Abstract
Increased pesticide use in rice agricultural ecosystems may alter competitive interactions between invasive and native amphibian species. We conducted an experiment with two rice paddy amphibians found in Luzon, Philippines, the invasive cane toad (Rhinella marina) and the endemic Luzon wart frog (Fejervarya vittigera), to determine whether exposure to a common herbicide, butachlor, drives competitive interactions in favor of the invasive amphibian. Our results revealed that competition had a strong effect on the development of both species, but in opposing directions; Luzon wart frog tadpoles were smaller and developed slower than when raised alone, whereas cane toad tadpoles were larger and developed faster. Contrary to our predictions, development and survival of endemic wart frog tadpoles was not affected by butachlor, whereas invasive cane toad tadpoles were affected across several endpoints including gene expression, body size, and survival. We also observed an interaction between pesticide exposure and competition for the cane toad, where survival declined but body size and expression of thyroid sensitive genes increased. Taken together, our findings indicate that the success of the cane toad larvae in rice fields may be best explained by increased rates of development and larger body sizes of tadpoles in response to competition with native Luzon wart frog tadpoles rather than lower sensitivity to a common pesticide. Our results for the cane toad also provide evidence that butachlor can disrupt thyroid hormone mediated development in amphibians, and further demonstrate that important species interactions such as competition can be affected by pesticide exposure in aquatic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amphibian populations are in critical decline worldwide (Wake and Vredenburg 2008). Knowledge of the status of Southeast Asian species is particularly limited, with approximately 36 percent currently classified as data deficient and 20 percent classified as threatened (Sodhi et al. 2004; Rowley et al. 2010). Rice agriculture comprises significant total landmass in Southeast Asia (GRiSP 2013) and provides native amphibians with important substitute wetland habitat (Bambaradeniya et al. 2004; Edirisinghe and Bambaradeniya 2006; Holzer et al. 2017). Amphibians utilize rice fields throughout larval, metamorphic, and adult life stages, and provide important ecosystem services. They consume arthropod pests (Attademo et al. 2005; Khatiwada et al. 2016), act as sentinels to reveal negative impacts of pesticide exposure, and are a staple food source for humans (Punhali 1995; Hocking and Babbitt 2014).
Lowland irrigated rice fields in the Philippines harbor native amphibian species, including Fejervarya vittigera, Occidozyga laevis, Polypedates leucomystax, and Kaloula picta (McLeod et al. 2011; Ramirez 2014). However, non-native species including Rhinella marina, Hoplobatrachus rugulosus, and Kalula pulchra have spread rapidly throughout the country, and may be outcompeting natives (Diesmos et al. 2006; Diesmos 2008). The larvae, juveniles, and adults of the Luzon wart frog (F. vittigera) and the cane toad (R. marina), in particular, co-occur at high densities in rice fields throughout the rainy season. The Luzon wart frog is endemic to the Philippines, widespread throughout lowland Luzon (Diesmos et al. 2015), and commonly consumed and sold by locals (Howard 2015). The cane toad is an opportunistic invasive species that has an ability to disperse rapidly across new environments and negatively impact native species, particularly in Australia (Molloy and Henderson 2006; Shine 2010). The cane toad was introduced in the Philippines in the 1930s as a biological control agent in sugar cane crops, and now occupies all major islands except Palawan (Diesmos et al. 2006). Its extensive distribution and high abundance suggest it may pose a threat to native fauna in the country (Diesmos 2008); however, no reported studies have addressed this threat in rice fields.
We conducted an experiment to determine whether cane toad tadpoles have a competitive advantage over the Luzon wart frog, and furthermore, whether pesticide use, which has increased over the past two decades in rice (Horgan et al. 2016), could drive competitive interactions in favor of the invasive species. Pesticide exposure is a threat to biodiversity and human health, and many pesticide products are endocrine disrupting chemicals (EDCs) that impair vertebrate development and reproduction (McKinlay et al. 2008). Comparative studies of amphibians have revealed interspecific differences in sensitivity to pesticides (Bridges and Semlitsch 2000; García-Muñoz et al. 2010), which may be attributed to differences in exposure risk or genetics.
Genetic variability within populations can allow some species to adapt to novel stressors and persist in changing environments (Lande and Shannon 1996), and may play a key role in the success of invasive species (Prentis et al. 2008). In the case of the cane toad, traits such as heightened metabolic and immune system response have allowed toads to respond successfully to challenges encountered along the invasion front in Australia (Rollins et al. 2015). If invasive amphibians such as the cane toad have traits that decrease their susceptibility to common pesticides, this may provide them a competitive edge over native species in rice agro-ecosystems.
Interactions between pesticide and ecological stressors are poorly understood, but have the potential to produce cascading consequences at multiple levels of biological organization. For example, pesticide use in rice fields can alter community composition of beneficial insects (Cohen et al. 2014) and migratory waterbirds (Parsons et al. 2010). Sub-lethal pesticide exposure can also indirectly affect population size by altering predation rates of aquatic insects on amphibian larvae or changing competitive outcomes between species (Relyea and Hoverman 2008). At the organismal and tissue levels, pesticide and ecological stressors affect development, reproduction, and gene expression by acting directly upon endocrine targets, and indirectly via crosstalk between the stress and thyroid endocrine systems (Denver 1997, 2009; Kulkarni and Buchholz 2012).
The goals of our experiment were three-fold: (1) to determine the effects of competition on the larvae of the cane toad and Luzon wart frog, (2) to determine if species-specific differences exist in response to exposure to butachlor, a common herbicide used in rice, and (3) to test for an interaction between pesticide exposure and interspecific competition. To establish the level of sensitivity for each species to our competition, pesticide, and pesticide x competition treatments, we examined several endpoints at the organismal (morphology, activity, development, and survival) and tissue levels (gene expression).
We hypothesized that cane toad tadpoles would be less affected by butachlor exposure across all measured endpoints, compared with native Luzon wart frog tadpoles. Moreover, we predicted that this difference in sensitivity would affect competitive interactions with the native species, incurring an advantage to the cane toad in terms of growth and survival. At the tissue level, we expected both butachlor and competition would have a larger effect on the expression of thyroid sensitive genes controlling development in the endemic species, the Luzon wart frog.
