Abstract
TiO2 nano particles (NPs) are one of the most produced nanoparticles in the world which are increasingly being released in to the soil. Soils are exposed to various level of concentration of TiO2 NPs, which has raised concern over the adverse influence on soil microbial community, in turn on ecosystem functions. Although, increasing number of studies on ecotoxicological effect of TiO2 NPs are coming up recently, however, a common conscience has yet to be reached regarding the impact of TiO2 NPs on soil microbial community and processes. Moreover, very few studies have targeted soil enzymes which are being considered as sensitive indicator of soil health. Therefore, the present study has been carried out to estimate the ecotoxicological effect of various doses of TiO2 NPs (5, 10, 20, 40, 80, 100 mg kg−1 soil) on different soil enzymes and microbial community structure. Results revealed that soil enzyme activities and microbial biomass had a uniform trend where the value increased up to the dose of 20 mg TiO2 NPs kg−1 soil and there onwards reduced drastically up to 100 mg TiO2 NPs kg−1 soil dose. On the contrary, soil respiration and metabolic quotient kept increasing up to 100 mg TiO2 NPs kg−1 soil dose indicating sub-lethal stress on microbial community. Nevertheless, the structure of microbial community had slightly different trend where the biomass of total phospho lipid fatty acid (PLFA), Gram positive, Gram negative bacteria, fungi, actinomyctetes and anaerobes were found to be increased up to dose of 80 mg TiO2 NPs kg−1 soil, but, significantly declined at 100 mg TiO2 NPs kg−1 soil dose. Furthermore, temperature effect on TiO2 NPs toxicity had exhibited a less negative impact at 40 °C rather than at 25 °C. In addition alteration index (AI3), an integrated indicator of C, N, P cycling of soils as well as a well-documented indicator of soil pollution, has been found to be regulated by soil respiration, clay content, anaerobe and eukaryote for AI3-Acid Phos. and by fungi to bacteria ratio, soil respiration, microbial biomass and Gram positive bacteria for AI3-Alk. Phos. Overall, the study provided valuable information regarding ecotoxicological impact of environmentally relevant concentrations of TiO2 NPs in clay loam soils as well as improved our perception regarding the impact of NPs on soil functioning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent decades have witnessed the rise of engineered nanoparticles (ENPs), as well as recognized their considerable benefits. Though, the production of nano-scale materials is not new but their large-scale production and application in industrial, medical and farming sector owing to their specific electrical, magnetic and catalytic traits has been intensified in recent years (Adhikari et al. 2015; Hou et al. 2018). TiO2 NPs are documented as one of the five kinds of NPs being employed widely as an anti-bacterial and water treatment agent, in cosmetics products owing to the higher stability, anticorrosive and photocatalytic properties (Hou et al. 2018). According to Keller et al. (2013) worldwide production of TiO2 NPs is approximately 88,000 t y−1. This large scale production, intensive use and indiscriminate disposal of TiO2 NPs have inevitably resulted in their discharge into the environment, where they might produce adverse effect on individual organisms together with the ecosystems (Minetto et al. 2016; Khosravi-katuli et al. 2017). Likewise, indirect discharges of TiO2-NPs in agricultural soils through irrigation, sewage-sludge application or as direct application as nano-fertilizers or nano-pesticides, could dramatically increase the TiO2 NPs load in the soils (Gogos et al. 2012). Eventually, there has been a growing concern regarding the harmful consequences of TiO2 NPs exposure to the environment which have instigated new research attempts aiming to perceive their eco-toxicological effects. Till now very limited information has been surfaced on the transport pathways of TiO2 NPs in different components of environment, such as waters, soils, sediments or their impacts on natural ecosystem. Although the concentrations of most ENPs and their exact transport pathways in the environments are yet to be deciphered; the exposure modeling depicts soil as a prime sink of most of the ENPS as compared to water and air (Chai et al. 2015).
Soil is a prime interface for sustaining plant and animal productivity, maintaining water and air quality, and supporting human health and inhabitation. Henceforth evaluation, monitoring and safeguarding of soil health is of utmost importance and requires immediate attention. Out of the various physical, chemical and biological soil health indicators, biological indicators such as soil organism, soil enzymes, soil respiration are being documented as utmost important as the ecosystem function is regulated mostly by dynamics of soil microbial biomass (Doran and Zeiss 2000). Soil microbes play crucial roles in nutrient cycling and organic carbon dynamics. The toxicity of NPs towards the living organisms, their metabolism as well as transportation are mostly governed by labile and resistant pools of organic carbon ionic strengths and pH of the soil under study (Zhu et al. 2014). Soil generally contains approximately 0.5 % geogenic TiO2, indicating a definite evolutionary adjustment of soil microorganisms towards TiO2; nonetheless, TiO2 as nanoparticles might distress soil organisms differently owing to their higher specific surface area, reactivity and solubility as compared to TiO2 naturally available in soils, and could disturb ecosystem functions (Scheffer et al. 2002; Nowack and Bucheli 2007; Gardea-Torresdey et al. 2014). It has already been documented that NPs can easily enter into the microbial cells and cause injuries at cellular and DNA level towing to their nano scale size (Auffan et al. 2012). Likewise, it is well perceived that any xenobiotic which enters the soil ecosystem is capable of altering the metabolic traits of the whole soil microbial community and biomass. Thus, it is presumed that if NPs have toxic effects on soil microbes, there would be significant decline in the soil microbial biomass and consequent shift in microbial community structure. Similarly, increased cell permeability and eventually cell death in Nitrosomonas europaea which is instrumental in soil nitrification process, have been reported due to the exposure of the TiO2 NPs (Fang et al. 2010).
