Abstract
Sedimentation of metals preserves historical records of contaminant input from local and regional sources, and measurement of metals in sediment cores can provide information for reconstruction of historical changes in regional water and sediment quality. Sediment core was collected from Stege Marsh located in central San Francisco Bay (California, USA) to investigate the historical input of trace metals. Aluminum-normalized enrichment factors indicate that inputs from anthropogenic sources were predominant over natural input for Ag, Cu, Pb, and Zn. Among these, lead was the most anthropogenically impacted metal with enrichment factors ranging from 32 to 108. Depth profiles and coefficients of variation show that As, Cd, and Se were also influenced by anthropogenic input. The levels of these anthropogenically impacted metals decline gradually towards the surface due to regulation of the use of leaded gasoline, municipal and industrial wastewater discharge control, and closure of point sources on the upland of Stege Marsh. Although trace metal contamination is expected to be continuously declining, the rates of decline have slowed down. For lead, it is estimated to take 44, 82, and 153 years to decrease to probable effects level (112 μg/g), the San Francisco Bay ambient surface sediment level (43.2 μg/g), and the local baseline levels (5 μg/g), respectively. Some metals in surface sediments (0–6 cm) are still higher than sediment quality guidelines such as the probable effects level. To further facilitate the recovery of sediment quality, more efficient management plans need to be developed and implemented to control trace metals from non-point sources such as stormwater runoff.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trace metals in the water column generally tend to be adsorbed onto suspended particulate matter and then deposited to the bottom as sediments. This sedimentation of particle-bound metals preserves historical records of contaminant input from local and regional sources. Therefore, measurements of metals in core sediments can provide information for reconstruction of historical changes in regional water and sediment quality (Hartmann et al. 2005; Cantwell et al. 2007). The reconstructed contaminant input history then helps evaluate efficiencies of management and regulatory actions implemented to reduce potential health effects.
Generally, the environmental quality of watersheds reflects urbanization and land use patterns of surrounding areas (Van Dolha et al. 2008). Growth of human population and industrialization in coastal areas has resulted in increased input of hazardous trace metals that degrade the quality of bays and estuaries. San Francisco Bay has experienced significant contamination due to population growth combined with industrial activities, which started more than 100 years ago and especially grew during and after World War II. Population in the San Francisco Bay area increased from 0.1 million in 1860 to 1.2 million in 1920, 2.7 million in 1950, 5.2 million in 1980, and 6.8 million in 2000 (Bay Area Census 2000). San Francisco Bay is known as one of the most contaminated estuaries for anthropogenically derived metals. Since the mid 1800s San Francisco Bay has received mercury from historic gold extraction and mercury mining (Nriagu 1994). Municipal and industrial wastewater discharges and urban and agricultural runoff have added enormous amounts of many toxic metals to the San Francisco Bay system (Conaway et al. 2007; Squire et al. 2002). Until the mid 1960s, raw or minimally treated sewage (approximately 1 million tons every day) containing large amounts of toxic metals was discharged into San Francisco Bay (SFBRWQCB 2000). In spite of population increase, input of toxic metals from municipal and industrial wastewater discharges has been decreasing since the Porter Cologne Water Quality Control Act was passed in 1969, and more significantly since the San Francisco Bay Regional Water Quality Control Board (SFBRWQCB) issued its first Water Quality Control Plan for the San Francisco Bay Basin in 1975 for the fulfillment of the Clean Water Act (SFBRWQCB 2000).
Coastal tidal salt marshes play important roles in sustaining ecological diversity by providing vital food and habitat for clams, crabs, and juvenile fish as well as offering food, shelter, and nesting sites for waterfowl and shorebirds. They also play critical buffering roles by slowing shoreline erosion and absorbing nutrients and contaminants before they reach the oceans and estuaries, resulting in excess levels of contaminants accumulated in marshes near urban and industrial areas. In San Francisco Bay, a vast majority (~80%) of the original wetlands has disappeared during the last 150 years and the remaining wetlands are fragmented so the distribution of certain wildlife is isolated (Goals Project 1999). When these fragmented wetlands are heavily contaminated by numerous toxic chemicals, as in the case of Stege Marsh, wetland dwelling animals, including endangered clapper rail and vegetarian mice and vole, would be substantially stressed and may disappear in the near future in the worst case.
