Abstract
In 2004, airborne particulate matter (PM) was collected for several aerosol episodes occurring in the southern region of Taiwan. The particulate samples were taken using both a MOUDI (Micro-orifice Uniform Deposit Impactor) and a nano-MOUDI sampler. These particulate samples were analyzed for major water-soluble ionic species with an emphasis to characterize the mass concentrations and distributions of these ions in the ambient ultrafine (PM0.1, diameter <0.1 μm) and nano mode (PMnano, diameter <0.056 μm) particles. Particles collected at the sampling site (the Da-Liao station) on the whole exhibited a typical tri-modal size distribution on mass concentration. The mass concentration ratios of PMnano/PM2.5, PM0.1/PM2.5, and PM1/PM2.5 on average were 1.8, 2.9, and 71.0%, respectively. The peak mass concentration appeared in the submicron particle mode (0.1 μm < diameter <1.0 μm). Mass fractions (percentages) of the three major water-soluble ions (nitrate, sulfate, and ammonium) as a group in PMnano, PM0.1, PM1, and PM2.5 were 18.4, 21.7, 50.0, and 50.7%, respectively. Overall, results from this study supported the notion that secondary aerosols played a significant role in the formation of ambient submicron particulates (PM0.1−1). Particles smaller than 0.1 μm were essentially basic, whereas those greater than 2.5 μm were neutral or slightly acidic. The neutralization ratio (NR) was close to unity for airborne particles with diameters ranging from 0.18 to 1 μm. The NRs of these airborne particles were found strongly correlated with their sizes, at least for samples taken during the aerosol episodes under study. Insofar as this study is exploratory in nature, as only a small number of particulate samples were used, there appears to be a need for further research into the chemical composition, source contribution, and formation of the nano and ultrafine mode airborne particulates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Particulate matter (PM) is the sum of all solid and liquid particles suspended in air. Recent epidemiological studies have suggested a statistical association of health effects with ambient concentrations of fine particulates, especially with the ultrafine and nano fractions (i.e., those with diameters <100 nm), which are considered (Samet et al. 2000; Katsouyanni et al. 2001) capable of easily penetrating the alveolar region of the lungs. For example, Pope et al. (2002) indicated that for each elevation of 10 μg m−3 in fine particulate air pollution, there were noticeable increases in mortality from cardiopulmonary disorders (∼6%), lung cancer (∼8%), and all causes (∼4%). These findings were consistent with those observed in other cities (Schwartz 2000; Krewski et al. 2003; Ostro et al. 2007). Toxicological studies also confirmed that inhalation of ultrafine particles could cause oxidative stress (Wilson et al. 2002) and inflammation (Brown et al. 2001) in rat lungs. Seaton et al. (1995) further advanced the hypothesis that inhalation exposure to ultrafine particles could result in an inflammatory response in the lungs that may be capable of altering blood coagulation. In recent years, more toxicological studies have been carried out in North America, Western Europe, and other countries with a focus on the ultrafine size fraction of vehicle exhaust aerosols or ambient air (Li et al. 2003; Bastain et al. 2003; Cho et al. 2004; Kreyling et al. 2004).
Ultrafine and nano particles are mostly emitted from mobile sources, which include primarily automobiles and diesel trucks, and from stationary combustion sources (Whitby and Svendrup 1980). These particles can be formed in the atmosphere through reaction and/or nucleation of vapor precursors, such as through condensation of supersaturated vapors (Seinfeld and Pandis 1998) or by radioactive decay (Kulmala 2003; Biswas and Wu 2005).
Numerous studies have been conducted to determine the mass concentrations, the distributions, and chemical components of ambient PM2.5 (diameter <2.5 μm) present in urban and heavily industrialized areas. Yet much less is known, and even less has been done about particles of nano and ultrafine size. Earlier Geller et al. (2002) demonstrated the use of a nano-MOUDI (Micro-orifice Uniform Deposit Impactor) sampler by collecting size-segregated (10–18, 18–32, and 32–56 nm) ambient ultrafine particles that could be further analyzed for chemical speciation. Their study indicated that in the Los Angeles basin, ultrafine particles were generated by emissions mostly during the morning hours, whereas the formation of secondary aerosols became more important as the day progressed. The nano-MOUDI technology was also used by Miguel et al. (2005) in California to analyze 12 polycyclic aromatic hydrocarbons (PAHs) in the Aitken size range (10 nm < diameter <32 nm). They found that the measured masses of PAH increased with decreasing particle size.
Air quality in the southern Taiwan air basin is the worst in the country and has become a serious health concern. The air basin has been classified as a non-attainment region for PM since the 1990s. Therefore, many studies have been conducted to characterize the origin of the ambient particles in this air basin (Lin 2002a; Lin and Lee 2004; Tsai et al. 2005). Results from these earlier studies collectively suggested the need for further research into the chemical composition, the source contribution, and the formation of ultrafine and nano particles present particularly in polluted days.
