Abstract
Biosorption properties of arsenate [As(V)] onto activated sludge were investigated in batch systems. The adsorption of As(V) onto sludge increased from 23 to 266 μg/g dry weight through the methylation of the activated sludge. This increase resulted from neutralization of carboxylic groups via the methylation process. The pH effect of As(V) uptake was also investigated and As(V) adsorption by methylated sludge decreased significantly at high pH (pH > 11) due to competition between As(V) and OH− ions for binding sites distributed on sludge surfaces. In contrast, low pH favored As(V) adsorption by methylated sludge because of the elevated quantities of positively charged functional groups. The results suggest that methylated activated sludge may provide promising applications for the simultaneous removal and separation of As(V) from aqueous effluents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is a widespread contaminant that occurs in soil and aqueous environments as a result of geochemical processes and anthropogenic activities (Nordstrom 2002). The properties of As compounds have been known since antiquity, and the toxicity of As has been well documented and reviewed (Oremland and Stolz 2003). Recent human exposure to As-contaminated groundwater in Bangladesh and West Bengal, India, has led the United States Environmental Protection Agency to revise the As maximum contaminant level (MCL) to 10 μg/l for drinking water (USEPA 2002).
Physicochemical technologies such as solidification/stabilization (Vandecasteele et al. 2002; Kim et al. 2003), ion exchange (Korngold et al. 2001), membrane filtration (Brandhuber and Amy 1998; Gecol et al. 2004), and soil washing processes (Alam et al. 2001; Ko et al. 2005) have been developed for the removal or stabilization of As at contaminated sites. However, the application of such processes is sometimes restricted due to technical or economical constraints, especially when concentrations of As in the effluents occur in low levels, such as occurs in groundwater (Volesky 1990).
Using adsorption processes, the appropriate MCL level can be achieved and limitations of conventional methods can be overcome (Lin and Wu 2001; Ko et al. 2004). Furthermore, the use of microorganisms as sorbents for adsorption of metal ions offers a potential alternative for the separation and recovery of toxic substances from wastewaters (Kang et al. 2005a, b). The biosorption process includes a fast and reversible reaction through the passive and physicochemical binding of metal ions to bacteria, such as an ion exchange reaction with no requirements for bacterial activity (Kim and Kang 2006). Biosorption of As has been investigated recently, and the results demonstrate that biomass represents an efficient and economic sorbent for the removal of As from aqueous solutions (Loukidou et al. 2003; Maity et al. 2005).
Perhaps the most abundant source of microbial biomass is activated sludge. Activated sludge can play an important role in the research and development of new biosorption materials for metal remediation due to its high capacity and selectivity (Aksu et al. 1991; Utgikar et al. 2000). Sağ et al. (2003) showed that activated sludge is an efficient material for the removal of copper and lead from wastewater. Furthermore, waste-activated sludge obtained from sewage treatment plants is of particular economical interest due to its relative abundance and low cost. Although metal adsorption by activated sludge has been studied, there has been no research concerning the bio-removal of As by activated sludge biomass to date, despite its high potential as a good sorbent.
The objective of this study was to investigate the biosorption characteristics of As in wastewater onto activated sludge. Arsenate, As(V), which can be reduced into more toxic arsenite, As(III), was used in this study because the predominant form of As in aerobic aqueous environments of South Korea is As(V) (Saxena et al. 2004). The effect of the methylation on the As(V) adsorption capacity of activated sludge was also studied. The main aim of this paper was to examine aspects of a possible strategy for the removal of As using activated sludge.
Materials and methods
Preparation of the biomass
Activated sludge was obtained from a sewage treatment plant in Cheonan, Korea. The sludge was used as a biosorbent in all the biosorption of As(V) experiments. Activated sludge was washed thoroughly with 0.01 M NaOH and then washed repeatedly with distilled water. After washing, the sludge was suspended in 1 l methyl alcohol and stirred at room temperature for 24 h. The sludge was separated in a centrifuge at 3,300 rpm for 30 min, freeze-dried, and ground to a fine powder. The resulting dried sludge was designated as the pristine activated sludge for use in this study and subjected to methylation treatment. The dried sludge was stored in desiccators.
