Abstract
Pesticides are highly toxic substances. Their toxicity may not be absolutely specific to the target organisms but can adversely affect different processes in the non-target host plants. In the present study, the effect of over application of four commonly used pesticides (emamectin benzoate, alpha-cypermethrin, lambda-cyhalothrin and imidacloprid) was evaluated on the germination, seedling vigor and photosynthetic pigments in tomato. The obtained results revealed that seed germination was decreased by the pesticides and this effect was more prominent at early stages of exposure. All the tested pesticides reduced the growth of tomato when applied in higher concentration than the recommended dose, but at lower doses the pesticides had some stimulatory effects on growth as compared to the control. A similar effect of pesticides was observed on the photosynthetic pigments, i.e. a decrease in pigments concentrations was caused at higher doses but an increase was observed at lower doses of pesticides. The calculation of EC50 values for different parameters revealed the lowest EC50 values for emamectin (ranged as 51–181 mg/L) followed by alpha-cypermethrin (191.74–374.39), lambda-cyhalothrin (102.43–354.28) and imidacloprid (430.29–1979.66 mg/L). A comparison of the obtained EC50 values for different parameters of tomato with the recommended doses revealed that over application of these pesticides can be harmful to tomato crop. In a few cases these pesticides were found toxic even at the recommended doses. However, a field based study in this regard should be conducted to further verify these results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The intensive and multiple cropping systems in modern agriculture are unavoidably associated with intensification of disease incidences because the optimal doses of water and fertilizer provided to the land almost throughout the year enhance the survival ability, multiplication and dissemination of pathogens (Yardim and Edwards 2003). The traditional practices including resistant varieties may fail to provide desired level of diseases and pest control. Consequently, pest eradication by chemical pesticides is a common measure to protect crops against pests (Siddiqui and Ahmed 2006). Different classes of pesticides like insecticides, herbicides, fungicides, rodenticides, molluscicides and nematicides are used to control different types of pests. According to Zhang et al. (2011), about 4.6 million tons of chemical pesticides with about 500 different types are annually used across the world. About 85 % of the total pesticides used in the world are applied on agricultural crops while the remaining 15 % are used for other purposes (Idrovo 2000; CEPIS/PAHO 2005).
The use of chemical pesticides has reduced crop losses caused by pests, but their intensive and large-scale application caused diverse adverse effects on the environment (Carvalho 2006; Haq et al. 2008; Akhtar et al. 2009). The toxicity of pesticides does not remain restricted to the target organisms only but can also affect non-target organisms in the environment (Dobsikova 2003; Rachid et al. 2008). The indiscriminate and unskillful use of pesticides affects the growth of plant and animal, increases pest resistant to pesticides, accumulates residue in fruits and vegetables, causes biodiversity losses and declines natural habitats (Baig et al. 2012). Pesticides application can also lead to demolition of micro-fauna and flora of soil and water (Edwards 1986; Martinez et al. 2015). Cardone (2015) observed that pesticides can disrupt the growth of testes in non-target animals like lizards. The environmental and health risks of pesticides application in agriculture are severe, which not only leads to the chemical buildup of pesticide residues in crops but also disrupts the biochemical processes in plants (Lee-Fook-Choy and Seeneevassen 1998). Pesticides can interfere with various processes in plants like cell growth, photosynthesis, respiration, biosynthetic reactions, and molecular composition (DeLorenzo et al. 2001). These elusive adverse effects can add up and lead to economic losses when multiple crops are grown. Pesticides caused visible toxicity to plants, animals and humans are often tested prior to their registration and recommendation for use, but their effects on parameters of non-target host plants such as flower production, stunted growth or production times are not often tested (Spiers et al. 2008). Several studies investigated the adverse effects of pesticides on non-target organisms, especially animals, but limited studies have been conducted regarding pesticides toxicity to non-target plants (Mitra et al. 2011). A few studies exist which investigated the effect of high dosage of pesticides on different parameters of plants such as pollen performance (Cali and Candan 2009; Tort et al. 2005), reproduction in potato (Rio et al. 2012), photosynthesis and enzyme activities in cucumber (Xia et al. 2006), growth in soybean (Aksoy et al. 2013) and morphology and physiology in maize (Kilic et al. 2015).
In advanced countries, strict monitoring and regulation of pesticides ensure the safe use and proper handling of pesticides. The control schemes further ensure their use on scientific basis that support their effectiveness against target pests and minimize the effect on the environment and human health (Glover-Amengor and Tetteh 2008). In contrast, the use of pesticide in most of the developing countries is solely based on the recommendation of manufacturer which is based on the data of toxicological and environmental properties of pesticides. No doubt these information are useful but may not be appropriate under local conditions (Glover-Amengor and Tetteh 2008). In developing countries, there is no proper monitoring system for pesticide application. Most of the growers are unaware and unskilled regarding pesticides usage. The pesticides dealers are also mostly untrained and usually devise pesticides unwisely in two or three times higher concentration than the recommended dose (Ecobichon 2001; Asogwa and Dongo 2009).