Methods
Species collection and care
We collected wild Luzon wart frog and invasive cane toad tadpoles at Gosner stages 25–27 from experimental rice fields at the International Rice Research Institute (IRRI) in Los Baños, Philippines. To capture genetic diversity, three tadpole clusters from each species were collected from isolated paddies (SI Table 1). At the time of collection on June 23rd, 2016 paddies were flooded with rainwater, but were not cultivated, and the egg masses and tadpoles had not been exposed to pesticides directly from spraying. Following collection, we combined tadpoles of the same species, transferred them to 16 L tanks (36.8 × 33.8 × 23.6 cm plastic rice storage bins), and allowed them to acclimate for 1 day. We maintained each tank with 10 L of tap water aged for 24 h to allow any possible chlorine to evaporate. Throughout the experiment, tadpoles were fed ad libitum with a 1:1 mixture of crushed rabbit pellets and fish flakes, and deceased individuals were removed every morning.
Pesticide
We evaluated the effects of the acetanilide herbicide butachlor, one of the most extensively used herbicides in rice agriculture throughout the Philippines (Snelder et al. 2008) and Southeast Asia (Abigail et al. 2015), and a suspected endocrine disruptor to which paddy amphibians are exposed during development in the wild (Liu et al. 2011; Li et al. 2016). In particular, butachlor exposure has been observed to disrupt thyroid hormone mediated pathways in the African clawed frog, Xenopus laevis (Li et al. 2016), and zebrafish, Danio rerio (Chang et al. 2011). We used a commercial formulation, Machete EC (68% active ingredient, 600 mg/mL 2-chloro-2′,6′-diethyl-N-butoxymethyl-acetanilide, Sinochem Crop Protection Inc., Philippines), to which paddy amphibians are realistically exposed in the field (Snelder et al. 2008; Yap and Demayo 2015). We exposed tadpoles to 0.2 mg/L, a concentration we estimated to exist in fields 6 to 7 days after spraying based upon the recommended application rate of 4.8 mg/L (Geng et al. 2005), and the observed half life of 1.7–2.5 days in paddy water (Huarong et al. 2010). Amphibian breeding occurs when rice fields are irrigated, and corresponds with the typical application of pre-emergence herbicides such as Machete 1–3 days after transplanting. We estimated that 0.2 mg/L should be a realistic concentration to which recently hatched tadpoles of Gosner stage 25–27 could be exposed.
Experimental design
We conducted a full factorial laboratory experiment at IRRI with the following treatments: (1) tadpoles of only R. marina, (2) tadpoles of R. marina exposed to butachlor (3) tadpoles of only F. vittigera, (4) tadpoles of F. vittigera exposed to butachlor 3) tadpoles of both R. marina and F. vittigera, and (5) tadpoles of both R. marina and F. vittigera exposed to butachlor (Fig. 1). We replicated each treatment four times for a total of 24 experimental units (tanks), each containing 20 individual tadpoles. Water temperature and pH was kept at 26˚ C and between 8.2–8.6, respectively, within the temperature and pH range of paddy water (Kuwagata et al. 2008, Ramirez 2014). The facility was kept on a 13-hour light: 11-hour dark cycle to simulate field conditions in Luzon during June and July.
To generate a nominal concentration of 0.2 mg/L in each pesticide treated tank we performed a serial dilution. Trained pesticide applicators at IRRI created a solution stock 1) with a nominal concentration of 2 mg/mL (2000 mg/L), consisting of 2 mL of concentrated 600 mg/mL Machete added to 598 mL of water, and a second solution (stock 2) with a nominal concentration of 0.002 mg/mL (2 mg/L), consisting of 66 mL of stock 1 added to each of six bins containing 65.934 L of aged tap water (66 L total volume). To start the experiment, we added 1 L of stock solution 2 to each of our treatment tanks pre-filled 9 L to yield a nominal concentration of 0.0002 mg/mL (0.2 mg/L). We added the same amount of aged tap water to our non-pesticide tanks. In order to mimic degradation of butachlor over time in the field, we stored water from stock solution 2 for the duration of the experiment and added 1 L of this water to our tanks every water change. Although we were unable to verify the actual concentrations of butachlor to which our animals were exposed, appropriate conclusions can still be drawn from our experiment because our primary aim was to determine if an environmentally relevant pesticide exposure might affect competitive interactions. Furthermore, IRRI pesticide applicators perform the same 2 step dilution series when they prepare Machete for application, suggesting that our exposure is a conservative estimate of a concentration in rice fields.
We began our experiment on June 24th after placing 20 tadpoles at Gosner stages 25–27 in each tank, and adding Machete to the pesticide treatment groups. We took daily measurements of tadpole stage using the Gosner staging chart (Gosner 1960) and recorded survival and activity level by counting the number of live and moving tadpoles in the tank prior to feeding. We recorded temperature and pH every 5 days using a HI9829 multi-parameter meter (Hannah Instruments, Woonsocket, RI, USA). We conducted water changes every 5 days, re-filling each tank with a total of 10 L of aged tap water. In the pesticide-treated tanks, 1.43 L of the final 10 L volume was from stock solution 2. We terminated the experiment on day fourteen when the first tadpoles reached metamorphosis climax (Gosner stage 41), and euthanized all individuals in 0.02% MS222 over a subsequent 36-hour period. We took lateral photographs of individuals (Fig. 1) for morphometric analysis, and harvested tissue samples from the intestine and gonad. Samples were placed in RNAlater solution within a half hour after death (Thermofisher Scientific Inc., Nepean, ON, Canada), shipped on dry ice to Northern Arizona University, and stored at −80 °C.
Quantitative polymerase chain reaction (qPCR)
We conducted a qPCR assay to determine the effects of the competition, pesticide, and competition x pesticide treatments on thyroid sensitive genes. We examined intestinal tissue because the intestine undergoes a comprehensive physiological transformation during amphibian metamorphosis that is mediated by thyroid hormone (Shi et al. 2012). We extracted RNA from the intestine of both species using AllPrep DNA/RNA Mini Kits (Qiagen, Hilden, Germany), and determined concentrations using a Nanodrop ND-1000 (ThermoFisher Scientific Inc., Nepean, ON, Canada). We validated RNA quality using a 2100 Bioanlayzer (Agilent) with an RIN cut-off of 8, and then used Invitrogen SuperScript® VILO™ Master Mix (ThermoFisher Scientific Inc., Nepean, ON, Canada) to convert to cDNA using the manufacturer’s instructions. All subsequent qPCR reactions were carried out using 19 uL iQ SYBR Green SuperMix (Bio-Rad Laboratories In., Hercules, CA, USA) containing 10 pmol of each primer and 1 ul sample cDNA on a Realplex 4 thermo-cycler (Eppendorf, Hamburg, Germany).