Methylation of activated sludge
To achieve maximal esterification of the biomass, methylation was performed according to the method of Fraenkel-Conrat and Olcott (1945). A 10-g sample of dried sludge was suspended in 1 l methyl alcohol containing 0.1 N of HCl. The sludge suspension was shaken in a shaker (180 rpm) at room temperature for periods ranging from 6 to 24, after which it was centrifuged at 6,000 rpm for 20 min. The supernatant was decanted, and the remaining solid washed two times with distilled water and freeze-dried for 24 h. It was ground to a fine powder, and particles less than 125 μm in size were collected. Three types of activated sludge were prepared for the Fourier Transform Infrared (FTIR) analysis and sorption studies: activated sludge (a) without methylation, and with methylation for (b) 6 h and (c) 24 h.
Surface characterization
To identify the nature of surface functional groups on the activated sludge, IR spectra of the three types of activated sludge were obtained using a FTIR spectrophotometer (Jasco; Japan) under ambient conditions. For IR studies, 1.6 mg biomass was encapsulated in 315 mg KBr. The pellet was obtained by pressing the ground material with the aid of a bench press (55 MPa for 5 min). Lyophilized sludge was examined in the wave number range 400–4000 cm−1.
Adsorption experiments
The As adsorption experiments were performed in a batch system at a temperature of 25°C and a mixing rate of 180 rpm. As solutions (Na2HAsO4; reagent grade; Aldrich) were prepared in the range of 0–2 mg/l in a background 0.01 M NaNO3 electrolyte solution. The initial solution pH value was adjusted to pH 5.0 using 0.1 N HNO3 or 0.1 N NaOH to minimize anion competition. To each As solution, pristine or methylated activated sludge was suspended and shaken in a shaker for 6 h. The solutions were then filtrated through a 0.2-μm syringe filter. The filtrate was analyzed for metal concentration by inductively coupled plasma-mass spectrometry (ICP-MS; Agilent; USA).
Adsorption experiments were also conducted to study the effect of pH on As biosorption by activated sludge after 24 h methylation under the same conditions as described above. The pH of the studies ranged from 3 to 9 and was adjusted using NaOH and HNO3. Methylated sludge was added to the solutions with 5 mg/l of As(V) in a background 0.01 M NaNO3 electrolyte solution. The final concentration of As was determined after 6 h, and the adsorbed amounts of As were calculated from the difference between As in solution and the concentration of As added initially in each case.
Results and discussion
Identification of functional groups
FTIR analysis of activated sludge was performed to identify the surface nature of activated sludge. As shown in Fig. 1, the IR spectrum of activated sludge displays a number of peaks indicating the complex nature of the biomass. The broad bands around 3,400 cm−1 are dominated by –OH and –NH stretching. The spectrum of activated sludge exhibited distinct bands around 2,929 cm−1 (C–H stretch), 1,660 cm−1 (amide I, mainly C=O stretch), 1,540 cm−1 (amide II, mainly N–H bend), 1,456 cm−1 (partly C–H deformation), 1,410 cm−1 (partly C=O stretch of COOH), 1,238 cm−1 (P=O, C–O–C stretch and amide III), and in the 1,100–1,000 cm−1 region (P–O and C–OH stretch). The IR spectrum indicates the presence of carboxylic, hydroxyl and amine groups on the surface of activated sludge. Interestingly, the peak position of the observed IR spectrum was similar to that of bacteria (Kang et al. 2005b), algae (Tüzün et al. 2005), and fungi (Kapoor and Viraraghavan 1997). This result suggests that microorganisms are organized as activated sludge, which is composed of varying components of protein, lipid, and polymeric compounds. The functional groups of components are able to react with metal cations or anions in aqueous solution.