Like other parts of the world, large quantities of different pesticides are used in Pakistan. According to an estimate, about 70,000 tons of pesticides are used in Pakistan annually and this quantity is continuously increasing with an annual rate of about 6 % (WWF 2007). As each crop is susceptible to attack by more than one type of pests, it is usually treated with several pesticides. In Pakistan, pesticides are often used indiscriminately and in excess amount (Khan 2004; Khan et al. 2012; Sheikh et al. 2013). The pesticide dealers always recommend pesticides in excessive dose because they are more interested in earning their profit rather than guiding the farmers properly (Rehman 1994).
Tomato (Lycopersicon esculentum) is a major horticultural crop with an estimated global production of over 120 million metric tons (FAO 2007) and has a high economic value worldwide. In Pakistan, tomato is the second major growing crop among vegetables (Mirza 2007). For example, in 2011 tomato was grown on an area of 39,918 hectares which gave an estimated annual production of 433,128 tones. In 2009–2010, Pakistan exported to a quantum of 5692 tons of tomato and earned 77 million PKR (Khokhar 2013). Due to the rapid increase in population, the demand for domestic consumption of tomato in Pakistan is increasing day by day. People use tomato in various ways like in the form of vegetables, salad, fruit, ketchup, and chatni etc. (Chohan and Ahmad 2008). In Pakistan the yield of tomato is lower as compared to developed countries because of various factors including pests attack as a major factor (Shakoor et al. 2010). To overcome the losses due to pests, farmers extensively use pesticides on tomato in higher concentrations which can probably affect its growth and yield. The extensive and over application of pesticides on vegetables and fruits also results in residue problems in vegetables and fruits which ultimately affects human health. However, the effect of over application of these pesticides on tomato has never been investigated in the country. The present study was therefore designed to investigate the effect of different doses of four commonly used pesticides in Pakistan (emamectin, alpha-ypermethrin, imidacloprid and lambda-cyhalothrin) on seed germination, plant vigor and photosynthetic pigments in tomato.
Materials and methods
Collection of seeds
The seeds of tomato (variety BSS-30) were obtained from a certified dealer at Bannu city, Khyber Pakhtunkhwa, Pakistan.
Sterilization of materials
The seeds of tomato were sterilized by rinsing with 80 % (v/v) ethanol for 5 min and then washed with distilled water for two to three times. Before using, all glass wares were autoclaved at 121 °C for 15 min.
Pesticides solutions
A total of four pesticides including imidacloprid, emamectin benzoate, alpha-cypermethrin and lambda-cyhalothrin were used in this study. Alpha-cypermethrin and lambda-cyhalothrin belong to the group pyrethroids while imidacloprid and emamectin belong to the neonicotinoids and abamectin groups of pesticides, respectively. All the used pesticides are broad spectrum insecticides, basically nerve poison affecting nerve impulses in the exposed insect. In emamectin, the active ingredient is avermectine which causes paralysis in the insects by activating chloride channels in nerves cells. Alpha-cypermethrin and lambda-cyhalothrin are pyrethroids which modulate the sodium channels causing hyperexcitation in nerves. The imidacloprid contains neonicotinoids which mimic the agonist action of acetylcholine and cause hyperexcitation in the nervous system of exposed insects. The selection of these pesticides was based on literature which reveals these to be among the most commonly used pesticides in Pakistan (Khooharo et al. 2008; Khan et al. 2011, 2012). A general informal discussion with farmers and pesticide dealers in a tomato growing area confirmed the same. Since the dealers usually prescribe higher doses of pesticides than the dose recommended by manufacturer as described in the introduction section of this manuscript, therefore, the concentrations tested for each pesticide were: recommended dose, two higher and two lower doses than the recommended dose (Table 1). In this way five concentrations of each pesticide were tested. Three independent replicates were tested for every concentration of each pesticide.
Experimental procedure
A total of ten seeds were placed with appropriate distance in each Petri plate lined with double layered filter paper and initially soaked with 10 ml of the respective solution. The experiments were conducted in a growth chamber. Initially the dark condition was provided for germination and thereafter a photoperiod of 16/08 h light/dark period with a light intensity of 500 µmol/m2/s was provided. A 30 °C temperature and 60 % humidity was maintained throughout the experiment. Each treatment was added with 3 ml of the respective solution after every 48 h. Germination percentage was recorded for 13 days at different intervals while the rest of parameters were determined at 21st day of experiment.
Determination of germination
The germination of seeds was observed at different intervals for 13 days. A seed was considered to be germinated if its radicle was emerged. The germination percentage was calculated from the number of total seeds and germinated seeds in a Petri plate.
