Abstract
Laboratory incubation trials were conducted to investigate the effects of several factors on the persistence as well as the dissipation of three synthetic pyrethroid pesticides in red soils obtained from the Yangtze River Delta region in China. The pyrethroids selected for investigation were cypermethrin, fenvalerate, and deltamethrin, which continue to be used extensively to control pests on farmland in the region despite the concern that they are highly toxic to certain vertebrate and mammalian species. Data from this exploratory study showed that the dissipation half-lives (T 1/2) tended to correlate with soil pH and soil organic matter contents, but not with soil cation-exchange capacity. The T 1/2 values were seen to be shorter in soil samples fertilized with glucose than without. The rates of pyrethroid dissipation also tended to increase with increasing initial soil concentration, but were largely unaffected by whether the pesticides were present in the soil separately or as a mixture. Another noteworthy observation is that microbial activity appeared to dominate the degradation process. Findings of this type could offer valuable clues for future research directions in reducing pesticide persistence in soil, which in turn could lead to the ultimate reduction of environmental pollution caused by pyrethroid application to farmland in the region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Beginning in the late 1970s, synthetic pyrethroid pesticides were successfully introduced into the agricultural market as a new generation of insecticides, owing much to their unique ability to knock down insects at lower application rates, to their lower mammalian toxicity, and to their longer stability in outdoor environments. These synthetic pyrethroids, including cypermethrin, fenvalerate, and deltamethrin, have been applied extensively for the control of mosquitoes and in the treatment of ectoparasitic disease. They also have been widely used as home and garden insecticides since 1978 (Katsuda 1999; Ying 1990).
With such extensive use, the accumulation of synthetic pyrethroids in the environment is bound to be substantial. Pesticide residues in soil can diffuse, evaporate, or leach, thus having the potential to cause water pollution and other ecological problems (Henry and Kishimba 2006; Köprücü and Aydin 2004; Oudou et al. 2004). Despite the fact that most synthetic pyrethroids have lower mammalian toxicity compared to other classes of insecticides (e.g., organochlorines or organophosphates), they can still be very harmful to certain vertebrate and mammal species, including bees (Zhu et al. 1999), chicks (Garg et al. 2004), and fish or shellfish (Pang et al. 2004; Pan et al. 2000). It has been reported (Andrew and Colin 2001) that sublethal levels of cypermethrin in the aquatic environment can have a long-term impact on Atlantic salmon by interference with some aspects of their reproduction (Köprücü and Aydin 2004). In recent years, there also has been increasing concern regarding the health risk from pesticide residues present in crop soil, as their uptake or translocation by plants can easily lead to food (e.g., crop) contamination. For example, fenvalerate residues are frequently detected in Chinese tea at levels that often reduce its export potential (Chen 1999).
Cypermethrin, fenvalerate, and deltamethrin are used extensively to control pests on farmland located in the Yangtze River Delta region in China (Fig. 1). These pyrethroids are used in the region at the rate of approximately 10.2 kg/hm2 per year, resulting in about 50–60% of their residues being preserved in farmland topsoil (Ma et al. 2005; He and Li 2003; Wu 2003). Certain soil properties under various environmental conditions are reported to have specific effects on the transformation, degradation, or dissipation of certain pesticides in the soil (Jin 2001; Jones and Norris 1998; Rangaswamy and Venkateswarlu 1992). An understanding of this type of dissipation effects is critical to ecological and human health, given that the potential for environmental or food contamination would increase with increasing pesticide persistence in the soil or other media.
Map of the Yangtze River Delta area in China showing the locations of sampling stations S01, S02, and S03 (see Table 1 for further description of sampling stations)
At present, few investigations of this type have been conducted for cypermethrin, fenvalerate, and deltamethrin (He et al. 2003; Qin et al. 2000). Accordingly, a series of laboratory experiments was performed in this study to further explore the effects of certain relevant factors on the persistence as well as the dissipation of these three pyrethroids in the Yangtze River Delta soils. In the present study, the dissipation of pyrethroids was quantified using gas chromatography equipped with an electron-capture detector (GC-ECD). The factors under investigation included microbial activity, three soil properties (pH, organic matter contents, cation-exchange capacity), external carbon source, and initial soil concentration.