Though, there have been reports on the impact of TiO2 NPs on soil microbial community, there are relatively fewer studies on the activity of soil enzymes which are considered as subtle predictors of the activities of soil microorganisms and ecosystem functions (Caldwell 2005; Padhan et al. 2020). Moreover, soil enzyme activity can be distressed by the harmful effects of contaminants on soil microbes, thus act as consistent indicator of the existing status of microbial biomass. Evidences of the adverse effects of contaminants on soil ecosystem functions could be generated by estimating soil enzyme activity which modulate biogeochemical cycling and nutrients and organic carbon dynamics (Wang et al. 2015). In spite of their significance in soil ecosystem function, there are still paucity of the information on the influence of TiO2NPs on soil microbial communities and their extracellular enzyme activities. Henceforth, it is of utmost importance to comprehend the behavior of nano particles in soil and to assess the threat of NPs in soil ecosystems. We hypothesized that the measurement of specific soil enzyme activities pertaining to the nutrient cycling along with alteration in overall microbial community structure would be appropriate to portray the noxious effect of NPs on soil microbes and biochemical pathways. To test this hypothesis, a combination of approaches which involve monitoring the alteration in microbial community composition and soil enzyme activities, have been employed to characterize the impact of TiO2 NPs.
Materials and method
Characterization of TiO 2 nanoparticles
TiO2 NPs, which were procured from Sigma Aldrich, USA, was 99.8 % pure (on metal basis) with a mean diameter of the particle is <50 nm (diameter ranged from 2–50 nm). The TiO2 NPs were utilized in the experiment without further purification and were stored in a refrigerator (T = 4 °C) until used. Transmission electron microscopy (TEM) was used to confirm the size and shape of TiO2 NPs. TEM instrument (100 CX, JEOL, Japan) was run at an accelerating voltage of 200 kV (Sun et al. 2006). Similarly, Field Emission Scanning Electron Microscopy instrument (JFM 6701 F, JEOL, Japan) was also run at an accelerating voltage of 20 kV (Cheng et al. 2012) to generate the micrograph of the TiO2 NPs and Energy-dispersive X-ray spectroscopy (EDAX) study was also conducted to ascertain the presence of Ti in TiO2 NPs.
Experimental soil
Soil sample for microcosm study has been collected from the experimental fields of ICAR-Indian Institute of Soil Science, Bhopal, India. The location is characterized by 23°18′N latitude, 77°24′E longitude, and 485 m altitude and falls under semi-arid to sub-tropical climate with dry summer and cold winter. The mean annual air temperature, annual rainfall and humidity are 25 °C, 1208 mm, and about 56%, respectively. The experimental soil belongs to Vertisols, with Hypothermic family and Typic Haplusterts great group. The soil samples were randomly collected from five different points at 0–15 cm soil depth. Collected soil samples were then thoroughly mixed to produce a composite samples which was then processed and homogenized by passing through 2 mm sieve. After that the composite soil samples were separated in to three subsets. One subset was kept at −20 °C for analysis of soil biological parameters. Second sub set was kept for measuring gravimetrical moisture content, and third set was used for soil chemical parameter analysis. The experimental soil sample was clay loam (sand 15.2%, silt 30.3%, clay 54.5%) in nature with pH 7.5, EC 0.43 dS m−1, organic carbon (5.9 g kg−1), available N, P and K (230 mg kg−1, 3.2 and 245 mg kg−1 respectively).
Soil microcosm and treatment imposition
Microcosm study was conducted in four replicated set up where each replication of microcosm was exposed to five graded doses (5, 10, 20, 40, 80 and 100 mg kg−1 soil) of TiO2 NP and a control (without TiO2 NP addition). To attain the desired concentration, 100 g soil was spiked with defined volume of TiO2 NP suspension thoroughly mixed using ultra sonicator and the final concentration was reached to 5, 10, 20, 40, 80 and 100 mg kg−1 soil along with 60% water holding capacity. Homogeneous TiO2-NP suspensions generated through ultra-sonication were added soil which was followed by thorough mixing for 10 min to achieve uniform spiking. In case of control, soils were mixed with same volume of ultrapure water only. The flasks were incubated at two temperatures; such as 25 and 40 °C for 45 days. When the incubation was terminated, the microcosm soil samples were sub divided in to two sets and kept at −20 °C. One set has been used for soil enzymes and microbial biomass analysis and other set has been lyophilized for extraction of phospholipids.
Soil enzymes, microbial biomass, soil respiration and metabolic quotient
Soil dehydrogenase (DHA), β-Glucosidase, acid and alkaline phosphatase (ACP and ALP, respectively) and urease activities were assessed by following the methods reported earlier (Bhattacharjya et al. 2016). p-nitrophenol Total microbial activity was measured by determining the fluorescein diacetate (FDA) hydrolyzing capacity of soil incubated with 60 mM potassium phosphate buffer (pH 7.6) and FDA at 30 °C for 20 min (Adam and Duncan 2001). Soil microbial biomass carbon (SMBC) has been determined by the method documented earlier (Padhan et al. 2020; Bhattacharjya et al. 2016). Soil respiration was estimated through alkali trap method (Anderson 1982). The metabolic quotient (qCO2) was enumerated as soil respiration per unit microbial biomass (Anderson and Domsch 1993; Bhattacharjya et al. 2017b).
Enzyme activity-based index enumeration: Alteration Index 3
Alteration index3 (AI3) has been computed as per the following equation (Puglisi et al. 2006).