Determination of roots and shoots length
After 20 days of growth, seedlings were separated into roots and shoots and their length was measured in millimeter (mm) with the help of a measuring tape.
Fresh and dry weight of roots and shoots
For the determination of fresh and dry weight of root and shoot, seedlings were separated into roots and shoots and were weighed with an electronic balance. The fresh roots and shoots were then separately placed in paper envelopes and were oven dried at 80 °C for 24 h and weighed for dry weight as described by Bibi et al. (2012).
Determination of photosynthetic pigments
For determination of photosynthetic pigments (chlorophyll a, b and total carotenoids), 25 mg of dried plant material was taken in a test tube and 25 mg of MgO was added to prevent the formation of pheophytin. A 5 ml of methanol was added in each sample and homogenized on shaker for 2 h followed by centrifugation for 5 min at 4000 rpm. After centrifugation, the supernatant was transferred to a 1-cm path length cuvette. The absorbance was measured using methanol solvent as a blank in a UV–Vis spectrophotometer at three different wavelengths: 666, 653 and 470 nm. Chlorophyll a, b and total carotenoids were calculated according to the method of Lichtenthaler and Wellburn (1983) as follow:
where C a , C b and Cx+c represent the concentration of chlorophyll a, b and total carotenoids, respectively while OD shows optical density (absorbance) at a given wavelength.
Statistical analysis
The student t test was applied to measure the significance of differences among different treatments and the control. The difference was considered to be significant if p value was smaller than or equal to 0.05 (p ≤ 0.05). For the calculation of dose-dependent response (EC50 values), linear regression methods and the equation Y = mx + c or Y = mx − c was used where Y = 50 % inhibition, x = concentration, c = constant and m = coefficient.
Results
Effect of pesticides on seed germination
The effect of different concentrations of the tested pesticides on seed germination in tomato is shown in Fig. 1a–d. A significant decrease in germination of seeds was observed with the increase in concentrations of pesticides as compared to the control. The concentrations of pesticides below the recommended dose promoted the germination capacity of seeds except in the case of imidacloprid where this effect was not significant as compared to the control. In early days of germination (up to 72 h), the highest concentrations of alpha-cypermethrin, lambda-cyhalothrin, imidacloprid and emamectin (500, 240 and 2000 and 160 mg/L, respectively) almost completely inhibited the germination. With the passage of time, a recovery of germination was observed; however, the germination at higher doses of pesticides was still lower in comparison to the control. The overall data on germination revealed that the effect of pesticides on seed germination was concentration and time dependent, i.e. a decrease with increasing concentration of pesticides was shown but a decrease in inhibitory effect was observed with increase in exposure time. The EC50 values obtained for germination after 312 h were 90.24, 344.10, 181.28, and 1624.91 mg/L for emamectin benzoate, alpha-cypermethrin, lambda-cyhalothrin and imidacloprid, respectively (Table 2).
Effect of the pesticides emamectin (a), cypermethrin (b), lambda-cyhalothrin (c) and imidacloprid (d) on seed germination in tomato. Each bar represents the mean value of three independent replicates and the error bar shows standard deviation. Asterisk (*) indicates significant difference (p ≤ 0.05) as compared to the control
Effect of pesticides on root-shoot length
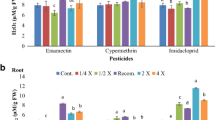
The response of root and shoot growth in tomato seedlings was different to different pesticides (Figs. 2 and 3). In case of shoot, the highest reduction in length was observed at 500 mg/L of alpha-cypermethrin where the shoot length was 7.3 mm as compared to 30.6 mm in the control (Fig. 2). At the recommended or lower concentrations, almost all pesticides improved the shoot length but with no significant difference as compared to the control. The longest shoot was found in seedlings treated with 60 mg/L of alpha-cypermethrin while the highest inhibition was caused by 500 mg/L of alpha-cypermethrin. For shoot length, the EC50 values calculated were 89.81, 274.80, 112.92, and 632.76 mg/L for emamictin benzoate, alpha-cypermethrin, lambda-cyhalothrin and imidacloprid, respectively. In case of root, the growth was adversely affected with increasing concentrations of each pesticide (Fig. 2). The most significant inhibition in root length was observed when the seedlings were subjected to 500 mg/L of alpha-cypermethrin, 120 mg/L of lambda-cyhalothrin and 2000 mg/L of imidacloprid (Fig. 2). For root length, the EC50 values of emamictin, alpha-cypermethrin, lambda-cyhalothrin and imidacloprid were 106.17, 241.79, 136.99 and 620.63 mg/L, respectively. By considering the application rate (recommended and double dose), imidacloprid seems to pose high risk to roots following by alpha-cypermethrin as their EC50 values (620.63 and 241.78, respectively) were found below their double doses (Tables 1 and 2).