Various industrial and munitions manufacturing operations on the upland portion and urban runoff from Carson Creek and Meeker Creek have significantly contaminated Stege Marsh for more than 100 years (URS 2000). Due to severe contamination, Stege Marsh has been included in the list of ten high-priority candidate toxic hot spots and has been the subject of several environmental investigations, remediation, and restoration under the direction of the SFBRWQCB (1999). These investigations mostly focused on surface sediment contamination in inner Stege Marsh located inside the bike path and paid less attention to outer marsh. Our previous studies (Hwang et al. 2006a, b, 2008a, b) revealed that surface sediments in outer Stege Marsh also had high levels of contaminants that may affect the health of wildlife that inhabits Stege Marsh. However, there is no detailed information regarding historical input of toxic metals to outer Stege Marsh. The purpose of the present study was to reconstruct historical changes in terms of trace metal pollution in the tidal flat of outer Stege Marsh. This type of information will help predict future trends of marsh sediment quality and decide if further actions are required to accelerate recovery of healthy ecosystem in outer Stege Marsh.
Study area
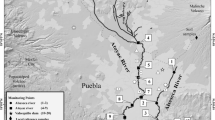
Stege Marsh is on the western margin of central San Francisco Bay (California, USA), located near the city of Richmond (Fig. 1). In the late 1940s, a railroad track was constructed along the southern portion of Stege Marsh and operated until 1969 to move munitions. The railroad track was changed into a bike trail in the 1970s. This bike trail separates inner and outer Stege Marsh. Through two channels (Carson Creek and Meeker Creek) the inner and outer marshes are connected and stream water and bay water are exchanged by tidal action. Over the last 100 years, Stege Marsh has become heavily contaminated by the adjacent historic industrial activities, including an agrochemical manufacturing facility, asphalt manufacturing plant, and shipbuilding facility (URS 2000). Stormwater runoff entering through Carson Creek and Meeker Creek from adjacent urban areas and highways also contributes to contamination. Other potential sources of contaminants in the vicinity of Stege Marsh include landfill operated during the 1940s through the 1960s in Hoffman Marsh located east of Stege Marsh. During this period, various filling materials, including substantial amounts of lead-containing crushed battery casings, were deposited (URS 2000). The majority of sediments deposited in Stege Marsh are transported from the two freshwater creeks (Carson Creek and Meeker Creek) and San Francisco Bay. Due to high levels of pollutants, the Bay Protection and Toxic Cleanup Program that was established under the San Francisco Bay Regional Water Quality Control Board included Stege Marsh in the list of ten high-priority candidate toxic hot spots and issued Cleanup and Abatement Orders (SFBRWQCB 1999). Stege Marsh is a habitat for endangered species such as the California clapper rail.
