Abstract
Neonicotinoid seed treatments are extensively used to systemically protect corn from invertebrate herbivory. Interseeding cover crops can promote beneficial insect communities and their ecosystem services such as predation on pests, and this practice is gaining interest from farmers. In this study, cereal rye (Secale cereale) and hairy vetch (Vicia villosa) were planted between rows of early vegetative corn that had been seed-treated with thiamethoxam. Thiamethoxam and its insecticidal metabolite, clothianidin were quantified in cover crop leaves throughout the growing season. Thiamethoxam was present in cereal rye at concentrations ranging from 0 to 0.33 ± 0.09 ng/g of leaf tissue and was detected on six out of seven collection dates. Cereal rye leaves contained clothianidin at concentrations from 1.05 ± 0.22 to 2.61 ± 0.24 ng/g and was present on all sampling dates. Both thiamethoxam and clothianidin were detected in hairy vetch on all sampling dates at rates ranging from 0.10 ± 0.05 to 0.51 ± 0.11 ng/g and 0.56 ± 0.15 to 9.73 ± 5.04 ng/g of leaf tissue, respectively. Clothianidin was measured at a higher concentration than its precursor, thiamethoxam, in both plant species on every sampling date. Neonicotinoids entering interseeded cover crops from adjacent treated plants is a newly discovered route of exposure and potential hazard for non-target beneficial invertebrates. Future research efforts should examine the effects of systemic insecticides on biological communities in agroecosystems whose goal is to diversify plant communities using methods such as cover cropping.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Under conventional agricultural management, corn grown as a monoculture is damaged by numerous invertebrate pests (SDSU 2018), which annually cost US farmers > $3 billion in control measures (Lundgren and Fausti 2015). Farmers typically react to corn pests once they reach a certain population threshold by applying insecticides (Bode and Calvin 1990). Alternatively, prophylactic control methods can be employed which typically involve use of genetically modified plants producing insecticidal proteins (Tabashnik 2010), or insecticidal seed treatments, such as neonicotinoids (Douglas and Tooker 2015).
Neonicotinoid seed treatments are commonly used by agricultural producers worldwide (>120 countries) on a large number of crops (Jeschke et al. 2011). It is estimated that 70–100% of corn seed planted in the U.S. (37.1 million ha) in 2011 were treated with neonicotinoids (NASS 2011; Douglas and Tooker 2015). Neonicotinoids are highly water soluble and enter a plant’s root system, eventually becoming systemic throughout all tissues and exudates (Bredeson and Lundgren 2018; Cowles and Eitzer 2017; Laurent and Rathahao 2003). The aim of systemically treating crops with insecticides is to kill herbivorous pests while limiting insecticide contact to non-pests. However, there are numerous cases describing detrimental effects of neonicotinoid seed treatments on non-target invertebrate species (Douglas et al. 2015; Gontijo et al. 2014, 2015; Mogren and Lundgren 2016; Moser and Obrycki 2009; Pisa et al. 2017) and communities (Bredeson and Lundgren 2018; Seagraves and Lundgren 2012). When exposed to systemic neonicotinoids through any of several possible routes (contaminated nectar (Bredeson and Lundgren 2018), pollen (Krupke et al. 2012), prey (Bredeson et al. 2015; Douglas et al. 2015), vegetation (Bredeson and Lundgren 2015), and dust (Krupke et al. 2012), etc.), susceptible non-targeted insects can experience direct mortality (Douglas et al. 2015) or altered behavior and possible indirect mortality if sublethal concentrations are encountered (Henry et al. 2012).
Cover crops are one way that agricultural producers can reduce their input costs, lower the environmental impact of their operations, and reduce pesticide costs. In fact, US producers increased the number of hectares planted to cover crops on their farms by over 84% between 2012 and 2016 (SARE-CTIC 2016). Typically, cover crops are established on farmland outside the period when cash crops are growing, but some farmers incorporate cover crops onto agricultural land while row crops such as corn are actively growing. Cover crops can be employed as a versatile tool to assist in meeting several agronomic goals (including, but not limited to: nutrient uptake (Li et al. 2014), nitrogen fixing (Ashworth et al. 2017), weed suppression (Khan et al. 2006), amelioration of microclimates (Orr et al. 1997), water infiltration (Haruna et al. 2018), erosion prevention (Alliaume et al. 2014), providing wildlife habitat (Wilcoxen et al. 2018), stimulation of microbial communities (Schmidt et al. 2018), and building organic matter (LaCanne and Lundgren 2018)).