Effect of pesticides on fresh and dry weight
A change in the biomass of seedlings measured as fresh and dry weight of root and shoot was observed upon exposure to pesticides. A signification reduction in shoot fresh and dry weight was found by exposing the seedlings to higher concentrations of pesticides (Figs. 4 and 5). In case of root fresh weight, lower concentrations of all the selected pesticides were found to increase the biomasses while at higher concentrations tremendous reduction was observed (Fig. 4). Like its fresh weight, the dry weight of root was also adversely affected when exposed to high doses of pesticides (Fig. 5). For the fresh and dry biomass of shoot, the lowest EC50 values were obtained for emamectin while the highest were obtained for imidacloprid. Almost similar trend in EC50 values was observed in the case of fresh and dry weight of root (Table 2). The overall results revealed that higher concentrations of pesticides caused hostile effects on tomato biomass (Figs. 4 and 5). Among the tested pesticides, imidacloprid can be found more dangerous to tomato when the recommended dose and obtained EC50 values for biomass are compared. For example, the EC50 of imidacloprid for root fresh weight (430.29 mg/L) was below its recommended dose of 500 mg/L.
Effect of pesticides on photosynthetic pigments
The effect of pesticides on chlorophyll a, b and total carotenoids was tested in shoot of tomato seedlings. The adverse effect of the tested pesticides on all pigments was found to increase with the increase in concentration of pesticides. The photosynthetic pigments were more susceptible to pesticide stress than other tested end points. At lower concentrations, no significant change was found in chlorophyll a content except in the case of alpha-cypermethrin where an increase was observed as compared to the control (Fig. 6). A significant reduction in chlorophyll a was observed in seedlings treated with the recommended or higher concentrations of all the four pesticides tested. The highest decrease in chlorophyll a content was observed when seedlings were exposed to 2000 mg/L of imidacloprid (Fig. 6d). The response of chlorophyll b to pesticides stress showed a similar trend as of chlorophyll a (Fig. 6). All the pesticides at all doses reduced the concentration of chlorophyll b except for lower concentrations of emamectin which had no significant effect as compared to the control (Fig. 6). The response of total carotenoids to pesticide exposure varied and depended upon the type and concentration of pesticides used. Lower concentrations of all the tested pesticides stimulated the production of total carotenoids. The recommended concentrations of all the four pesticides showed no significant effect on total carotenoids as compared to the control. However, the higher concentrations (above the recommended doses) of pesticides caused a prominent decrease in total carotenoids (Fig. 7). Like other parameters, a variation in EC50 values of the four pesticides for different pigments was observed with emamectin giving the lowest and imidacloprid the highest EC50 value for all the three pigments (Table 2). The overall results of photosynthetic pigments revealed that chlorophyll b was more susceptible to pesticides stress than chlorophyll a and total carotenoids as three of the four pesticides gave lower EC50 values for chlorophyll b as compared to the other two pigments (Table 2). In the case of chlorophyll a and b, the toxicity order of pesticides was found to be alpha-cypermethrin > imidacloprid > emamectin > lambda-cyhalothrin (Fig. 6).
Discussion
Pesticides are the modern tools used to control pests, weeds and diseases so as to increase crop yield. The pesticide manufacturing companies recommend pesticides at a particular dose. However, pesticides dealers in developing countries like Pakistan usually recommend higher doses to farmers than the recommended dose of a pesticide prescribed by the manufacturer (Ecobichon 2001; Asogwa and Dongo 2009; Rehman 1994). These higher doses can probably harm the host crop. Therefore, the present study was conducted to evaluate the effect of over doses of pesticides on different parameters of tomato.
In the present study, lower concentrations of pesticides stimulated seed germination in tomato but higher doses inhibited it. Similar results were reported by Sammaiah et al. (2011) that high doses of the pesticides endosulfan and kitazin inhibited germination in brinjal (Solanum melongena L.). Similarly, different pesticides have been found to inhibit seed germination in different plants species like Solanum melongena, Chilli, Pisum, Brassica nigra, tomato, Typha latofolia and maize (Chahid et al. 2013; Coskun et al. 2015; Kilic et al. 2015; Moore et al. 1999; Murthy and Rao 1980; Muthuswamy et al. 1983; Krishnasamy and Pandian 1992; Maheswari et al. 1993). It was also observed in the present study that in early days of exposure, germination was more susceptible to pesticide stress than in the later stages. It reflects that at certain concentration, a pesticide may not completely inhibit germination but may delay it. Among the pesticides tested in the present study, imidacloprid had the most severe effect on seed germination in tomato while emamectin caused the least effect. This difference among the pesticides’ effect on germination might be due to different nature of compounds in different pesticides and their applied dose. The company recommended dose of imidacloprid is higher than emamectin, and hence its higher concentration was used here accordingly, which can be another explanation for the higher effect of imidacloprid.
