Table 1) when the adsorption process was carried out in column with circulation. One can explain this based on the reduction of mass transfer resistance in the boundary layer of agglomerates.
Static uptakes of arsenic by fly ash agglomerates were determined to be 5.4, 4.5, 3.6, 2.2 and 1.4 mg As/g adsorbent for adsorbent/arsenic ratios of 0.1, 0.15, 0.2, 0.25 and 0.5 g/mL of 1000 ppm As solution, respectively. Overload of the sorbent has not been reached under these conditions. For non-agglomerated fly ash, maximum uptake was achieved for 0.025 adsorbent-to-arsenic ratio, being 74.4 mg/g (unpublished results). Literature provides numerous data of maximum uptake for As(III) adsorption onto various adsorbents (Mohan and Pittman 2007), such as for char-carbon extracted from coal fly ash (89.2 mg/g) and soot (29.9 mg/g; Pattanayak et al. 2000), modified red mud (68.5 mg/g; Zhang et al. 2008), fungal biomass (51.9 mg/g; Sari and Tuzen 2009), ArsenXnp resin (38 mg/g), chars obtained from pine and oak (1.2–12.15 mg/g) and Carbon F-400 (0.2 mg/g; Mohan et al. 2007) and iron-oxide-coated cement (0.15–0.3 mg/g; Kundu and Gupta 2007). Maximum adsorption values of As(V) onto fly ash achieved by Diamadopoulos et al. (1993) and Wang et al. (2008) were 30 mg/g and 0.8 mg/g, respectively.
Leaching experiments were carried out in static mode, i.e. treating agglomerates with deionized water for 72 h, with shaking every 12 h. The arsenic content was below the detection limit of the method, i.e. 1 ppm. This indicates that puzzolanic reaction results in strong retention of arsenic in fly ash agglomerates.
Conclusions
Fly ash can be efficiently granulated with water into spherical agglomerates of sufficient strength without additives. Granulated materials can be easily handled and stored, including packing in adsorption columns. Experimental data of arsenic adsorption onto fly ash agglomerates can be fitted by a Freundlich isotherm, while kinetics follows a PSO model. The circulation mode is preferable to the batch mode, based on the kinetic parameters, which are remarkably higher in the former. Our studies confirm that arsenic can be removed from wastewater, lowering its concentration below 1 ppm. Unfortunately, the analytical method used did not allow us to test whether this was acceptable for drinking water, i.e. below 0.01 ppm. Fly ash from brown-coal burning in the form of agglomerates can be used as a cheap and disposable adsorbent for arsenic removal from water, especially for arsenic-rich leaches generated during regeneration of ion-selective resins, such as ArsenXnp and Lewatit FO36.
References
Aguilar-Carrillo, J., Garrido, F., Barrios, L., & Garcia-Gonzalez, M. T. (2006). Sorption of As, Cd and Tl as influenced by industrial by-products applied to an acidic soil: Equilibrium and kinetics experiments. Chemosphere, 65, 2377–2387.
Baykal, G., & Döven, A. G. (2000). Utilization of fly ash by pelletization process; theory, application areas and research results. Resourses, Conservation and Recycling, 30, 59–77.
Cho, H., Dalyoung, O., & Kwanho, K. (2005). A study on removal characteristics of heavy metals from aqueous solution by fly ash. Journal of Hazardous Materials, B127, 187–195.
Deliyanni, E. A., Nalbandian, L., & Matis, K. A. (2006). Adsorptive removal of arsenites by a nanocrystalline hybrid surfactant-akaganeite sorbent. Journal of Colloid and Interface Science, 302, 458–466.
Diamadopoulos, E., Ioannidis, S., & Sakellaropoulos, G. P. (1993). As(V) removal from aqueous solutions by fly ash. Water Research, 27, 1773–1777.
Fan, Y., Zhang, F.-S., & Feng, Y. (2008). An effective adsorbent developed from municipal solid waste and coal co-combustion ash for As(V) removal from aqueous solution. Journal of Hazardous Materials, 159, 313–318.
Giergiczny, Z. (2006). The role of calcium and silicous fly ash in the formulation of modern binders and cementous materials’ properties. Cracow, Poland: Cracow University of Technology.
Ho, Y. S., Ng, J. C. Y., & McKay, G. (2000). Kinetics of pollutant sorption by biosorbents: Review. Separation and Purification Methods, 29, 189–232.
Hycnar, J. J. (2006). Factors influencing physical, chemical and functional properties of solid products from fluidized bed fuels combustion. Katowice, Poland: Wydawnictwo Górnicze.
