Abstract
Cadmium (Cd) reduces plant growth by interfering with important plant metabolic processes at the physiological, biochemical, and molecular levels. Here, the effects of foliar application of zinc oxide nanoparticles (ZnO-NPs) on growth, antioxidant enzymes, glyoxalase system, and macro- and micro-elements levels of purslane (portulaca oleracea L.) under Cd toxicity were investigated. The results revealed that Cd toxicity increased the levels of hydrogen peroxide (H2O2), methylglyoxal (MG) and malondialdehyde (MDA), resulting in oxidative stress and the induction of electrolyte leakage (EL). Cd stress enhanced the leaf concentration of Cd and declined the leaf concentrations of macro- and micro-elements, resulting in a decrease in the content of photosynthetic pigments and plant growth. However, the foliar application of ZnO-NPs improved the activity of antioxidant enzymes and the glyoxalase system and, consequently, reduced the levels of H2O2, MG, MDA, and EL in Cd-stressed plants. ZnO-NPs decreased the leaf concentration of Cd and restored the leaf concentrations of macro- and micro-elements, thereby improving photosynthetic pigments and the growth of Cd-stressed purslane plants. In general, the results revealed that the foliar application of ZnO-NPs improved the growth of purslane plants under Cd phytotoxicity by maintaining nutrient homeostasis, improving the defense mechanisms (antioxidant enzymes and glyoxalase cycle), and increasing the accumulation of proline and glutathione. Therefore, the results of the present study strongly recommend that ZnO-NPs could be used effectively in the cultivation of plants in areas contaminated with toxic Cd metal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, serious concerns have been raised about the negative effects of environmental pollutants, including heavy metals, on plant quality and yield (Ghorbani et al. 2022). Cadmium (Cd) is known as one of the most important environmental pollutants that is released into ecosystems through metal working industries, power stations, heating systems, urban traffic, waste incinerators, cement factories, and phosphorus fertilizers (di Toppi and Gabbrielli 1999; Nolan et al. 2003). Cd is easily absorbed by the plant and accumulates in different parts of the plant, which not only has adverse impacts on plant growth and development but can also have toxic effects on the organisms that consume them (Hasan et al. 2009; Liu et al. 2007). Cd, as an anti-metabolite, inhibits the activity of enzymes and disrupts the function of biochemical and physiological processes in plants (Stroin´ski 1999). It has been shown that cadmium stress induces oxidative stress by increasing the generation of active oxygen species and thus diminishes plant growth and yield (Rizwan et al. 2016; Ali et al. 2015).
Nanotechnology as an emerging discipline is known as an ideal solution to overcome the adverse effects of heavy metals in agriculture. Recently, the application of zinc oxide nanoparticles (ZnO-NPs) has been shown to improve plant nutrition, which has been considered to increase plant growth and yield (Rizwan et al. 2019; Faizan et al. 2021). Zinc is one of the most important essential micronutrients that is involved in the activity of important enzymes such as superoxide dismutase, transphosphorylases, isomerases, aldolases, tryptophan synthetase, dehydrogenases, and RNA and DNA polymerases (Sturikova et al. 2018; Broadley et al. 2007). ZnO-NPs are widely used in biomedical and catalysis applications as well as in agriculture as part of sunscreens, solar cells, ceramics, and wall paints (Santhoshkumar et al. 2017; Suliman et al. 2007). Rizwan et al. (2019) and Singh et al. (2019) revealed that the application of ZnO-NPs has positive impacts on the physiological, nutritional, and quantitative parameters of maize and wheat plants. The application of ZnO-NPs has also been shown to effectively improve plant tolerance to abiotic stresses, especially heavy metal toxicity. Faizan et al. (2021) indicated that the foliar application of ZnO-NPs improved the tolerance of tomato plants to Cd toxicity. Ahmad et al. (2020) revealed that ZnO-NPs modulated the activity of antioxidant enzymes, the glyoxalase system, and the ascorbate-glutathione cycle and reduced arsenic (As) accumulation in the roots and leaves of soybeans, thereby improving plant growth under As stress. The decreased Cd accumulation induced by the application of ZnO-NPs has also been reported in Triticum aestivum (Hussain et al. 2018) and Gossypium hirsutum (Venkatachalam et al. 2017).
Although several studies have shown that the application of ZnO-NPs can reduce the accumulation of heavy metals, especially Cd, in plants, more research is needed to better understand the role of ZnO-NPs in improving plant tolerance under Cd toxicity. Therefore, in the present study, the effect of foliar application of ZnO-NPs on Cd uptake as well as photosynthetic apparatus, antioxidant defense system, and ionic homeostasis in purslane (portulaca oleracea L.) as a model medicinal plant under Cd phytotoxicity was investigated.
Material and methods
Materials and treatments
Purslane seeds germinated after sterilization with mercuric chloride (0.01%) and washing with double distilled water (DDW) (Ghorbani et al. 2019b). Then, 10-day-old purslane seedlings were transferred to pots containing half-strength Hoagland solution (pH 6.0). Every 5 days, the pots were refilled with fresh Hoagland solution. The pots were retained in a growth chamber at 25 °C, 16 h light (150 μmol m−2 s−1) and 65–70% relative humidity. Cd treatments and foliar sprays of ZnO-NPs were applied to 20-day-old purslane seedlings (after 10 days of adaptation). Cd treatments were prepared at concentrations of 50 and 100 μM by adding CdCl2 to 1/2-strength Hoagland solution. ZnO-NPs were purchased from USA-Nano with properties of 20–30 nm size, 99% purity, and 5.61 g/cm3 density and were prepared in concentrations of 50 and 100 mg/L. Plant foliage was sprayed twice (before treatments and 7 days after the start of treatments) with ZnO-NPs. The control plants were sprayed with DDW. 34-day-old seedlings (2 weeks after the start of treatments) were sampled and stored at −80 °C after recording the plant height. To determine the total dry weight, the samples were incubated for 24 h at 70 °C and then weighed (Ghorbani et al. 2009).