Although these ultrafine and nano particulates contribute to negligible mass, they often dominate the atmospheric particle concentrations in number. In fact, it has been said (Harrison et al. 2000) that due to their high number concentrations, a given mass of ultrafine particles has 100–1,000 times more surface area than an equal mass of fine particles (0.1 μm < diameter <2.5 μm), and approximately 105 times more surface area than an equal mass of coarse particles (2.5 μm < diameter <10 μm). Accordingly, these ultrafine particles may be considered as more toxic to humans. Many studies actually suggested that upon deposition, those ultrafine and nano particles when coated with strong acids would especially cause tissue damage simply due to the acidity involved (Lippman et al. 1982; Schlesinger 1989; Chen et al. 1991). With the availability of the nano-MOUDI technology, these suggestions have thus now led to the special interest in, as well as the urgent need for, measuring the water-soluble ions in these particles.
In line with such a special interest, the present study was undertaken to quantify as well as to characterize the ultrafine and nano particles collected in the southern Taiwan air basin. As expected, the ambient particle samples in the present study were collected using a nano-MOUDI sampler. These particulate samples were actually part of an intensive sampling program carried out for aerosol episodes where the maximum 24-h PM10 concentration exceeded 183 μg m−3. The particulate mass concentrations were measured for several size ranges (0.01–18 μm). The collected particulate samples were also analyzed for water-soluble ionic species with an emphasis to characterize the concentrations and distributions of the ions in ambient ultrafine and nano particles. The neutralization ratio (NR) indices were used to characterize the acidity of these particles. All the data from this study were ultimately used to distinguish the particle characteristics in samples taken between the episode (polluted) and non-episode (normal) days.
Materials and methods
Study area and sampling site
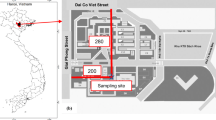
The ambient aerosol particle measurements were taken at the Da-Liao ambient air quality monitoring station, which is part of the Taiwan Air Quality Monitoring Network established by the Taiwan Environmental Protection Administration (TEPA) in 1993. This monitoring station was located immediately downwind from the Kao-Ping ambient air quality basin, where the air quality has been the worst in the country primarily due to the high emissions of air pollutants in the area. Further information on this air basin, which is located in southern Taiwan, was given in Lin (2002a). It is of note that the Da-Liao monitoring site was also located less than 10 km from the Kaoshiung coastal area, and southwest of a major mountain (Central Mountain, peak 3,000 m) where the air was relatively stable during the sampling periods. The monitoring station per se was physically installed on the roof of a building, at an effective inlet height of 10 m above ground. Hourly ambient PM10 concentrations were monitored following the standard method of the (Taiwan) National Institute of Environmental Analysis (NIEA) A206.10C, which counts on the use of a beta attenuation monitor.
Sampling program
Between January and March 2004, there were 46 polluted days (with an average PM10 concentration of 150 ± 19 μg m−3) and 45 normal days (with an average PM10 concentration of 100 ± 22 μg m−3) in which TEPA monitored for PM10 levels. For the present study, seven samplings were taken randomly between February and March 2004, with each lasting 48 h, and only on days without rain. The sampling system used in this study consisted of a MOUDI and a nano-MOUDI sampler. This system was basically the same one used previously by many other investigators (e.g., Geller et al. 2002; Miguel et al. 2005; Lin et al. 2005). The designed cut-off sizes were 18, 3.2, 1.8, 1.0, 0.56, 0.32, 0.18, 0.10, 0.056, 0.032, 0.018, and 0.010 μm. The flow rates for MOUDI and nano-MOUDI were 30 and 10 L min−1, respectively. Particulates were collected using 47-mm Teflon filters (Zefluor™-supported PTFE) for the nano-MOUDI sampler, and 37 mm for the MOUDI sampler.
The particle concentrations measured in these study samples were considered representative of those of the local ambient particles. Hourly meteorological data (wind speed and direction) and PM10 concentrations were extracted from the Da-Liao station. The minimum and maximum hourly wind speeds obtained from the station during each sampling period were then determined, as shown in Table 1.
Chemical analysis
The filters were weighted by using a high-precision six-digit electronic balance (Mettler MX5) after they had been conditioned at 40±3% relative humidity and 17 ±1°C for over 24 h. The filters were then immersed in water and extracted by ultrasonic agitation. The extractions were sealed and stored at 4°C until further analysis. A Dionex-120 ion chromatography unit was employed to analyze the inorganic ions under consideration (Cl−, NO −3 , SO 2−4 , Na+, NH +4 , K+, Ca2+, and Mg2+). For anion analysis, the mobile phase was a mixture of 2.7 mM sodium carbonate and 0.3 mM sodium bicarbonate at a flow rate of 1.5 ml min−1. The cations were analyzed using methane sulfonic acid as the eluent at 1 ml min−1.
Quality assurance and quality control tests (e.g., analysis of blanks, method detection limit, recovery efficiency) were additionally conducted. Method detection limit (MDL) was estimated from repeated analyses of predefined quality control solutions. The MDLs (all in ng m−3 for a sampling duration of 48 h) of Cl−, NO −3 , SO 2−4 , Na+, NH +4 , K+, Mg2+, and Ca2+ were 16, 14, 20, 4, 9, 4, 3, and 9, respectively. The recoveries ranged from 89% for Na+ to 116% for Cl−, with an overall average of 103%. The relative standard deviations of the fraction recovered were 6%, thus reflecting a high degree of reproducibility (based on 10% being set as the benchmark).
