Abstract
Pollution due to heavy metals is a serious global environmental problem, particularly in China. It is thus important to study the effects of heavy metal pollution, especially in mining areas. Cadmium(Cd) and lead(Pb) severely damage the microbial life in soil. The concentration of heavy metals and their toxic effects on microbes and enzymes in soil were examined in this study using contaminated soil samples. The Biolog method was used to analyze the characteristics of the microbial community. The results showed that the addition of Cd2+ and Pb2+ in different concentrations has a significant impact on microbial and enzyme activity in soil. With an increase in their concentrations, activities of the microbial community and enzymes decreased gradually. Each index related to the structure of the microbial community in soil decreased, indicating that pollution due to Cd and Pb reduced its size and functional activity. This study provides a reference for future research on the functional diversity of the microbial community in soil and plays its role in their environmental management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
China has a vast territory containing a large area of cultivated land and mineral resources. However, with rapid industrialization and excessive mining for minerals, problems such as soil pollution due to heavy metals have arisen (Xu et al. 2020). According to Wei and Yang (2010), heavy metals, especially lead(Pb) and cadmium(Cd), have accumulated in China’s urban and agricultural soil owing to anthropogenic activities.
Heavy metal pollution in soil occurs mainly in mining areas (Fakayode and Onianwa 2002). Heavy metals accumulate in soil from weathering, industrial effluents, agricultural chemicals, and toxic gases in the air that eventually mix with the soil due to rain (Wang et al. 2004). Moreover, heavy metals are diffused through the atmosphere and water bodies in the surrounding areas. Acosta et al. (2011) analyzed two tailings ponds of an abandoned Pb–zinc(Zn) mine and found that the concentration of heavy metals was higher than in distant areas. High concentrations of soluble Zn2+ and Cd2+ have seeped into streams and groundwater Meshalkina et al. (1996) observed high levels of S, V, and As in soil 1–2 km from a Russian sulfuric acid production plant.
According to Huang et al. (2018), more than 70% of land in suburbs contaminated by mines has been used for agriculture. Heavy metals in soil affect the local ecology and agricultural products. Papaioannou et al. (2019) treated soil samples with wastewater containing heavy metals to reduce the beet dry matter yield. Antoniadis et al. (2017) found that heavy metals tend to bioaccumulate in the food chain and gradually affect the safety of residents in the surrounding areas. Heavy metals tend to settle in polluted areas for a long time owing to their long half-lives, which poses daunting challenges to the ecological restoration of mining areas (Chen and Guo 2014). Chao et al. (2016) found that the structure of the microbial community in mining areas was significantly different from that in unmined areas. To protect the ecology of mining areas and the health of local residents, it is imperative to study pollution due to heavy metals in soil.
The microbial communities in soil are very sensitive to heavy metal stress. The volume of microbes and the structure of the community change when the soil is stressed by heavy metals. Therefore, the microbial diversity in soil is an important characteristic of soil quality and a sensitive indicator of soil ecology (Vinhal-Freitas et al. 2017). Zhao et al. (2019) analyzed the microbial community in the soil of a mining area and found that Proteobacteria and Firmicutes were highly resistant to heavy metals. The absorption and adsorption of microorganisms can help reduce the mobility and biological toxicity of heavy metals. The microorganisms in soil thus have a certain restorative effect on soil contaminated by heavy metals. This has practical significance for research on changes to the microbial population, plant population, and the structure and metabolic function of the microbial community when polluted by heavy metals in soil. By researching these issues, the resistance of microorganisms to heavy metals in soil can be better understood.
In the present work, we prepared the contaminated soil samples with lead chloride (PbCl2) and cadmium chloride (CdCl2) solution, and examined the effect of Pb2+ and Cd2+ on the microbial communities and enzyme activities in soil.
Materials and methods
Medium
The Martin medium was used to culture fungi in 1 L of distilled water, 10 g glucose, 5 g peptone, 1 g K2HPO4, 0.5 g MgSO4·7H2O, 18 g agar, 3.3 mL of 10 g/L Bengal red solution, and 3 mL of 10 g/L streptomycin solution prepared just before use. The improved Gause medium was used to culture the actinomycete in 1 L of distilled water, 20 g starch, 0.5 g NaCl, 1 g KNO3, 0.01 g FeSO4·7H2O, 0.5 g K2HPO4, 0.5 g MgSO4·7H2O, and 18 g agar. The beef extract-peptone agar medium was used to culture the bacteria in 1 L of distilled water, 5 g peptone, 3 g beef paste, and 18 g agar.