Photosynthetic pigments
The contents of chlorophyll a, b, and carotenoids were determined following the procedure of Arnon (1949). After homogenizing the fresh leaves in 80% ice-cold acetone and centrifuging at 5000 rpm for 10 min, the supernatants were read spectrophotometrically at 470, 645, and 663 wavelengths and calculated as mg/g FW.
Proline, glutathione (GSH) and electrolyte leakage (EL)
To determine the leaf content of free proline, leaf tissues were extracted with sulphosalicylic acid (3%, v/v). After centrifugation, the supernatants were mixed with a ninhydrin solution. The mixtures were incubated in a hot water bath (90 °C) for 30 min and then transferred to an ice bath. Toluene was added to the samples and vortexed for 15 s. After incubating the mixtures in the dark (room temperature) for 30 min, the absorbance of the upper phase was read at 520 nm and the proline content was calculated following the procedure of Bates et al. (1973). Extraction of total GSH was performed using meta-phosphoric acid (6%, pH 2.8) containing EDTA (1 M), and after centrifugation, polyvinylpolypyrrolidone was added to the supernatants and centrifuged at 10,000 rpm for 15 min. The supernatants were mixed with potassium phosphate buffer (0.1 M, pH 7.0), EDTA (5 mM), GR (20 IU mL–1), 5,5′-dithiobis(2-nitrobenzoic acid) (2.4 mg mL–1) and NADPH (1.9 mg mL–1). By recording the absorbance change at 412 nm, the leaf content of GSH was achieved according to Yu et al. (2003). To determine EL, the leaf pieces were immersed in DDW for 24 h. After recording the electrolyte conductivity (C1), they were boiled for 30 min. After recording the electrolyte conductivity (C2) of the cooled solution, EL was obtained according to Huo et al. (2016) and the following equation: EL = C1/C2 × 100.
Hydrogen peroxide (H2O2), lipid peroxidation and methylglyoxal (MG)
After extracting the leaves in 0.1% trichloroacetic acid and centrifuging at 10,000 rpm for 20 min, the supernatants were mixed with 1 M potassium iodide and 10 mM potassium phosphate buffer (pH 6.8). After reading the reaction mixture at 390 nm, H2O2 content was calculated according to Sinha et al. (2005). The method of Hodges et al. (1999) was utilised to quantify MDA content. Fresh leaf tissue was extracted with trichloroacetic acid (10%) and centrifuged at 10,000 rpm for 15 min. The supernatants were mixed with thiobarbituric acid (0.6%) in trichloroacetic acid (10%) and incubated in a hot water bath for 30 min. After placing the samples in an ice path, the mixtures were centrifuged and the absorbance of the supernatants was read at 532 and 600 wavelengths. To measure MG, fresh leaves were extracted with perchloric acid (5%) and centrifuged at 10,000 rpm for 20 min at 4 °C. Then, the supernatants were decolorized and neutralized using charcoal and saturated potassium carbonate, respectively. By reading the neutralized solution at 288 nm, MG content was determined according to the method previously described by Wild et al. (2012).
Extraction and assay of enzymes
To prepare the enzymatic extract, fresh purslane leaves were homogenized with sodium phosphate buffer (50 mM, pH 7.0), polyvinylpyrrolidone (1%) and EDTA (0.2 mM) and centrifuged at 10,000 rpm for 15 min at 4 °C. Supernatants were stored at 4 °C to measure enzyme activity.
By monitoring the increase in the absorbance of the reaction mixture, including enzyme extract, potassium phosphate buffer (pH 6.8, 10 mM), riboflavin (20 µM), NaCa3 (1.5 M), distilled water, nitro blue tetrazolium (750 µM), EDTA (3 mM) and methionine (0.2 M) at 240 nm, the activity of the superoxide dismutase (SOD) enzyme was calculated as per Beauchamp and Fridovich (1971). Catalase (CAT) activity was obtained by measuring the reduction in absorbance of the reaction solution, which consisted of potassium phosphate buffer (100 mM, pH 7.0), enzyme extract, and H2O2 (75 mM) at 240 nm as per the method proposed by Aebi (1984). By recording the change in absorbance of the mixture including potassium phosphate buffer (pH 6.8, 10 mM), enzyme extract, H2O2 (10 mM), EDTA (0.4 mM), and ascorbate (1 mM) at 290 nm, the activity of ascorbate peroxidase (APX) was calculated according to the method previously described by Nakano and Asada (1981). The activity of glutathione reductase (GR) was determined based on the Hasanuzzaman et al. (2011) method and recording the change in the absorbance of the reaction solution (enzyme solution, potassium phosphate buffer (pH 7.0, 0.1 mM), NADPH (0.2 mM), GSSG (1 mM) and EDTA (1 mM) at 340 nm.
By monitoring the decline in the absorbance of the assay mixture containing potassium phosphate buffer (pH 7.0, 100 mM), enzyme extract, MG (3.5 mM), GSH (1.7 mM) and magnesium sulfate (15 mM) at 240 nm, glyoxalase (Gly) 1 activity was measured by the method of Hasanuzzaman et al. (2011). The activity of Gly II enzyme was measured by reading the absorbance of the assay solution (Tris-HCl buffer (pH 7.2, 100 mM), enzyme solution, S-D-lactoylglutathione (1 mM) and DTNB (0.2 mM)) at 412 nm as previously published by Principato et al. (1987).