A signification decline was noticed in the fresh and dry weight of shoot and root when exposed to increased pesticide stress. Reduction in biomass production by pesticides like triadimenole and triticonazole was also observed previously in wheat (Montfort et al. 1996). They concluded that pesticides affect biomass by affecting seedling growth, shoot development and production of root axis. Similarly, the adverse effects of pesticides on physiology and yield of other crops have been observed. For example, Picea sitchensis treated with dimethoate, malathion, primicarb (Straw et al. 1996) and tomato exposed to abamectin and cartap at higher doses (Picanco et al. 1998) were negatively affected. Higher doses of different pesticides have inhibited the overall plant growth in different plants species as in soybean (Siddiqui and Ahmed 2006), maize (Coskun et al. 2015; Kilic et al. 2015), tomatoes (Chahid et al. 2013), and chickpea (Tiyagi et al. 2004).
The changes in photosynthetic pigments of plants are usually used as a tool for the assessment of stressful conditions (Ashraf and Harris 2013). The analysis of pigments in the present study showed a decrease in photosynthetic pigments in tomato with increasing concentration of pesticides. Literature survey reveals that the application of pesticides like fludioxonil and carbendazim reduced the chlorophyll and carotenoids contents in Vitis vinifera and Nicotiana tabacum, respectively (Garcia et al. 2003; Saladin et al. 2003). Similarly, the application of pyriprixifen reduced photosynthetic pigments in maize (Coskun et al. 2015), aldicarb, carbufurane, phrate fensulsothion and fenamiphos in cheackpea (Tiyagi et al. 2004), chlorantraniliprole in maize (Kilic et al. 2015), and difenoconazole and tricyclazole in tomato (Shanmugapriya et al. 2013). A systemic fungicide, benomyl, was also reported to inhibit biosynthesis of pigments in Helianthus annuus (Ahmed et al. 1983). The impairment of photosynthetic pigments by pesticides can lead to a reduced photosynthetic efficiency in plants. For example, Xia et al. (2006) observed significant reduction in the net photosynthetic rate of cucumber (Cucumis sativus L.) upon exposure to nine different pesticides. Chauhan et al. (2013) reported that exposure to pesticide caused several biochemical changes in potato. The mechanism of photosynthesis is greatly affected due to inefficient biosynthesis of chlorophyll contents that results in appearance of leaf chlorosis (Mitra and Raghu 1998). In addition to their adverse effects on higher plants, pesticides like carbufuran have also been reported to affect photosynthesis and photosynthetic pigments in the freshwater flagellate Euglena gracilis (Azizullah et al. 2011).
The obtained results revealed that pesticides application at higher concentration caused inhibitory effects in tomato seedlings. Similar trends of plant responses to pesticides exposure were reported by Siddiqui and Ahmed (2006) who demonstrated that higher doses of pesticides declined but lower doses enhanced the growth of soybean. Several other studies reported similar observations (Montfort et al. 1996; Siddiqui et al. 1997; Siddiqui and Ahmed 2000). Different explanations have been suggested for the adverse effects of pesticides on non-target crops. They may retard plant growth by inhibiting various physiological processes like seed germination, seedling growth, cell division, cell elongation and enlargement, and tissue and organ differentiation (Akobundu 1987). The presence of pesticides in soil may also affect take up of essential nutrients by plant root, which causes nutrients deficiency and retorted growth (Siddiqui and Ahmed 2006). Functional groups like –OH, –NH2, –CO.NH2, –COOR and –NR–, in pesticides can accelerate the process of adsorption in the soil which may affect the growth and development of plant via disrupting the soil water plant relationship (Misra and Mani 1994). Glover-Amengor and Tetteh (2008) reported that pesticides application may decline growth and yield of vegetables by affecting the beneficial microflora of soil. Pesticides may also reduce plant growth and development by reduction of hydroxyl phenyl pyruvate dehydrogenase which plays a vital role in the meristematic growth and development (Luscombe et al. 1995; Parween et al. 2011; Yildiztekin et al. 2015). Recently it has been reported that pesticides induced growth suppression in plants can be attributed to the increased electrolyte leakage (Yildiztekin et al. 2015) as well as to oxidative stress caused by pesticides (Yildiztekin et al. 2015). The slight stimulatory effects on growth of tomato caused by the lower concentrations of pesticides might be due to the utilization of some organic compounds present in pesticides by plants or it might be a response of plants to exposure to low doses of toxic substances as is generally observed in plants.