Materials and methods
Samples and sites
Topsoil (0–20 cm deep, red) samples were collected from farmland in the following three locations in the Yangtze River Delta region: a farm located in Shuangqiao village (in Jiaxing city), Zhejiang province (S01, ortho red soil); the Changshu agriculture and ecology experimental station in Xinzhuang town (in Changshu city), Jiangsu province (S02, yellow-red soil); and the Xieqiao experimental station in Xieqiao town (in Changshu tity), Jiangsu province (S03, brown red soil). After collection, these soil field samples were air-dried, sieved through a 60-mesh sieve, and then stored in the dark at 20°C until analysis. The physical and chemical properties of these field samples, which were detected not to contain any pyrethroid residues, are summarized in Table 1.
Test materials and reference standards
Reference standards (98% purity) for cypermethrin, fenvalerate, and deltamethrin were obtained from Sigma-Aldrich, Ltd. (USA). Florisil (60/100 mesh) and anhydrous Na2SO4 were used for extract clean-up were obtained from Sigma-Aldrich and Chinese Nanjing Chemical Reagent Co., respectively. In this study, florisil was activated at 650°C for 4 h, cooled in a desiccator, and then kept inside an airtight vase until use, as per product use instructions. Na2SO4 was baked at 500°C also for 4 h and stored in an airtight glass bottle until use.
Other laboratory apparatuses used in this study included: a rotary evaporator and a vacuum pump (both manufactured by the Shanghai Yarong Biochemistry Apparatus Factory, China) for pre-concentration use; an ultrasonic water bath (manufactured by the Kunshan Ultrasonic Apparatus, Ltd., China) for extraction; and a temperature-regulated biochemical cultivation box (manufactured by the Shanghai Senxin Experimental Apparatus, Ltd., China) for incubation of soil microbes.
Experiments on pesticide persistence
Pesticides present in soil separately versus a mixture
In the laboratory, red soil samples from the three fields (i.e., the field samples) were each allocated into four batches for treatment later with the three pyrethroids separately and as a mixture, all at the product label-specified maximum application rate of 2 mg kg−1. The lab samples treated with pyrethroids were kept in an incubation box at 25°C throughout the course of the entire investigation. Water was added to each lab sample to maintain a moisture content of 25% by weight. Certain portions (10 g dry weight each) of the lab samples were monitored (quantified) for residue contents at intervals of 0, 7, 14, 21, 28, 35, 49, 70, 91, and 112 days following pyrethroid treatment. Additional allocations were made from the lab samples (i.e., from the batches) for investigation of the other effects described below.
Sterilized versus unsterilized soil
Lab samples allocated for this experiment were each subdivided into two groups to investigate the dissipation rates under sterilized versus unsterilized conditions. Each portion (10 g dry weight) of the sample for sterilization was autoclaved three times (24 h apart) each for 30 min in a capped 100 ml Erlenmeyer flask at 121°C. Axenic water and tap water were added to the germ-free (autoclaved) and the original (unautoclaved) soils, respectively, to obtain a water content of 25% by weight. These moistened subsamples were placed in an incubation box at 25°C in the dark for a week prior to pyrethroid treatment (at the label-specified maximum application rate of 2 mg kg−1). Residue contents in these subsamples were monitored at regular intervals as described above.
External carbon source
Glucose solution was prepared at 200 g l−1 as a source for external carbon. Lab samples allocated for this experiment were treated with the three pyrethroids separately, again at the maximum field concentration of 2 mg kg−1. About 1 ml of the glucose solution was then added to each of the subsamples treated with the pyrethroids. These subsamples were incubated at 25°C in the dark and maintained at a water content of 25% by weight. Residue contents in these fertilized subsamples, along with those unfertilized, were monitored at regular intervals as described earlier.
Pesticide concentration
For exploratory purposes and due to limited resources at this time, only the Changshu field sample (S02) was allocated for this part of the study. Two higher soil concentrations, one at 5 mg kg−1 and the other at 20 mg kg−1, were used to explore the effects of pesticide concentration on persistence. These subsamples, together with the ones treated at the maximum application rate of 2 mg kg−1, were incubated at 25°C in the dark and kept at a water content of 25% by weight. Residue contents in these subsamples were again monitored regularly as described earlier.