Mineral nutrients
The leaf concentrations of calcium (Ca), potassium (K), magnesium (Mg), iron (Fe), manganese (Mn), copper (Cu), zinc (Zn) and cadmium (Cd) were achieved by ICP-MS (Agilent 7500 cx) and after acidic digestion of dried leaves in a mixture of HNO3–H2O (5:1). The leaf contents of nitrogen (N) and phosphorus (P) were determined according to the micro-Kjeldahl (Jackson 1967) and the molybdovanado phosphate (Kitson and Mellon 1944) methods, respectively.
Statistical analysis
The SAS software (V. 9.1.3) was employed for data analysis and the mean comparison was performed based on the least significant difference (LSD) test (p < 0.05) (Ghorbani et al. 2011). The values are mean ± SD and n = 5.
Results
Effects of Cd and ZnO-NPs on growth and photosynthetic pigments
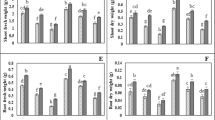
Cd treatments statistically reduced the morphological traits of purslane plants compared to controls. At 50 and 100 μM Cd the height (9.8 and 26.1%) and the total dry weight (8.7 and 23.3%) declined compared to non-treated plants. However, at both Cd levels, foliar application of ZnO-NPs improved the height and total dry weight, and the maximum improvement was observed in plants treated with 100 mg/L ZnO-NPs (Fig. 1A, B). Chlorophyll a and b content decreased under Cd toxicity. The highest decrease in chlorophyll a (45.8%) and b (60.1%) content was observed in plants treated with 100 μM Cd. However, the application of ZnO-NPs restored the contents of chlorophyll a and b in Cd-subjected plants. The application of 50 and 100 mg/L ZnO-NPs enhanced chlorophyll a by 12 and 14.9% in 50 μM Cd-stressed plants and 35.2 and 43.8% in 100 μM Cd-stressed plants, respectively compared to Cd treatment alone (Fig. 1C, D). The carotenoids content was found to be decreased to 21.6 and 55.2% for 50 and 100 μM Cd treatments, respectively, that the controls. The exogenous application of 50 and 100 mg/L ZnO-NPs increased carotenoids by 16.7 and 17.2% in 50 μM Cd-stressed plants and 43.6 and 55.8% in 100 μM Cd-stressed plants, respectively, compared to Cd treatment alone (Fig. 1E).
Effects of zinc oxide nanoparticles (ZnO-NPs, 0, 50 and 100 mg/L) on height (A), total dry weight (B), chlorophyll (Chl) a (C), Chl b (D) and carotenoids (Car, E) in purslane plants subjected to cadmium (Cd, 0, 50 and 100 µM). Values (means ± SD, n = 5) followed by the same letter are not significantly different (P < 0.05; LSD test)
Effects of Cd and ZnO-NPs on proline and GSH contents
Cd treatment at concentrations of 50 and 100 μM enhanced leaf proline content (2.4- and 3.9-fold) compared with controls. However, the application of ZnO-NPs significantly increased the proline content in the leaves of Cd-stressed plants, and the highest increase was recorded under the treatment of 100 mg/L ZnO-NPs (Fig. 2A). The GSH content of the leaves increased by 19% under 50 μM Cd treatment, while it decreased by 20.3% under 100 μM Cd treatment. However, foliar application of 50 and 100 mg/L ZnO-NPs increased GSH content by 29.5 and 35.6% in 50 μM Cd-stressed plants and 61.7 and 66.5% in 100 μM Cd-stressed plants compared to Cd treatments alone (Fig. 2B).
Effects of zinc oxide nanoparticles (ZnO-NPs, 0, 50 and 100 mg/L) on proline (A), glutathione (B), hydrogen peroxide (H2O2, C), methylglyoxal (MG, D) and malondialdehyde (MDA, E) contents and electrolyte leakage (EL, F) in purslane plants subjected to cadmium (Cd, 0, 50 and 100 µM). Values (means ± SD, n = 5) followed by the same letter are not significantly different (P < 0.05; LSD test)
Effects of Cd and ZnO-NPs on levels of H2O2, MG, MDA and EL
The data revealed that H2O2 content in purslane leaves showed a significant increase of 28 and 102.4% under 50 and 100 μM Cd, respectively, over the controls. In Cd-stressed plants, the foliar application of ZnO-NPs decreased the leaf H2O2 content (Fig. 2C). Treatments of 50 and 100 μM Cd raised leaf MG content by 2.2- and 29-fold, respectively, when compared with controls. Leaf spray of 50 and 100 mgL ZnO-NPs diminished leaf MG content in 50 μM Cd-stressed plants by 24.8 and 31.2%, and in 100 μM Cd-stressed plants by 33.9 and 44.8%, respectively, compared to Cd treatment alone (Fig. 2D). Treatment with 50 and 100 μM Cd led to a meaningful increase in leaf MDA content compared to the controls (49.1 and 152.3%). However, at both Cd levels, foliar application of ZnO-NPs significantly lessened leaf MDA content (Fig. 2E). Compared to untreated plants, Cd stress significantly enhanced leaf EL and the maximum EL was observed in plants treated with 100 μM Cd (94.7%). However, foliar spray with ZnO-NPs decreased leaf EL in Cd-treated plants (Fig. 2F).