In order to contrast the levels of field-sampled PM between the normal and polluted days, the mean and standard deviation (SD) for wind speeds and PM10 concentrations during each sampling period were calculated (Table 1). For the same purposes, the mean and SD were likewise calculated for major inorganic ion compositions (Table 2).
Results and discussion
Mass concentration
In Taiwan the ambient air quality standard, which was based on the beta gauge method, is 125 μg m−3 (24-h average) for PM10 (TEPA 2004). Accordingly, all the particulate samples collected in this study were classified as either of normal days or of polluted days, depended on whether their PM10 concentrations were lower or higher than 125 μg m−3. In each sample, the measured airborne particulates were divided into the following four groups: nano particles (PMnano, diameter <0.056 μm); ultrafine particles (PM0.1, diameter <0.1 μm); fine particles (PM0.1–2.5, 0.1 μm < diameter <2.5 μm); and coarse particles (PM2.5–10, 2.5 μm < diameter <10 μm). These four particle groups were determined by linear interpolation, which yielded a fairly smooth size distribution for each group.
The particle concentrations classified into the polluted and normal days are shown in Table 1. The first 6 days (three samples) were polluted days and the remaining 8 days (four samples) were normal days. Linear regression was performed for PM10 concentrations measured in this study (using the beta attenuation technique) on those levels (on dry particle weight basis) monitored at the station. The strong correlation (correlation coefficient = 0.99) demonstrated that the ambient samples obtained in this study were representative of the local ambient air quality.
A slight northeast monsoon started to influence the study area after the first 6 days. During the polluted (i.e., the first 6) days, the wind speeds were 0–4.0 m s−1, and for 5 of these 6 days, the wind speeds were below 2 m s−1. However, the prevailing wind direction changed from the northeast to southwest during the remaining 8 days. The wind speeds (0.1–5.3 m s−1) during these normal days were slightly higher than those on the polluted days, while 6 of the normal days also had a wind speed below 2 m s−1. There was a notion that the higher ratio of calm winds during the polluted days tended to yield poorer air dispersion thus leading to higher PM10 concentrations. In both periods, the diurnal relative humidities (40–90%) and temperatures (15–32°C) were similar.
As shown in Table 1, the PM2.5 concentrations measured on the polluted days (65–92 μg m−3) were much higher than those on the normal days (31–55 μg m−3). The PM1 concentrations on the polluted days (mean = 53 μg m−3) were also somewhat higher than those for the normal days (mean = 34 μg m−3). For a better appreciation of these mass concentrations, all individual particle fractions of the total suspended particulates (TSP) for both the polluted and normal days are depicted in Fig. 1a. The cumulative (integrated) PM0.1, PM1, PM2.5, and PM10 fractions were 2, 38, 54, and 75% of the TSP concentration, respectively. The results for PM2.5 (approaching 60%) and PM0.1 (an insignificant portion) were basically in agreement with those observed by others in other areas (Keywood et al. 1999; Tsai et al. 2005; Lin 2002b). In the present study, the fractions from the polluted days were higher than those from the normal days for particle size greater than 1 μm, as depicted in Fig. 1b. This finding suggested that the PM1 (submicron aerosol particles) fraction played an important role in the ambient atmosphere on the polluted days. The data also indicated that in addition to particle concentrations (Table 1), both the ratios of PM1/TSP and PM2.5/TSP increased over time during the polluted days.
The particle mass concentrations over various particle sizes showed a tri-modal size distribution (Fig. 2). The major peak appeared in the fine particle size mode, with other peaks showing in the coarse and ultrafine modes.
For a more effective visual appreciation, the scales were expanded for the mass-size distributions of ultrafine and nano particles in the samples, as illustrated in Fig. 3. Although the patterns of particle size distribution for the polluted and normal days were similar, the airborne particulate concentrations from the polluted day samples appeared to be slightly higher. Actually, for each particle size range under study, the PM concentrations during the polluted days were higher than those during the normal days (Table 1).
Water-soluble ionic species
Ammonium in particles contributed to about 60–90% of the water-soluble cations analyzed, whereas sulfate and nitrate in particles were about 80–99% of the water-soluble anions analyzed. Table 2 summarizes the mass fractions (percentages) of these three ions according to the various particle size ranges under study (PMnano, PM0.1, PM1, PM2.5, PM10, and TSP), and by sample classification (normal vs. polluted days). Mass fractions of the three water-soluble ions as a group during the polluted and normal days were as follows, respectively: 59% and 57% in PM2.5; 33% and 30% in PM2.5–10; and 24% and 24% in PM0.1. In the fine particles (0.1 μm < diameter <2.5 μm), secondary formed compounds (sulfate, nitrate, and ammonium) were the major components, accounting for 51% and 48% of the total mass concentration on the polluted and normal days, respectively. The mass fractions of water-soluble ions in PM2.5 and PM2.5–10 on the polluted days were slightly higher than those on the normal days.