Theoretically, biomass can adsorb anions through positively charged groups such as amine groups and can also remove metal cations by negatively charged groups such as the carboxyl, phosphoryl, and sulfate groups of the biomass. However, Lee and Park (2005) showed that As was not effectively adsorbed by Pseudomonas aeruginosa because of the electrostatic repulsion between anionic compounds of aqueous As(V) and the cell surfaces of bacteria. Therefore, a pretreatment process is needed before the biosorption of anions such as As to remove the negative charge of the cell surface. The methylation treatment was performed to investigate the potential of activated sludge in the removal of As from aqueous solution in this study.
Methylation of activated sludge
Figure 2 shows the IR spectra obtained from activated sludge treated with methanol and hydrochloric acid for 6 h (Fig. 2b) and 24 h (Fig. 2c). The IR spectrum of the pristine activated sludge (Fig. 2a) was used for comparison to verify a change of spectrum in response to methylation. The decrease in intensity of the IR band at 2,929 cm−1 and 1,410 cm−1 was attributed to the carboxylic groups of activated sludge. This suggests that the neutralization of negatively charged groups may have occurred in activated sludge as a result of the esterification reaction during methylation. The new peaks at 1,388 cm−1 were assigned to the asymmetric bending of the CH3 groups of the acetyl moiety (Yee et al. 2004). The broad overlapping region in the range 3,200–3,600 cm−1 also presents some changes, but it is difficult to determine the group causing the shift.
The present methylation reaction is specific to only the carboxylic group; other functional groups are unaffected (Kapoor and Viraraghavan 1997). It causes esterification of carboxylic groups of the biomass as follows (Gardea-Torresdey et al. 1990):
where R is the carbon chain of organic matter. The carboxyl group is the main functional group of bacteria representing negative charge (Drake et al. 1996). From this reaction, it can be assumed that neutralization of the negatively charged groups by methylation allows the approach of As anions onto the positively charged functional groups of activated sludge.
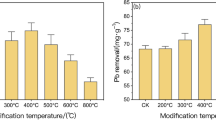
Adsorption of As(V) onto activated sludge
The results of As(V) adsorption experiments are displayed in Fig. 3. An increase of the amount of As(V) adsorbed onto activated sludge was observed after increasing the methylation time of the sludge, whereas As(V) was scarcely adsorbed onto the pristine sludge. These results show that biosorption of As(V) was severely inhibited when carboxyl groups existed on the biomass surface, indicating their importance in the biosorption of anions. The amount of adsorbed As(V) increased significantly when negatively charged functional groups of activated sludge were esterified. This suggests that positively charged adsorption sites take part in the biosorption of As(V) after methylation of the biomass. As ions can easily approach the surface of activated sludge after methylation and, as a consequence, the capacity of As(V) is enhanced.
Effect of pH on As(V) biosorption
The pH value of a solution substantially affects the capacity of the surface of biosorbents to interact with metal ions. Figure 4 shows speciation of As(V) calculated as a function of pH from thermodynamic data using MINEQL+ (Shecher and McAvoy 1998). In the pH range 3–9, which was of interest in this study, MINEQL + results show that the main species are H2AsO4 − and HAsO4 2− in the form of oxyanions in solution. These anions were expected to interact with adsorption sites carrying positive charges.
The results of sorption experiments obtained after contacting the methylated sludge with As(V) solution under various levels of pH are shown in Fig. 5. The maximum uptake of As(V) reached 180 μg/g at pH 4. However, As(V) uptake decreased when the pH level was greater than pH 4. The difference in As(V) binding capacity at different pH can be attributed to the nature of the chemical interactions of As with the activated sludge in relation to the surface charge of the biomass. As the pH value decreased, the overall surface charge on the biomass became less negative or even positive, which one would expect to increase the interaction of As with the biomass. Moreover, pretreatment of activated sludge with methylation made more positive surface charges, and hence the adsorption capacity of As(V) was improved. When the pH level was above 4, hydroxyl ions started to compete strongly with As ions for the positively charged sites, and the As(V) uptake was therefore decreased.