An emerging practice among these producers is the use of cover crops to improve habitat suitability for insect diversity, predator communities, and improve the biological control of pest arthropods. (Barbosa 1998) (LaCanne and Lundgren 2018; Landis et al. 2000) (Lundgren and Fergen 2014). This works in part by providing habitat for natural enemies and non-prey foods (Lundgren 2009), alternative prey or hosts (Manandhar and Wright 2016), and a variety of microclimates (Orr et al. 1997). Within interseeded cover crops, Manandahar and Wright (2016) observed an increase in parasitization of Helicoverpa zea eggs by Trichogramma spp. when cowpeas (Vigna unguiculata) and sunnhemp (Crotalaria juncea) were established between rows of sweetcorn. The same study also revealed that interseeded buckwheat (Fagopyrum esculentum) increased predator Orius spp. populations in relation to pest abundance compared to sweetcorn monocultures, likely due to the provisioning of nectar by flowering plants. Similarly, in corn fields where the stem borer, Chilo partellus (Swinhoe) is of economic concern, interseeding lablab (Lablab purpurens) effectively reduces pest infestations (Maluleke et al. 2005).
Systemic neonicotinoids unintentionally present in the leaves and flowers of cover crop species may increase the likelihood that beneficial invertebrates attracted to cover crops are exposed to the toxins. Scenarios have recently been described where the neonicotinoid clothianidin was detected in untreated milkweed (Asclepias syriaca) (Pecenka and Lundgren 2015) and dandelion (Taraxacum officinale) (Krupke et al. 2012) tissue growing along the margins of seed-treated cornfields. The purpose of the present study was to quantify neonicotinoids thiamethoxam and metabolite, clothianidin, in cereal rye (Secale cereal L.) and hairy vetch (Vicia villosa Roth) leaf tissue growing between rows of seed-treated corn under field conditions.
Methods
Treated cornfield establishment
Experimental plots were established on a farm located near Toronto, South Dakota, USA (44.585211, −96.579910; latitude, longitude). Field corn (Zea mays) (Legend Seeds Inc.; variety mixture: A10946, A10258; 95% Bt, 5% refuge seed; Maturity: 97 days) was planted in a 8-ha field on May 5, 2017 at a rate of 79,074 seeds per ha with 76-cm between rows. The field was planted to untreated spring wheat in 2016. Corn seed was pre-treated with 0.25 mg of thiamethoxam per seed (CruiserMaxx® 250, Syngenta, Greensborough, NC, USA).
Plots were fertilized prior to corn planting according to soil test results. Urea, monoammonium phosphate, potash, ammonium sulfate and zinc were applied at rates of 162.6, 84.0, 84.0, 11.1, and 3.5 kg/ha, respectively. A pre-emergent herbicide mixture was applied on May 6, 2017 following corn planting. The mixture consisted of acetochlor, mesotrione, clopyralid MEA salt (3 L/ha; Resicore®, Dow AgroSciences, Indianapolis, IN, USA), atrazine (1.2 L/ha; Syngenta), glyphosate (1.2 L/ha; Roundup PROMAX® glyphosate; Monsanto Company, St. Louis, MO, USA) and a surfactant containing ammonium sulfate, corn syrup, and alkyl polyglucoside (1.2 L/ha; Class Act®, Winfield, St. Paul, MN, USA). Urea was broadcasted into plots on June 17, 2017 at a rate of 51.6 kg N/ha. No post-emergent herbicides were used.