Jösson, B., Wennerström, H., Nonat, A., & Cabane, B. (2004). Onset in cement paste. Langmuir, 20, 6702–6709.
Kundu, S., & Gupta, A. K. (2007). As(III) removal from aqueous medium in fixed bed using iron oxide-coated cement (IOCC): Experimental and modeling studies. Chemical Engineering Journal, 129, 123–131.
Lim, J.-W., Chang, Y.-Y., Yang, J.-K., & Lee, S.-M. (2009). Adsorption of arsenic on the reused sanding wastes calcinated at different temperatures. Journal of Colloid and Interface Science A, 345, 65–70.
Lorenzen, L., van Deventer, J. S. J., & Landi, W. M. (1995). Factors affecting the mechanism of the adsorption of arsenic species on activated carbon. Minerals Engineering, 8, 557–569.
Mohan, D., & Pittman, C. U. (2007). Arsenic removal from water/wastewater using adsorbents—A critical review. Journal of Hazardous Materials, 142, 1–53.
Mohan, D., Pittman, C. U., Bricka, M., Smith, F., Yancey, B., Mohammad, J., et al. (2007). Sorption of arsenic, cadmium and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. Journal of Colloid and Interface Science, 310, 57–73.
Moon, D. H., Dermatas, D., & Menounou, N. (2004). Arsenic immobilization by calcium-arsenic precipitates in lime treated soils. Science of Total Environment, 330, 171–185.
Papandreou, A., Stounaras, C. J., & Panias, D. (2007). Copper and cadmium adsorption on pellets made from fired coal fly ash. Journal of Hazardous Materials, 148, 538–547.
Pattanayak, J., Mondal, K., Mathew, S., & Lalvani, S. B. (2000). A parametric evolution of the removal of As(V) and As(III) by carbon-based adsorbents. Carbon, 38, 589–596.
Phenrat, T., Marhaba, T. F., & Rachakornkij, M. (2005). A SEM and X-ray study for investigation of solidified/stabilized arsenic-iron hydroxide sludge. Journal of Hazardous Materials, B118, 185–195.
Polowczyk, I., Bastrzyk, A., Koźlecki, T., Rudnicki, P., Sawiński, W., Sadowski, Z., et al. (2007). Application of fly ash agglomerates in the sorption of arsenic. Polish Journal of Chemical Technology, 9, 37–41.
Polowczyk, I., Drąg, E., Bastrzyk, A., & Sadowski, Z. (2006). Application of spherical agglomeration process in formation of adsorbents from fly ash. Polish Journal of Chemical Technology, 8, 95–99.
Reijnders, L. (2005). Disposal, use and treatments of combustion ashes: A review. Resources, Conservation and Recycling, 43, 313–336.
Sari, A., & Tuzen, M. (2009). Biosorption of As(III) and As(V) from aqueous solution by macrofungus (Inonotus hispidus) biomass: Equilibrium and kinetic studies. Journal of Hazardous Materials, 164, 1372–1378.
Singh, T. S., & Pant, K. K. (2006). Solidification/stabilization of arsenic containing solid wastes using portland cement, fly ash and polymeric materials. Journal of Hazardous Materials, B131, 29–36.
Vandecasteele, C., Dutre, V., Geysen, D., & Wauters, G. (2002). Solidification/stabilisation of arsenic bearing fly ash from the metallurgical industry. Immobilisation mechanism of arsenic. Waste Management, 22, 143–146.
Vassilev, V. V., & Vassileva, C. G. (2005). Methods for characterization of composition of fly ashes from coal-fired power stations: A critical overview. Energy & Fuels, 19, 1084–1098.
Wang, J., Wang, T., Burken, J. G., Chusuei, C. C., Ban, H., Ladwig, K., et al. (2008). Adsorption of arsenic(V) onto fly ash: A speciation based approach. Chemoshere, 72, 381–388.
Zhang, S., Liu, C., Luan, Z., Peng, X., Ren, H., & Wang, J. (2008). Arsenate removal from aqueous solutions using modified red mud. Journal of Hazardous Materials, 152, 486–492.
Acknowledgments
Authors are grateful to Dr Przemysław Szklarz (Faculty of Chemistry, University of Wrocław) for XRD analyses and Mr. Rafał Mech (Faculty of Mechanics, Wrocław University of Technology) for mechanical strength measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Polowczyk, I., Bastrzyk, A., Koźlecki, T. et al. Use of fly ash agglomerates for removal of arsenic. Environ Geochem Health 32, 361–366 (2010). https://doi.org/10.1007/s10653-010-9306-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-010-9306-x