Effects of Cd and ZnO-NPs on the activity of antioxidant enzymes and glyoxalase system
The results revealed that Cd treatments upregulated the activity of SOD and CAT enzymes in purslane leaves over the controls, and the highest activity was recorded in plants treated with 100 μM Cd. However, ZnO-NPs treatments caused a greater increase in SOD and CAT activity in Cd-exposed plants than Cd treatments alone (Fig. 3A, B). APX activity was found to be increased by 2.2- and 2.3-fold under 50 and 100 μM Cd toxicity, respectively, when compared to controls. In 50 and 100 μM Cd-stressed plants, foliar application of ZnO-NPs further raised APX activity, although no significant difference was found between the two concentrations of ZnO-NPs (Fig. 3C). GR activity was enhanced by 77.1% under 50 μM Cd and by 105.9% under 100 μM Cd when compared to controls. GR activity was further increased in Cd-treated plants when they were sprayed with ZnO-NPs (Fig. 3D).
Effects of zinc oxide nanoparticles (ZnO-NPs, 0, 50 and 100 mg/L) on the activity of superoxide dismutase (SOD, A), catalase (CAT, B), ascorbate peroxidase (APX, C) and glutathione reductase (GR, C) in purslane plants subjected to cadmium (Cd, 0, 50 and 100 µM). Values (means ± SD, n = 5) followed by the same letter are not significantly different (P < 0.05; LSD test)
The activity of Gly I and Gly II enzymes was found to be raised by 26.2 and 64.3% in 50 μM Cd-exposed plants and by 41.8 and 51.5% in 100 μM Cd-exposed plants, respectively when compared to the controls. At both levels of Cd, foliar application of ZnO-NPs further upregulated the activity of Gly I and Gly II, although no significant difference was found between the two nanoparticle ZnO-NPs (Except for Gly I activity in 100 μM Cd-stressed plants, which had the highest activity under the application of 100 mg/L ZnO-NPs) (Fig. 4A, B).
Effects of zinc oxide nanoparticles (ZnO-NPs, 0, 50 and 100 mg/L) on the activity of glyoxalase 1 (Gly I, A) and glyoxalase II (Gly II, B) in purslane plants subjected to cadmium (Cd, 0, 50 and 100 µM). Values (means ± SD, n = 5) followed by the same letter are not significantly different (P < 0.05; LSD test)
Effects of Cd and ZnO-NPs leaf macro- and micro-nutrient concentrations
Treatments with 50 and 100 μM Cd lessened the leaf N concentration by 13 and 30.3% and the leaf Ca concentration by 5.2 and 31.9%, respectively, over the control samples. In plants stressed with 50 μM Cd, the foliar application of ZnO-NPs was found to have no significant effect on the leaf concentrations of N and Ca. However, ZnO-NPs significantly enhanced the leaf concentrations of N and Ca in plants exposed to 100 μM Cd, and the highest concentrations of N and Ca were obtained under 100 mg/L ZnO-NPs (Fig. 5A, B). Leaf P concentration diminished by 42.8% under 100 μM Cd treatment compared to the controls. However, the application of 100 mg/L ZnO-NPs caused a significant rise in the leaf P concentration of 100 μM Cd-stressed plants compared to 100 μM Cd treatment alone (Fig. 5C). Leaf K concentration lowered with increasing Cd concentration by 12.8 and 21.8% under 50 and 100 μM Cd treatments, respectively, compared to the controls. However, treatments with ZnO-NPs restored the leaf K concentration in Cd-stressed plants, although no significant difference was found between the two levels of ZnO-NPs (Fig. 5D). Treatments with 50 and 100 μM Cd were found to diminish the leaf concentration of Mg by 17.7 and 35.3%, respectively, over the controls. In plants treated with 50 μM Cd, 50 mg/L ZnO-NPs application did not show a significant effect on the leaf concentration of Mg. However, in 100 μM Cd-plants, treatments with 50 and 100 mg/L ZnO-NPs increased the leaf concentration of Mg by 25.6 and 32.6%, respectively, compared to plants treated with 100 μM Cd alone (Fig. 5E).
Effects of zinc oxide nanoparticles (ZnO-NPs, 0, 50 and 100 mg/L) on the leaf concentrations of nitrogen (N, A), calcium (Ca, B), phosphorus (P, C), potassium (K, D), magnesium (Mg, E) and cadmium (Cd, F) in purslane plants subjected to cadmium (Cd, 0, 50 and 100 µM). Values (means ± SD, n = 5) followed by the same letter are not significantly different (P < 0.05; LSD test)
Cd treatments enhanced the leaf concentration of Cd over the controls and the highest Cd concentration was obtained in plants treated with 100 μM Cd. However, in plants treated with 50 and 100 μM Cd, foliar application of ZnO-NPs diminished Cd accumulation and the lowest Cd concentration was observed in plants treated with 100 mg/L ZnO-NPs (Fig. 5F).
Treatments with 50 and 100 μM Cd significantly decreased the leaf concentrations of Fe and Mn when compared to the control samples, and the lowest concentrations of Fe and Mn were recorded in plants treated with 100 μM Cd. However, foliar application of ZnO-NPs was found to restore leaf concentrations of Fe and Mn in 50 and 100 μM Cd-stressed plants, although no significant difference was found between the two ZnO-NPs levels (Fig. 6A, B). The results also revealed that 50 μM Cd did not have a significant effect on the leaf concentration of Cu compared to the controls. However, 100 μM Cd stress significantly decreased the leaf concentration of Cu by 41.6% over the controls. In control plants and 50 μM Cd-treated plants, foliar application of ZnO-NPs did not induce a significant effect on the leaf concentration of Cu. However, in 100 μM Cd-treated plants, 50 and 100 mg/L ZnO-NPs applications enhanced Cu concentration by 28.9 and 50.1%, respectively, compared to plants treated with 100 μM Cd alone (Fig. 6C). Cd toxicity significantly lessened leaf Zn concentration over the controls, and the lowest Zn concentration was observed in plants treated with 100 μM Cd. However, the application of ZnO-NPs enhanced Zn concentration in non-Cd-treated plants and plants treated with 50 and 100 μM Cd, and the maximum zinc concentration was obtained under 100 mg/L ZnO-NPs treatment (Fig. 6D).