Ammonium
Figure 4 depicts the single fine mode for ammonium peaking around 0.56–1 μm, which is consistent with the observation made by Zhuang et al. (1999). According to Zhuang et al. (1999), fine particle ammonium would originate from ammonia vapor as a result of the latter reacting or condensing on a particle surface and accumulating in the droplet mode (with an aerodynamic diameter of 0.57±0.10 μm). In other words, ammonium size distributions, particularly in the fine particulate range, would follow a mode similar to that of sulfate or nitrate. Therefore, the results from this study implied certain reactions of nitric acid with ammonia in the particle phase, and certain mass accumulation of SO2 in the droplet phase as well.
In the present study, the average concentration ratios of PM1/PM2.5, PM2.5/PM10, and PM1/PM10 for ammonium were 0.98, 0.95, and 0.93, respectively. These data suggested that the ammonium in the PM1 fraction played an important role in the ambient atmosphere. Concentrations of NH +4 in the fine particles were about 1.7 times higher on the polluted days than on the normal days.
The mass fraction of ammonium in PMnano was the largest when compared to the cation’s fractions in other particle size modes, and reduced with particle size increment (as shown in Fig. 5). The mass fractions of ammonium in PM1–2.5 and PM2.5–10 on the polluted days were larger than those on the normal days. However, in smaller particle sizes, the mass fractions of ammonium on the polluted days were smaller than those on the normal days.
Nitrate
The mass-size distribution of nitrate was bimodal, with higher amounts seen in the fine mode than in the coarse mode. The fine and coarse modes of nitrate ranged from 0.56 to 1 μm and from 3.2 to 18 μm, respectively, as shown in Fig. 4. These findings were consistent with those observed in other cities (Mehlmann and Warneck 1995; Yao et al. 2003). The cause of this bimodal distribution may be explained as follows.
Fine mode nitrate is formed by the homogeneous gas-phase transformation of NO x to HNO3, which later reacts with pre-existing fine particles (Seinfeld and Pandis 1998). When NO x is oxidized to HNO3 in the atmosphere, nitrate particles are formed (Matsumoto and Tanaka 1996). Also, HNO3 can react with NH3 in atmosphere to form NH4NO3. In the present study, a similar mode, ranging from 0.56 to 1 μm (i.e., a droplet mode), was found between nitrate and ammonium. The nitrate droplet mode in this study apparently supported the condensation mode of ammonium nitrate.
Several studies showed that coarse mode nitrate could be formed through the reaction of gaseous HNO3 with coarse sea-salt particles (Wall et al. 1988; Harrison et al. 1994; Pakkanen 1996; Jonson et al. 2000). Other studies also suggested that the existence of coarse mode nitrate could be through the reaction of gaseous HNO3 with coarse soil particles (Wolff 1984; Dasch and Cadle 1990; Zhuang et al. 1999). The Da-Liao sampling site in this study is situated within an industrial district surrounded by cultivated lands, and is close to the Kaohsiung coastal area. Therefore, the coarse mode nitrate might have been formed by reactions of gaseous HNO3 with either sea-salt or soil particles.
The mass concentration ratios of PM1/PM2.5, PM2.5/PM10 and PM1/PM10 for nitrate were 0.92, 0.74, and 0.68, respectively. The nitrate concentrations on the polluted days were higher than those on the normal days, by a factor of 1.8 and 1.5 for the fine and coarse modes, respectively. For samples taken on the normal days, only 40% of nitrate was in particles larger than PM2.5. Most of the nitrate ions were present in the fine mode particles.
The mass percentage of nitrate in PM0.056–0.1 was the smallest when compared to the anion’s fractions in other particle size modes, and increased with particle size increment (as shown in Fig. 5). The largest percentage for nitrate ions was in particles of 2.5 to 10 μm. The mass fractions of nitrate in PM0.1–1 and PM1–2.5 on the polluted days were smaller than those on the normal days.
Sulfate
Sulfate was tri-modal with peaks appearing at 0.056 to 0.1 μm (ultrafine), 0.56 to 1 μm (fine), and around 3.2 to 18 μm (coarse), as shown in Fig. 4. These findings were consistent with those observed in other cities (Zhuang et al. 1999; Yao et al. 2003; Cabada et al. 2004), all showing a fine mode domination. The cause of this tri-modal distribution may be explained as follows.
Insofar as sulfate dominates in the droplet mode, the in-cloud processes are likely to be a major path for the formation of this anion (Hegg and Hobbs 1982). The weather conditions at the Da-Liao monitoring site were therefore believed to have played an important role in the contribution of sulfate seen in the fine mode. During the sampling period, fog was frequently observed near the ground surface at the Da-Liao site during the nights and mornings of the polluted days. According to Pandis et al. (1992) and Kerminen and Wexler (1995), relatively high concentrations of sulfate could be formed by aqueous-phase reactions in fog or cloud droplets.
The ultrafine mode sulfate observed in the Da-Liao area were likely to have been formed through heterogeneous nucleation of ammonium sulfate particles (Zhang et al. 2000). The coarse mode sulfate, on the other hand, might have been formed by reactions of gas-phase SO2 or H2SO4 on the wet surface of sea salt or soil particles (Wall et al. 1988; Zhuang et al. 1999).