Equilibrium among three extracellular microbial enzymes and alteration in respective soil characteristics and function could be tracked down by enumerating the AI3 index as it cumulatively represent the biogeochemical cycling of three C, N and P in soil and how this ecological function is being hampered due to the adverse influence of TiO2 NPs (Ghosh et al. 2019).
Soil microbial community analysis
Structure and composition of soil microbial community was determined by following phospholipid fatty acid (PLFA) analysis (Bhattacharjya et al. 2017a; Padhan et al. 2020).
Nomenclature of the fatty acids were as per the formula (i/a/cyc)X: YωZ(c/t) (Frostegård et al. 1993).The extracted PLFAs were grouped into different biomarker categories as reported in previous studies (Bhattacharjya et al. 2017a). The abundance of each PLFAs was enumerated by the total concentration of PLFAs in nano mol g−1.
Statistical analysis
Data were analyzed through two way analysis of variance using SPSS 20.0 (SPSS Inc., Chicago, USA). Duncan’s Multiple Range Test was followed for mean separations at p < 0.05. Causal relationship among the variables was derived by employing multiple regressions, followed by path analysis. A non-significant χ2 value (P > 0.05) reflects “goodness of fit” of the path model. The path coefficients were determined by correlation matrices. Paths of the model having a P-value < 0.05 were considered as significant.
Results
Characterization of TiO2 NPs
A TEM micrograph (Fig. 1) showed NPs were oblate spherical with clear edge of crystal and lattice structure, which supports the crystalline nature of TiO2 NPs. FESEM-EDAX showed the undulating topology of TiO2 nano-particle analysis (Fig. 2) and also confirmed the presence of Ti in nano material.
Effect on soil enzyme activity and AI3
To assess the impact of TiO2 NPs, temperature and their interaction on soil biological activities and nutrient cycling different enzyme activities viz DHA, acid and alkaline phosphatase, β-glucosidases, urease and FDA hydrolysis of soil were measured. Results revealed similar pattern of response in case of all the enzymes where the enzyme activities and FDA hydrolysis were increased with higher doses of TiO2 NPs up to 20 mg kg−1 soil, and thereafter drastically reduced from 40 mg kg−1 dose onwards (Table 1). The highest decline was noticed in the dose of 100 mg kg−1 soil where the activity of DHA (31.3 %), Acid Phos (18.6 %), urease (21.4 %) and β-glucosidases (10.2 %) was significantly (p < 0.05) lower than the control treatment. It was followed by 80 mg kg−1 soil dose where also the reduction was significantly lower as compared to control. Similarly, in case of Alk. Phos (8.4 %) and FDA hydrolysis (9.7 %) the highest reduction was also found in 100 mg kg−1 soil dose which was significantly lower than the control. The response of soil enzyme activities to the effect of temperature was similar in all the cases where the significantly higher rates of enzyme activities were noticed (irrespective of doses) with increasing incubation temperature from 25 to 40 °C. For DHA, Acid Phos and Alk. Phos, β-glucosidases and urease, increase in enzyme activities were in the range of 5.6–12.5 %. However, in case of FDA hydrolysis, 41.7 % higher activity was noticed with increase in temperature from 25 °C to 40 °C. Furthermore, significant interactions effect between TiO2 NPs doses and temperature on soil enzyme activities were recorded in the present study.
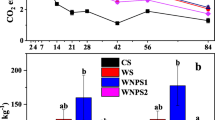
TiO2 NPs also had substantial influence on enzyme-based index i.e AI3. AI3 was derived with both the Acid Phos and Alk. Phos (Fig. 3a). The lowest value (more negative) of AI3-Acid Phos. has been noticed in 20 mg kg−1 soil dose and there onwards drastically increased to the highest (less negative) value at 100 mg kg−1 soil (recorded 48.8 % changes in AI3 from 0 mg kg−1 soil). Likewise, the lowest value of AI3-Alk. Phos. was recorded at 20 mg kg−1 soil dose and subsequent increase was noticed in 100 mg kg−1 soil as compared to 0 mg kg−1 soil (Fig. 3b).
Effect on soil microbial biomass carbon
Graded doses of TiO2 NPs and temperature had significant influence on SMBC. SMBC also had the comparable response like soil enzyme activities, where it enhanced with higher doses of TiO2 NPs up to 20 mg kg−1 soil, and drastically declined from 40 mg kg−1 soil onwards (Table 2). The lowest SMBC was registered in 100 mg kg−1 soil TiO2 NPs dose which is 3.8% significantly less than the control. On the contrary, the SMBC was magnified (irrespective of doses) with respect to increase in temperature from 25 to 40 °C. However, no significant interaction was noticed between TiO2 NPs doses and temperature on SMBC.
Effect on soil respiration and metabolic quotient
In contrast to the reaction of soil enzymes and SMBC discussed above, soil respiration and qCO2 were found to be augmented with increase in TiO2 NPs doses and the highest soil respiration and metabolic quotient were recorded under 100 mg kg−1 soil TiO2 NPs dose. Likewise, 20, 40 and 80 mg kg−1 soil doses also had significantly (p < 0.05) higher soil respiration in comparison to control (Table 2). qCO2, all the doses of TiO2 NPs had registered significantly higher value than control indicating stress on soil microbial community (Table 2). Similarly, significantly (p < 0.05) higher soil respiration and qCO2were noticed at 40 °C temperature as compared to 25 °C. However, no significant interaction effect was noticed between TiO2 NPs doses and temperature on soil respiration and qCO2.