We tested nine genes (SI Table 2) for potential use in our wild species using a two-tiered approach for quality control (Veldhoen et al. 2014). We selected genes that had been previously established as thyroid hormone sensitive in X. laevis and Rana catesbiana (Veldhoen et al. 2014; Wolff et al. 2015). To confirm primer specificity, we first conducted qPCR using pooled intestinal cDNA across treatments for each species under the thermocycler conditions used by Veldhoen et al. (2014), and examined products on 1% agarose gels stained with ethidium-bromide. For primers that yielded products of the expected molecular weight, we then performed a 1:2 serial dilution with pooled cDNA to test for parallel amplification efficiency against the housekeeping gene, rpl8 (Bustin et al. 2010). Transcript levels of rpl8 were tested, and were not affected by experimental treatments.
For our final qPCR assay we used three primers that yielded the correct product and ran with parallel efficiency to our reference gene: thyroid hormone receptors alpha and beta (thra and thrb) and Krüppel-like factor 9 (klf9). Thra and thrb mediate the effects of thyroid hormone on amphibian metamorphosis (Shi et al. 2012). Klf9 regulates expression of thrb in the model amphibian X. laevis (Kanamori and Brown 1992), and mediates actions of thyroid hormone on differentiation of nerve cells (Knoedler and Denver 2014). We used intestinal cDNA diluted 20-fold, and ran quadruplicate reactions using the following thermocycler sequence (1) enzyme activation at 95 °C for 3 min, (2) 38 cycles of DNA template denaturation at 95 °C for 15 s, primer annealing at 62 °C (klf9, thra) and 60 °C (rpl8, thrb) for 30 s, and elongation at 72 °C for 45 s, and (3) a melt curve. We used the comparative cycle threshold (Ct) method (ΔΔCt) to calculate fold change in transcript levels for thra, thrb, and klf9 relative to our reference gene, rpl8 (Schmittgen and Livak 2008).
Morphometric analyses
For morphometric analyses we enhanced and screened photos, discarding images in which tadpole features were not clearly visible. One individual performed analysis on all randomized photos, without knowledge of the associated treatments. We used ImageJ (NIH, Bethesda, Maryland) to obtain measures of tail width and lengths of total body, snout–vent, tail, and hind limb.
Statistical analysis
We used R for all statistical analyses. We examined data for normality using qqplots and Shapiro-Wilk tests, and tested for homogeneity of variance using Levene’s tests. To visualize differences in survival over time, we computed nonparametric Kaplan-Meier survival curves. To determine increases/decreases in hazard ratios between our control and treatment groups, we then calculated Cox proportional-hazards regression coefficients for each species. Significant positive coefficients indicated a higher risk of tadpole death in a given treatment compared with the control. We used the R package ‘survival’ for both steps in our survival analysis (Therneau 2016).
Components of developmental stage and morphometric data were non-normally distributed or exhibited unequal variances. Activity and gene expression data met assumptions. Transformations did not improve the distribution of stage data, so we performed non-parametric Kruskal–Wallis tests followed by Dunn’s post hoc tests with Bonferroni correction for multiple comparisons (kruskal.test and dunn.test functions in R) to test for differences across treatments. Morphometric data met assumptions following log transformations. We used the nlme package in R to examine the effects of butachlor exposure crossed with competition on tadpole morphology, gene expression, and activity using mixed effects models that accounted for variance in our experimental unit by including tank as a random effect. For activity, we included day of measurement (1–10) as a fixed effect, to determine whether activity levels changed throughout the course of the experiment. We used the multcomp package in R to test for significant differences in treatment groups by setting our model reference (intercept) as control, butachlor, and competition to generate all possible comparisons.
Results
Survival analysis
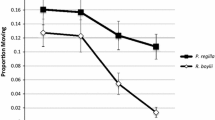
We observed interspecific differences in survival across the butachlor and butachlor x competition treatments (Fig. 2). Across treatments, 93% of all Luzon wart frog tadpoles survived the two-week experimental period, and we observed no significant differences in survivorship of those in control water, exposed to butachlor, competition, or both. In contrast, 80% of cane toad tadpoles survived across all treatments, and those exposed to butachlor were 27% more likely (Cox Hazard Ratio = 2.74, 95% CI 6.61, 1.14, P < 0.05) to experience mortality than control tadpoles over the two-week experimental period. Competition alone did not affect survival of cane toad tadpoles, but cane toad larvae in the competition x butachlor treatment were 58% more likely (Cox Hazard Ratio = 5.78, 95% CI 14.06, 2.38, P < 0.05) to experience mortality than control animals, and 4.7% more likely (Cox Hazard Ratio = 0.45, 95% CI 0.94, 0.24, P < 0.05) to experience mortality than animals exposed to only butachlor (Table 1).
Nonparametric Kaplan–Meier survival curves depicting percentages of Luzon wart frog (Fejervarya vittigera) and cane toad (Rhinella marina) tadpoles surviving over the course of the experiment in response to competition, butachlor, or competition × butachlor. Errors bars depict SE and stars represent treatments where survival was significantly reduced compared to control (P < 0.05)
Developmental stage
Cane toad tadpoles developed 1.4 times faster than Luzon wart frog tadpoles. All individuals began at Gosner stages 25–27, but after 14 days control cane toad tadpoles were approaching metamorphosis (Gosner stages 40–41) whereas control wart frog larvae were still growing limb buds (Gosner stages 26–32). Development of wart frog and cane toad tadpoles was significantly affected by treatment, but in opposing directions (Fig. 3; Kruskal–Wallis, P < 0.001 and P < 0.001, respectively). Cane toad tadpoles developed faster in response to competition, but were not affected by butachlor or the combined competition x butachlor treatment (Fig. 3 and Table 2). In contrast, Luzon wart frog tadpoles developed slower in the competition treatment compared with control animals. Butachlor alone had no effect on development, but in the competition x butachlor treatment, Luzon wart frog tadpoles were observed at a developmental stage intermediate to both control and competition treatments. In this treatment, decreased survivorship of the cane toad alleviated some, but not all, competitive pressure on the Luzon wart frog (Fig. 3 and Table 2).