The response of tomato to the tested pesticides varied possibly due to different chemical nature of pesticides and different tested concentrations of pesticides (the tested concentrations of a pesticide were based on its recommended dose). The overall trend of EC50 values showed that emamectin showed the lowest EC50 and imidacloprid gave the highest EC50 values for almost all parameters. A general order of phytotxicity of the tested pesticides for tomato based on EC50 values can be emamectin < alpha-cypermethrin < lambda-cyhalothrin < imidacloprid. However, the recommended dose of a pesticide should be taken into account while considering these EC50 values. For example, the highest EC50 values (showing lowest toxicity in term of mg/L) were obtained for imidacloprid but this pesticide could affected some parameters in tomato even at the recommended dose as its recommended dose is much higher than the other tested pesticides. In most of the cases, the EC50 values were above the recommended dose but well below the double dose. For example, in case of emamectin the EC50 obtained for the root and shoot dry weight (51.48 and 80.6 mg/L, respectively) were above the recommended concentration (40 mg/L) but below the double dose (80 mg/L). The EC50 values of alpha-cypermethrin for chlorophyll b and root length were above the recommended dose and below the double dose. Similarly, in many other cases, the EC50 values obtained were below the double dose of pesticides. It can be concluded that over application of these pesticides, which is a common practice in countries like Pakistan as discussed above in this manuscript, can adversely affect tomato growth. Most of the studied parameters were adversely affected by double or higher dose of pesticides, but in some cases even the recommended doses were found to cause phytotoxicity.
Conclusions
It can be concluded from the present study that pesticides application above the recommended dose can adversely affect tomato growth. At higher doses, all the tested pesticides caused toxic effects on all the studied parameters of tomato. Since pesticide dealers usually suggest farmers to apply pesticides in doses doubled to the recommended dose, it can be harmful and affect tomato growth and yield. The application of pesticides above the recommended dose should be discouraged. There is a need to educate pesticide dealers and farmers about the proper and optimal applications of pesticides. The effects of these pesticides on non-target host plants should be further investigated at anatomical, biochemical and molecular level to identify the mechanism by which they cause toxicity in non-target plants.
References
Ahmed AM, Heikal MD, Hindawy OS (1983) Side effects of benomyl (Fungicide) treatments on sunflower, cotton and cowpea plants. Phyton 23:185–195
Akhtar MW, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdisc Toxicol 2:1–12
Akobundu (1987) Safe use of herbicides. Weed science in the tropics: principles and practices. Wiley, New York, pp 318–334
Aksoy O, Deveci A, Kizilirmak S, Akdeniz GB (2013) Phytotoxic effect of quizalofop-P-ethyl on soybean (Glycine max L.). J Biol Environ Sci 7:49–55
Ashraf M, Harris PJC (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51:163–190
Asogwa EU, Dongo LN (2009) Problems associated with pesticide usage and application in Nigerian cocoa production: a review. Afr J Agric Res 4:675–683
Azizullah A, Richter P, Häder DP (2011) Comparative toxicity of the pesticides carbofuran and malathion to the freshwater flagellate Euglena gracilis. Ecotoxicology 20:1442–1454
Baig SA, Akhter NA, Ashfaq M, Asi MR, Ashfaq U (2012) Imidacloprid residues in vegetables, soil and water in the southern Punjab, Pakistan. J Agric Technol 8:903–916
Bibi A, Sadaqat HA, Tahir MHN, Akram HM (2012) Screening of sorghum (Sorghum bicolor var Moench) for drought tolerance at seedling stage in polyethylene glycol. J Anim Plant Sci 22:671–678
Cali IO, Candan F (2009) The effect of fungicide application on pollen structure in tomato (Lycopersicon esculentum Mill.) plant. J Appl Biol Sci 3:37–40
Cardone A (2015) Imidacloprid induces morphological and molecular damages on testis of lizard (Podarcis sicula). Ecotoxicology. doi:10.1007/s10646-014-1361-0
Carvalho FP (2006) Agriculture, pesticides, food security and food safety. Environ Sci Policy 9:68–692
CEPIS/PAHO (2005) Pan-American center for sanitary engineering and environmental Sciences. Self taught course on diagnosis, treatment and prevention of acute pesticide poisoning