Effect on soil microbial community
Significant effects of both the doses of TiO2 NPs as well as temperature were noticed on soil microbial community as studied by PLFA analysis. Total PLFA biomass increased with increasing doses of TiO2 NPs up to 80 mg kg−1 soil however reduced drastically at 100 mg kg−1 soil (Table 3). Similarly with increasing incubation temperature total PLFA biomass was reduced from 708.78 to 637.14 nano mole g−1 soil. The biomass of fungi, actinomycetes and eukaryotes was also enhanced from 20 - 80 mg kg−1 of TiO2 NPs doses following a substantial reduction at 10 mg kg−1 soil. The lowest PLFA of fungi, actinomycetes and eukaryotes were noted at 100 mg kg−1 soil dose. Besides, the ratio of Gram +ve/Gram –ve bacteria (G + ve/G-ve) and ratio of fungi/bacteria (F/B) showed two different tend. G + ve/G-ve decreased with higher doses of TiO2 NPs up to 80 mg kg−1 soil (lowest), then sharply increased at 100 mg kg−1 soil (Fig. 4a). On the contrary, F/B after initial reduction at 10 mg kg−1 soil, augmented up to 80 mg kg−1 soil and then declined at 100 mg kg−1 dose (Fig. 4b).
The effect of temperature on PLFA revealed a significant reduction of all the PLFA corresponding to fungi, Gram +ve bacteria, Gram −ve bacteria, actinomycetes, eukaryotes, and anaerobes at higher temperature (Table 3). In case of the ratio, G +ve/G −ve decreased while F/B increased at higher temperature (Fig. 4a, b). In addition, TiO2 NPs dose and temperature also had significant interaction effect on different PLFA.
Relationship among AI3 index and microbial community
In order to find out an interrelationship among AI3 and soil microbial community, linear multiple regression has been done where AI3-Acid Phos. and AI3-Alk. Phos. were represented as the dependent variables. Regression model (Adjusted R2 = 0.90; Eq. (2)) predicts that soil respiration, clay content of the soil, anaerobes and eukaryotes are the key regulator of AI3-Acid Phos. The biggest contributor is found to be soil respiration followed by clay content, anaerobes and eukaryotes where each of the independent variables has negative slope. i.e they bear a negative relationship with the dependent variable. Likewise, another regression model (Adjusted R2 = 0.87; Eq. (3)) with reference to AI3-Alk. Phos. predicts that SMBC, soil respiration, Gram +ve bacteria and F/B were the major controller. Here the biggest contributor is found to be F/B ratio followed by soil respiration, MBC and Gram +ve bacteria where each of the independent variables except Gram +ve bacteria, has negative slope. i.e they bear a negative relationship with the dependent variable and Gram +ve bacteria bear positive relationship with AI3-Alk. Phos.
In addition, path analysis, that estimates the magnitude and implication of presumed causal relationship among a group of variables, was done among AI3-Acid Phos., soil respiration, clay, eukaryote, and anaerobe (Fig. 5a). The path model has shown “Goodness of fit” on the basis of χ2 (p = 0.052; CMIN/DF = 2.575), NFI (0.933), CFI (0.953), IFI (0.958), AIC (Supplementary File: S1). The unit less path coefficients are represented by the values on the arrows of the path diagram. Here clay content has been found to impact AI3-Acid Phos. directly along with having indirect effect through soil respiration only. The standardized total effect of clay content on AI3-Acid Phos. was 0.288 succeeded by anaerobes (−0.208;) > eukaryotes (−0.321;) > soil respiration (−0.982,). Similarly, the path model comprising of AI3-Alk. Phos., F/B ratio, soil respiration, SMBC and Gram +ve bacteria (Fig. 5b) was also a “good fit” on the basis of χ2 (p = 0.098; CMIN/DF = 2.097), NFI (0.958), CFI (0.976), TLI (0.879), IFI (0.978), AIC (Supplementary file: S2). Here MBC has been found to exert direct impact on AI3-Alk. Phos. as well indirect impact through the soil respiration and Gram +ve bacteria. The standardized total effect of MBC on AI3-Alk. Phos. was −0.832 succeeded by soil respiration (−0.446;) > Gram +ve (−0.418;) > F/B ratio (−0.163,). It signifies that one unit variation in SMBC would result in 0.832 unit variation in AI3-Alk. Phos.