Effects of zinc oxide nanoparticles (ZnO-NPs, 0, 50 and 100 mg/L) on the leaf concentrations of iron (Fe, A), manganese (Mn, B), copper (Cu, C) and zinc (Zn, D) in purslane plants subjected to cadmium (Cd, 0, 50 and 100 µM). Values (means ± SD, n = 5) followed by the same letter are not significantly different (P < 0.05; LSD test)
Discussion
Although the effective role of NPs in cleaning up environmental pollution is well established, the exact role of NPs in improving the growth and yield of plants under heavy metal phytotoxicity is not fully understood (Tripathi et al. 2015). The main purpose of this study was to investigate the hypothesis that foliar application of ZnO-NPs could be employed as an eco-friendly and effective amendment to reduce the toxic effects of Cd on purslane plants with respect to mineral nutrient homeostasis and antioxidant defense systems. Improving the growth of Triticum aestivum (Rizwan et al. 2019) and Leucaena leucocephala (Venkatachalam et al. 2017) by the application of ZnO-NPs under Cd toxicity has already been reported. Sharifan et al. (2020) indicated that the application of 100 mg/L ZnO-NPs for two weeks improved the growth of Spinaciae oleracea under Cd toxicity by increasing the leaf concentration of Fe, Cu and Zn and diminishing the uptake of Cd. Hussain et al. (2018) showed that the leaf foliar of ZnO-NPs (50, 75, and 100 mg/L) by upregulating the activity of antioxidant enzymes and reducing oxidative stress, increased photosynthetic pigments and, as a result, improved the growth of wheat under Cd toxicity. Improving the tolerance of Oryza sativa (Wu et al. 2020) and Gossypium hirsutum (Priyanka et al. 2021) plants by ZnO-NPs under the toxicity of As and lead has also been reported, which indicates the positive role of ZnO-NPs in enhancing the tolerance of plants under the toxicity of heavy metals. Therefore, the improvement in the growth and biomass of purslane by foliar application of ZnO-NPs under Cd toxicity may be due to increased concentrations of nutrients such as Zn and reduced leaf Cd accumulation as well as an improved antioxidant defense system (Venkatachalam et al. 2017; Rizwan et al. 2019). However, most studies on the exogenous application of ZnO-NPs on plant resistance to Cd toxicity have been conducted under greenhouse or controlled conditions, and field studies are required for more realistic and practical results.
Estimation of photosynthetic pigment content can indicate the level of oxidative stress induced by heavy metal toxicity (Ghorbani et al. 2018b). The results showed that Cd stress reduced the contents of chlorophyll a, b, and carotenoids, which is consistent with the results reported by Gerami et al. (2018) in Salvia officinalis and Wang et al. (2014) in Oryza sativa. Cd stress has been reported to induce oxidative stress and damage the structure of chloroplasts and thylakoid membranes, thereby reducing photosynthetic pigments (Fagioni et al. 2009; Wang et al. 2014). However, the foliar application of ZnO-NPs restored photosynthetic pigments in Cd-exposed plants, which according to the results previously published by Hussain et al. (2018) and Venkatachalam et al. (2017) in wheat and L. leucocephala, respectively. Sharifan et al. (2020) reported that ZnO-NPs improve chlorophyll pigments and protect the photosynthetic apparatus from Cd toxicity by reducing the translocation of Cd to photosynthetic organs. It has been reported that increasing the content of photosynthetic pigments induced by metal NPs may be due to their ability to induce photosynthetic pigment biosynthesis, improve chemical energy production and quantum yield of the photosynthetic system (Juhel et al. 2011; Rico et al. 2015).
Proline and GSH have been shown to play an important role in reducing the damage induced by stressful conditions (Ghorbani et al. 2018a; Ghasemi-Omran et al. 2021). Therefore, increasing the accumulation of these compounds can play an important role in improving plant tolerance under Cd toxicity (Ramezani et al. 2021). However, the leaf concentration of GSH decreased under 100 μM Cd compared to non-Cd and 50 μM Cd-treated plants, which could be due to the toxic effects of Cd on GSH-synthesizing enzymes or reduced sulfur availability for GSH synthesis (Lu et al. 2019). The results revealed that the foliar application of ZnO-NPs enhanced the levels of proline and GSH in Cd-stressed plants, which is consistent with the results previously reported by Faizan et al. (2021) and Ahmad et al. (2020) under the toxicity of As and Cd, respectively. Ghorbani et al. (2021) indicated that GHS reduces the toxicity of heavy metals by chelating the toxic metal. Kaya et al. (2020) indicated that increasing proline and GSH accumulation can maintain enzyme function and redox homeostasis, improve the scavenging of toxic radicals, and protect biochemical processes. Ahmad et al. (2020) reported that the exogenous application of 50 and 100 mg/L ZnO-NPs increases the accumulation of proline under the toxicity of heavy metals by inducing the pathway of proline synthesis. Therefore, the application of ZnO-NPs increased the leaf accumulation of proline and GSH, which can play an important role in improving the tolerance of purslane under Cd toxicity.