Box plots depicting mean plus lower (25%) and upper (75%) quartiles of the effect of competition, butachlor, and competition × butachlor on developmental stage (Gosner stage) of Luzon wart frog (Fejervarya vittigera) and cane toad (Rhinella marina) tadpoles at day 14. Whiskers extend to lower and upper values excluding outliers, which are represented as points
Activity
The activity of Luzon wart frog tadpoles was not affected by exposure to butachlor, but was significantly reduced when in competition with the cane toad. We observed no interactions between competition and butachlor, and cane toad activity levels were not affected by any treatment (SI Fig. 2, SI Table 3). Activity levels for both species varied significantly over the course of the exposure period.
Morphology
We observed no differences in the measurements of Luzon wart frog tadpoles exposed to butachlor alone across morphological endpoints, with the exception of tail width, which was wider in exposed tadpoles (Table 3). Total body, tail, and hind limb lengths of Luzon wart frog tadpoles were affected by competition, and comparisons revealed tadpoles in the competition treatments were shorter across all morphological endpoints compared with controls (Fig. 4). We observed no interactions between competition x butachlor on morphology of the Luzon wart frog (Table 3).
Box plots depicting mean plus lower (25%) and upper (75%) quartiles of the effect of competition, butachlor, and competition × butachlor on morphometric measurements (body, snout-vent, and hind limb lengths and tail width) of a, Luzon wart frog (Fejervarya vittigera), and b, cane toad (Rhinella marina) tadpoles at day 14. Whiskers extend to lower and upper values excluding outliers, which are represented as points. Unique letters depict significant differences (P < 0.05) between treatments as determined using mixed effect model accounting for variance in our experimental unit, tank. Data were log transformed prior to statistical analysis
Butachlor exposure affected the snout-vent length of cane toad tadpoles (Table 3), and comparisons revealed exposed tadpoles exhibited longer snout-vent lengths compared with control animals (Fig. 4). The same morphological endpoints that were affected by competition for the Luzon wart frog (total body, tail, and hind limb lengths) were also affected in the cane toad. However, cane toad tadpoles responded in the opposite direction, and exhibited longer total body lengths compared to control animals. For tail width, we observed a significant interaction in the competition x butachlor treatment (Table 3).
Gene Expression
We observed no differences in the relative expression of klf9 and thra in Luzon wart frog tadpoles exposed to butachlor alone, or in the competition x butachlor treatment. Competition significantly affected expression of both genes, but in opposite directions; expression of klf9 increased and expression of thra decreased relative to control. For the cane toad, exposure to butachlor increased expression of thrb but did not affect expression of klf9. Competition alone did not affect expression of either gene, but we observed an interaction in our competition x butachlor treatment that resulted in increased expression of both klf9 and thrb in cane toad tadpoles (Fig. 5).
Box plots depicting mean plus lower (25%) and upper (75%) quartiles of the effect of competition, butachlor, and competition × butachlor on relative expression of thyroid dependent genes (thra, thrb, and klf) in intestinal tissue of Luzon wart frog (Fejervarya vittigera) and cane toad (Rhinella marina) tadpoles. Points represent samples included in analysis, which consisted of n = 9 individual tadpoles per treatment, chosen at random from replicate tanks. Results of two-way ANOVAs are presented for each gene, and unique letters depict significant differences (P < 0.05) between treatments as determined using mixed effect models accounting for variance in our experimental unit, tank
Discussion
Contrary to our predictions, we demonstrated that invasive cane toad tadpoles were more sensitive to exposure to the common pesticide, butachlor, than endemic Luzon wart frog tadpoles. The population of cane toad larvae in our experiment also exhibited traits, including faster development and larger body size, which may lend them a competitive edge over the native Luzon wart frog in rice agro-ecosystems. Larger body sizes at metamorphosis improve chances of survival later in life (Johansson et al. 2010; Cabrera-Guzmán et al. 2013a), and faster development can limit the period of time larva are directly exposed to pesticide contaminants. Competition with the cane toad negatively affected growth and development of several native amphibian species in Queensland, and higher baseline activity levels and aggressive feeding behaviors have been suggested as a possible mechanism of success (Williamson 1999). However, exceptions exist. For instance, competition with one native Australian species has been found to negatively impact growth and development of cane toad larvae (Cabrera-Guzmán et al. 2013b), which suggests that the effects of competition on development are both plastic and species-dependent.
Exposure to bufadienolide chemical cues may be partially responsible for the reduced activity levels and slowed growth and development of the Luzon wart frog in the competition treatments. Cane toads contain bufadienolide toxins that are present in eggs, in early larval developmental stages, and in adults (Hayes et al. 2009). Bufadienolides are cardioactive steroids known to inhibit Na+/K+-ATPase, but also have been observed to suppress immune system function (Cunha-Filho et al. 2010) and blood vessel growth (Lee et al. 1997). Future research should test the possibility that bufadienolides disturb developmental processes of native tadpoles; particularly given our results that competition with cane toad larvae slowed development and affected expression of two thyroid sensitive genes, thra and klf9, in the native species.
Intraspecific competition can also affect the development, growth, and morphology of amphibian larvae (Relyea 2002). In the wild, cane toad larvae are often found in aggregates consisting of thousands of tadpoles, and intense intraspecific competition including cannibalism of conspecific eggs and dead tadpoles has been well documented (Pizzatto and Shine 2008). Our findings that control cane toad tadpoles developed slower and were, on average, smaller compared with those in competition with the Luzon wart frog suggest that competition with conspecifics is more intense than interspecific competition at the same density.
The effects of both inter and intraspecific competition observed in the laboratory can be dampened in the field by interactions with predators and by abiotic factors (Loman 2001). For instance, differences in microhabitat use may dampen interspecific competition because wart frog larvae appear to stay close to the bottom sediment, whereas cane toad larvae forage higher in the water column (M. Shuman-Goodier, personal observation). Differences in predation pressure could also plausibly affect the magnitude and direction of competitive interactions in rice fields. For example, aquatic insect predators have been observed to prefer cane toad tadpoles over native Australian species (Cabrera-Guzmán et al. 2012), perhaps because they are obvious, slow swimmers. Field studies are needed to determine whether the strong effects of interspecific competition we observed in the laboratory persist in rice agro-ecosystems.