The mass concentration ratios for sulfate in PM1/PM2.5, PM2.5/PM10, and PM1/PM10 were 0.97, 0.92, and 0.90, respectively. These data suggested that the PM1 fraction of sulfate played an important role in the ambient atmosphere. The fine mode SO 2−4 concentrations on average were about 1.6 times higher on the polluted days than on the normal days.
As with the other two ions, the mass fraction for sulfate in PM0.056–0.1 was the largest among those in all particle size modes, and these percentages were reduced with particle size increment (as shown in Fig. 5). The mass percentages of sulfate in PM0.1–1 and PM1–2.5 were smaller on the polluted days than on the normal days. The mass percentages of sulfate in PM2.5 were about 22% for both the polluted and normal day periods.
Nano/ultrafine particle mass composition
As shown in Table 1, the mean mass concentrations for ultrafine (1.9 ± 0.7 μg m−3) and nano (1.4 ± 0.7 μg m−3) particles in the study samples were comparable. These findings were by and large consistent with those observed in the literature, except in a few places (e.g., Hughes et al. 1998; Pakkanen et al. 2001; Geller et al. 2002). Sulfate, nitrate, and ammonium ions contributed 9, 5, and 8 to the ultrafine mass, respectively. Each of the other inorganic ions studied here (i.e., K+, Na+, Ca2+, Mg2+, and Cl−) contributed less than 4% of the ultrafine mass. The contents of inorganic ions in the ultrafine mass were lower than those reported by Cabada et al. (2004).
For the nano particulates, sulfate, nitrate, and ammonium ions accounted for approximately 5, 6 and 7% of the total mass, respectively (Table 2). Each of the other inorganic ions contributed less than 5% of the nano mass. The contents of inorganic ions in the nano mass were higher than those reported by Pakkanen et al. (2001). In the present study, the inorganic water-soluble ions were found to be insignificant in terms of their mass compositions in the ultrafine and nano particulates. These observations were also consistent with those reported in the literature.
Neutralization ratio of airborne particles
Several studies (Querol et al. 2001; Parmar et al. 2001; Kim and Park 2001; Shrestha et al. 2002; Hu et al. 2002) estimated the acidity of airborne particulates by calculating the relationship among the NH +4 , NO −3 , and non-sea salt SO 2−4 (nss SO 2−4 ) ions present in the particulates. The neutralization ratio (NR) index was used to express this interrelationship, which was defined as follows (Colbeck and Harrison 1984):
where eq m−3 denotes normal concentration and eq = equivalent.
The content of nss.SO 2−4 in airborne particulates was calculated by subtracting the amount of SO 2–4 in sea salt from that measured in the atmosphere, whereas the amount of sulfate in seawater (sea salt) was estimated from the ratio of SO 2–4 to Na+ in bulk seawater. According to Ohta and Okita (1990), aerosol Na+ suspended in the atmosphere comes from sea salt particles only. The Na+ in suspended aerosol was thus assumed to be all contributed from sea salt particles. Under this assumption, the value for nss.SO 2–4 could hence be derived using the following equation (Parmar et al. 2001):
For the case where NR>1, the excess in NH +4 and the airborne particulates were considered to be basic. For NR=1, all three ions and the airborne particulates were considered to be neutral. For NR<1, the excess in NO –3 and SO 2–4 and the airborne particulates were considered to be acidic. Colbeck and Harrison (1984) further suggested the possible presence of NH4Cl in airborne particulates for cases where N>1, and the presence of acidic or metal sulfates where NR < 1.
Table 3 summarizes the comparison of the NRs determined in the present study with those in other studies. As shown in this table, the NRs were close to unity in the fine mode for several cities (Tsai and Cheng 1999; Querol et al. 2001; Parmar et al. 2001; Shrestha et al. 2002; Hu et al. 2002), although in general the values from the present study appeared to be somewhat higher (excepted for Dayalbagh, Agra). The higher concentrations of ammonium seen in the southern Taiwan atmosphere might have been due to the dense applications of fertilizer and livestock waste usually seen in the region. The data also indicated that the formation of NH +4 in aerosol was not limited by the availability of NH3. In the fine mode, NH +4 was richer compared to nss-SO 2–4 . This suggested that the dominant compounds of inorganic water-soluble species were (NH4)2SO4 and NH4NO3 in the fine mode, and CaSO4, NaNO3, and Ca(NO3)2 in the coarse mode.
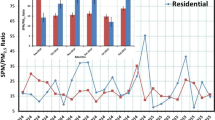
The NRs in the various particle size ranges for the polluted and normal day samples are shown in Fig. 6. The airborne particulates smaller than 0.1 μm were essentially basic, whereas those larger than 2.5 μm were either neutral or slightly acidic. The lower NR value seen for PM10 in the present study suggested the possibility of additional neutralization of sulfate and nitrate by calcium or sodium distributed in the coarse mode particles (Harrison et al. 1994; Wakamatsu et al. 1996). The estimated NR was close to unity for airborne particulates in the 0.18–1 μm range. All in all, the NRs for airborne particulates measured in the present study were seen to be strongly correlated with particle sizes, at least for the aerosol episodes under study.