Soil samples
The soil samples were collected from Buxian Mountain in uncontaminated form with abundant vegetation. The samples were collected from four corners and the center of a piece of land 20 m × 20 m at a depth of 10–15 cm to avoid biases. They were mixed thoroughly, air dried, crushed in a ceramic mortar, and sieved through a <2 mm mesh. Then, they were divided into two parts and put in aseptic polythene bags. The first sample was used to prepare soils contaminated by heavy metals, and determine the structural characteristics of the microbial community and enzyme activity in soil. The second sample was used as control check (CK). The two samples were stored in refrigerator at 4 °C for further use.
Simulating heavy metal pollution
The concentration gradients of the added Cd2+ were 11.2, 16.8, 22.4, and 28.0 mg/kg. The concentration gradients of the added Pb2+ were 10.35, 20.70, 31.05, and 41.40 mg/kg.
Soil samples added with Cd2+ at 280 mg/kg and Pb2+ at 414 mg/kg were prepared by using 100 g of air-dried soil soaked in 0.5 mol/L of a cadmium chloride (CdCl2) solution and a 0.5 mol/L lead chloride (PbCl2) solution, respectively. The samples were then mixed with the air-dried soil in proportion to the above-mentioned amount of contaminated soil samples as shown in Table 1.
The eight soil samples were examined for the microbial analysis of soil, enzyme activity, and the structure of the microbial community. All experiments were carried out in triplicate.
Microbial analysis of soil
For microbial analysis, 10 g of soil was put into 100 mL of distilled water in a 250 mL flask. It was stirred with a magnetic stirrer for 30 min to thoroughly suspend the soil particles. The suspension was then diluted to obtain dilutions of 10−5, 10−6, and 10−7. These suspensions were then added to the previously prepared medium according to the required concentration, respectively. The 10−5 soil suspension was inoculated into the Martin medium, the 10−6 suspension was inoculated into the improved Gause medium, and the 10−7 suspension was inoculated into the beef extract-peptone agar medium. These media were then inoculated with 200 μL of inoculum in a shaker incubator for 4 days at 30 °C.
Assays of enzyme activity in soil
Different assays were followed to detect enzyme activity in soil, including urease, sucrase, and acid phosphatase. Urease activity in soil was assayed by the indophenol blue colorimetric method (Huang et al. 2015). For sucrase, the 3,5-dinitrosalicylic acid colorimetric method was used (Li and Zheng et al. 2016), whereas acid phosphatase was determined by the phenyl phosphate disodium colorimetric method (Li and Geng et al. 2016).
To quantitatively reflect the degree of inhibition of enzyme activity in soil by different concentrations of heavy metals, the enzyme activity inhibition rate was used, which was estimated as follows (Fang et al. 2017):
Analysis of structural characteristics of microbial community
The structural characteristics of the microbial community in soil were analyzed by using Biolog-ECO microplate analysis (Garland 1996). The moisture content of soil was calculated by the drying method. Soil samples were first activated by incubation at 25 °C for 24 h, and 10 g (dry weight) of the activated soil was added to 90 mL of a 0.145 mol/L sodium chloride (NaCl) solution in a 250 mL flask. The flask was shaken for 30 min. The supernatant was diluted to 10−3, inoculated at 150 μL per well to the ECO plate, and incubated in the dark at 25 °C for 7 days. The aseptic water was added into the control well instead of the supernatant.
The metabolic activity of microorganisms is shown by the 590 nm value minus the 750 nm value. Well optical density values under 0.06 were set to zero (Classen et al. 2003). The average well color development (AWCD) was calculated using the following equation (Wang et al. 2019):
where C is the absorbance of each well, and R is the absorbance of the control well in the plate (i.e., blank). The AWCD value reflected the overall utilization of 31 carbon sources (Choi and Dobbs 1999; Kumar et al. 2017; Shrestha et al. 2019).