Cane toad survival was reduced in the pesticide and pesticide x competition treatments, which alleviated some, but not all, competitive stress from the native species. The variation in sensitivity we observed may be attributed to differences in the rate each species bioaccumulates, biotransforms, and eliminates butachlor and its metabolites (Leney et al. 2006). Amphibian populations have been observed to exhibit tolerance to pesticides, particularly those that have been used for a long time period (Hua et al. 2015a, b), as is the case with the herbicide butachlor. It is also possible that the species introduction in the 1940’s produced a founder effect, and that low genetic diversity of cane toad populations may have limited their ability to adapt to pesticide exposure compared with the Luzon wart frog. Alternatively, the Luzon wart frog may be vulnerable to butachlor exposure at later stages of development, which we did not test in our 15-day experiment. For example, survival of Fejervarya limnocharis tadpoles, a congener found in Taiwan, was negatively affected by butachlor exposure at the same concentration of 0.2 mg/L (Liu et al. 2011), but effects were only observed after 21 days of exposure. Realistically, however, exposure risk is likely to be highest at young developmental life stages for all amphibian species because butachlor is typically applied early in the rice-cropping season, as a pre-emergence herbicide, 1–3 days after transplanting.
A growing body of evidence indicates that butachlor exposure at environmentally relevant concentrations can disturb vertebrate development by disrupting the thyroid axis (Zhu et al. 2014; Li et al. 2016). The hypothalamic pituitary thyroid (HPT) axis plays a critical role in amphibian metamorphosis, and changes to levels of circulating thyroid hormones (THs), and expression of TH sensitive genes can disturb normal development (Opitz et al. 2006; Fort et al. 2007). Exposure to butachlor increased levels of circulating Ths, T3 and T4, in zebra fish (Danio rerio) at 50 and 100 ug/L (Chang et al. 2011), female rare minnows (Gobiocypris rarus) at 0.1, 1, and 10 ug/L (Zhu et al. 2014), and in X. laevis tadpoles at 100 ug/L. THs act at the level of the receptor (thra and thrb) to regulate gene expression. Increased expression of thrb, as we observed in our experiment for R. marina exposed to butachlor, can parallel increased levels of circulating THs, and result in a faster rate of metamorphosis (Fort et al. 2007). Our gene expression results for R. marina corroborate work done with X. laevis, which found that butachlor up-regulated thrb, increased total body and hind limb length, and promoted metamorphosis (Li et al. 2016). Moreover, corticosterone hormones typically act synergistically with THs to speed up metamorphosis (Denver 1997, 2009; Kulkarni and Buchholz 2012), and cross talk between the stress and HPT axes may explain the interaction between competition x butachlor we observed at the tissue level in R. marina.
Disruption of the HPT axis by endocrine disrupting chemicals can cause developmental and reproductive disorders in humans similar to those observed in wildlife (Choksi et al. 2003). The above studies observed interference with the HPT axis using butachlor concentrations within the range of those in the environment (Li et al. 2016), where concentrations ranging from 0.05 to 1.4 μg/L in surface water (Mamun et al. 2009; Toan et al. 2013) and 0.01 to 17.8 μg/L in paddy water (Li et al. 2016) have been detected. Butachlor exposure thus poses a realistic threat to both wildlife and human health, and the greatest risks are for organisms, such as amphibians, that utilize rice paddies during developmental and reproductive life stages.
Our experiment also demonstrates that exposure to a common pesticide can indirectly affect competitive interactions between native and invasive tadpole larvae, albeit in a direction contrary to our hypotheses. The sub-lethal effects of pesticides on species interactions, such as predation and competition, are understudied, but have the potential to shape the life history of a variety of taxa, including amphibians (Campero et al. 2007; Pestana et al. 2009; Janssens and Stoks 2013; Rasmussen et al. 2013). For example, competition decreased survival of bullfrog tadpoles exposed to the herbicide glyphosate at high densities (Jones et al. 2011), and exposure to the insecticide carbaryl promoted survival but increased competition between woodfrog tadpoles by killing off insect predators and reducing availability of food resources (Mills and Semlitsch 2004). As our own results demonstrate, the direction of these interactions is not always intuitive, and reflects a pressing need to incorporate ecological knowledge and complexity into ecotoxicological studies (Chapman 2002; Köhler and Triebskorn 2013).
Conclusion
We found that endemic Luzon wart frog larvae were negatively affected by competition with cane toad larvae, but contrary to our predictions, cane toad larvae were more susceptible to butachlor exposure. Our findings suggest that the success of the cane toad in rice fields may be best explained by increased rates of development and larger body sizes of tadpoles in response to competition with the native wart frog rather than species-specific sensitivity to butachlor. Our results for the cane toad also support evidence that butachlor can disrupt thyroid hormone mediated development in vertebrates, and further demonstrate that species interactions such as competition can be affected by pesticide exposure in aquatic ecosystems. To increase the environmental relevancy of the hypotheses we tested, we suggest that future work explore interactions between pesticide exposure and competition at additional juvenile and adult life stages, as well as use longer exposures to encompass species with slower developmental rates. There is also a need for studies that test for relationships between amphibian diversity and pesticide use in rice fields at the landscape level.