Chemical analysis
A sediment core (54 cm) was collected using a piston core sampler from the center of eastern outer Stege Marsh in June 2004 (Fig. 1). The collected sample was immediately transported to the laboratory and sliced at 2 cm intervals. Then each layer was split for analysis of metals and organic contaminants. Analytical procedures for the measurement of metals in core sediments were identical to those used for surface sediments in a previous study (Hwang et al. 2006b). This method is similar to those used by others to measure metals in soils and sediments (Schiff and Weisberg 1999). To measure metals (aluminum, arsenic, cadmium, chromium, cobalt, copper, iron, lead, lithium, magnesium, manganese, nickel, selenium, silver, zinc, vanadium), an aliquot of wet samples (~500 mg) was placed into tall glass tubes and 2 mL concentrated trace-metal-grade HClO4 (70%) and HNO3 (65%) mixture (1:5) was added. Compared to very strong acid (HF), extraction efficiencies of the digestion acid mixture used in the present study are lower for some metals such as Al, Cr, and V by up to 30% (Hornberger et al. 1999). A batch of samples included one procedural blank, two sample duplicates, and one standard reference material (National Institute of Standards and Technology SRM 1646a, estuarine sediment) to validate quantification data. Tubes were kept at room temperature overnight and then were placed in the digestion block. The temperature of the digestion block was increased to 200°C in several steps over 4 h to enhance digestion. Tubes were then centrifuged at 2,000 rpm for 10 min. An aliquot (1 mL) of extract was removed from the tubes and diluted 500-fold for instrumental analysis. Quantification of metals was performed using an Agilent 7500i inductively coupled plasma-mass spectrometer (ICP-MS, Palo Alto, CA, USA). Calibration standards for ICP-MS were prepared by serial dilution from National Institute of Standards and Technology (NIST)-traceable stocks purchased from Spex CertiPrep (Methuen, NJ, USA) and verified against standard reference materials (SRMs) such as SLRS-4 (National Research Council, Ottawa, Canada) and NIST 1643e (Gaithersburg, MD, USA). Concentrations in the blank were not compensated. Relative percentage differences for duplicate samples were within 25%. The levels of target compounds in the SRMs were within 30% of certified values except for Al, Cr, and V, which are known to be incompletely recovered using out digestion method (Cook et al. 1997; Hornberger et al. 1999). Metal concentrations are reported on a dry weight basis. To determine water content, an aliquot (~1 g) of wet sediment was dried to constant weight (60°C for 24 h).
Results and discussion
Concentrations of metals in core sediments are given in Table 1. The highest and median concentrations of toxic metals such as Ag, Cd, Cu, Pb, and Zn in Stege Marsh core sediments were much higher than those found in sediments from the subtidal open waterway of San Francisco Bay (Hornberger et al. 1999) by up to tenfold, presumably because of the proximity of Stege Marsh to source areas. The levels found in the present study, however, were much lower than those in inner Stege Marsh by up to two orders of magnitude (URS 2000). Concentrations of Al, Co, Cr, Fe, Li, Mg, Mn, Ni, and V were nearly constant throughout the whole sediment core layers and their depth profiles were quite similar (Fig. 2). Near-surface enrichment of Mn and Fe due to upward migration of reduced species and precipitation in oxic surface layers is well known (Farmer and Lowell 1984), however, this diagenetic subsurface enrichment of Fe and Mn was not found in the present study. Depth profiles of As, Cd, Cu, and Se were almost identical, with highest concentrations at depths around 40 cm and gradual decline towards the surface (Fig. 2). Similar depth profiles and high correlation coefficients (discussed below) indicate that these metals were associated with similar types of sources. Zinc was found highest at the deepest section (approximately 55–60 cm) and exhibited a continuously decreasing pattern towards the surface. Depth profiles of metals (Fig. 2) showed that the levels of metals of concern such as Ag, Cd, Cu, Pb, and Zn had been significantly influenced by anthropogenic input and declining following the regulations on the use of leaded gasoline and municipal and industrial wastewater discharge control. However, their levels in surface sediments (0–6 cm) were still higher than sediment quality guidelines such as the threshold effects level (TEL) and probable effects level (PEL), meaning that the health of wildlife species dwelling in Stege Marsh could not be fully supported (Hwang et al. 2008b). The average concentration of lead (416 μg/g) in the surface layer (0–6 cm) was higher than the PEL (112 μg/g) by a factor of 3.7. The concentrations of copper and zinc were very close to their PELs (Fig. 2). The levels of arsenic, chromium, and silver were lower than their PELs but higher than their TELs. When the concentration of a metal in the sediment is lower than its TEL or higher than its PEL, adverse biological effects are observed rarely or frequently, respectively.