As stated in the Introduction, particles with a diameter of less than 0.1 μm were considered to be capable of penetrating the alveolar region of the lungs easily. In the present study, the majority of the alkaline load was found as delivered by particles of this size. This finding thus suggested that areas deep in the human lungs (that preferentially collect particles in the ultrafine size range) could indeed be vulnerable to the alkaline conditions prevailing in the southern Taiwan region.
Conclusions
This study investigated the concentrations and distributions of nano- to coarse-size airborne particulates for several aerosol episodes occurring in the southern Taiwan region. The particulate samples were taken with the MOUDI and the nano-MOUDI sampler. The ambient particles on the whole exhibited a typical tri-modal size distribution on mass concentration. The highest peak appeared in the fine particle mode (0.1 μm < diameter <2.5 μm), with the other two showing in the coarse (2.5 μm < diameter <10 μm) and the ultrafine (diameter <0.1 μm) modes.
Sulfate, nitrate, and ammonium were the major components seen in the fine particle mode. These three water-soluble inorganic ions collectively accounted for 51 and 48% of the mass composition for particle samples taken on the polluted and normal days, respectively. On the polluted days, the total mass compositions of the three major water-soluble ion species were 59, 33, and 24% in PM2.5, PM2.5–10, and PM0.1, respectively. On the normal days, the total mass compositions of the three ions in the three particle size modes were, respectively, 57, 30, and 24%. The total mass fractions of the three water-soluble ions in PM2.5 were the largest (57−59%) among all particulate sizes, regardless of sampling day.
Particles smaller than 0.1 μm were essentially basic, whereas those larger than 2.5 μm were either neutral or slightly acidic. The value of NR was close to unity for airborne particulates in the 0.18–1 μm range. This finding suggested that areas deep in the human lungs, which preferentially collect particles in the ultrafine size range, could indeed be vulnerable to the alkaline conditions prevailing in the southern Taiwan region. Insofar as this study is exploratory in nature, as only a small number of particulate samples were used, there appears to be a continuous need for further research into the chemical composition, the source contribution, and the formation of nano and ultrafine airborne particulates in Taiwan, if not in other countries as well.
References
Bastain, T. M., Gilliland, F. D., Li, Y. F., Saxon, A., & Diaz-Sanchez, D. (2003). Intraindividual reproducibility of nasal allergic responses to diesel exhaust particles indicates a susceptible phenotype. Clinical Immunology, 109, 130–136.
Biswas, P., & Wu, C. Y. (2005). Nanoparticles and the environment. Journal of Air & Waste Management Association, 55, 708–746.
Brown, D. M., Wilson, M. R., MacNee, W., Stone, V., Donaldson, K. (2001). Size-dependent proinflammatory effects of ultra fine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultra fines. Toxicology and Applied Pharmacology, 175, 191–199.
Cabada, J. C., Rees, S., Takahama, S., Khlystov, A., Pandis, S., Davidson, C. I., & Robinson, A. L. (2004). Mass size distributions and size resolved chemical composition of fine particulate matter at the Pittsburgh Supersite. Atmospheric Environment, 38, 3127–3141.
Chen, L. C., Peoples, S. M., & Amdur, M. O. (1991). Pulmonary effects of sulfur oxides on the surface of copper oxide aerosol. American Industrial Hygiene Association Journal, 52, 187–191.
Cho, A., Stefano, E., You, Y., Rodriguez, C., Schmitz, D., Kumagai, Y., Miguel, A., Eiguren-Fernandez, A., Kobayashi, T., Avol, E., & Froines, J. (2004). Determination of four quinones in diesel exhaust particles, SRM 1649a, and atmospheric PM2.5. Aerosol Science and Technology, 38, 68–81.
Colbeck, I., & Harrison, R. M. (1984). Ozone-secondary aerosol-visibility relationships in north-west England. The Science of the Total Environment, 34, 87–100.
Dasch, J. M., & Cadle, S. H. (1990). The removal of nitric acid to atmospheric particles during a wintertime field study. Atmospheric Environment, 24A, 2557–2562.
Geller, M. D., Kim, S., Misra, C., Sioutas, C., Olson, B. A, & Marple, V. A. (2002). A methodology for measuring size-dependent chemical composition of ultrafine particles. Aerosol Science and Technology, 36, 748–762.
Harrison, R. M., Msibi, M. I., Kitto, A. M. N., & Yamulki, S. (1994). Atmospheric chemical transformations of nitrogen compounds measured in the North Sea Experiment, September 1991. Atmospheric Environment, 28, 1593–1599.
Harrison, R. M., Shi, J. P., Xi, S., Khan, A., Mark, D., Kinnersley, R., & Yin, J. (2000). Measurement of number, mass and size distribution of particles in the atmosphere. Philosophical Transactions of the Royal Society A, 358, 2567–2579.
Hegg, D.A., Hobbs, P.V., 1982. Measurement of sulfate production in natural clouds. Atmospheric Environment, 16, 2663–2668.
Hu, M., He, L. Y., Zhang, Y. H., Wang, M., Kim, Y. P., & Moon, K. C. (2002). Seasonal variation of ionic species in fine particles at Qingdao, China. Atmospheric Environment, 36, 5853–5859.