References
Abigail MEA, Samuel SM, Ramalingam C (2015) Addressing the environmental impacts of butachlor and the available remediation strategies: a systematic review. Int J Environ Sci Technol 12:4025–4036. https://doi.org/10.1007/s13762-015-0866-2
Attademo AM, Peltzer PM, Lajmanovich RC (2005) Amphibians occurring in soybean and implications for biological control in Argentina. Agric Ecosyst Environ 106:389–394. https://doi.org/10.1016/j.agee.2004.08.012
Bambaradeniya C, Edirisinghe JP, De Silva DN, Ranawana KB, Wijekoon S (2004) Biodiversity associated with an irrigated rice agro-ecosystem in Sri Lanka. Biodivers Conserv 12:1715–1753
Bridges CM, Semlitsch RD (2000) Variation in pesticide tolerance of tadpoles among and within species of Ranidae and patterns of amphibian decline. Conserv Biol 14:1490–1499
Bustin SA, Beaulieu J-F, Huggett J et al. (2010) MIQE précis: practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol Biol 11:74. https://doi.org/10.1186/1471-2199-11-74
Cabrera-Guzmán E, Crossland MR, Brown GP, Shine R (2013a) Larger body size at metamorphosis enhances survival, growth and performance of young cane toads (Rhinella marina). PLoS ONE. 8:7. https://doi.org/10.1371/journal.pone.0070121
Cabrera-Guzmán E, Crossland MR, Shine R (2013b) Competing tadpoles: Australian native frogs affect invasive cane toads (Rhinella marina) in natural waterbodies. Austral Ecol 38:896–904. https://doi.org/10.1111/aec.12029
Cabrera-Guzmán E, Crossland MR, Shine R (2012) Predation on the eggs and larvae of invasive cane toads (Rhinella marina) by native aquatic invertebrates in tropical Australia. Biol Conserv 153:1–9. https://doi.org/10.1016/j.biocon.2012.04.012
Campero M, Slos S, Ollevier F, Stoks R. (2007) Sublethal pesticide concentrations and predation jointly shape life history: behavioral and physiological mechanisms. Ecol Appl 17:2111–2122
Chang J, Liu S, Zhou S et al (2011) Effects of butachlor on reproduction and hormone levels in adult zebrafish (Danio rerio). Exp Toxicol Pathol 65:205–209. https://doi.org/10.1016/j.etp.2011.08.007
Chapman PM (2002) Integrating toxicology and ecology: putting the ‘eco’ into ecotoxicology. Mar Pollut Bull 44:7–15. https://doi.org/10.1016/S0025-326X(01)00253-3
Choksi NY, Jahnke GD, St. Hilaire C, Shelby M (2003) Role of thyroid hormones in human and laboratory animal reproductive health. Birth Defects Res Part B–Dev Reprod Toxicol 68:479–491. https://doi.org/10.1002/bdrb.10045
Cohen JE, Schoenly K, Heong KL, Justo H, Arida G, Barrion AT, Litsinger JA (2014) A food web approach to evaluating the effect of insecticide spraying on insect pest population dynamics in a Philippine irrigated rice ecosystem. J Appl Ecol 31:747–763
Cunha-Filho GA, Resck IS, Cavalcanti BC, Pessoa CÓ, Moraes MO, Ferreira JRO, Rodrigues FAR, dos Santos ML (2010) Cytotoxic profile of natural and some modified bufadienolides from toad Rhinella schneideri parotoid gland secretion. Toxicon 56:339–348. https://doi.org/10.1016/j.toxicon.2010.03.021
Denver RJ (1997) Environmental stress as a developmental cue: corticotropin-releasing hormone is a proximate mediator of adaptive phenotypic plasticity in amphibian metamorphosis. Horm Behav 31:169–179. https://doi.org/10.1006/hbeh.1997.1383
Denver RJ (2009) Stress hormones mediate environment-genotype interactions during amphibian development. Gen Comp Endocrinol 164:20–31. https://doi.org/10.1016/j.ygcen.2009.04.016
Diesmos AC (2008) Ecology and diversity of herpetofaunal communities in fragmented lowland rainforests in the Philippines. PhD Thesis, National University of Singapore.
Diesmos AC, Diesmos ML, Brown RM (2006) Status and distribution of alien invasive frogs in the Philippines. J Environ Sci Manag 9:41–53
Diesmos AC, Watters JL, Huron NA, Davis DR, Alcala AC, Crombie RI, Afuang LE, Gee-Das G, Sison RV, Sanguila MB, Penrod ML, Labonte MJ, Davey CS, Leone EA, Diesmos ML, Sy EY, Welton LJ, Brown RM, Cameron DS (2015) Amphibians of the Philippines, Part I: checklist of the Species. Proc Calif Acad Sci 62:457–539
Edirisinghe JP, Bambaradeniya CNB (2006) Rice fields: an ecosystem rich in biodiversity. J Natl Sci Found Sri Lanka 34:57–59. https://doi.org/10.4038/jnsfsr.v34i2.2084
Fort DJ, Degitz S, Tietge J, Touart LW (2007) The hypothalamic-pituitary-thyroid (HPT) axis in frogs and its role in frog development and reproduction. Crit Rev Toxicol 37:117–161. https://doi.org/10.1080/10408440601123545
García-Muñoz E, Guerrero F, Parra G (2010) Intraspecific and interspecific tolerance to copper sulphate in five Iberian amphibian species at two developmental stages. Arch Environ Contam Toxicol 59:312–321. https://doi.org/10.1007/s00244-010-9473-x
Geng BR, Yao D, Xue QQ (2005) Acute toxicity of the pesticide dichlorvos and the herbicide butachlor to tadpoles of four anuran species. Bull Environ Contam Toxicol 75:343–349. https://doi.org/10.1007/s00128-005-0759-z
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetol Leag 16:183–190
GRiSP (Global Rice Science Partnership) (2013) Rice Almanac, 4th edn. International Rice Research Institute, Los Baños
Hayes RA, Crossland MR, Hagman M, Hagman M, Capon RJ, Shine R (2009) Ontogenetic variation in the chemical defenses of cane toads (Bufo marinus): toxin profiles and effects on predators. J Chem Ecol 35:391–399. https://doi.org/10.1007/s10886-009-9608-6
Hocking DJ, Babbitt KJ (2014) Amphibian contributions to ecosystem services. Herpetol Conserv Biol 9:1–17
Holzer KA, Bayers RP, Nguyen TT, Lawler SP (2017) Habitat value of cities and rice paddies for amphibians in rapidly urbanizing Vietnam. J Urban Ecol 3:1–12. https://doi.org/10.1093/jue/juw007
Horgan FG, Ramal AF, Bernal CC et al (2016) Applying ecological engineering for sustainable and resilient rice production systems. Procedia Food Sci. 6:7–15. https://doi.org/10.1016/j.profoo.2016.02.002
Howard BD (2015) Catching evidence with frogs through a focused ethnographic research with a Filipino rice farming community. MSc Thesis. Northern Arizona University