Interseeded cover crops
Six, single-row plots (10 m long) of interseeded cover crops were planted between corn rows on June 6, 2017; corn was in the two-leaf stage of development. Each plot was separated by 2.3 m of corn and bare soil. Cover crop species used in the mixture were hairy vetch (Vicia villosa, 3.5 kg/ha), lentil (Lens culinaris, 3.5 kg/ha), Japanese millet (Echinochloa esculenta, 3.5 kg/ha), sorghum × sudangrass (Sorghum bicolor ssp. Drummondii, 5.7 kg/ha), cereal rye (Secale cereale 14.6 kg/ha), field pea (Pisum sativum 14.6 kg/ha) and flax (Linum usitatissimum 8.9 kg/ha) (rate recommendations advised by Greencover Seed®, Bladen, NE, USA). Cover crop seeds were dispersed by hand into a 3-cm deep furrow that was created directly in between the corn rows.
Plant tissues were collected from thiamethoxam seed-treated fields on June 19, 28, July 6, 14, 25, August 17, and October 6. On each collection date, three hairy vetch and three cereal rye samples were taken from each of the six plots for a total of 18 samples of each species on each collection date. For consistent tissue collection the most recently developed hairy vetch trifoliates were removed, and approximately 8 cm of rye tissue from the most apical leaves were taken for analysis. Forceps sterilized in 70% ethanol were used to remove leaves from individual plants, and all leaf tissues were weighed. Samples were frozen at −20 °C until insecticide quantification.
Greenhouse-grown control plants
Untreated plants and soils in nature are routinely contaminated with neonicotinoids in the field (Ainsley et al. 2014; Botías et al. 2015; Krupke et al. 2012; Mogren and Lundgren 2016; Pecenka and Lundgren 2015), and so we produced cover crop plants in the greenhouse to create untreated controls. Plastic flower pots (n = 5, 150 mm tall × 105 mm wide at the base, Kord Traditional Std.; Brantford, Ontario, Canada) were filled to a depth of 10 cm with potting soil (Master Garden Premium Garden Soil: Flower and Vegetable; Premier Horticulture Ltd.; Riviere-du-Loup, Quebec, Canada). Cover crop seed mixture (15 mL) was spread onto the soil surface and covered with 1 cm of soil. Pots were watered to soil saturation every other day. Greenhouse conditions were set to 16:8 h (light:dark) photoperiod (300 W; Viparspectra V300 LED lights; Shenzhen Bailuo Technology Co., Ltd., 638 Block C Baoyuan, Shenzhen, China), 24 °C, with variable humidity. Samples of hairy vetch and cereal rye leaf tissue were harvested from greenhouse-grown plants after 5 weeks. Forceps sterilized in 70% ethanol were used to remove leaves from individual plants, and all leaf tissue was weighed. Samples were frozen at −20 °C until analysis.
Insecticide analysis
Leaf tissue was homogenized using a plastic pestle in distilled water at a ratio of 600 µL water/0.1 g tissue. Samples were agitated for 1 h at room temperature using an orbital shaker set to 210 rpm (orbit diameter: 22 mm). Samples were vortexed for 10 s, and then centrifuged at 10,000 × g for 5 min. Supernatant was separated from solid materials, diluted to 20% using distilled water and placed into a new 1.5 mL centrifuge tube.
Standard curves (n = 3) of known insecticide concentrations were run on each ELISA plate. Supernatant from untreated control plants was mixed with distilled water to achieve a 40% concentration (4:6, v:v; supernatant:water). Thiamethoxam (Thiamethoxam PESTANAL®, Sigma-Aldrich®, Product number: 37924, St. Louis, MO, USA) and clothianidin (Sigma-Aldrich®, Product number: 33589) dilutions were established in distilled water at 2X the final desired concentrations (0, 0.0625, 0.125, 0.25, 0.5, 1.0, 2.0 and 4.0 ng/mL). Final standard concentrations were made by mixing equal parts 40% control supernatant and 2X thiamethoxam or clothianidin mixtures, yielding standards used directly in ELISA analyses composed of 20% supernatant at 0, 0.03, 0.06, 0.13, 0.25, 0.5, 1.0 and 2.0 ng/mL thiamethoxam or clothianidin, depending on which insecticide was being quantified.