Hughes, L. S., Cass, G. R., Gone, J., Ames, M., & Olmez, I. (1998). Physical and chemical characterization of atmospheric ultrafine particles in the Los Angeles area. Environmental Science & Technology, 32, 1153–1161.
Jonson, J. E., Semb, A., Barrett, K., Grini, A., & Tarrason, L. (2000). On the distribution of sea salt and sodium nitrate particles in Europe. Transport and chemical transportation in the troposphere, Proceedings of the EUROTRAC Symposium, Sixth, Gaimisch-Partenkirchen, Germany, 27–31 March 2000, pp. 695–699.
Katsouyanni, K., Touloumi, G., Samoli, E., Gryparis, A., Le Tertre, A., Monopolis, Y., Rossi, G., Zmirou, D., Ballester, F., Boumghar, A., Anderson, H.R., Wojtyniak, B., Paldy, A., Braunstein, R., Pekkanen, J., Schindler, C., Schwartz, J. (2001). Confounding and effect modification in the short term effects of ambient particles on total mortality: results from 29 European cities within APHEA2 project. Epidemiology, 12, 521–531.
Kerminen, V. M., & Wexler, A. S. (1995). Growth laws for atmospheric aerosol particles: An examination of bimodality of the accumulation mode. Atmospheric Environment, 29, 3263–3275.
Keywood, M. D., Ayers, G. P., Gras, J. L., Gillett, R. W., & Cohen, D. D. (1999). Relationships between size segregated mass concentration data and ultrafine particle number concentrations in urban areas. Atmospheric Environment, 33, 2907– 2913.
Kim, B. G., & Park, S. U. (2001). Transport and evolution of a winter-time yellow sand observed in Korea. Atmospheric Environment, 35, 3191–3201.
Krewski, D., Burnett, R., Goldberg, M., Hoover, B. K., Siemiatycki, J., Jerrett, M., Abrahamowicz, M., & White, W. (2003). Overview of the reanalysis of the Harvard six cities study and American Cancer Society study of particulate air pollution and mortality. Journal of Toxicology and Environmental Health. Part A, 66, 1507–1551.
Kreyling, W. G., Semmler, M., & Moller, W. (2004). Dosimetry and toxicology of ultrafine particles. Journal of Aerosol Medicine, 17, 140–152.
Kulmala, M. (2003). How particulates nucleate and growth. Science, 302, 1000–1001.
Li, N., Sioutas, C., Cho, A., Schmitz, D., Misra, C., Sempf, J., Wang, M. Y., Oberley, T., Froines, J., & Nel, A. (2003). Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environmental Health Perspectives, 111, 455–460.
Lin, C. C., Chen, S. J., Huang, K. L., Hwang, W. I., Chang-Chien, G. P., & Lin, W. Y. (2005). Characteristics of metals in nano/ultrafine/fine/coarse particles collected beside a heavily trafficked road. Environmental Science & Technology, 39, 8113– 8122.
Lin, J. J. (2002a). Characterization of the major chemical species in PM2.5 in the Kaohsiung City, Taiwan. Atmospheric Environment, 36, 1911–1920.
Lin, J. J. (2002b). Characterization of water-soluble ion species in urban ambient particles. Environment International, 28, 55–61.
Lin J. J., & Lee L. C. (2004). Characterization of the concentration and distribution of urban submicron (PM1) aerosol particles. Atmospheric Environment, 38, 469–475.
Lippman, M., Schlesinger, R. B., Leikauf, G., Spektor, D., & Albert, R. E. (1982). Effects of sulphuric acid aerosols on respiratory tract airways. The Annals of Occupational Hygiene, 26, 677–690.
Matsumoto, K., & Tanaka, H. (1996). Formation and dissociation of atmospheric particulate nitrate and chloride: An approach based on phase equilibrium. Atmospheric Environment 30, 639–648.
Mehlmann, A., & Warneck, P. (1995). Atmospheric gaseous HNO3, particulate nitrate, and aerosol size distributions of major ionic species at a rural site in western Germany. Atmospheric Environment, 29, 2359–2373.
Miguel, A. H., Eiguren-Fernandez, A., Sioutas, C., Fine, P. M., Geller, M., & Mayo, P. R. (2005). Observations of twelve USEPA priority polycyclic aromatic hydrocarbons in the Aitken size range (10–32 nm Dp). Aerosol Science and Technology, 39, 415–418.
Ohta, S., & Okita, T. (1990). A chemical characterization of atmospheric aerosol in Sapporo. Atmospheric Environment 24A, 815–822.
Ostro, B., Feng, W. Y., Broadwin, R., Green, S., Lipsett, M. (2007). The effects of components of fine particulate air pollution on mortality in California: results from CALFINE. Environmental Health Perspectives, 115, 13–19.
Pakkanen, T. A. (1996). Study of formation of coarse particle nitrate aerosol. Atmospheric Environment, 30, 2475–2482.
Pakkanen, T. A., Kerminen, V. M., Korhonen, C. H., Hillamo, R. E., Aarnio, P., Koskentalo, T., & Maenhaut, W. (2001). Sources and chemical composition of atmospheric fine and coarse particles in the Helsinki area. Atmospheric Environment, 35, 5381–5391.