To minimize the toxicity potential of metals, their levels in environmental media need to be reduced through source control and/or treatment control. For better source control, it is important to know the sources of metals and, as the first step of the source identification, the contribution of anthropogenic input should be determined. Contrary to organic contaminants such as polychlorinated biphenyls (PCBs) and chlorinated pesticides, which have anthropogenic origins only, trace metals can come from both anthropogenic and natural sources. Natural occurrence of metals and geographical mineralogical variation can hamper the accurate assessment of anthropogenic input of metals. Generally, the anthropogenic and natural contribution of metals can be distinguished by comparing metals in environmental samples with a representative background, which is obtained from regional soil data or sediment core data. To minimize the effects of geographical variations and sediment grain size, concentrations of each metal in samples and background are normalized with reference elements (e.g., Fe, Al, Li). Among them, Fe and Al have been used most frequently (Velinsky et al. 1994; Daskalakis and O’Connor 1995; Hornberger et al. 1999; Lu et al. 2005). Anthropogenic input of metals can be suspected when the normalized concentrations of metals in samples are higher than background levels. The enrichment factor of this study is defined as
where C M is the concentration of metal (M) and C R is the concentration of a reference metal (Al). Iron- or lithium-normalized enrichment factors were similar to aluminum-normalized enrichment factors. The enrichment factors of metals were calculated using the baseline concentrations reported by Hornberger et al. (1999).
Aluminum-normalized enrichment factors (Fig. 3) indicate that inputs from anthropogenic sources were predominant over natural input for Ag, Cu, Pb, and Zn. Among these, lead was the most anthropogenically impacted metal with enrichment factors ranging from 32 to 108 (Fig. 3). The enrichment factors of Cu varied from 3.6 to 19. The enrichment factors of Ag and Zn were slightly lower than those of Cu. Chromium, nickel, and vanadium did not show enrichment compared with their baseline concentrations. Their enrichment factors were slightly lower than 1. The enrichment factors of As, Cd, and Se cannot be calculated because their baseline concentrations were not available. In this case, coefficients of variation might be used as a less conservative indicator for anthropogenic input of metals (Hornberger et al. 1999; Hwang et al. 2006b). The anthropogenically enriched metals (Ag, Cu, Pb, and Zn) had greater variation in their core sediment concentrations (Fig. 2). The coefficients of variation (percentage of the standard deviation against the mean) of these metals were 35% (Zn), 34% (Pb), 39% (Cu), and 20% (Ag). Metals such as Ni, V, and Cr that are dominated by natural inputs had much lower coefficients of variation (6.3–12%), which are the same as those of Al (7.4%) and Fe (10%). As a less conservative approach, the high coefficients of variation for As (55%), Cd (48%), and Se (45%) indicate that these metals were likely enriched by anthropogenic input. Low coefficients of variation (8–11%) for Co, Li, Mg, and Mn indicate that these metals were not likely enriched by anthropogenic input.
Metal data were subject to linear regression analysis to identify any correlation among metals measured in the present study. High regression coefficients (r 2) indicate that they have similar sources and geochemical fate. Generally regression coefficients were higher when a metal in one group (anthropogenically contaminated or dominated by natural input) was compared with another in the same group, while they were lower when metals were compared between different groups (Table 2). This pattern can be seen clearly in the cases of Al and Cu. The regression coefficients between Al and anthropogenically enriched metals (Ag, As, Cd, Cu, Pb, Se, and Zn) were 0.01–0.12, which is much lower than those (0.42–0.83) between Al and naturally dominant metals (Cr, Fe, Li, Mn, Ni, and V). Copper had lower coefficients (0.01–0.06) with naturally dominated metals (Al, Li, Mn, Ni, and V) and higher coefficients (0.52–0.82) with anthropogenically enriched metals (As, Cd, Se, and Zn). Regression coefficients for Pb with the other anthropogenically enriched metals were not significantly different from those with naturally dominated metals; presumably Pb had additional unique input sources such as leaded gasoline.