Insecticide analysis via ELISA was conducted based on kit instructions (Thiamethoxam HS plate kit, lot no. 10031; Beacon Analytical Systems Inc., Saco, ME, USA, and clothianidin, Product No. 500800, Abraxis LLC®, 54 Steamwhistle Drive, Warminster, PA, USA) and closely followed the procedures described previously (Bredeson and Lundgren 2018; Bredeson et al. 2015). Sample absorbances (at 450 nm) were read using a spectrophotometer (SpectraMAXX®, Molecular Devices, LLC. San Jose, CA, USA). Quantities of thiamethoxam or clothianidin were calculated based on the plate-specific standard curve series.
Data analysis
Sample absorbances were deemed outliers if they fell above or below established bounds. To establish upper and lower bounds the first and third quartiles and interquartile range (IQR) were found for a plant species on a given sampling date (n = 18 samples). An upper bound was set at 1.5 × IQR above quartile three, while a lower bound was 1.5 × IQR below quartile one. Sample sizes used to calculate mean neonicotinoid concentrations are noted in Figs 1 and 2. To avoid reporting false-positives, a sample was considered to have no detectable insecticide if its absorbance reading was a greater value than one standard deviation below the mean of negative control samples (n = 5 per ELISA plate) (sample absorbance in direct competitive ELISA is inversely correlated with insecticide concentration). Kruskal–Wallis one-way analysis of variance was used to determine differences between mean insecticide levels across sampling dates for each plant species and neonicotinoid (α = 0.05). If insecticide concentrations varied across sampling dates Dunn’s all-pairwise comparison tests were used for post-hoc analysis between sampling dates (α = 0.05). Kruskal–Wallis and Dunn’s tests were conducted using Statistix® 10 software (Analytical Software, Tallahassee, FL, USA).
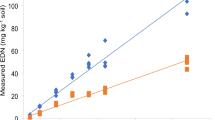
Seasonal pattern of mean ± SEM nanograms thiamethoxam and clothianidin per gram of cereal rye (Secale cereale) leaf tissue interseeded between two-leaf corn treated with CruiserMaxx 250® (Syngenta, US) seed treatment. Corn and cereal rye were planted on the 125 and 157 days of the year, respectively. Numbers above error bars represent sample sizes used to calculate mean ± SEM
Seasonal pattern of mean ± SEM nanograms thiamethoxam and clothianidin per gram of hairy vetch (Vicia villosa) leaf tissue interseeded between two-leaf corn treated with CruiserMaxx 250® (Syngenta, US) seed treatment. Corn and hairy vetch were planted on the 125 and 157 days of the year, respectively. Numbers above error bars represent sample sizes used to calculate mean ± SEM
Results
Cereal rye
Thiamethoxam was present in interseeded cereal rye leaf tissue on all sampling dates except for August 17. Thiamethoxam concentrations per date ranged from 0 to 0.33 ± 0.09 ng/g tissue with the highest being in samples collected on June 28 (Supplementary Table S1). Mean concentrations of thiamethoxam varied among sampling dates (χ26 < 0.01, P = 0.03). Thiamethoxam’s primary insecticidal metabolite, clothianidin, was found in cereal rye tissue on all collection dates and varied in mean concentration among dates (χ26 < 0.01, P < 0.01). Levels of clothianidin were found at higher levels than that of its precursor on all dates, ranging from 1.05 ± 0.22 to 2.61 ± 0.24 ng/g tissue (Supplementary Table S1), with the highest level of clothianidin collected on July 14. Both neonicotinoids in cereal rye leaf tissue tended to follow a pattern of higher concentrations for the first five sampling dates, followed by the lowest amount being recorded on the sixth date (August 17), and then a subsequent increase on the final sampling date (October 6) (Fig. 1a, b).
Hairy vetch
Thiamethoxam varied throughout the growing season (χ26 < 0.01, P < 0.01), but was present in hairy vetch leaf tissue from all sampling dates ranging from 0.10 ± 0.05 to 0.51 ± 0.11 ng/g tissue, with the lowest concentration recorded from August 17, and the highest from June 28 (Supplementary Table S1). Clothianidin within hairy vetch leaf tissue was measurable in samples from all collection dates and accounted for the three highest neonicotinoid concentrations across both plant species (Supplementary Table S1). Levels of clothianidin varied across sampling dates (χ26 < 0.01, P < 0.01) and ranged from a low of 0.56 ± 0.15 ng/g (August 17) to as high as 9.73 ± 5.04 ng/g collected during the earliest sampling date (June 16). Neonicotinoid concentrations in hairy vetch leaf tissue tended to be higher toward the beginning of the growing season, gradually tapering to a season low on the sixth sampling (August 17), followed by a slight increase on the final collection date (Fig. 2a, b).