Chahid K, Laglaoui A, Zentar S, Ennabili A (2013) Effect of alpha-cypremethrin on morphological parameters in tomato plants (Lycopersicon esculentum Mill.). Am J Environ Prot 2:149–153
Chauhan SS, Agrawal S, Srivastava A (2013) Effect of imidacloprid insecticide residue on biochemical parameters in potatoes and its estimation by HPLC. Asian J Pharma Clin Res 6:114–117
Chohan TZ, Ahmad S (2008) An assessment of tomato production practices in Danna Katchely, Azad Jammu Kashmir. Pak J Life Soc Sci 6:96–102
Coskun Y, Kilic S, Duran RE (2015) The effects of the insecticide pyriproxyfen on germination, development and growth responses of maize seedlings. Fresenius Environ Bull 24:278–284
DeLorenzo ME, Scott GI, Ross PE (2001) Toxicity of pesticides to aquatic microorganisms: a review. Environ Toxicol Chem 20:84–98
Dobsikova R (2003) Acute toxicity of carbofuran to selected species of aquatic and terrestrial organisms. Plant Protect Sci 39:103–108
Ecobichon DJ (2001) Pesticide use in developing countries. Toxicology 160:27–33
Edwards CA (1986) Agrochemicals as environmental pollutants. In: Van Hofsten B, Eckstrom G (eds) Control of pesticide applications and residues in food. A guide and directory. Swedish Science Press, Uppsala
FAO (2007) Food and Agricultural Organization Stat, core production 2005. http://faostat.fao.org/site/340/default.aspx
Garcia PC, Rivero RM, Ruiz JM, Romero L (2003) The role of fungicides in the physiology of higher plants: implications for defense responses. Bot Rev 69:162–172
Glover-Amengor M, Tetteh FM (2008) Effect of pesticide application rate on yield of vegetables and soil microbial communities. W Afr J Appl Eco 12:1–7
Haq QU, Ali T, Ahmad M, Nosheen F (2008) An analysis of pesticide usage by cotton growers: a case study of district Multan, Punjab-Pakistan. Pak J Agric Sci 45:133–137
Idrovo A (2000) Surveillance of pesticide poisonings in Colombia. Public health 2:36–46
Khan AF (2004) Hazards of pesticide spray on vegetables. The Daily Dawn Pakistan, Internet Edition, June 12
Khan MS, Shah MM, Mahmood Q, Hassan A, Akbar K (2011) Assessment of pesticide residues on selected vegetables of Pakistan. J Chem Soc Pak 33:816–821
Khan BA, Zubair A, Khan SA, Ud-Din Z (2012) Monitoring pesticide residues in fruits and vegetables grown in Khyber Pakhtoonkhwa. Int J Green Herb Chem 1:302–313
Khokhar KM (2013) Present status and prospects of tomatoes in Pakistan. http://www.agricorner.com/present-status-and-prospects-of-tomatoes-in-pakistan/
Khooharo AA, Memon RA, Mallah MU (2008) An empirical analysis of pesticide marketing in Pakistan. Pak Eco Soc Rev 46:57–74
Kilic A, Duran RE, Coskun Y (2015) Morphological and physiological responses of maize (Zea mays L.) seeds grown under increasing concentrations of chlorantraniliprole insecticides. Pol J Environ Stud 3:1069–1075
Krishnasamy V, Pandian IRS (1992) Effect of fungicidal seed treatment in Brinjal. South Indian Hortic 40:207–212
Lee-Fook-Choy LH, Seeneevassen S (1998) Monitoring insecticide residues in vegetables and fruits at the market level. AMAS, Food and Agricultural Research Council, Reduit 95
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Luscombe BM, Pallett KE, Loubiere P, Millet JC, Melgarejo J, Vrabel TE (1995) RPA 201772 A novel herbicide for broad leaf and grass weed control in maize and sugar cane. British Crop Protection Council. Brighton Crop Prot Conf Weeds 2:35–42
Maheswari DK, Gupta M, Swahney R, Khandelwal A (1993) Dual behaviour of carbaryl and 2,4-dichlorophenoxyacetic acid in Rhizobium leguminosarum under explanta conditions. Xentrablatt Microbiol 148:588–592
Martinez RS, Di Marzio WD, Saenz ME (2015) Genotoxic effects of commercial formulations of Chlorpyrifos and Tebuconazole on green algae. Ecotoxicology 24:45–54
Mirza I (2007) Tomato paste plant to be set up at Killa Saifullah. http://www.pakissan.com/english/news/newsDetail.php?newsid=15041. Accessed 31 Aug 2007
Misra SG, Mani D (1994) Adverse effects of Pesticides. In: Misra SG, Mani D (eds) Agricultural pollution II. Ashish Publisher, New Delhi
Mitra J, Raghu K (1998) Pesticides non target plants interactions: an overview. Arch Agron Soil Sci 43:445–500
Mitra A, Chatterjee C, Mandal FB (2011) Synthetic chemical pesticides and their effects on birds. Res J Environ Toxicol 5:81–96
Montfort F, Klepper BL, Smiley RW (1996) Effects of two triazole seed-treatments, triticonazole and triadimenol, on growth and development of wheat. Pest Sci 46:315–322
Moore MT, Huggett DB, Huddleston GM, Rodgers JH, Cooper CM (1999) Herbicide effects on Typha latifolia (Linneaus) germination and root and shoot development. Chemosphere 38:3637–3647