For the present study, sediment core samples were not dated using radioisotopes (e.g., 210Pb, 137Cs) so accurate sedimentation rates are unknown. However, though less accurate, sedimentation rates can be estimated using depth profiles of PCBs and lead, which have a unique history of use and release (Hartmann et al. 2005; Cantwell et al. 2007). Cantwell et al. (2007) demonstrated that sediment deposition rates could be obtained using PCBs and lead as proxies. The production of PCBs and leaded gasoline peaked around 1970 (Nriagu 1990; Brinkmann and de Kok 1980) before regulations were implemented. In 1971, Monsanto, the sole domestic commercial producer of PCBs, voluntarily restricted sales of PCB to closed-system uses. Maximum deposition of PCBs in San Pablo Bay, which is the northern part of a San Francisco estuarine system, was found in the sediment deposited around 1975 (Venkatesan et al. 1999). So the layers (12–14 cm) having the highest PCB concentration found in the same core analyzed for the present study were likely deposited around that time (Hwang et al. in preparation). Hence the estimated average sediment deposition rate since 1975 is 0.45 cm/year.
The enrichment factors of Ag, Cu, and Zn in surface sediment (0–6 cm) are 3.5–8 and their decreasing trends in the top 30 cm layer are exponential (Fig. 4). Assuming that the declines of these metals continue following the current trends with continuous sediment deposition at a rate of 0.45 cm/year, it is estimated that it will take 10, 2.5, and 25 years for Ag, Cu, and Zn, respectively, for the surface layer to reach the San Francisco Bay ambient sediment levels (SFBRWQCB 1998) and 82, 46, and 54 years, respectively, to reach background levels. Lead concentration had been increasing until it reached its highest concentration (750 μg/g) at a depth of 11 cm, then declining toward the surface. Changes of the enrichment factors in the upper 30 cm layer are very similar to the trend of historical consumption of lead in gasoline in the USA (Nriagu 1990). The enrichment factor of lead in the surface layer (0–6 cm) is 68, indicating that lead is still being significantly influenced by anthropogenic sources. The current decreasing rate of lead from the peak layer seems linear. However, as found in the cases of Ag, Cu, and Zn, the decreasing rate of lead will also level off as the concentration approaches the background level. Assuming that lead will follow the same decrease pattern as Cu, it is estimated that it will take 44, 82, and 153 years to reach the PEL (112 μg/g), the San Francisco Bay ambient surface sediment level (43.2 μg/g), and the local baseline levels (5 μg/g), respectively.
The highest enrichment factor of Ag was found at a depth of 19 cm and enrichment factors have been declining gradually towards the surface due to wastewater discharge control. The depth profile of Ag is different from those of As, Cd, Cu, and Se, suggesting that Ag in Stege Marsh had additional sources. San Francisco Bay has unusually high levels of Ag in its sediments, biota, and water (Flegal et al. 2007). In the mid 1980s, silver concentrations in San Francisco Bay sediments were about 30 times higher than baseline concentration. Atmospheric and fluvial sources of silver are considered relatively small in San Francisco Bay, so industrial and municipal effluents were likely the major sources. In South San Francisco Bay, most of the anthropogenic silver was traced to effluent from a photographic processing plant (Squire et al. 2002).
Sedimentary concentrations of Ni in the San Francisco Bay (SFB) watershed have often exceeded the sediment quality guidelines (Topping and Kuwabara 2003; Yee et al. 2007). Nickel concentrations in core sediments measured in the present study were also higher than sediment quality guidelines and anthropogenic input of Ni can be suspected. However, Ni in sediments from all depths was very constant and enrichment factors were less than 1, indicating that Ni measured in the present study is not likely from anthropogenic sources. Compared with other metals such as Cu, Pb, and Zn, which have many anthropogenic sources, Ni in sediment cores collected from clean (Tomales Bay) and contaminated (Central San Francisco Bay and Stege Marsh) areas showed the same depth profile (no difference between surface and 200 cm), confirming that the majority of Ni has been coming from natural sources (Hornberger et al. 1999). Soils in SFB watersheds contain Ni-rich source rocks such as serpentinite, in which Ni concentrations reach up to 3,000 μg g−1 (Topping and Kuwabara 2003), which is 150-fold higher than average continental crust in the range of 20 μg g−1 (Taylor and McLennan 1985).
The results of the present study indicate that the levels of trace metals in Stege Marsh sediments have been declining. The toxicity potential of surface sediment is, however, still high and adverse effects are likely to be found frequently. Although trace metal contamination is expected to decline continuously, the rates of decline have been slowing down. It may take many decades for sediment quality to recover to levels that fully support the health of wildlife species that inhabit Stege Marsh. To further facilitate the recovery of the sediment quality, more efficient management plans need to be developed and implemented to control trace metals from non-point sources such as stormwater runoff.