Fortification
Recovery of cypermethrin, fenvalerate, and deltamethrin was determined using soil samples at fortification levels of 0.01, 0.8, and 4.0 mg kg−1. Each solution used to provide fortification was prepared by measuring an appropriate amount of the pyrethroid reference standard into a known quantity of a petroleum ether/acetone (2:1 v/v) solution. An appropriate amount (20.0 ml) of the fortification solution was then evenly pipetted into a 250-ml Erlenmeyer flask containing 50.0 g of the soil sample. After equilibration for 2 h, the fortified samples were air-dried overnight, mixed thoroughly, and frozen at −40°C. In an effort to simulate the field samples as closely as practical, these fortified samples were thawed, air-dried overnight again, and then re-mixed prior to analysis.
Extraction and clean-up procedures
For each experiment (trial) described above, pyrethroid residues in the subsamples were extracted as follows (for further details see the following papers: Chen et al. 2006; Shao and Tan 2006; Wu et al. 2006; Lin and Liu 2005; Wang et al. 2004; Musumeci and Ostiz 1994). The subsamples were each kept for 24 h in a 100-ml centrifuge tube containing 50 ml of petroleum ether/acetone (2:1, v/v). Afterwards, the test tube was agitated in an ultrasonic bath for 30 min and then centrifuged for 15 min at 3,000 rev min−1. This extraction procedure was repeated three times. At each time, 30 ml of the supernatant (i.e., the organic extract phase) was collected into a different test tube. Supernatants collected from the three extractions were then combined and concentrated to about 20 ml using a rotary evaporator at 40–45°C.
The 20 ml extract solution was further dried by anhydrous Na2SO4 and pre-concentrated in the rotary evaporator to nearly 2 ml. The resultant extract was then cleaned up by filtering through a florisil column (20 × 1.5 cm, absorbent cotton, 2 cm anhydrous Na2SO4, 3 g florisil) at 4 ml min−1. The analytes were eluted with 60 ml petroleum ether/ethyl acetate (9:1 v/v), with the eluate being collected into another test tube. Finally, the eluate was concentrated in the rotary evaporator until nearly dry. The dried eluate was redissolved in 1 ml petroleum ether upon analysis by GC-ECD.
Gas chromatography
Chemical analysis was performed using a HP-5890 GC equipped with a 63Ni microcell ECD and a capillary HP-5 column (30 m in length, 0.32 mm i.d., and 0.25 μm film thickness). Nitrogen was used as the carrier gas at 40 ml min−1. With a column head pressure of 50 kPa, the column oven temperature was programmed from 210°C (initial time, 1 min) to 285°C, at a rate of 10°C min−1 and thereupon held for 10 min. The injector and detector temperatures were set at 270 and 320°C, respectively. Samples were injected at 1 μl for analysis. Retention time was utilized to identify the correct peak in the chromatogram (Fig. 2). Concentrations of individual pyrethroids were quantified according to the peak heights of the respective reference standards. All pyrethroid residues were calculated on a soil dry weight basis and reported without correction for recovery loss, as the recoveries (89.7–93.0%) were well within the acceptable range. The detection limits of cypermethrin, fenvalerate, deltamethrin by the above method are each 1.0 pg.
Results
Pyrethroids present in the soil separately versus as a mixture
As shown in Figs. 3–5, the pyrethroid residues decreased gradually with cultivation (incubation) time at approximately the same rate, whether the pyrethroids were present in the soil separately or present together as a mixture. In all cases, as evident from the correlation coefficients r listed in Table 2, the pyrethroid dissipation in the soil tended to follow closely the first-order exponential decay process:
where C 0 is the initial soil concentration (e.g., in mg kg−1), −k is the dissipation rate constant = −ln[2] × (t 1/2)−1, and t is the time (e.g., day) since treatment (with pyrethroid). Note that the above first-order exponential equation may be equivalently expressed as the log-linear regression: ln[C t] = ln[C 0] + (−k)t. And here t 1/2 is simply the dissipation half-life, when 50% of the initial amount of residues is left in the soil.
In addition to the correlation coefficient r, the dissipation statistics generated from the six log-linear regressions (with two for each pyrethroid) are summarized in Table 2. A r value greater than 85% is generally considered to be indicative of a good correlation between two variables (e.g., dissipation over time), provided that the data points (sample size) are not fewer than five.
Sterilized versus unsterilized conditions
Dissipations of the three pyrethroids under sterilized versus unsterilized soil conditions are also characterized in Figs. 3–5, with regression statistics likewise listed in Table 2. The data showed that in all cases, the residues were dissipated at a faster rate when the soil was not sterilized (i.e., not autoclaved). More specifically, the dissipation rate constant −k in each case was about four to five times smaller (slower) for the residues incubated under sterilized conditions than when they were present in unsterilized soil.