Discussion
Risk associated with a toxicant is characterized by hazard and exposure to a particular species. Here we document that untreated cover crops are contaminated with neonicotinoid insecticides, which represents a potential risk to non-target species. Despite using the lowest commercially available rate of seed-treatment, CruiserMaxx®, and planting cover crops in a single row at a maximum distance from adjacent corn rows, neonicotinoids thiamethoxam and clothianidin were present in both cover crop species on all sampling dates except for one (Fig. 1a, Supplementary Table S1). Additional research will need to substantiate the degree to which non-target beneficial insects are exposed via cover crops and whether the concentrations documented here are harmful. An additional risk factor requiring further attention which is not addressed in this research is the potential for additive and synergistic effects between neonicotinoids and other types of pesticides often applied concurrently (David et al. 2016).
Concentrations of neonicotinoid insecticides observed in cover crop leaf tissue are near to, or exceed amounts previously found in contaminated plant tissues (Goulson 2013; Krupke et al. 2012; Pecenka and Lundgren 2015), and are at levels that can cause direct harm to beneficial insects (Prabhaker et al. 2017). For example, a recent laboratory study of monarch butterfly (Danaus plexippus) larval susceptibility to neonicotinoids revealed LC10 and LC20 values for clothianidin to be 7.72 and 9.89 parts per billion (ppb), respectively (Pecenka and Lundgren 2015). The same study also found that first instar monarchs were shorter, weighed less, and were slower to develop compared to control larvae at clothianidin concentrations as low as 0.5 ppb (Pecenka and Lundgren 2015). When Sandrock et al. (2014) exposed solitary red mason bees (Osmia bicornis) to artificial nectar spiked with sub-lethal dosages of thiamethoxam (2.78 ng/g) and clothianidin (0.45 ng/g) reproductive success was significantly altered. Specifically, neonicotinoid-exposed bees produced 47.7% fewer offspring, and at a male dominated sex ratio, in comparison to unexposed bees (Sandrock et al. 2014). Susceptibility to these insecticides varies among insects (Pisa et al. 2017), and additional toxicological work on the majority of important species in the corn system remain to be quantified.
The observed pattern of steadily decreasing insecticide concentration in systemically treated plant tissue throughout the growing season (Figs 1 and 2) represents a fluctuating exposure scenario that should be reflected in a risk analysis. This diminishing insecticide content has been documented in previous studies on neonicotinoid fate (Bredeson and Lundgren 2015). Interestingly, interseeded cereal rye and hairy vetch possessed the smallest amount of thiamethoxam and clothianidin within their tissues on the penultimate sampling date (August 17) before again trending upward for the final collection (October 6) (Figs 1 and 2). It is possible that an increase in insecticide concentration could have occurred because of factors related to corn maturity. By the final sampling date corn leaves had desiccated, and the previously dense canopy created by corn leaves had senesced allowing light necessary for cover crop growth to reach interrow spaces (den Hollander et al. 2007). Additional light penetration resulted in noticeable late-season cover crop growth which may have also increased cover crop transpiration (McNaughton and Jarvis 1991) and uptake of dissolved neonicotinoids.
Though thiamethoxam was treated to corn seeds in this study the toxic metabolite, clothianidin, was ubiquitous in both cereal rye and hairy vetch on all sampling dates and was always measured at a higher concentration compared to thiamethoxam on the same date (Figs 1 and 2). Special attention must be given to such metabolites when performing environmental risk assessments and when considering agrichemical usage where non-targets may become exposed. Pesticides metabolized into additional compounds through largely unknown processes in plants and soils can show similar or elevated toxicity (Nauen et al. 2003; Simon-Delso et al. 2015) and even persist for extended periods (Goulson 2013) when compared to their parent molecules. For example, under field conditions thiamethoxam seed-treated sunflowers possess clothianidin within leaf tissue even after thiamethoxam is no longer measurable, possibly contributing to reductions in pollinator and predatory populations in treated fields (Bredeson and Lundgren 2015, 2018). Uptake and persistence of neonicotinoids and their metabolites by interseeded cover crops pose a risk to beneficial organisms attracted to the resources provided by additional plant diversity.