References
Bay Area Census. (2000). Population by County, 1860–2000, Bay Area Census. http://www.bayareacensus.ca.gov. Assessed 20 January 2008.
Brinkmann, U. A. T., & de Kok, A. (1980). Production, properties, and usage. In R. D. Kimbrough, (Ed.), Halogenated bipheynyls, terpenyls, naphthalenes, dibenzodioxins and related products (2nd ed., Vol. 4). Topics in environmental health. Amsterdam: Elsevier/North-Holland Biomedical Press.
Cantwell, M. G., King, J. W., Burgess, R. M., & Appleby, P. G. (2007). Reconstruction of contaminant trends in a salt wedge estuary with sediment cores dated using a multiple proxy approach. Marine Environmental Research, 64, 225–246. doi:10.1016/j.marenvres.2007.01.004.
Cook, J. M., Gardner, M. J., Griffiths, A. H., Jessep, M. A., Ravenscroft, J. E., & Yates, R. (1997). The comparability of sample digestion techniques for the determination of metals in sediments. Marine Pollution Bulletin, 34, 637–644.
Daskalakis, K. D., & O’Connor, T. P. (1995). Normalization and elemental sediment contamination in the coastal United States. Environmental Science & Technology, 29, 470–477. doi:10.1021/es00002a024.
Farmer, J. G., & Lovell, M. A. (1984). Massive diagenetic enhancement of manganese in Loch Lomond sediments. Environmental Technology Letters, 5, 257–262.
Flegal, A. R., Brown, C. L., Squire, S., Ross, J. R. M., Scelfo, G. M., & Hibdon, S. (2007). Spatial and temporal variations in silver contamination and toxicity in San Francisco Bay. Environmental Research, 105, 34–52. doi:10.1016/j.envres.2007.05.006.
Goals Project. (1999). Baylands ecosystem habitat goals: A report of habitat recommendation. Oakland, CA: U.S. Environmental Protection Agency/San Francisco Bay Regional Water Quality Control Board.
Hartmann, P. C., Quinn, J. G., Cairns, R. W., & King, J. W. (2005). Depositional history of organic contaminants in Narragansett Bay, Rhode Island, USA. Marine Pollution Bulletin, 50, 388–395. doi:10.1016/j.marpolbul.2004.11.020.
Hornberger, M. I., Luoma, S. N., van Green, A., Fuller, C., & Anima, R. (1999). Historical trends of metals in the sediments of San Francisco Bay, California. Marine Chemistry, 64, 39–55. doi:10.1016/S0304-4203(98)80083-2.
Hwang, H.-M., Green, P. G., Grosholz, E. D., Carr, R. S., Higashi, R. M., & Anderson, S. A. (2008a). Quality assessment of sediments in tidal salt marshes in California using a sediment quality triad approach. Journal of Environmental Science and Health Part A (submitted).
Hwang, H.-M., Green, P. G., Higashi, R. M., & Young, T. M. (2006a). Tidal salt marsh sediment in California, USA. Part 2: Occurrence and anthropogenic input of trace metals. Chemosphere, 64, 1899–1909. doi:10.1016/j.chemosphere.2006.01.053.
Hwang, H.-M., Green, P. G., & Young, T. M. (2006b). Tidal salt marsh sediment in California, USA. Part 1: Occurrence and sources of organic contaminants. Chemosphere, 64, 1383–1392. doi:10.1016/j.chemosphere.2005.12.024.
Hwang, H.-M., Green, P. G., & Young, T. M. (2008b). Tidal salt marsh sediment in California, USA. Part 3: Current and historic toxicity potential of contaminants and their bioaccumulation. Chemosphere, 71, 2139–2149. doi:10.1016/j.chemosphere.2008.01.005.