The data for the 72 h culture were selected for analysis, and the Simpson index (D), Shannon diversity index (H), and McIntosh index (U) were calculated to characterize the functional diversity of the microbial community in soil. The parameter was estimated as follows (Zak et al. 1994; Yang et al. 2013):
Where ni = Ci − R, Ci is the absorbance of each well and R is the absorbance of the control well; S is the number of wells in which color changes occurred, i.e., 31; Pi is the ratio of the relative absorbance of each well (Ci − R) to the sum of the relative absorbance of the entire plate. When calculating the Simpson index (D), the data were expanded 1000 times to prevent from having a negative value (Wang et al. 2010).
Results and discussion
Microbial culture in soil contaminated with Cd2+ and Pb2+
The results in Table 2 show that the highest microbial growth occurred in CK, and the addition of heavy metals significantly affected the growth of the microorganisms. After the addition of the heavy metals, the growths of the three colonies were affected. With increasing concentration of heavy metals, the impact became more significant.
The number of fungal communities in Cd-contaminated soil decreased by more than 75% and that of actinomycetes decreased by more than 88%. It is clear that Cd pollution had a greater impact on the growth of actinomycetes in soil. The number of fungi communities in Pb-contaminated soil decreased by more than 31%, that of actinomycetes decreased by more than 88%, and that of bacterial communities decreased by more than 27%. It is clear that Pb pollution had the greatest influence on the growth of actinomycetes in soil and the smallest impact on the growth of bacteria.
Cd pollution had a greater impact on the growth of microorganisms than Pb pollution. The two heavy metal had the greatest influence on the growth of the actinomycetes and the smallest impact on that of the bacteria.
The most deleterious effect was observed on the bacteria as no growth was determined in the highest Cd-contaminated soils. The experiment was repeated to eliminate operational errors. The results were also confirmed by using the following Cd concentrations: 3.4, 6.8 and 19.6 mg/kg.
Figure 1 shows that at low Cd concentrations, the number of bacteria generally decreased with an increase in the added amount. When the added Cd2+ was 22.4 mg/kg, the number of bacteria increased but was still lower than that in untreated soil samples.
Heavy metal-resistant microorganisms are often found in soil, and low concentrations of Cd may promote their growth. The number of bacteria increased at 22.4 mg/kg of Cd, and a Cd-resistant bacterial group became the dominant species at this concentration. Research has shown that the number of bacteria in the soil treated by low concentrations of Cd2+ does not exhibit any regularity, but high concentrations of Cd have a significant inhibitory effect on microorganisms in soil.
Effects of heavy metals on enzyme activities in soil
The absorbance of each sample was measured by the colorimetric method and the activity of various enzymes was calculated by a standard curve. The results are shown in Table 3.
The above results indicate that different concentrations of Cd2+ and Pb2+ had posed different degrees of inhibition on the activities of the tested enzymes, and this inhibition increased with the concentration of heavy metal ions.
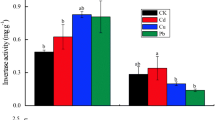
Urease was more strongly inhibited by Cd than by Pb as 94.3% of its activity was inhibited at 28.0 mg/kg of Cd. With an increase in the concentration of heavy metal ions, the activity of urease in soil decreased significantly. Urease activity decreased by nearly 50% as Cd2+ increased and from 0.205 to 0.167 mg as Pb2+ increased. Therefore, urease in soil is highly sensitive to Cd2+. Gao et al. (2008) have shown that the activity of urease can be used as an indicator for pollution due to mercury (Hg) and Cd.
Sucrase in soil acts as a hydrolase and its activity can affect the carbon cycle. It is also an important indicator of nutrient supply capacity, and soil fertility and quality. The data showed that Cd2+ and Pb2+ had an inhibitory effect on sucrase activity in soil, but Cd2+ were more toxic. 11.2 mg/kg of Cd2+ led to a reduction in sucrose from 11.64 to 3.74 mg, which inhibited its activity by 67.9%. Therefore, Cd2+ had a significant influence on sucrase activity in soil. Similarly, when Pb2+ was at 10.35 mg/kg, the sucrase in soil decreased by 59.8% and enzyme activity recorded a significant drop.
The inhibitory effects of Cd2+ and Pb2+ on acid phosphatase activity in soil were similar, but the inhibition of Pb2+ was greater than that of Cd2+. When the additive amounts of Pb2+ were from 20.70 to 41.40 mg/kg, acid phosphatase activity in soil was nearly constant. It may be that 31.05 mg/kg of Pb2+ was the maximum inhibitory concentrations of acid phosphatase in soil. In addition, Pb2+ inhibited acid phosphatase in soil, whereas the inhibition range of Cd2+ was wider.