a Path diagram of the causal relationship among AI3_Acid Phos. as dependent variable and soil respiration, clay content of the soil, anaerobes and eukaryotes as modulator. b Path diagram of the causal relationship among AI3_Alk. Phos. as dependent variable and MBC, soil respiration, Gram +ve bacteria and F/B ratio as modulator. # Values on the arrows indicate the unit-less path coefficients (standardized regression weights); e1, e2, e3 and e4 indicate the associated error term
Discussion
Response of soil enzymes and soil microbes to TiO2 NPs
Soil microbial activities especially soil enzyme are perceived to be sensitive indicators of soil health, as they modulate the alteration as well as decomposition of naturally occurring compounds and xenobiotics (Josko et al. 2014). Likewise, the microbial community lead the decomposition processes, nutrient cycling, along with soil decontamination process. Therefore, dysfunction of the soil enzymatic activity along with the alteration in microbial community structure might distress the soil biological stability, which in turn impact environmental and economic outcomes. The consequences of environmental distresses on soil microbial activity is classically approached through analysis of soil respiration or C, N, P, S cycling enzyme activities (Schimel and Schaeffer 2012). In the present study enzymes have been chosen to represent the biogeochemical cycling of C, N, and P as well as overall microbial activity, where all the enzyme activity resulted in initial increase up to 40 mg kg−1 soil followed by drastic decline at 100 mg kg−1 soil TiO2 NP doses. This stimulation-suppression mechanism of soil enzymes could be explained in the light of the behavior of TiO2 NPs in clay loam soil suspension having monmorillonite type of clay minerals. When the TiO2 NPs get exposed to soil colloids mainly two types of aggregation may take place: homoaggregation among the similar NPs, or heteroaggregation among NP and other colloidal particle in the suspension. Usually, the higher concentration of environmental particles than NPs will lead to formation of heteroaggregates (Lowry et al. 2012; Wang et al. 2015) due to Brownian motion. Heteroaggregation of NPs occurs as a result of interaction between two particles mainly through attractive and repulsive forces. Colloidal interactions could also take place due to van der Waals forces, Hydrogen bonding, Lewis acid-base interactions that eventually result in to heteroaggregation of NPs in aqueous and non-aqueous systems (Zhao et al. 2014; Wang et al. 2015). Aggregation can thus diminish the toxicity of NPs as the toxic effect is the result of a surface area related reaction such as reactive oxygen species (ROS) generation or dissolution (Hotze et al. 2010). This condition seems to be responsible in the present work where no harmful effect of TiO2 NPs at lower concentration (5, 10, 20 mg kg−1 soil) were noticed on soil microbial biomass and soil enzymes. Nevertheless, relatively higher concentration of TiO2 NPs (40, 80 100 mg kg−1 soil) had shown adverse influence on soil enzymes and microbial biomass. Similar negative effects of TiO2 NPs on soil enzymes and microbial biomass have been reported in earlier studies (Du et al. 2011; Ge et al. 2011, Pawlett et al. 2013). With increasing concentration of TiO2 NPs, the surfaces of the clay colloids might get saturated while lower organic matter content of Vertisol also could not supply sufficient surface for increasing heteroaggregation. In this scenario the TiO2 NPs, which could not form heteroaggregates, remain dissolved in the suspension through their electrostatic repulsion with each other. In addition, lower dissolved organic carbon concentration in clay loam-low organic matter soil also contributes to the smaller hydrodynamic diameter of TiO2 NPs (Simonin et al. 2015). These TiO2 NPs in turn get attached with microbial cell wall through metal-complexation with surface functional groups (Carboxyl, amide, phosphate and hydroxyl groups) and the extracellular polymer substances (Ma and Lin 2013) and in turn cause toxicity toward microbes by membrane disruption and ROS production (Simonin et al. 2015; Hou et al. 2018). ROS cause oxidative stress in microbial cells that could results in to damage of the cells and eventually leads to decline in the production and secretion of soil enzymes, in turn affecting the biogeochemical cycling of nutrients in soil.
Although, the soil microbial biomass and soil enzyme activities got supressed after initial spike, soil respiration and qCO2 continues to increase along with increasing doses of TiO2 NPs. This finding corroborates with the earlier reports (Hänsch and Emmerling 2010; Dinesh et al. 2012) where higher doses of Ag NPs increased soil respiration and qCO2 and reduced soil microbial biomass. This is actually a circumstantial evidence as well as classical example of the sub-lethal stress in soil microbial community in polluted soils condition where lower substrate use efficiency is expected (Hänsch and Emmerling 2010).
Application of TiO2 NPs at different level of doses also had similar effect on PLFA biomass, which is regarded as a predictor of soil microbial biomass that excretes extracellular enzymes pertinent to nutrient cycling in soil (Padhan et al. 2020). Decrement of overall PLFA biomass corroborates with the reduction of soil microbial biomass as mentioned above. Nevertheless, soil microbial community displayed slightly different pattern as compared to the soil enzymes. Here, total PLFA biomass, biomass of fungi, actinomycetes, anaerobes, eukaryotes, Gram +ve and Gram –ve bacteria were amplified with higher doses of TiO2 NPs up to 80 mg kg−1 soil, then significantly reduced at 100 mg kg−1 soil. This scenario enables us to envision the scenario how the TiO2 NPs will affect the ecosystem functions (Ge et al. 2012). Although, previous studies reported no or less harmful impact of TiO2 NPs on bacterial abundance, diversity and community structure and composition; nevertheless, the higher concentration (500 mg kg−1 soil, 1000 mg kg−1 soil, 2000 mg kg−1 soil, 20000 mg kg−1 soil) of TiO2 NPs tested on those studies has to be taken in to consideration (Ge et al. 2012; Simonin et al. 2015, 2017; Zhai et al. 2019). As compared to these reported higher concentration we used comparatively less concentration of TiO2 NPs which might have been bioavailable to soil microorganisms causing toxicity. However, the popular indicator of shift or alteration in microbial community, the ratio of G +ve/G −ve and the ratio of fungi/bacteria (F/B) showed two different trends. Reduction in G +ve/G −ve ratio with increasing doses of TiO2 NPs reflected the inefficient substrate use leading to rise of the population of fast growing Gram −ve bacteria. However, at higher TiO2 NPs concentration (100 mg kg−1 soil) drastic reduction of Gram −ve bacteria resulted in wider ratio of G +ve/G −ve, indicating higher tolerance of Gram +ve bacteria towards the TiO2 NPs. It could be ascribed to the protective cell wall structure of Gram +ve bacteria that has a thicker peptidoglycan layer (~80 nm) with covalently bonded teichoic and teichuronic acids. Damage of the cell wall resulting from physical interaction between NPs and the cell wall is more injurious to Gram −ve bacteria due to the absence of dense peptidoglycan layer present in Gram +ve bacteria which function as a protective layer (Slavin et al. 2017). On the contrary, reduction of F/B ratio at 100 mg kg−1 soil indicated susceptibility of fungi as compared to bacteria.