It has been well established that the phytotoxicity of Cd boosts the accumulation of reactive oxygen species (ROS) and induces oxidative stress, resulting in damage to bio-membranes and increased EL (Mittler 2002; Ghorbani et al. 2020). The results showed that Cd stress increased H2O2, MG, MDA, and EL in the leaves, which indicates the induction of oxidative stress and damage to membrane lipids. Increases in H2O2, MG, and MDA levels as well as enhanced EL have also been reported in wheat (Hussain et al. 2018), cotton (Priyanka et al. 2021) and tomato (Faizan et al. 2021) under Cd toxicity. However, ZnO-NPs upregulated the leaf activity of SOD, CAT, APX, GR, Gly I and Gly II under Cd toxicity, which was associated with decreased levels of H2O2, MG and MDA as well as EL, indicating the ability of ZnO-NPs to protect bio-membranes against Cd-induced oxidative stress. These results are consistent with the results previously revealed in other plants (Rizwan et al. 2019; Venkatachalam et al. 2017; Priyanka et al. 2021). Ahmad et al. (2020) reported that the foliar application of 50 and 100 mg/L ZnO-NPs for two weeks reduced the levels of H2O2 and MDA by upregulating the activity of CAT, SOD, APX, GR, Gly I and Gly II, thereby improving the tolerance of soybean under As toxicity. These results demonstrated that the application of ZnO-NPs increased the activity of antioxidant enzymes and the glyoxalase cycle, which could effectively help the plant to scavenge toxic radicals under Cd toxicity.
The results confirmed that Cd stress lessened the leaf concentration of macro (N, Ca, P, K, and Mg) and micro (Fe, Mn, Cu, and Zn) nutrients and increased the leaf concentration of Cd in purslane, suggesting the interference of Cd with the translocation of nutrients to the aerial part of the plant. It has been shown that Cd ions may compete with elements such as Ca, Fe, Mn, and Zn to cross the membrane (Llamas et al. 2000). Ca transporters and channels, as well as NRAMP and ZIP members that are involved in nutrient uptake, have been reported to be involved in Cd uptake (Perfus-Barbeoch et al. 2002). Cd-induced nutrient imbalances can be caused by competition between nutrients and Cd ions for binding sites in various compartments, including the cell wall and plasma membrane. Sun and Shen (2007) indicated that Cd toxicity reduced photosynthesis and, as a result, plant growth by reducing the leaf concentrations of P, Mg, S, Fe, and Mn. However, foliar application of ZnO-NPs increased the leaf concentrations of macro- and micro-nutrients and reduced the leaf accumulation of Cd in Cd-stressed plants, which could play an important role in increasing plant tolerance under Cd toxicity. The positive effects of ZnO-NPs on the nutritional parameters have been reported in several plants (Zhao et al. 2014; Kolenčík et al. 2019). However, the interaction between ZnO-NPs and Cd stress on the uptake and transport of nutrients in plants has not been well studied. Palusińska et al. (2020) indicated that the high supply of Zn in the plant could reduce the translocation of Cd from the roots to the shoots by changing the expression pattern of ZIP genes. Zn has been shown to induce the synthesis of S-containing compounds including GSH and phytochelatins, therefore, increasing the endogenous concentration of Zn induced by the application of ZnO-NPs can increase the levels of phytochelatins and GSH, sequestering Cd in the vacuole and reducing its translocation to the shoots. Sharifan et al. (2020) showed that the application of ZnO-NPs by increasing the leaf concentration of Fe, Zn, and Cu improved the growth of leafy greens under Cd stress. Ahmad et al. (2020) indicated that ZnO-NPs decreased the As uptake and increased the Zn uptake in soybean plants under As toxicity. Rizwan et al. (2019) found that the application of ZnO-NPs reduced Cd uptake and enhanced Zn uptake, thereby improving the growth and yield of wheat under Cd toxicity. Although the results of the present study confirmed that the foliar application of ZnO-NPs improved the leaf concentrations of macro- and micro-elements under Cd stress, more studies at the molecular and biochemical levels are needed to better understand the role of ZnO-NPs in the uptake and translocation of nutrients to leaves (Ghorbani et al. 2019a).
Conclusions
The results revealed that the foliar application of ZnO-NPs increased the leaf concentrations of macro- and micro-nutrients and thus improved photosynthetic pigments and the growth of purslane under Cd stress. By improving the activity of antioxidant enzymes and the glyoxalase cycle, ZnO-NPs reduced the levels of toxic compounds H2O2 and MG, protected bio-membranes and improved plant tolerance under Cd toxicity. The results indicated that the foliar application of ZnO-NPs at appropriate concentrations (100 mg/L) could reduce Cd translocation to the leaves and improve plant height and biomass under Cd toxicity. Although the results showed that the foliar application of ZnO-NPs significantly increased purslane resistance to Cd toxicity, additional field studies and comparisons with these results are needed for more practical outcomes.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmad P, Alyemeni MN, Al-Huqail AA, Alqahtani MA, Wijaya L, Ashraf M, Kaya C, Bajguz A (2020) Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants (Basel) 9(7):825
Ali B, Gill RA, Yang S, Gill MB, Farooq MA, Liu D, Daud MK, Ali S, Zhou W (2015) Regulation of cadmium-induced proteomic and metabolic changes by 5-aminolevulinic acid in leaves of Brassica napus L. PLoS ONE 10:1–23
Arnon DI (1949) Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol 24:1–15
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702
Fagioni M, D’Amici GM, Timperio AM, Zolla L (2009) Proteomic analysis of multiprotein complexes in the thylakoid membrane upon cadmium treatment. J Proteome Res 8(1):310–326
Faizan M, Faraz A, Mir AR, Hayat S (2021) Role of zinc oxide nanoparticles in countering negative effects generated by cadmium in Lycopersicon esculentum. J Plant Growth Regul 40:101–115
Gerami M, Ghorbani A, Karimi S (2018) Role of salicylic acid pretreatment in alleviating cadmium-induced toxicity in Salvia officinalis L. Iranian. J Plant Biol 10(1):81–95