Murthy PK, Rao D (1980) Effect of two fungicides on the germination and growth in Brassica nigra Koach. Geobios 7:160–161
Muthuswamy S, Padmanabhan D, Nagarajan R (1983) Efficacy of seed dressing fungicides on the viability of chili seeds. Pesticides 17:23–28
Parween T, Jan S, Fatma T (2011) Alteration in nitrogen metabolism and plant growth during different developmental stages of green gram (Vigna radiata L.) in response to chlorpyrifos. Acta Physiol Plant 33:2321–2328
Picanco M, Leite GL, Guedes RN, Silva EA (1998) Yield loss in trellised tomato affected by insecticidal sprays and plant spacing. Crop Prot 17:447–452
Rachid R, Djebar-Berrebbah H, Djebar MR (2008) Growth, chitin and respiratory metabolism of Tetrahymena pyriformis exposed to the insecticide Novaluron. Am-Eurasian J Agric Environ Sci 3:873–881
Rehman K (1994) Pesticides causing environmental pollution. In: Habib N (ed.) A workshop on people and pesticides. Khoj-Research and Publication Center, Lahore, Pakistan, pp 39–40
Rio AD, Bamberg J, Centeno-Diaz R, Salas A, Roca W, Tay D (2012) Effects of the pesticide furadan on traits associated with reproduction in wild potato species. Am J Plant Sci 3:1608–1612
Saladin G, Magne C, Clement C (2003) Effects of fludioxonil and pyrimethanil, two fungicides used against Botrytis cinerea, on carbohydrate physiology in Vitis vinifera L. Pest Manag Sci 59:1083–1092
Sammaiah D, Shekar ChC, Prasad VR, Reddy JK (2011) Pesticides induced alterations in physiological responses in Solanum melongena L. Int J Pharma Bio Sci 2:374–384
Shakoor A, Sabri MA, Afzal M, Bashir MH (2010) Role of plant morphological characters towards resistance of some cultivars of tomato against phytophagous mites (Acari) under greenhouse conditions. Pak J Life Soc Sci 8:131–136
Shanmugapriya AK, Sivakumar T, Panneerselvam R (2013) Difenoconazole and Tricyclazole induced changes in photosynthetic pigments of Lycopersicon esculentum L. Int J Agric Food Sci 3:72–75
Sheikh SA, Nizamani SM, Panhwar AA, Mirani BN (2013) Monitoring of pesticide residues in vegetables collected from markets of Sindh, Pakistan. Food Sci Technol Lett 4:41
Siddiqui ZS, Ahmed S (2000) Effect of systemic fungicide on nutritive composition of diseased and healthy plant of Triticum aestivum L. Pak J Biol Sci 3:2148–2150
Siddiqui ZS, Ahmed S (2006) Combined effects of pesticide on growth and nutritive composition of soybean plants. Pak J Bot 38:721–733
Siddiqui ZS, Ahmed S, Gulzar S (1997) Effect of topsin-M (methyl-thiophenate) and Bayleton (Triademifon) on seedling growth, biomass, nodulation and phenolic content of Sesbania sesban. Bang J Bot 26:127–130
Spiers JD, Davies FT, Che JR, Heinz KM, Bogran CE, Starman TW (2008) Do insecticides affect plant growth and development? Greenhouse Grower. http://aggie-horticulture.tamu.edu/faculty/davies/research/abstracts/pdfs
Straw NA, Fielding NJ, Waters A (1996) Phytotoxicity of insecticides used to control aphids on Sitka spruce, Picea sitchensis (Bong.) Carr. Crop Prot 15:451–459
Tiyagi SA, Ajaz S, Azam MF (2004) Effect of some pesticides on plant growth, root nodulation and chlorophyll content of chickpea. Arch Agron Soil Sci 50:529–533
Tort N, Oztork I, Guvensen A (2005) Effects of some fungicides on pollen morphology and anatomy of tomato (Lycopersicon esculentum mill.). Pak J Bot 37:23–30
Xia XJ, Huang YY, Wang L, Huang LF, Yu YL, Zhou YH, Yu JK (2006) Pesticides-induced depression of photosynthesis was alleviated by 24-epibrassinolide pretreatment in Cucumis sativus L. Pestic Biochem Physiol 86:42–48
Yardim EN, Edwards CA (2003) Effects of organic and synthetic fertilizer sources on pest and predatory insects associated with tomatoes. Phyto Parasit 4:324–329
Yildiztekin M, Kaya C, Tuna AL, Ashraf M (2015) Oxidative stress and antioxidative mechanisms in tomato (Solanum lycopersicum L.) plants sprayed with different pesticides. Pak J Bot 47:717–721
Zhang WJ, Jiang FB, Ou JF (2011) Global pesticide consumption and pollution: with China as a focus. Proc of the Int Acad Eco Environ Sci 1:125–144
Acknowledgments
The authors are thankful to Kohat University of Science and Technology (KUST), Kohat, Pakistan for supporting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shakir, S.K., Kanwal, M., Murad, W. et al. Effect of some commonly used pesticides on seed germination, biomass production and photosynthetic pigments in tomato (Lycopersicon esculentum). Ecotoxicology 25, 329–341 (2016). https://doi.org/10.1007/s10646-015-1591-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-015-1591-9