In summary, Cd2+ inhibited urease and sucrase in soil more strongly than Pb2+, and Pb2+ inhibited acid phosphatase activity more strongly than Cd2+. The order of inhibition of Cd2+ on the three enzymes was urease > acid phosphatase > sucrose. Because urease is the most sensitive to contamination by Cd2+, it can be used as an indicator of Cd pollution. The order of inhibition of Pb2+ on the three enzymes was acid phosphatase > sucrose > urease. Because acid phosphatase in soil is the most sensitive to pollution from Pb2+, it can be used as an indicator of Pb pollution.
Correlation between enzyme activities in soil and heavy metal pollution
Soil microbes are an important part of the soil ecosystem, and there is a correlation between the degree of heavy metal pollution and enzyme activities in soil. The correlation and regression analyses of the microbial community and enzyme activity in soil with Cd and Pb are shown in Table 4 and Table 5.
The results show that there is significant negative correlation between the number of fungal communities in soil and the degree of pollution due to the two heavy metals, and the fitting effect was good. This shows that fungi in soil were sensitive to pollution due to two heavy metals. The number of bacterial communities in soil was significantly negatively correlated only with the degree of Pb pollution, whereas the correlation with Cd content was not significant and the fitting effect was poor. This indicates that the mechanism of Cd affecting the bacterial community is complex, and resistant bacteria might have multiplied under specific concentrations of Cd2+ (Griffiths and Philippot 2013).
The correlation between the heavy metal ions and enzymes in soil did not reach a significant level, but the enzymes were more significantly affected by Cd2+ than by Pb2+. The effect of heavy metals on the enzymes is complex. Heavy metals can inhibit the growth of microorganisms, which in turn affects the synthesis of the enzymes. In addition, enzyme activity in soil increases due to “resistant-enzyme activity“ caused by the proliferation of partial resistant- microorganisms (Griffiths and Philippot 2013). Enzyme activity in soil is also affected by other physical and chemical properties of the soil. Therefore, the results of different soil samples affected by heavy metals are different as reported in the literature. For example, Angelovičová et al. (2014) found the urease was significantly negatively affected by Pb and Zn, but the negative correlation between Pb and acid phosphatase was not significant. Zhao et al. (2015) found that heavy metals had no significant effect on urease activity, and Zhang et al. (2015) found no significant correlation between Pb content and enzymes in soil.
Effect of heavy metals on metabolic activity of microbial community in soil
Figure 2 shows that the AWCD value increased with culturing time, but hardly changed within 24 h after the inoculation because microbes require a certain response time to utilize different carbon sources. In the next 24 to 96 h, the AWCD value increased most rapidly. Thus, the microbial biomass in soil was abundant during this period. The growth rate from 96 to 192 h declined, possibly because of the depletion of carbon sources.
Within the first 72 h, only bacteria was visible, which is evident from the rapid rise in the AWCD value with time. After 72 h, the growth of fungi was evident and the curve was still on the rise. However, the growth rate was slow over time because the carbon source in the plate had been consumed.
Compared with uncontaminated soil, the AWCD values of microbes in soil with Cd and Pb decreased. Except for those of 10.35 mg/kg of Pb and 28.0 mg/kg of Cd, the curves showed that the AWCD value gradually decreased with an increase in the concentration of heavy metals.
Effect of heavy metals on functional diversity of microbial community in soil
Table 6 shows the effect of Cd and Pb on functional diversity of microbial community.The Simpson index (D) is used to reflect the most common and dominant species in the treated samples (Xu et al. 2008). It was found to increase with the concentration of Cd2+. Therefore, there was no significant effect on community dominance at 22.4 and 28.0 mg/kg of Cd2+. Only the Simpson index at 31.05 mg/kg of Pb2+ decreased by 5.5%, whereas other amounts of Pb2+ had no significant effect on community dominance.
The Shannon diversity index (H) is used to characterize the richness of microbial communities (Garland 1996; Dan et al. 2019). The data show that the addition of heavy metal influenced the richness of microbial communities, but there was no obvious effect of concentration gradient. The greatest impact on microbial richness was found at 11.2 mg/kg of Cd2+ and 20.70 mg/kg of Pb2+, with a decrease of 7.8 and 14%, respectively.