Ghasemi-Omran VO, Ghorbani A, Sajjadi-Otaghsara SA (2021) Melatonin alleviates NaCl-induced damage by regulating ionic homeostasis, antioxidant system, redox homeostasis, and expression of steviol glycosides-related biosynthetic genes in in vitro cultured Stevia rebaudiana Bertoni. In Vitro Cell Dev Biol- Plant 57:319–331
Ghorbani A, Zarinkamar F, Fallah A (2009) The effect of cold stress on the morphologic and physiologic characters of tow rice varieties in seedling stage. J Crop Breed 1:50–66
Ghorbani A, Zarinkamar F, Fallah A (2011) Effect of cold stress on the anatomy and morphology of the tolerant and sensitive cultivars of rice during germination. J Cell Tissue 2(3):235–244
Ghorbani A, Razavi SM, Ghasemi Omran VO, Pirdashti H (2018b) Piriformospora indica inoculation alleviates the adverse effect of NaCl stress on growth, gas exchange and chlorophyll fluorescence in tomato (Solanum lycopersicum L.). Plant Biol 20:729–736
Ghorbani A, Razavi SM, Ghasemi Omran VO, Pirdashti H (2018a) Piriformospora indica alleviates salinity by boosting redox poise and antioxidative potential of tomato. Russ J Plant Physiol 65:898–907
Ghorbani A, Razavi SM, Ghasemi Omran V, Pirdeshti H (2019b) Effects of endophyte fungi symbiosis on some physiological parameters of tomato plants under 10 day long salinity stress. J Plant Proc Func 7(27):193–208
Ghorbani A, Ghasemi Omran VO, Razavi SM, Pirdashti H, Ranjbar M (2019a) Piriformospora indica confers salinity tolerance on tomato (Lycopersicon esculentum Mill.) through amelioration of nutrient accumulation, K+/Na+ homeostasis and water status. Plant Cell Rep 38:1151–1163
Ghorbani A, Pishkar L, Roodbari N, Pehlivan N, Wu C (2021) Nitric oxide could allay arsenic phytotoxicity in tomato (Solanum lycopersicum L.) by modulating photosynthetic pigments, phytochelatin metabolism, molecular redox status and arsenic sequestration. Plant Physiol Biochem 167:337–348
Ghorbani A, Tafteh M, Roudbari N, Pishkar L, Zhang W, Wu C (2020) Piriformospora indica augments arsenic tolerance in rice (Oryza sativa) by immobilizing arsenic in roots and improving iron translocation to shoots. Ecotoxicol Environ Saf 209:111793
Ghorbani A, Pishkar L, Roodbari N, Ali Tavakoli S, Moein Jahromi E, Chu W (2022) Nitrate reductase is needed for methyl jasmonate-mediated arsenic toxicity tolerance of rice by modulating the antioxidant defense system, glyoxalase system and arsenic sequestration mechanism. J Plant Growth Regul. https://doi.org/10.1007/s00344-022-10616-2
Hasan SA, Hayat S, Ahmad A (2009) Screening of tomato (Lycopersicon esculentum) cultivars against cadmium through shotgun approach. J Plant Inter 4:187–201
Hasanuzzaman M, Hossain MA, Fujita M (2011) Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol Trace Elem Res 143(3):1704–1721
Hodges DM, Deiong JM, Forney CF, Prange R (1999) Improving the thiobarbituric acid-recative–substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Huo Y, Wang M, Wei Y, Xia Z (2016) Overexpression of the maize psbA gene enhances drought tolerance through regulating antioxidant system, photosynthetic capability, and stress defense gene expression in tobacco. Front Plant Sci 6:1223
Hussain A, Ali S, Rizwan M, Rehman MZ, Javed MR, Imran M, Chatha SAS, Nazir R (2018) Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ Pollut 242:1518–1526
Jackson ML (1967) Soil chemical analysis, 1st edn. Prentice Hall of India Pvt. Ltd, New Delhi, p 144–197
Juhel G, Batisse E, Hugues Q, Daly D, van Pelt FNAM, O’Halloran J, Jansen MAK (2011) Alumina nanoparticles enhance growth of Lemna minor. Aquat Toxicol 105:328–336
Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2020) Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol Plant https://doi.org/10.1111/ppl.13012
Kitson R, Mellon M (1944) Colorimetric determination of phosphorus as molybdivanado phosphonic acid. Ind Eng Chem Res 16:379–383
Kolenčík M, Ernst D, Komár M, Urík M, Šebesta M, Dobročka E, Černý I, Illa R, Kanike R, Qian Y, Feng H, Orlová D, Kratošová G (2019) Effect of Foliar Spray application of zinc oxide nanoparticles on quantitative, nutritional, and physiological parameters of Foxtail Millet (Setaria italica L.) under Field Conditions. Nanomaterials (Basel) 9(11):1559
Liu Y, Wand X, Zeng G, Qui D, Gu J, Zhou M, Chau L (2007) Cadmium induced oxidative stress and response of the ascorbate glutathione cycle in Bechmeria nivea (L.) Gaud. Chemosphere 69:99–107
Llamas A, Ullrich CI, Sanz A2+ effects on transmembrane electrical potential difference, respiration and membrane permeability of rice (Oryza sativa L) roots. Plant Soil 219(1–2):21–28
Lu Y, Wang, Qf Li,J, Xiong J, Zhou L, He SL, Zhang JQ, Chen ZA, He SG, Liu H (2019) Effects of exogenous sulfur on alleviating cadmium stress in tartary buckwheat. Sci Rep 9:7397
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nakano Y, Asada K (1981) Hydrogen peroxide scavenged by ascor- bate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nolan AL, McLaughlin MJ, Mason SD (2003) Chemical speciation of Zn, Cd, Cu, and Pb in pore waters of agricultural and contaminated soils using donnan dialysis. Environ Sci Technol 37(1):90–98
Palusińska M, Barabasz A, Kozak K, Papierniak A, Maślińska K, Antosiewicz DM (2020) Zn/Cd status-dependent accumulation of Zn and Cd in root parts in tobacco is accompanied by specific expression of ZIP genes. BMC Plant Biol 20:37
Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C (2002) Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J 32(4):539–548
Principato GB, Rosi G, Talesa V (1987) Purification and characterization of two forms of glyoxalase II from the liver and brain of Wistar rats. Biochim Biophys Acta 911(3):349–355
Priyanka N, Geetha N, Manish T, Sahi SV, Venkatachalam P (2021) Zinc oxide nanocatalyst mediates cadmium and lead toxicity tolerance mechanism by differential regulation of photosynthetic machinery and antioxidant enzymes level in cotton seedlings. Toxicol Rep 8:295–302
Ramezani M, Enayati M, Ramezani M, Ghorbani A (2021) A study of different strategical views into heavy metal (oid) removal in the environment. Arab J Geosci 21:1–16
Rico CM, Peralta-Videa JR, Gardea-Torresdey JL (2015) Chemistry, Biochemistry of Nanoparticles, and Their Role in Antioxidant Defense System in Plants. In Nanotechnology and Plant Sciences; Springer International Publishing: Cham, Switzerland, 1–17.