The present experiment also studied the effect of two different temperatures (25 and 40 °C) on soil enzymes, MBC, soil respiration and microbial community irrespective of the doses of TiO2 NPs. Increase in enzyme activities at higher temperature supports the well-known Arrhenius theory according to which the rate of the reaction of enzyme activities doubles with each 10 °C temperature rise. Moreover, all the studied enzymes are having their optima at 37 °C which is near to 40 °C thus requiring to cross lesser energy barrier. Likewise, soil respiration is a biochemical reaction which is also found to be increased at higher temperature. This scenario is possible when there is reduced substrate use efficiency which could be possible due to shift in bacterial community from “k” to “r” strategist which eventually results in stress in microbial community (Bhattacharjya et al. 2017b). This finding corroborates with the increase in abundance of fast growing Gram –ve bacteria as compared to Gram +ve bacteria at higher temperature, resulting in reduced substrate use efficiency. Although there is increase in abundance of Gram –ve bacteria, fungi, actinomycetes and eukaryotes, however, total PLFA biomass has been found to be declined at higher temperature which is presumably contributed by reduction in the abundance of Gram +ve bacteria and anaerobes. Furthermore, the significant interaction effect of TiO2 NPs doses and temperature revealed that higher temperature might have exerted less toxic effect of TiO2 NPs on soil enzymes and soil microbial community.
Multi-causality of effect of TiO2 NPs on soil microbial community and functions
Alterations or changes in the soil system due to various detrimental practices and soil contamination is accountable for high AI3 values in soil systems having balance between input and output (Puglisi et al. 2006). AI3 has also been considered indicator of soil degradation due to imbalanced nutrient application (Ghosh et al. 2019). However, Hinojosa et al. (2004) used the AI3 index to differentiate between highly polluted (by mining effluent spills leading to heavy-metal contamination) and non-polluted soil from a soil regained by intensive remediation. This index clearly distinguished the polluted sites from the non-polluted ones; where polluted sites had the highest value (less negative) of AI3. Similar trend has been noticed in the present study where the highest value (significantly lower than 0 mg TiO2 NPs kg−1 soil) of AI3-Acid phos. and AI3Aalk. Phos. have been found in 100 mg TiO2 NPs kg−1 soil dose. Furthermore, AI3 can be considered as an integrative index representing soil health and biogeochemical cycling of major nutrients i.e C, N and P (Ghosh et al. 2019).
Multiple regression followed by path analysis have become a popular choice of analytical methods to find out the dynamics and causal relationship among the indicators of soil biological and ecological process (Padhan et al. 2020). It has been noticed that the major regulator of AI3-Acid Phos. are soil respiration, clay content of the soil, anaerobes and eukaryotes whereas for AI3-Alk. Phos. the major controllers are MBC, soil respiration, Gram +ve bacteria and F/B. Soil respiration represents the metabolic status of soil microbial community and the metabolism of the soil microbes are highly regulated by the availability of different nutrients which are modulated by C, N, P cycling enzymes. Therefore, soil respiration is found to be a great modulator of AI3-Acid Phos. as well as AI3-Alk. Phos. where soil respiration bears an inverse relationship with the later one (correlation among soil respiration × AI3-Acid Phos. = −0.88; p < 0.01; soil respiration × AI3-Alk. Phos. = −0.75; p < 0.01). Similarly, soil clay content may also exert great effect on AI3-Acid Phos. through heteroaggregates formation at lower concentration of TiO2 NPs, thus have little effect on the soil enzymes leading to more negative value of AI3-Acid Phos. However, the opposite scenario has been noticed at higher concentration of TiO2 NPs. In addition, other important contributors are anaerobes and eukaryote, where the abundances of them were found to be higher at lower concentration of TiO2 NPs (i.e higher negative value of AI3-Acid Phos.) and the opposite trend was found at higher concentration TiO2 NPs. Likewise, the signature PLFA representing the microbial groups such as anaerobes, eukaryotes, Gram +ve bacteria and the F/B ratio were appeared to be key regulator of AI3-Acid Phos. and AI3-Alk. Phos. which could clearly differentiate the toxic and less toxic effect of TiO2 NPs doses. Similar result has been documented by Puglisi et al. (2006) where the alteration index developed based on signature PLFA had aptly discriminated the high chromium contaminated soil with lower index value from low contaminated soil. Besides, contribution of SMBC which is the indicator of overall abundance and activity of the microbial community, is quite similar to the response of anaerobes, eukaryotes, Gram +ve bacteria in regulating AI3-Alk. Phos. where soil microbial biomass produces extracellular C, N, P cycling enzymes for depolymerization of organic residues for microbial metabolism (Padhan et al. 2020). Altogether the path analysis method had characterized the multi-causal effect of TiO2-NPs on the integrated soil index AI3-Acid Phos. and AI3-Alk. Phos.