Pandis, S. N., Seninfeld, J. H., & Pilinis, C. (1992). Heterogeneous sulfate production in an urban fog. Atmospheric Environment, 26A, 2509–2522.
Parmar, R. S., Satsangi, G. S., Kumari, M., Lakhani, A., Srivastava, S. S., & Prakash, S. (2001). Study of size distribution of atmospheric aerosol at Agra. Atmospheric Environment, 35, 693–702.
Pope, C. A., Burnett, R. T., Thun, M. J., Calle, E. E., Krewski, D., Ito, K., & Thurston, G. D. (2002). Lung cancer, cardiopulmonary mortality and long- term exposure to fine particulate air pollution. Journal of the Air & Waste Management Association, 287, 1132–1141.
Querol, X., Alastuey, A., Rodriguez, S., Plana, F., Ruiz, C. R., Cots, N., Massague, G., & Puig, O. (2001). PM10 and PM2.5 source apportionment in the Barcelona Metropolitan area, Catalonia, Spain. Atmospheric Environment, 35, 6407–6419.
Samet, J.M., Dominici, F., Curriero, F.C., Coursac, I., Zeger, S.L. (2000). Fine particulate air pollution and mortality in 20 US cities, 1987–1994. The New England Journal of Medicine, 343, 1742–1799.
Schlesinger, R. B. (1989). Factors affecting the response of lung clearance systems to acid aerosols: Role of exposure concentration, exposure time, and relative acidity. Environmental Health Perspectives, 79, 121–126.
Schwartz, J. (2000). Harvesting and long term exposure effects in the relation between air pollution and mortality. American Journal of Epidemiology, 151, 440–448.
Seaton A, MacNee W, Donaldson K, & Godden D. (1995). Particulate air pollution and acute health effects. Lancet, 345, 176–178.
Seinfeld, J. H., & Pandis, S. N. (1998). Atmospheric chemistry and physics: From air pollution to global change. New York: John Wiley & Sons.
Shrestha, A. B., Wake, C. P., Dibb, J. E., & Whitlow, S. I. (2002). Aerosol and precipitation chemistry at a remote Himalayan site in Nepal. Aerosol Science and Technology, 36, 441–456.
Taiwan Environmental Protection Administration. (2004) National Ambient Air Quality Standards. Taipei, Taiwan.
Tsai, J. H., Chang, K. L., Lin, J. J., Lin, Y. H., & Chiang, H. L. (2005). Mass-size distributions of particulate sulfate, nitrate, and ammonium in a particulate matter nonattainment region in southern Taiwan. Journal of the Air & Waste Management Association, 55, 502–509.
Tsai, Y. I., & Cheng, M. T. (1999). Visibility and aerosol chemical compositions near the coastal area in central Taiwan. The Science of the Total Environment, 231, 37–51.
Wakamatsu, S., Utsunomiya, A., Han, J. S., Mori, A., Uno, I., & Uehara, K. (1996). Seasonal variation in atmospheric aerosols concentration covering northern Kyushu, Japan and Seoul, Korea. Atmospheric Environment, 30, 2343–2354.
Wall, S. M., John, W., & Ondo J. L. (1988). Measurement of aerosol size distributions for nitrate and major ionic species. Atmospheric Environment, 22, 1649–1656.
Whitby, K. T., & Svendrup, G. M. (1980). California aerosols: Their physical and chemical characteristics. Advanced Environmental Science and Technology, 10, 477.
Wilson, M. R., Lightbody, J. H., Donaldson, K., Sales, J., & Stone, V. (2002). Interactions between ultrafine particles and transition metals in vivo and in vitro. Toxicology and Applied Pharmacology, 184, 172–179.
Wolff, G. T. (1984). On the nature of nitrate in coarse continental aerosols. Atmospheric Environment, 18, 977–981.
Yao, X., Lau, A. P. S., Fang, M., Chan, C. K., & Hu, M. (2003). The size dependence of chloride depletion in fine and coarse sea-salt particles. Atmospheric Environment, 37, 743–751.
Zhang, D., Shi, G., Iwasaka, Y., Hu, M. (2000). Mixture of sulfate and nitrate in coastal atmospheric aerosols: individual particle studies in Qingdao (36°04′ N, 120°21′ E), China. Atmospheric Environment, 34, 2669–2679.
Zhuang, H., Chan, C. K., Fang, M., & Wexler, A. S. (1999). Size distributions of particulate sulfate, nitrate, and ammonium at a coastal site in Hong Kong. Atmospheric Environment, 33, 843–853.
Acknowledgements
The authors gratefully acknowledge the National Science Council of the R.O.C. (Taiwan) for financial support under Project No. NSC.92-2621-Z-006-002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, LP., Tsai, JH., Chang, KL. et al. Water-soluble inorganic ions in airborne particulates from the nano to coarse mode: a case study of aerosol episodes in southern region of Taiwan. Environ Geochem Health 30, 291–303 (2008). https://doi.org/10.1007/s10653-007-9122-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-007-9122-0