The McIntosh index (U) is a diversity index based on the multidimensional space of the microbial community, and is used to reflect the uniformity of microbial communities in soil (Xu et al. 2008). Except for 10.35 mg/kg of Pb2+ and 28.0 mg/kg of Cd2+, the amount of heavy metal added influenced the spatial diversity of microbes in soil compared to untreated soil, but there was no prominent effect of concentration gradient. The McIntosh index was minimum at 11.2 mg/kg of Cd2+ and 20.70 mg/kg of Pb2+, with a decrease of 27.4 and 26.1%, respectively.
Conclusion
The results of this study show that the microbial communities in soil were increasingly inhibited over time and the number of such colonies decreased with an increase in the concentration of Cd2+ and Pb2+. The two heavy metals had the greatest influence on actinomycetes in soil, followed by fungi, and had the smallest influence on bacterial growth. However, Cd contamination had odd effects on the microbial communities, and this requires further investigation.
Cd2+ and Pb2+ also affected the activities of urease, invertase, and acid phosphatase in soil. Urease showed the highest sensitivity to Cd2+ and can be used as an indicator of Cd pollution. Acidic phosphatase had the highest sensitivity to Pb and can be used as an indicator of Pb pollution. The environmental quality of the contaminated soil needs further examination.
The AWCD value gradually increased with incubation time, and Cd and Pb inhibited the metabolic activities of the microorganisms in soil. The addition of heavy metals affected the microbial diversity index, but there was no prominent effect of gradient concentration. The Biolog method is effective and easy to perform, but only culturable microorganisms that can utilize carbon sources on the board can be reflected through the method. In the future, a variety of analytical methods should be used to assess the functional diversity of microbial communities in soil.
References
Acosta JA, Faz A, Martínez-Martínez S, Zornoza R, Carmona DM, Kabas S (2011) Multivariate statistical and GIS-based approach to evaluate heavy metals behavior in mine sites for future reclamation. J Geochem Explor 109(1):8–17
Angelovičová L, Lodenius M, Tulisalo E, Fazekašová D (2014) Effect of heavy metals on soil enzyme activity at different field conditions in Middle Spis mining area (Slovakia). Bull Environ Contam Toxicol 93(6):670–675
Antoniadis V, Shaheen SM, Boersch J, Frohne T, Laing GD, Rinklebe J (2017) Bioavailability and risk assessment of potentially toxic elements in garden edible vegetables and soils around a highly contaminated former mining area in Germany. J Environ Manag 186(2):192–200
Chao Y, Liu W, Chen Y, Chen W, Zhao L, Ding Q, Wang S, Tang Y, Zhang T, Qiu R (2016) Structure, variation, and co-occurrence of soil microbial communities in abandoned sites of a rare earth elements mine. Environ Sci Technol 50(21):11481
Chen Q, Guo X (2014) The current research progress on the plant response to cadmium enriched in soil. Bot. Res 3:111–116
Choi KH, Dobbs FC (1999) Comparison of two kinds of Biolog microplates (GN and ECO) in their ability to distinguish among aquatic microbial communities. J Microbiol Method 36(3):203–213
Classen AT, Boyle SI, Haskins KE, Overby ST, Hart SC (2003) Community-level physiological profiles of bacteria and fungi: plate type and incubation temperature influences on contrasting soils. FEMS Microbiol Ecol 44:319–328
Dan X, Xiao S, Ye Y, Wei Z, He X, Wang K (2019) Microbial biomass, metabolic functional diversity, and activity are affected differently by tillage disturbance and maize planting in a typical karst calcareous soil. J Soil Sediment 19(2):809–821
Fakayode SO, Onianwa PC (2002) Heavy metal contamination of soil, and bioaccumulation in Guinea grass (Panicum maximum) around Ikeja Industrial Estate, Lagos, Nigeria. Environ Geol 43(1–2):145–150
Fang L, Liu Y, Tian H, Chen H, Wang Y, Huang M (2017) Proper land use for heavy metal-polluted soil based on enzyme activity analysis around a Pb-Zn mine in Feng County, China. Environ Sci Pollut Res 24:28152–28164
Gao D, Hao J, Jin J, Cui X, Li D, Hong S, Liu H (2008) Effects of single stress and combined stress of Hg and Cd on soil enzyme activities. J Agric Environ Sci 27(3):73–78