References
Ainsley J, Paul H, Gordon T (2014) Neonicotinoid concentrations in arable soils after seed treatment applications in preceding years. Pest Manag Sci 70:1780–1784. https://doi.org/10.1002/ps.3836
Alliaume F, Rossing WAH, Tittonell P, Jorge G, Dogliotti S (2014) Reduced tillage and cover crops improve water capture and reduce erosion of fine textured soils in raised bed tomato systems. Agric Ecosyst Environ 183:127–137. https://doi.org/10.1016/j.agee.2013.11.001
Ashworth A et al. (2017) N2 fixation of common and hairy vetches when intercropped into switchgrass. Agronomy 7:39
Barbosa P (1998) Conservation biological control. Academic Press, San Diego, London, Boston, New York, Sydney, Tokyo, Toronto
Bode WM, Calvin DD (1990) Yield-loss relationships and economic injury levels for European corn borer (Lepidoptera: Pyralidae) populations infesting Pennsylvania field corn. J Econ Entomol 83:1595–1603. https://doi.org/10.1093/jee/83.4.1595
Botías C, David A, Horwood J, Abdul-Sada A, Nicholls E, Hill E, Goulson D (2015) Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ Sci Technol 49:12731–12740. https://doi.org/10.1021/acs.est.5b03459
Bredeson MM, Lundgren JG (2015) Thiamethoxam seed treatments have no impact on pest numbers or yield in cultivated sunflowers. J Econ Entomol. https://doi.org/10.1093/jee/tov249
Bredeson MM, Lundgren JG (2018) Thiamethoxam seed treatments reduce foliar predator and pollinator populations in sunflowers (Helianthus annuus), and extra-floral nectaries as a route of exposure for seed treatments to affect the predator, Coleomegilla maculata (Coleoptera: Coccinellidae). Crop Prot 106:86–92. https://doi.org/10.1016/j.cropro.2017.12.019
Bredeson MM, Reese RN, Lundgren JG (2015) The effects of insecticide dose and herbivore density on tri-trophic effects of thiamethoxam in a system involving wheat, aphids, and ladybeetles. Crop Prot 69:70–76. https://doi.org/10.1016/j.cropro.2014.12.010
Cowles RS, Eitzer BD (2017) Residues of neonicotinoid insecticides in pollen and nectar from model plants. J Environ Hortic 35:24–34. https://doi.org/10.24266/0738-2898-35.1.24
David A, Botias C, Abdula-Sada A, Nicholls E, Rotheray E, Hill E, Goulson D (2016) Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ Int 88:169–178
den Hollander NG, Bastiaans L, Kropff MJ (2007) Clover as a cover crop for weed suppression in an intercropping design: II. Competitive ability of several clover species. Eur J Agron 26:104–112. https://doi.org/10.1016/j.eja.2006.08.005
Douglas MR, Rohr JR, Tooker JF (2015) EDITOR’S CHOICE: neonicotinoid insecticide travels through a soil food chain, disrupting biological control of non-target pests and decreasing soya bean yield. J Appl Ecol 52:250–260. https://doi.org/10.1111/1365-2664.12372
Douglas MR, Tooker JF (2015) Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in US field crops. Environ Sci Technol 49:5088–5097
Gontijo PC, Moscardini VF, Michaud JP, Carvalho GA (2014) Non-target effects of chlorantraniliprole and thiamethoxam on Chrysoperla carnea when employed as sunflower seed treatments. J Pest Sci 87:711–719. https://doi.org/10.1007/s10340-014-0611-5
Gontijo PC, Moscardini VF, Michaud JP, Carvalho GA (2015) Non-target effects of two sunflower seed treatments on Orius insidiosus (Hemiptera: Anthocoridae). Pest Manag Sci 71:515–522. https://doi.org/10.1002/ps.3798