Effects of different soil types
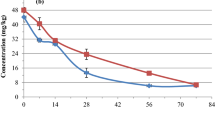
Soil organic matter contents (OMC) and soil cation-exchange capacity (CEC) were determined according to the methods by Bao (2000, b). Regressions of dissipation half-lives on pH, OMC, and CEC were performed for the three pyrethroids, with the resultant correlation coefficients r listed in Table 3. The half-lives appeared to correlate with soil pH and soil OMC, but not with soil CEC. The dissipation half-lives of cypermethrin (under unsterilized conditions) were determined as 14.4, 17.1, and 19.5 days in the S01, S02, and S03 samples, respectively (Table 4). Similar effects were observed for fenvalerate and deltamethrin. Figs. 6–8 provide further graphic details on soil type effects for the three pyrethroids. These data all supported the observation that the characteristics (e.g., the physicochemical properties, as seen in Table 1) of S01 exerted a greater effect on the dissipation of pyrethroids than those of the other two soil types.
Dissipation of cypermethrin in three soil types (with properties and sampling stations as described in Table 1)
Dissipation of fenvalerate in three soil types (with properties and sampling stations as described in Table 1)
Dissipation of deltamethrin in three soil types (with properties and sampling stations as described in Table 1)
Effects of external carbon source
For all three soil types, the three pyrethroids consistently dissipated at a faster rate when glucose was added to the soil, with half-lives being reduced by about 2–7 days (Table 4). The greatest effect was observed in the S01 sample treated with fenvalerate.
Effects of pesticide concentration
Dissipation statistics for the effects of pesticide concentration are summarized in Table 5. The data showed that, for all three pyrethroids, the dissipation rates reduced considerably when the maximum field concentration increased 10-fold to 20 mg kg−1. On the other hand, the rates decreased only slightly when the soil concentration increased 2.5−fold (to 5 mg kg−1). The slowest dissipation rate of 0.02 mg kg−1 day−1 (and hence the longest half-life of 34.4 days) observed in this experiment was from the soil sample treated with fenvalerate at 20 mg kg−1.
Discussion
This study involved trials of limited data points. As such, several inherent statistical issues remain to be resolved and its data must be interpreted with caution. For example, even though the calculated correlation coefficients were as high as 0.97 (Table 3) for the three pyrethroids between their dissipation half-lives in three soil types and the organic matter contents in soil, such correlations were each based on only three data points. Yet with a sample size of three, the coefficient value should be above 0.99 in order for it to be considered statistically significant. It is also important to note that a better fitting procedure for the dissipation seen in Figs. 3–6 may be nonlinear fitting to the untransformed data. Nonetheless, the use of such a more-complex fitting procedure was not deemed necessary at this time, as the study was exploratory in purpose.
Despite such statistical limitations, the data from the present study, as summarized in Table 2 (as well as graphically in Figs. 3–5), tend to suggest that pyrethroid dissipation in soil is not affected by whether the pesticides are present in soil separately or together as a mixture. This notion is further supported by what was observed in the investigation of the effects of pesticide concentration. When the three pyrethroids were added to the soil together as a mixture, the overall soil concentration of pyrethroids increased threefold only, to 6 mg kg−1. Yet as shown in Table 5, the half-life of each pyrethroid in soil was not affected until the pesticide concentration was increased well beyond 2.5-fold.
The study data further reveal that microbial activity played a dominant role in the degradation of pyrethroids in soil, at least up to the first 70 days of cultivation (Figs. 6–8). That is, it is likely that, after the first 70 days of cultivation, microbial activity in a confined soil environment would decline significantly to the point that other factors could play a more-active role in pesticide degradation (Lin et al. 2006).
On the other hand, in the present study, soil pH (Table 3), soil organic matter contents (Table 3), and external carbon source (Table 4) were all seen to affect the half-lives of the three pyrethroids in soil somewhat, although to a lesser extent compared to microbial activity and apparently subject to statistical challenge due to the small number of data points used. Nonetheless, such degradation or dissipation effects are not unexpected, as soil organic matter often provides a good carbon and energy source for soil microbes to grow and function vigorously. The soil carbon content apparently increases considerably when glucose is deliberately added to a soil sample. Some pesticides are also seen to persist longer in acidic than alkaline soils. Meanwhile, the data in Table 5 unsurprisingly showed that the dissipation rate decreased considerably when pesticide concentration increased substantially, such as by 10-fold (to 20 mg kg−1). This latter observation, though also preliminary in nature, supports the general assertion that microbial activity can become saturated if the pesticide concentration reaches a certain level.