Garland JL (1996) Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biol Biochem 28(2):213–221
Griffiths BS, Philippot L (2013) Insights into the resistance and resilience of the soil microbial community. Fems Microbiol Rev 37(2):112–129
Huang Y, Chen Q, Deng M, Japenga J, Li T, Yang X, He Z (2018) Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in southeast China. J Environ Manag 207:159–168
Huang J, Li Z, Zhang J (2015) Improvement of indophenol blue colorimetric method on activity of urease in soil. J Civil Archit Environ Eng 34(1):102–107
Kumar U, Shahid M, Tripathi R, Mohanty S, Kumar A, Bhattacharyya P, Lal B, Gautam P, Raja R, Panda BB, Jambhulkar NN, Shukla AK, Nayak AK (2017) Variation of functional diversity of soil microbial community insub-humid tropical rice-rice cropping system under long-termorganic and inorganic fertilization. Ecol Indic 73(73):536–543
Li Y, Geng Y, Zhou H, Yang Y (2016) Comparison of soil acid phosphatase activity determined by different methods. Chin J Ecol Agric 24(1):98–104
Li Z, Zheng L (2016) Soil sucrase: detection conditions based on DNS colorimetric. Chin Agric Sci Bull 27(32):171–176
Meshalkina JL, Stein A, Makarov OA (1996) Spatial variability of soil contamination around a sulphureous acid producing factory in Russia. Water Air Soil Pollut 92(3–4):289–313
Papaioannou D, Kalavrouziotis IK, Koukoulakis PH, Papadopoulos F, Psoma P, Mehra A (2019) Simulation of soil heavy metal pollution environmental stress on plant growth characteristics in the presence of wastewater. Glob Nest J 21(1):23–29
Shrestha P, Gautam R, Ashwath N (2019) Effects of agronomic treatments on functional diversity of soil microbial community and microbial activity in a revegetated coal mine spoil. Geoderma 338:40–47
Vinhal-Freitas IC, Corrêa GF, Wendling B, Bobuľská L, Ferreirab AS (2017) Soil textural class plays a major role in evaluating the effects of land use on soil quality indicators. Ecol Indic 74:182–190
Wang Q, Kim D, Dionysiou DD, Sorial GA, Timberlake D (2004) Sources and remediation for mercury contamination in aquatic systems—a literature review. Environ Pollut 131(2):323–336
Wang S, Li T, Zheng Z, Chen H (2019) Soil aggregate-associated bacterial metabolic activity and community structure indifferent aged tea plantations. Sci Total Environ 654:1023–1032
Wang X, Wang Y, Yan H, Chu X, Yu Y (2010) Effects of repeated application of carbendazim on its persistence and functional diversity of soil microbial communities. Acta Pedol Sin 47:131–137
Wei B, Yang L (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94(2):99–107
Xu P, Du H, Peng X, Tang Y, Zhou Y, Chen X et al. (2020) Degradation of several polycyclic aromatic hydrocarbons by laccase in reverse micelle system. Sci Total Environ 708:134970
Xu Q, Jiang P, Xu Z (2008) Soil microbial functional diversity under intensively managed bamboo plantations in southern China. J Soil Sediment 8(3):177
Yang Q, Wang X, Shen Y, Philp JNM (2013) Functional diversity of soil microbial communities in response to tillage and crop residue retention in an eroded Loess soil. Soil Sci Plant Nutr 59(3):311–321
Zak J, Willig MR, Moorhead DL, Wildman HG (1994) Functional diversity of microbial communities: a quantitative approach. Soil Biol Biochem 26(26):1101–1108
Zhang F, Luo X, Wang J (2015) Effects of uranium and associated heavy metals Mn and Pb on soil enzyme activities. Environ Sci Technol 38(03):44–49
Zhao X, Huang J, Lu J, Sun Y (2019) Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotoxicol Environ Saf 170:218–226
Zhao Y, Zhang J, Zhou D, Wang C (2015) Mixed heavy metals contamination of tungsten mine area soil in south of Jiangxi province. China Environ Sci 35(08):2477–2484
Funding
This study was supported by the Fundamental Research Funds for the Central Universities (2019XKQYMS60).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiao, L., Yu, Z., Liu, H. et al. Effects of Cd and Pb on diversity of microbial community and enzyme activity in soil. Ecotoxicology 29, 551–558 (2020). https://doi.org/10.1007/s10646-020-02205-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-020-02205-4