Goulson D (2013) REVIEW: an overview of the environmental risks posed by neonicotinoid insecticides. J Appl Ecol 50:977–987. https://doi.org/10.1111/1365-2664.12111
Haruna S, Nkongolo N, Anderson S, Eivazi F, Zaibon S (2018) In situ infiltration as influenced by cover crop and tillage management. J Soil Water Conserv 73:164–172
Henry M, Béguin M, Requier F, Rollin O, Odoux J, Aupinel P, Aptel J, Tchamitchian S, Decourtye A (2012) A common pesticide decreases foraging success and survival in honey bees. Science 336:348–350. https://doi.org/10.1126/science.1215039
Jeschke P, Nauen R, Schindler M, Elbert A (2011) Overview of the status and global strategy for neonicotinoids. J Agric Food Chem 59:2897–2908. https://doi.org/10.1021/jf101303g
Khan ZR, Pickett JA, Wadhams LJ, Hassanali A, Midega CAO (2006) Combined control of Striga hermonthica and stemborers by maize—Desmodium spp. intercrops. Crop Prot 25:989–995. https://doi.org/10.1016/j.cropro.2006.01.008
Krupke CH, Hunt GJ, Eitzer BD, Andino G, Given K (2012) Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS One 7:e29268. https://doi.org/10.1371/journal.pone.0029268
LaCanne CE, Lundgren JG (2018) Regenerative agriculture: merging farming and natural resource conservation profitably. PeerJ 6:e4428. https://doi.org/10.7717/peerj.4428
Landis DA, Wratten SD, Gurr GM (2000) Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu Rev Entomol 45:175–201. https://doi.org/10.1146/annurev.ento.45.1.175
Laurent FM, Rathahao E (2003) Distribution of [14C]Imidacloprid in sunflowers (Helianthus annuus L.) following seed treatment. J Agric Food Chem 51:8005–8010. https://doi.org/10.1021/jf034310n
Li L, Tilman D, Lambers H, Zhang F-S (2014) Plant diversity and overyielding: insights from belowground facilitation of intercropping in agriculture. New Phytol 203:63–69. https://doi.org/10.1111/nph.12778
Lundgren JG (2009) Relationships of natural enemies and non-prey foods, vol 7. Springer Science & Business Media, Dordrecht, The Netherlands.
Lundgren JG, Fausti SW (2015) Trading biodiversity for pest problems. Sci Adv. 1. https://doi.org/10.1126/sciadv.1500558
Lundgren JG, Fergen JK (2014) Predator community structure and trophic linkage strength to a focal prey. Mol Ecol 23:3790–3798
Maluleke MH, Addo-Bediako A, Ayisi KK (2005) Influence of maize/Lablab intercropping on Lepidopterous stem borer infestation in maize. J Econ Entomol 98:384–388. https://doi.org/10.1603/0022-0493-98.2.384
Manandhar R, Wright MG (2016) Effects of interplanting flowering plants on the biological control of corn earworm (Lepidoptera: Noctuidae) and thrips (Thysanoptera: Thripidae) in sweet corn. J Econ Entomol 109:113–119. https://doi.org/10.1093/jee/tov306
McNaughton KG, Jarvis PG (1991) Effects of spatial scale on stomatal control of transpiration. Agric For Meteorol 54:279–302. https://doi.org/10.1016/0168-1923(91)90010-N
Mogren CL, Lundgren JG (2016) Neonicotinoid-contaminated pollinator strips adjacent to cropland reduce honey bee nutritional status. Sci Rep. 6:29608. https://doi.org/10.1038/srep29608 https://www.nature.com/articles/srep29608#supplementary-information
Moser SE, Obrycki JJ (2009) Non-target effects of neonicotinoid seed treatments; mortality of coccinellid larvae related to zoophytophagy. Biol Control 51:487–492. https://doi.org/10.1016/j.biocontrol.2009.09.001
[NASS] National Agricultural Statistics Service (2011). Quick stats. US Department of Agriculture, NASS, Washington, DC. Accessed 3 May 2018.