Lu, X. Q., Werner, I., & Young, T. M. (2005). Geochemistry and bioavailability of metals in sediments from northern San Francisco Bay. Environment International, 31, 593–602. doi:10.1016/j.envint.2004.10.018.
Nriagu, J. O. (1990). The rise and fall of leaded gasoline. The Science of the Total Environment, 92, 13–28. doi:10.1016/0048-9697(90)90318-O.
Nriagu, J. O. (1994). Mercury pollution from the past mining of gold and silver in the Americas. The Science of the Total Environment, 149, 167–181. doi:10.1016/0048-9697(94)90177-5.
Schiff, K. C., & Weisberg, S. B. (1999). Iron as a reference element for determining trace metal enrichment in southern California coastal shelf sediments. Marine Environmental Research, 48, 161–176.
SFBRWQCB. (1998). Staff report: Ambient concentrations of toxic chemicals in San Francisco Bay sediments. Oakland, CA: San Francisco Bay Regional Water Quality Control Board.
SFBRWQCB. (1999). Regional toxic hot spot cleanup plan. Oakland, CA: San Francisco Bay Regional Water Quality Control Board.
SFBRWQCB. (2000). Fifty years of protecting Bay area waters. Oakland, CA: San Francisco Bay Regional Water Quality Control Board.
Squire, S., Scelfo, G. M., Revenaugh, J., & Flegal, A. R. (2002). Decadal trends of silver and lead contamination in San Francisco Bay surface waters. Environmental Science & Technology, 36, 2379–2386. doi:10.1021/es015746r.
Taylor, S. R., & McLennan, S. M. (1985). The continental crust: Its composition and evolution. Blackwell Scientific: Oxford.
Topping, B. R., & Kuwabara, J. S. (2003). Dissolved nickel and benthic flux in South San Francisco Bay: a potential for natural sources to dominate. Bulletin of Environmental Contamination and Toxicology, 71, 46–71. doi:10.1007/s00128-003-0129-7.
URS. (2000). Filed sampling and analyses results, University of California, Berkeley Richmond Field Station/Stege Marsh, Richmond, California. Oakland, CA: URS.
Van Dolha, R. F., Riekerk, G. H. M., Bergquist, D. C., Felber, J., Chestnut, D. E., & Holland, A. F. (2008). Estuarine habitat quality reflects urbanization at large spatial scales in South Carolina’s coastal zone. The Science of the Total Environment, 390, 142–154. doi:10.1016/j.scitotenv.2007.09.036.
Velinsky, D. J., Wade, T. L., Schlekat, C. E., McGee, B. L., & Presley, B. J. (1994). Tidal river sediments in the Washington, D.C. area. I. Distribution and sources of trace metals. Estuaries, 17, 305–320. doi:10.2307/1352665.
Venkatesan, M. I., de Leon, R. P., van Geen, A., & Luoma, S. N. (1999). Chlorinated hydrocarbon pesticides and polychlorinated biphenyls in sediment cores from San Francisco Bay. Marine Chemistry, 64, 85–97. doi:10.1016/S0304-4203(98)90086-X.
Yee, D., Grieb, T., Mills, W., & Sedlak, M. (2007). Synthesis of long-term nickel monitoring in San Francisco Bay. Environmental Research, 105, 20–33. doi:10.1016/j.envres.2007.02.005.
Acknowledgements
We are thankful to Dr. Frances Malamud-Roam for her help in field sampling. We would like to thank Rodelia Busalpa, Yun Lu, William Schilling, and Marlene Relja for their help in laboratory chemical analysis. This research has been supported by a grant from the US Environmental Protection Agency’s Science to Achieve Results (STAR) Estuarine and Great Lakes (EaGLe) Coastal Initiative through funding to the Pacific Estuarine Ecosystem Indicator Research (PEEIR) Consortium, US EPA Agreement #EPA/R-82867601.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hwang, HM., Green, P.G. & Young, T.M. Historical trends of trace metals in a sediment core from a contaminated tidal salt marsh in San Francisco Bay. Environ Geochem Health 31, 421–430 (2009). https://doi.org/10.1007/s10653-008-9195-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-008-9195-4