Conclusion
The present work shows that TiO2 NPs at environmentally relevant concentration can supress the soil enzyme activities and soil microbial biomass in a clay-loam Vertisol. Increased soil respiration along with the increased metabolic quotient from 0 to 100 mg TiO2 NPs kg−1 soil dose reflected a circumstantial evidence of the sub-lethal stress in soil microbial community in NPs polluted soils. However, the microbial community structure had slightly different trend where the biomass of total PLFA, Gram +ve, Gram −ve bacteria, fungi, actinomyctetes, anaerobes were found to be increased up to TiO2 NPs dose of 80 mg kg−1 soil, but, significantly reduced at 100 mg kg−1 soil dose. Gram +ve bacteria appeared to be the least affected among all the microbial groups. Alteration index (AI3), an integrated indicator of C, N, P cycling of soils and well known indicator of soil pollution, has been found to be highest (least negative) at 100 mg kg−1 soil of TiO2 NPs dose, substantiating the fact that TiO2 NPs have detrimental effect on soil health and function. Furthermore, multiple regression and path analysis with respect to AI3 as dependent variable has revealed that AI3-Acid Phos. has been regulated by soil respiration, clay content, anaerobe and eukaryote while AI3-Alk. Phos. is being modulate by F/B ratio, soil respiration, MBC and Gram +ve bacteria. Altogether, our results provided valuable information regarding ecotoxicological effect of environmentally relevant concentrations of TiO2 NPs in clay loam soils that appeared to be a pertinent step towards better perception of the effect of NPs on soil ecosystem functions.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Adam G, Duncan H (2001) Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol Biochem 33(7):943–951
Adhikari T, Sarkar D, Mashayekhi H, Xing B (2015) Growth and enzymatic activity of maize (Zea mays L.) plant: solution culture test for copper dioxide nano particles. J Plant Nutr, https://doi.org/10.1080/01904167.2015.1044012
Anderson TH, Domsch KH (1993) The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol Biochem 25:393–395
Anderson JPE (1982) Soil respiration. In: Page AL, et al., eds. Methods of soil analysis. Part 2. Chemical and microbiological properties, 2nd ed. Agronomy Monograph 9. ASA and SSSA, Madison, WI, p 837–871
Auffan DM, Santaella DC, Thiéry PA, Paillès C, Rose J, Achouak DW, Thill DA, Masion A, Wiesner M, Bottero JY (2012) Ecotoxicity of inorganic nanoparticles: from unicellular organisms to invertebrates. In: Bhushan PB (Ed.) Encyclopedia of nanotechnology. Springer, Netherlands, p 623–636
Bhattacharjya S, Bhaduri D, Chauhan S, Chandra R, Raverkar KP, Pareek N (2017b) Comparative evaluation of three contrasting land use systems for soil carbon, microbial and biochemical indicators in North-Western Himalaya. Ecol Eng 103:21–30
Bhattacharjya S, Chandra R, Pareek N, Raverkar KP (2016) Biochar and crop residue application to soil: effect on soil biochemical properties, nutrient availability and yield of rice (Oryza sativa L.) and wheat (Triticum aestivum L.). Arch Agron Soil Sci 62(8):1095–1108
Bhattacharjya S, Chandra R, Sharma MP, Sharma SK, Agnihotri R (2017a) Biochar and crop residue amendments on soil microbial and biochemical properties. Proc Natl Acad Sci India Sect B Biol Sci 87(3):975–983
Caldwell BA (2005) Enzyme activities as a component of soil biodiversity: a review. Pedobiologia 49:637–644
Chai H, Yao J, Sun J, Zhang C, Liu W, Zhu M, Ceccanti B (2015) The effect of metal oxide nanoparticles on functional bacteria and metabolic profiles in agricultural. Soil Bull Environ Contam Toxicol 94:490–495
Cheng Z, Tan ALK, Tao Y, Shan D, Ting KE, Yin XJ (2012) Synthesis and characterization of iron oxide nanoparticles and applications in the removal of heavy metals from industrial waste water. Intl J Photoener 5. https://doi.org/10.1155/2012/608298
Dinesh R, Anandaraj M, Srinivasan V, Hamza S (2012) Engineered nanoparticles in the soil and their potential implications to microbial activity. Geoderma 173–174:19–27
Doran JW, Zeiss MR (2000) Soil health and sustainability: managing the biotic component of soil quality. Appl Soil Ecol 15:3–11. https://doi.org/10.1016/s0929-1393(00)00067-6
Du W, Sun Y, Ji R, Zhu J, Wu J, Guo H (2011) TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J Environ Monit 13:822–828
Fang X, Yu R, Li B, Somasundaran P, Chandran K (2010) Stresses exerted by ZnO, CeO2 and anatase TiO2 nanoparticles on the Nitrosomonas europaea. J Colloid Interface Sci 348(2):329–334. https://doi.org/10.1016/j.jcis.2010.04.075
Frostegård Å, Bååth E, Tunlid A (1993) Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol Biochem 25:723–730
Gardea-Torresdey JL, Rico CM, White JC (2014) Trophic transfer, transformation, and impact of engineered nanomaterials in terrestrial environments. Environ Sci Technol 48:2526–2540
Ge Y, Schimel JP, Holden PA (2011) Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ Sci Technol 45:1659–1664
Ge Y, Schimel JP, Holden PA (2012) Identification of soil bacteria susceptible to TiO2 and ZnO nanoparticles. Appl Environ Microbiol 78:6749–6758
Ghosh A, Singh AB, Kumar RV, Manna MC, Bhattacharyya R, Rahman MM, Sharma P, Rajput PS, Misra S (2019) Soil enzymes and microbial elemental stoichiometry as bio-indicators of soil quality in diverse cropping systems and nutrient management practices of Indian Vertisols. Appl Soil Ecol 145:103304