All in all, the above findings appear to offer valuable clues for future research directions in reducing pesticide persistence in soil, which in turn could lead to the ultimate reduction of environmental pollution caused by pyrethroid application to farmland in the Yangtze River Delta region. For example, in order to accelerate the degradation or dissipation of pyrethroids in soil, compost and mulch could be added to farmland topsoil to enrich its organic matter content. At the same time, the farmland could be aerated to improve microbial activity, as more sunlight would increase the oxygen content and the temperature in soil (Qin et al. 2000; Demoute 1989). In the Yangtze River Delta region, the soil pH for most farmland ranges from 4.5 to 7. He et al. (2003) reported that the dissipation half-lives of cypermethrin have an inverse correlation with the pH of soil. Lin et al. (2005) also observed that neutral pH condition is helpful in decreasing the residues of cypermethrin in soil. Alkaline fertilizer and gypsum could therefore be added to increase the soil pH in that region. Finally, efforts should also be made to avoid treating farmlands with pyrethroids at application rates higher than required.
Conclusion
The data in this exploratory study showed that there may be substantial variability in the fates of cypermethrin, fenvalerate, and deltamethrin and in their dissipation rates in the Yangtze River Delta soils. Implicated in such variability is the potential for pyrethroids to accumulate considerably within farmland soils following repeated applications over the years. These pesticides could also contaminate other areas of the environment, such as the groundwater and surface-water systems nearby, simply through leaching or runoff. Yet it is fair to say that all of the laboratory incubation trials conducted here were at best exploratory in nature. Accordingly more soil samples from more sampling stations are needed in order to adequately assess the environmental risk from pyrethroid use in this region.
References
Andrew, M., & Colin, P. W. (2001). The effects of a synthetic pyrethroid pesticide on some aspects of reproduction in Atlantic salmon (Salmo salar L.). Aquatic Toxicology, 52, 1–12.
Bao, S. D. (2000). Analysis of soil and agricultural chemistry. In S. D. Bao (Ed.), Analysis of organic matter contents in soil (pp. 30–34). Beijing, China: Chinese Agricultural Press (in Chinese).
Bao, S. D. (2000). Analysis of soil and agricultural chemistry In F. R. Zhang (Ed.), Analysis of cation-exchange capacity in soil (pp. 156–158). Beijing, China: Chinese Agricultural Press (in Chinese).
Chen, Z. M. (1999). Prohibit using fenvalerate in tea as soon as possible. Chinese Tea, 21(6), 6–7 (in Chinese).
Chen, L., Zhang, G. Y., Jin, W., & Hu, F. (2006). Determination of residues of pyrethroid insecticides in soil by capillary gas chromatography. Acta Pedologica Sinica, 43(5), 764–771.
Demoute, J. P. (1989). A brief review of the environmental fate and metabolism of pyrethroids. Pesticide Science, 27(4), 375–385.
Garg, U. K., Pal, A. K., Jha, G. J., & Jadha, S. B. (2004). Haemato-biochemical and immuno-pathophysiological effects of chronic toxicity with synthetic pyrethroid, organophosphate and chlorinated pesticides in broiler chicks. International Immunopharmacology, 4, 1709–1722.
He, L. L., & Li, Y. (2003). Comprehensive control of soil polluted by pesticides in farmland. Journal of Yunnan Agricultural University, 18(4), 430–434 (in Chinese).
He, H., Xu, C. H., Sun, C., Wang, X. R., & Shan, Z. J. (2003). The degradation trends of high effect on cypermethrin in soils. Chinese Environmental Science, 23(5), 490–492 (in Chinese).
Henry, L., & Kishimba, M. A. (2006). Pesticide residues in Nile tilapia (Oreochromis niloticus) and Nile perch (Lates niloticus) from Southern Lake Victoria, Tanzania. Environmental Pollution, 140, 348–354.
Jin, Q. (2001). The absorption and degradation of pesticides in soil and the effects on the soil ecosystem. Journal of Zunyi Normal College, 3(2), 89–90 (in Chinese).
Jones, R. J., & Norris, F. R. (1998). Factors affecting degradation of aldicarb and ethoprop. Journal of Nematology, 30(1), 45–55.