Summary
The FTIR results of this study revealed that dried activated sludge has various functional groups that react with As ions in aqueous solution. Additionally, esterification of carboxylic groups was demonstrated by comparing the IR spectra of pristine and methylated sludge. The adsorbed amount of As(V) increased markedly with increasing methylation time, while little As(V) was adsorbed by unmethylated sludge. This result can be explained by the surface of activated sludge being modified to produce positively charged functional groups, thus resulting in increased adsorption of negatively charged As ions with an increasing degree of methylation. Furthermore, methylated activated sludge adsorbed anionic As(V) under acidic conditions. We demonstrated the considerable potential of methylated activated sludge in the removal of As. This might be used as an effective biosorbent for the treatment of wastewater containing As ions, such as that associated with acid mine drainage.
References
Aksu, Z., Kutsal, T., Gün, S., Haciosmanoglu, N., & Gholaminejad, M. (1991). Investigation of biosorption of Cu(II), Ni(II) and Cr(VI) ions to activated sludge bacteria. Environmental Technology, 12, 915–921.
Alam, M. G. M., Tokunaga, S., & Maekawa, T. (2001). Extraction of arsenic in a synthetic arsenic-contaminated soil using phosphate. Chemosphere, 43, 1035–1041.
Brandhuber, P., & Amy, G. (1998). Alternative methods for membrane filtration of arsenic from drinking water. Desalination, 117, 1–10.
Drake, L. R., Lin, S., Rayson, G. D., & Jackson, P. J. (1996). Chemical modification and metal binding studies of Datura innoxia. Environmental Science & Technology, 30, 110–114.
Fraenkel-Conrat, H., & Olcott, H. S. (1945). Esterification of proteins with alcohols of low molecular weight. The Journal of Biological Chemistry, 161, 259–268.
Gardea-Torresdey, J., Becker-Hapak, M. K., Hosea, J. M., & Darnall, D. W. (1990). Effect of chemical modification of algal carboxyl groups on metal ion binding. Environmental Science & Technology, 24, 1372–1378.
Gecol, H., Ergican, E., & Fuchs, A. (2004). Molecular level separation of arsenic (V) from water using cationic surfactant micelles and ultrafiltration membrane. Journal of Membrane Science, 241, 105–119.
Kapoor, A., & Viraraghavan, T. (1997). Heavy metal biosorption sites in Aspergillus niger. Bioresource Technology, 61, 221–227.
Kang, S. Y., Lee, J. U., & Kim, K. W. (2005a). Metal removal from wastewater by bacterial biosorption: Kinetics and competition studies. Environmental Technology, 26, 615–624.
Kang, S. Y., Bremer, P. J., Kim, K. W., & McQuillan, A. J. (2005b). Monitoring metal ion binding in single layer Pseudomonas aeruginosa biofilms using ATR-IR spectroscopy. Langmuir, 22, 286–291.
Kim, J. Y., Davis, A., & Kim, K. W. (2003). Stabilization of available arsenic in highly contaminated mine tailings using iron. Environmental Science & Technology, 37, 189–195.
Kim, K.W., & Kang, S.Y. (2006). Bacterial biosorption of trace elements, In: Trace Elements in the Environment: Biogeochemistry, Biotechnology, and Bioremediation (pp. 325–340). Boca Raton, FL: CRC Press.
Ko, I. W., Kim, J. Y., & Kim, K. W. (2004). Arsenic speciation and sorption kinetics in the As-hematite-humic acid system. Colloid Surface A, 234, 43–50.