Gogos A, Knauer K, Bucheli TD (2012) Nanomaterials in plant protection and fertilization: current state, foreseen applications, and research priorities. J Agric Food Chem 60:9781–9792
Hänsch M, Emmerling C (2010) Effects of silver nanoparticles on the microbiota and enzyme activity in soil. J Plant Nutr Soil Sci 173:554–558
Hinojosa MB, Carreira JA, García-Ruíz R, Dick RP (2004) Soil moisture pre-treatment effects on enzyme activities as indicators of heavy metal-contaminated and reclaimed soils Soil Biol Biochem 36:1559–1568
Hotze EM, Phenrat T, Lowry GV (2010) Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment. J Environ Qual 39:1909
Hou J, Wang L, Wang C, Zhang S, Liu H, Li S, Wang X (2018) Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J Environ Sci 75:40–53
Jośko I, Oleszczuk P, Futa B (2014) The effect of inorganic nanoparticles (ZnO, Cr2O3, CuO and Ni) and their bulk counterparts on activities in different soils. Geoderma 232–234:528–537
Keller AA, McFerran S, Lazareva A, Suh S (2013) Global life cycle releases of engineered nanomaterials. J Nanopart Res 15:1–17. https://doi.org/10.1007/s11051-013-1692-4
Khosravi-katuli K, Prato E, Lofrano G, Guida M (2017) Effects of nanoparticles in species of aquaculture interest. Environ Sci Pollut Res 24:17326–17346
Lowry GV, Gregory KB, Apte SC, Lead JR (2012) Transformations of nanomaterials in the environment. Environ Sci Technol 46:6893–9
Ma S, Lin D (2013) The biophysicochemical interactions at the interfaces between nanoparticles and aquatic organisms: adsorption and internalization. Environ Sci Processes Impacts 15:145
Minetto D, Ghirardini MAV, Libralato G (2016) Saltwater ecotoxicology of Ag, Au, CuO, TiO2, ZnO and C 60 engineered nanoparticles: an overview. Environ Int 92:189–201
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150(1):5–22. https://doi.org/10.1016/j.envpol.2007.06.006
Padhan K, Bhattacharjya S, Sahu A, Manna MC, Sharma MP, Singh M, Wanjari R, Sharma RP, Sharma GK, Patra AK (2020) Soil N transformation as modulated by soil microbes in a 44 years long term fertilizer experiment in a sub-humid to humid Alfisol. Appl Soil Ecol 145:103355
Pawlett M, Ritz K, Dorey RA, Rocks S, Ramsden J, Harris JA (2013) The impact of zero-valent iron nanoparticles upon soil microbial communities is context dependent. Environ Sci Pollut Res 20:1041–1049
Puglisi E, Del Re AAM, Rao MA, Gianfreda L (2006) Development and validation ofnumerical indexes integrating enzyme activities of soils. Soil Biol Biochem 38:1673–1681
Scheffer F, Schachtschabel P, Blume HP, Brümmer GW, Schwertmann U, Horn R, Kögel-Knabner I, Stahr KBMW (2002) Scheffer/Schachtschabel: Lehrbuch der Bodenkunde. Spektrum Akademischer Verlag GmbH, Heidelberg, Berlin
Schimel JP, Schaeffer SM (2012) Microbial control over carbon cycling in soil. Front Microbiol. https://doi.org/10.3389/fmicb.2012.00348
Simonin M, Guyonnet JP, Martins JMF, Ginot M, Richaume A (2015) Influence of soil properties on the toxicity of TiO2 nanoparticles on carbon mineralization and bacterial abundance. J Hazard Mater 283:529–35
Simonin M, Martins JM, Le Roux X, Uzu G, Calas A, Richaume A (2017) Toxicity of TiO2 nanoparticles on soil nitrification at environmentally relevant concentrations: Lack of classical dose-response relationships. Nanotoxicology 11(2):247–255. https://doi.org/10.1080/17435390.2017.1290845
Slavin YN, Asnis J, Häfeli UO et al. (2017) Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol 15:65. https://doi.org/10.1186/s12951-017-0308-z
Sun Y-P, Li X-q, Cao J, Zhang W-x, Wang HP (2006) Characterisation of zero-valent iron nanoparticles Adv Colloid Inter Sci 120:47–56. https://doi.org/10.1016/j.cis.2006.03.001
Wang H, Adeleye AS, Huang Y, Li F, Keller AA (2015) Heteroaggregation of nanoparticles with biocolloids and geocolloids. Adv Coll Interface Sci 226:24–36
Zhai Y, Hunting ER, EliseBaas GL, Willie JGM, Peijnenburg WJGM, Vijver MJ (2019) Compositional alterations in soil bacterial communities exposed to TiO2 nanoparticles are not reflected in functional impacts. Environ Res 178:108713
Zhao J, Wang Z, White JC, Xing B (2014) Graphene in the aquatic environment: adsorption, dispersion, toxicity and transformation. Environ Sci Technol 48:9995–10009
Zhu JJ, Xu YQ, He JH, Yu HP, Huang CJ, Gao JM, Li CQ (2014) Human cardiotoxic drugs delivered by soaking and microinjection induce cardiovascular toxicity in zebrafish. J Appl Toxicol 34(2):139–148. https://doi.org/10.1002/jat.2843
Acknowledgements
Financial support provided by the CRP Platform of Nanotechnology, Indian Council of Agricultural Research, New Delhi, India is duly acknowledged.
Funding
CRP Platform of Nanotechnology, Indian Council of Agricultural Research, New Delhi,
Author contributions
SB conceptualized and executed the experiment, analyzed the soil samples for different soil parameters, processed and interpreted the data and prepared the manuscript. TA conceptualized and executed the experiment, analysed the soil samples for different soil parameters, processed and interpreted the data. AS contributed in data processing, result interpretation and manuscript preparation. AKP edited and corrected the final draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bhattacharjya, S., Adhikari, T., Sahu, A. et al. Ecotoxicological effect of TiO2 nano particles on different soil enzymes and microbial community. Ecotoxicology 30, 719–732 (2021). https://doi.org/10.1007/s10646-021-02398-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-021-02398-2