Rizwan M, Ali S, Abbas T, Rehman MZ, Hannan F, Keller C, Al-Wabel MI, Ok YS (2016) Cadmium minimization in wheat: a critical review. Ecotoxicol Environ Saf 130:43–53
Rizwan M, Ali S, Ali B, Adrees M, Arshad M, Hussain A, Zia ur Rehman M, Waris AA (2019) Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214:269–277
Santhoshkumar J, Kumar SV, Rajeshkumar S (2017) Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour-Effic Technol 3:459–465
Sharifan H, Moore J, Ma X (2020) Zinc oxide (ZnO) nanoparticles elevated iron and copper contents and mitigated the bioavailability of lead and cadmium in different leafy greens. Ecotoxicol Environ Saf 191:110177
Singh J, Kumar S, Alok A, Upadhyay SK, Rawat M, Tsang DCW, Bolan N, Kim KH (2019) The potential of green synthesized zinc oxide nanoparticles as nutrient source for plant growth. J Clean Prod 214:1061–1070
Sinha S, Saxena R, Singh S (2005) Chromium induced lipid peroxidation in the plants of Pistia stratiotes L.: role of antioxidants and antioxidant enzymes. Chemosphere 58:595–604
Stroin´ski A (1999) Some physiological and biochemical aspects of plant resistance to cadmium effect. I. Antioxidative system. Acta Physiol Plant 21(2):175–188
Sturikova H, Krystofova O, Huska D, Adam V (2018) Zinc, zinc nanoparticles and plants. J Hazard Mater 349:101–110
Suliman AE, Tang YW, Xu L (2007) Preparation of ZnO nanoparticles and nanosheets and their application to dye-sensitized solar cells. Sol Energy Mater Sol Cells 91:1658–1662
Sun JY, Shen ZG (2007) Effects of Cd stress on photosynthetic characteristics and nutrient uptake of cabbages with different Cd-tolerance. Chinese. J Appl Ecol 18(11):2605–2610
di Toppi SL, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41(2):105–130
Tripathi DK, Singh VP, Prasad SM, Chauhan DK, Dubey NK (2015) Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol Biochem 96:189–198
Venkatachalam P, Jayaraj M, Manikandan R, Geetha N, Rene ER, Sharma NC, Sahi SV (2017) Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: A physiochemical analysis. Plant Physiol Biochem 110:59–69
Wang Y, Jiang X, Li K, Wu M, Zhang R, Zhang L, Chen G (2014) Photosynthetic responses of Oryza sativa L. seedlings to cadmium stress: physiological, biochemical and ultrastructural analyses. Biometals 27:389–401
Wild R, Ooi L, Srikanth V, Münch G (2012) A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: the N-acetyl-L-cysteine assay. Anal Bioanal Chem 403:2577–2581
Wu F, Fang Q, Yan S, Pan L, Tang X, Ye W (2020) Effects of zinc oxide nanoparticles on arsenic stress in rice (Oryza sativa L.): germination, early growth, and arsenic uptake. Environ Sci Pollut Res 27:26974–26981
Yu CW, Murphy TM, Lin CH (2003) Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol 30(9):955–963
Zhao L, Peralta-Videa JR, Rico CM, Hernandez-Viezcas JA, Sun Y, Niu G, Servin A, Nunez JE, Duarte-Gardea M, Gardea-Torresdey JL (2014) CeO2 and ZnO nanoparticles change the nutritional qualities of cucumber (Cucumis sativus). J Agric Food Chem 62(13):2752–2759
Author information
Authors and Affiliations
Contributions
SY and LP conceived the idea and wrote the manuscript. LP and AI corrected the language of the manuscript. SY and LP conducted the literature survey.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pishkar, L., Yousefi, S. & Iranbakhsh, A. Foliar application of Zinc oxide nanoparticles alleviates cadmium toxicity in purslane by maintaining nutrients homeostasis and improving the activity of antioxidant enzymes and glyoxalase system. Ecotoxicology 31, 667–678 (2022). https://doi.org/10.1007/s10646-022-02533-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-022-02533-7