Hua J, Jones DK, Mattes BM, Cothran RD, Relyea RA, Hoverman JT (2015b) The contribution of phenotypic plasticity to the evolution of insecticide tolerance in amphibian populations. Evol Appl 8:586–596. https://doi.org/10.1111/eva.12267
Hua J, Jones DK, Mattes BM, Cothran RD, Relyea RA, Hoverman JT (2015a) Evolved pesticide tolerance in amphibians: predicting mechanisms based on pesticide novelty and mode of action. Environ Pollut 206:56–63. https://doi.org/10.1016/j.envpol.2015.06.030
Huarong GUO, Licheng YIN, Shicui Z, Wenrong F (2010) The toxic mechanism of high lethality of herbicide butachlor in marine flatfish flounder, Paralichthys olivaceus. Ocean Coast Sea Res 9:257–264. https://doi.org/10.1007/s11802-010-1693-1
Johansson F, Lederer B, Lind MI (2010) Trait performance correlations across life stages under environmental stress conditions in the common frog Rana temporaria. PLoS One 5:e11680. https://doi.org/10.1371/journal.pone.0011680
Janssens L, Stoks R (2013) Exposure to a widespread non-pathogenic bacterium magnifies sublethal pesticide effects in the damselfly Enallagma cyathigerum: from the suborganismal level to fitness-related traits. Environ Pollut 177:143–149
Jones DK, Hammond JI, Relyea RA (2011) Competitive stress can make the herbicide Roundup® more deadly to larval amphibians. Environ Toxicol Chem 30:446–454. https://doi.org/10.1002/etc.384
Kanamori A, Brown DD (1992) The regulation of thyroid hormone receptor beta genes by thyroid hormone in Xenopus laevis. J Biol Chem 267:739–745
Khatiwada JR, Ghimire S, Khatiwada PS, Paudel B, Bischof R, Jiang J, Haugaasen T (2016) Frogs as potential biological control agents in the rice fields of Chitwan, Nepal. Agric Ecosyst Environ 230:307–314. https://doi.org/10.1016/j.agee.2016.06.025
Knoedler JR, Denver RJ (2014) Kruppel-like factors are effectors of nuclear receptor signaling. Gen Comp Endocrinol 203:49–59. https://doi.org/10.1016/j.ygcen.2014.03.003
Köhler HR, Triebskorn R (2013) Wildlife ecotoxicology of pesticides: can we track effects to the population level and beyond? Science 80:759–765. https://doi.org/10.1126/science.1237591
Kulkarni SS, Buchholz DR, (2012) Beyond synergy: corticosterone and thyroid hormone have numerous interaction effects on gene regulation in Xenopus tropicalis tadpoles. Endocrinology 153:5309–5324. https://doi.org/10.1210/en.2012-1432
Kuwagata T, Hamasaki T, Watanabe T (2008) Modeling water temperature in a rice paddy for agro-environmental research. Agric For Meteorol 148:1754–1766. https://doi.org/10.1016/j.agrformet.2008.06.011
Lande R, Shannon S (1996) The role of genetic variation in adaptation and population persistence in a changing environment. Evolution 50:434–437. doi: 134.114.101.46
Lee DY, Yasuda M, Yamamoto T, Yoshida T, Kuroiwa Y (1997) Bufalin inhibis endothelial cell proliferation and angiogenesis in vitro. Life Sci 60:127–134
Leney JL, Drouillard KG, Haffner D (2006) Metamorphosis increases biotransformation of polychlorinated biphenyls: a comparative study of polychlorinated bipheny metabolism in green frogs (Rana clamitans) and leopard frogs (Rana pipiens) at various life stages. Environ Toxicol Chem 25:2971–2980. https://doi.org/10.1897/05-561r1.1
Li S, Li M, Wang Q, Gui W, Zhu G (2016) Exposure to butachlor causes thyroid endocrine disruption and promotion of metamorphosis in Xenopus laevis. Chemosphere 152:158–165. https://doi.org/10.1016/j.chemosphere.2016.02.098
Liu W-YY, Wang C-YY, Wang T-SS, Fellers GM, Lai BC, Kam YC (2011) Impacts of the herbicide butachlor on the larvae of a paddy field breeding frog (Fejervarya limnocharis) in subtropical Taiwan. Ecotoxicology 20:377–384. https://doi.org/10.1007/s10646-1010
Loman J (2001) Intraspecific competition in tadpoles of Rana arvalis: does it matter in nature? A field experiment. Popul Ecol 43:253–263. https://doi.org/10.1007/s10144-001-8189-1
Mamun MIR, Park JH, Choi J-H, Kim HK, Choi WJ, Han SS, Hwang K, Jang NI, Assayed ME, El-Dib MA, Shin HC, Abd EA, Shim JH (2009) Development and validation of a multiresidue method for determination of 82 pesticides in water using GC. J Sep Sci 32:559–574. https://doi.org/10.1002/jssc.200800606
McKinlay R, Plant Ja, Bell JNB, Voulvoulis N (2008) Endocrine disrupting pesticides: implications for risk assessment. Environ Int 34:168–183. https://doi.org/10.1016/j.envint.2007.07.013
Mcleod DS, Siler CD, Diesmos AC et al (2011) Amphibians and reptiles of Luzon island, v: the herpetofauna of Angat dam watershed, Bulacan province, Luzon island, philippines. Asian Herpetol Res 2:177–198. https://doi.org/10.3724/SP.J.1245.2011.00177
Mills NE, Semlitsch RD (2004) Competition and predation mediate the indirect effects of an insecticide on southern leopard frogs. Ecol Appl. 14:1041–1054. https://doi.org/10.1890/02-5134
Molloy K, Henderson W (2006) Science of cane toad invasion and control. Proceedings of the Invasive Animals CRC/ CSIRO/Qld NRM&W cane toad workshop. In: Animals. Invasive Animals Cooperative Research Centre, Canberra
Opitz R, Lutz I, Nguyen N-H et al (2006) Analysis of thyroid hormone receptor betaA mRNA expression in Xenopus laevis tadpoles as a means to detect agonism and antagonism of thyroid hormone action. Toxicol Appl Pharmacol. 212:1–13. https://doi.org/10.1016/j.taap.2005.06.014
Parsons KC, Mineau P, Renfrew RB (2010) Effects of esticide use in rice fields on birds. Waterbirds. 33:193–218. https://doi.org/10.1675/063.033.s115
Pestana JLT, Loureiro S, Baird DJ, Soares AMVM (2009) Fear and loathing in the ben- thos: responses of aquatic insect larvae to the pesticide imidacloprid in the presence of chemical signals of predation risk. Aquat Toxicol 93:138–149. https://doi.org/10.1016/j.aquatox.2009.04.008
Pizzatto L, Shine R (2008) The behavioral ecology of cannibalism in cane toads (Bufo marinus). Behav Ecol Sociobiol 63:123–133. https://doi.org/10.1007/s00265-008-0642-0