Katsuda, Y. (1999). Development of and future prospects for pyrethroid chemistry. Pesticide Science, 55(8), 775–782.
Köprücü, K., & Aydin, R. (2004). The toxic effects of pyrethroid deltamethrin on the common carp (Cyprinus carpio L.) embryos and larvae. Pesticide Biochemistry and Physiology, 80, 47–53.
Lin, G., & Liu, F. B. (2005). Study on characteristics of biodegradation of cypermethrin by mixed culture microbes. Chemistry & Bioengineering, 22(8), 40–42 (in Chinese).
Lin, G., Wu, C. B., & Yan, H. (2006). Study on characteristics of biodegradation of fenpropathrin by mixed microbes. Journal of Anhui Agricultural Sciences, 34(4), 704–705 (in Chinese).
Ma, G. L., Bo, L. Y., & Liu, Z. S. (2005). Prospect and control measures of pesticide pollution in soil–plant system. Modern Agriculture, 11, 10–13 (in Chinese).
Musumeci, M. R., & Ostiz, S. B. (1994). Binding of cypermethrin residue in Brazilian soils and its release by microbial activity. Revista de Microbiologia, 25(4), 216–219.
Oudou, H. C., Alonso, R. M., & Hansen, H. C. B. (2004). Voltammetric behaviour of the synthetic pyrethroid lambda-cyhalothrin and its determination in soil and well water. Analytica Chimica Acta, 523, 69–74.
Pan, H. J., Wu, S. Q., Hang, Z. B., Shi, C. B., & Li, K. B. (2000). Study the sensitivity of organophosphorous and pyrethroid pesticides on fish. Freshwater Fishery, 30(7), 44–45 (in Chinese).
Pang, S. X., Wei, T. L., Lan, Z. N., & Yang, W. L. (2004). Study the toxicity of three synthetic pyrethroid pesticides on Xiphophorus helleri and Tilapin. Freshwater Fishery, 34(4), 15–16 (in Chinese).
Qin, S., Qiao, X. W., Zhu, J. S., & Wang, J. (2000). Degradation of cypermethrin in soils under laboratory conditions. Chinese Journal of Pesticide Science, 2(3), 68–73 (in Chinese).
Rangaswamy, V., & Venkateswarlu, K. (1992). Degradation of selected insecticides by bacteria isolated from soil. Bulletin of Environmental Contamination and Toxicology, 49, 797–804.
Shao, C. B., & Tan, D. M. (2006). Biochemical degradation of cypermethrin residues in cabbage. Agrochemicals, 45(4), 265–268 (in Chinese).
Wang, L. G., Jiang, X., Yan, D. Y., Forster, S., & Martens, D. (2004). Comparison of two procedures for extraction and clean-up of organophosphorus and pyrethroid pesticides in sediment. Pedosphere, 14(2), 229–234.
Wu, J. l., Zou, Y. H., & Li, L. Q. (2006). Multi-residue analysis of fifteen organochlorine and pyrethroid insecticides in Raidix paeoniae Alba and Rhizoma coptidis. Chinese Journal of Pesticide Science, 8(1), 65–70 (in Chinese).
Wu, M. (2003). Controlled countermeasures against insecticide pollution in soil. Farming and Planting, 6, 49–50 (in Chinese).
Ying, S. H. (1990). Economic effect of synthetic pyrethroid pesticides in prevention and cure of pests. Translated Collection of Pesticides, 12(5), 21 (in Chinese).
Zhu, L. S., Wang, G. Z., & Xu, Y. X. (1999). Effect and toxicity of fenpropathrin, phoxim and their mixture on bees. Agricultural Environment Protection, 18(4), 165–167 (in Chinese).
Acknowledgements
The authors would like to thank Mr. W. Jin at the Analysis Center of Soil and Environment, Institute of Soil Science, Chinese Academy of Sciences, Nanjing, for his generous and helpful assistance in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by Chinese Academy of Sciences (Project No. KSCX2-YW-N-038) and the National Key Basic Research Support Foundation (973), China (No. 2002CB4108010).
Rights and permissions
About this article
Cite this article
Gu, Xz., Zhang, Gy., Chen, L. et al. Persistence and dissipation of synthetic pyrethroid pesticides in red soils from the Yangtze River Delta area. Environ Geochem Health 30, 67–77 (2008). https://doi.org/10.1007/s10653-007-9108-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-007-9108-y