Nauen R, Ebbinghaus-Kintscher U, Salgado VL, Kaussmann M (2003) Thiamethoxam is a neonicotinoid precursor converted to clothianidin in insects and plants. Pestic Biochem Physiol 76:55–69. https://doi.org/10.1016/S0048-3575(03)00065-8
Orr DB, Landis DA, Mutch DR, Manley GV, Stuby SA, King RL (1997) Ground cover influence on microclimate and Trichogramma (Hymenoptera: Trichogrammatidae) augmentation in seed corn production. Environ Entomol 26:433–438
Pecenka JR, Lundgren JG (2015) Non-target effects of clothianidin on monarch butterflies. Sci Nat 102:1–4. https://doi.org/10.1007/s00114-015-1270-y
Pisa L, Goulson D, Yang E, Gibbons D, Sanchez-Bayo F, Mitchell E, Aebi A, van der Sluijs J, MacQuarrie C, Giorio C, Yim Long E, McField M, van Lexmond M, Bonmatin J (2017). An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 2: impacts on organisms and ecosystems. Environ Sci Pollut Res 1–49. https://doi.org/10.1007/s11356-017-0341-3
Prabhaker N, Naranjo S, Perring T, Castle S (2017) Comparative toxicities of newer and conventional insecticides: against four generalist predator species. J Econ Entomol 110:2630–2636. https://doi.org/10.1093/jee/tox202
Sandrock C, Tanadini LG, Pettis JS, Biesmeijer JC, Potts SG, Neumann P (2014) Sublethal neonicotinoid insecticide exposure reduces solitary bee reproductive success. Agric For Entomol 16:119–128
SARE-CTIC (2016). Cover crop survey: 2015–2016 annual report. Conservation Technology Information Center, Sustainable Agriculture Research and Education, American Seed Trade Association. http://www.sare.org/Learning-Center/Topic-Rooms/Cover-Crops/Cover-Crop-Surveys. Accessed 3 May 2018.
Schmidt R, Gravuer K, Bossange AV, Mitchell J, Scow K (2018) Long-term use of cover crops and no-till shift soil microbial community life strategies in agricultural soil. PLoS One 13:e0192953. https://doi.org/10.1371/journal.pone.0192953
[SDSU] South Dakota State University (2018) South Dakota pest management guide: corn. http://igrow.org/up/resources/03-3041-2017.pdf. Accessed 3 May 2018.
Seagraves MP, Lundgren JG (2012) Effects of neonicitinoid seed treatments on soybean aphid and its natural enemies. J Pest Sci 85:125–132. https://doi.org/10.1007/s10340-011-0374-1
Simon-Delso N et al. (2015) Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res 22:5–34. https://doi.org/10.1007/s11356-014-3470-y
Tabashnik BE (2010) Communal benefits of transgenic corn. Science 330:189–190. https://doi.org/10.1126/science.1196864
Wilcoxen CA, Walk JW, Ward MP (2018) Use of cover crop fields by migratory and resident birds. Agric Ecosyst Environ 252:42–50. https://doi.org/10.1016/j.agee.2017.09.039
Acknowledgements
We thank Kassidy Weathers, Nicole Schultz, Cedric Gentils, Liz Adee, Tommy Fenster and Alex Nikolaus for their assistance in plant tissue collection and sample processing. Mark Longfellow assisted with ELISA development.
Funding
This research was supported by general funds from Ecdysis Foundation, and grant support from Threshold Foundation, Globetrotter Foundation, and by support from farmers, ranchers, and beekeepers around the world.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MMB is employed by Ecdysis Foundation and South Dakota State University. JGL was formerly employed by USDA and is the Director for Ecdysis Foundation and CEO for Blue Dasher Farm. We have not received any research support or financial contributions from companies mentioned in this manuscript.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bredeson, M.M., Lundgren, J.G. Neonicotinoid insecticidal seed-treatment on corn contaminates interseeded cover crops intended as habitat for beneficial insects. Ecotoxicology 28, 222–228 (2019). https://doi.org/10.1007/s10646-018-02015-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-018-02015-9