Prentis PJ, Wilson JRU, Dormontt EE et al (2008) Adaptive evolution in invasive species. Trends Plant Sci 13:288–294. https://doi.org/10.1016/j.tplants.2008.03.004
Punhali PL, PAR (1995) Impact oPlease check collab name “PAR” in ref. Punhali 1995f pesticides on farmer health and the rice environment. Kluwer Academic Publishers, Norwell, MA
Ramirez NEC (2014) The Influence of the frequency of rice crops per year on anuran abundance and diversity in Candaba Swamp, central Luzon, Philippines. MSc Thesis, University of the Philippines Diliman
Rasmussen JJ, Nørum U, Jerris MR et al (2013) Pesticide impacts on predator-prey interactions across two levels of organisation. Aquat Toxicol 140–141:340–345. https://doi.org/10.1016/j.aquatox.2013.06.019
Relyea RA (2002) Competitor-induced plasticity in tadpoles: consequences, cues, and connections to predator-induced plasticity. Ecol Monogr 72:523–540. https://doi.org/10.1890/0012-9615(2002)072[0523:CIPITC]2.0.CO;2
Relyea RA, Hoverman JT (2008) Interactive effects of predators and a pesticide on aquatic communities. Oikos 117:1647–1658. https://doi.org/10.1111/j.1600-0706.2008.16933.x
Rollins LA, Richardson MF, Shine R (2015) A genetic perspective on rapid evolution in cane toads (Rhinella marina). Mol Ecol 24:2264–2276. https://doi.org/10.1111/mec.13184
Rowley J, Brown R, Bain R et al (2010) Impending conservation crisis for Southeast Asian amphibians. Biol Lett 6:336–338. https://doi.org/10.1098/rsbl.2009.0793
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108. https://doi.org/10.1038/nprot.2008.73
Shi Y-B, Matsuura K, Fujimoto K, Wen W, Fu L (2012) Thyroid hormone receptor actions on transcription in amphibia: the roles of histone modification and chromatin disruption. Cell Biosci 2:42. https://doi.org/10.1186/2045-3701-2-42
Shine BR (2010) The ecological impact of invasive cane toads (Bufo Marinus) in Australia. Q Rev Biol 85:253–291. https://doi.org/10.1086/655116
Snelder DJ, Masipiquen MD, De Snoo GR (2008) Risk assessment of pesticide usage by smallholder farmers in the Cagayan Valley (Philippines). Crop Prot 27:747–762. https://doi.org/10.1016/j.cropro.2007.10.011
Sodhi NS, Koh LP, Brook BW, Ng PKL (2004) Southeast Asian biodiversity: an impending disaster. Trends Ecol Evol 19:654–660. https://doi.org/10.1016/j.tree.2004.09.006
Therneau T (2016) A package for survival analysis in S. (R package version 2.36–12, 2012)
Toan PV, Sebesvari Z, Bläsing M, Rosendahl I, Renaud FG (2013) Pesticide management and their residues in sediments and surface and drinking water in the Mekong Delta, Vietnam. Sci Total Environ 452–453:28–39. https://doi.org/10.1016/j.scitotenv.2013.02.026
Veldhoen N, Propper CR, Helbing CC (2014) Enabling comparative gene expression studies of thyroid hormone action through the development of a flexible real-time quantitative PCR assay for use across multiple anuran indicator and sentinel species. Aquat Toxicol 148:162–173. https://doi.org/10.1016/j.aquatox.2014.01.008
Wake DB, Vredenburg VT (2008) Colloquium paper: are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci USA 105:Suppl:11466–Suppl:11473. https://doi.org/10.1073/pnas.0801921105
Williamson I (1999) Competition between the larvae of the introduced cane toad Bufo marinus (Anura: Bufonidae) and native anurans from the Darling Downs area of southern Queensland. Aust J Ecol 24:636–643. https://doi.org/10.1046/j.1442-9993.1999.00993.x
Wolff SE, Veldhoen N, Helbing CC, Ramirez CA, Malpas JM, Propper CR (2015) Estrogenic environmental contaminants alter the mRNA abundance profiles of genes involved in gonadal differentiation of the American bullfrog. Sci Total Environ 521–522:380–387. https://doi.org/10.1016/j.scitotenv.2015.02.033
Yap SMS, Demayo CG (2015) Farmers’ knowledge and understanding of pesticide use and field spraying practices: a case study of rice farmers in the municipality of Molave, Zamboanga Del Sur, Philippines. Adv Environ Biol 9:134–142. https://doi.org/10.3390/bs5030384
Zhu L, Li W, Zha J et al (2014) Butachlor causes disruption of HPG and HPT axes in adult female rare minnow (Gobiocypris rarus). Chem Biol Interact 221:119–126. https://doi.org/10.1016/j.cbi.2014.07.016
Acknowledgements
We thank Frank von Hippel and Rachel Rubin for their editorial support. We are grateful to Linsey Benally, Alexander McCain, Renee Lorica, and Calsey Richardson for their assistance in field and laboratory data collection. We thank the anonymous reviewers who helped to improve the manuscript. Funding was provided by the Merriam Powell Center for Environmental Research’s Integrative Graduate Education, Research, and Traineeship Program (IGERT) Fellowship, the Achievement Rewards for College Scientists (ARCS) Foundation, the Closing Rice Yield Gaps in Asia with a Reduced Environmental Footprint (CORIGAP) funded by the Swiss Agency for Development and Cooperation, the National Institute on Minority Health and Health Disparities of the NIH, Award Number T37MD008626 to CRP, and the National Cancer Institute of the NIH award for the Partnership of Native American Cancer Prevention U54CA143925 to Northern Arizona University.
Author contributions
M.S.G., G.S., and C.R.P. conceived and designed the study. M.S.G. executed the study, analyzed the data, and wrote the manuscript. G.S. and C.R.P. contributed significant editorial guidance on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests associated with this study, and that all applicable institutional and/or national guidelines for the care and use of animals were followed and approved by Northern Arizona’s Institutional Animal Care and Use Committee (IACUC).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Shuman-Goodier, M.E., Singleton, G.R. & Propper, C.R. Competition and pesticide exposure affect development of invasive (Rhinella marina) and native (Fejervarya vittigera) rice paddy amphibian larvae. Ecotoxicology 26, 1293–1304 (2017). https://doi.org/10.1007/s10646-017-1854-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-017-1854-8