Ko, I. W., Chang, Y. Y., Lee, C. H., & Kim, K. W. (2005). Assessment of pilot-scale acid washing of soil contaminated with As, Zn and Ni using the BCR three-step sequential extraction. Journal of Hazardous Materials, 127, 1–13.
Korngold, E., Belayev, N., & Aronov, L. (2001). Removal of arsenic from drinking water by anion exchangers. Desalination, 141, 81–84.
Lee, J. -U., & Park, H. S. (2005). Arsenic adsorption onto Pseudomonas aeruginosa cell surface. Economical Environmental Geology, 38, 525–534.
Lin, T. F., & Wu, J. K. (2001). Adsorption of arsenite and arsenate within activated alumina grains: Equilibrium and kinetics. Water Resource, 35, 2049–2057.
Loukidou, M. X., Matis, K. A., Zouboulis, A. I., & Liakopoulou-Kyriakidou, M. (2003). Removal of As(V) from wastewaters by chemically modified fungal biomass. Water Resource, 37, 4544–4552.
Maity, S., Chakravarty, S., Bhattacharjee, S., & Roy, B. C. (2005). A study on arsenic adsorption on polymetallic sea nodule in aqueous medium. Water Resource, 39, 2579–2590.
Nordstrom, D. K. (2002). Worldwide occurrences of arsenic in ground water. Science, 296, 2143–2144.
Oremland, R. S., & Stolz, J. F. (2003). The ecology of arsenic. Science, 300, 939–944.
Sağ, Y., Tatar, B., & Kutsal, T. (2003). Biosorption of Pb(II) and Cu(II) by activated sludge in batch and continuous-flow stirred reactors. Bioresource Technology, 87, 27–33.
Saxena, V. K., Kumar, S., & Singh, V. S. (2004). Occurrence, behavior and speciation of arsenic in groundwater. Current Science, 86, 281–284.
Schecher, W. D., & McAvoy, D. C. (1998). MINEQL+: A Chemical Equilibrium Modeling System. Environmental Research Software. Maine: Hallowell.
Tüzün, İ., Bayramoğlu, G., Yalçin, E., Başaran, G., Çelik, G., & Arica, M. Y. (2005). Equilibrium and kinetic studies on biosorption of Hg(II), Cd(II) and Pb(II) ions onto microalgae Chlamydomonas reinhardtii. Journal of Environmental Management, 77, 85–92.
USEPA. (2002). National Primary Drinking Water Regulations. United States Environmental Protection Agency No. 815-z−02–001.
Utgikar, V., Chen, B. Y., Tabak, H. H., Bishop, D. F., & Govind, R. (2000). Treatment of acid mine drainage: I. Equilibrium biosorption of zinc and copper on non-viable activated sludge. International Biodeterioration & Biodegradation, 46, 19–28.
Vandecasteele, C., Dutré, V., Geysen, D., & Wauters, G. (2002). Solidification/stabilization of arsenic bearing fly ash from the metallurgical industry. Immobilisation mechanism of arsenic. Waste Management, 22, 143–146.
Volesky B. (1990). Removal and recovery of heavy metals by biosorption. In B. Volesky (Ed.), Biosorption of Heavy Metals (pp. 7–43) Boca Raton, FL: CRC Press.
Yee, N., Benning, L. G., Phoenix, V. R., & Ferris, F. G. (2004). Characterization of metal-cyanobacteria sorption reactions: A combined macroscopic and infrared spectroscopic investigation. Environmental Science & Technology, 38, 775–782.
Acknowledgements
This study was supported by the Gwangju Institute of Science and Technology (GIST) Research Fund and National Research Laboratory Project (Arsenic Geoenvironment Laboratory) to K.-W. Kim.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, SY., Kim, DW. & Kim, KW. Enhancement of As(V) adsorption onto activated sludge by methylation treatment. Environ Geochem Health 29, 313–318 (2007). https://doi.org/10.1007/s10653-007-9096-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-007-9096-y