Abstract
The amount of copper in natural ecosystems is steadily increasing, due to human activities. It accumulates in plants, posing a threat to herbivores. In polluted areas the population density of small rodents is observed to be lower. The decline in rodent numbers may be caused by increased mortality or diminished fertility. This study examined the effect of copper on the reproductive activity of the bank vole (Myodes glareolus), a small rodent which during foraging often wanders into fields where it might be exposed to pollution. The animals were treated with solutions of 0, 150 or 600 ppm Cu. After 12 weeks of exposure the quality and quantity of the male’s sperm was tested. To assess morphological development we compared the experimental groups for body weight, the weight of the male’s testes and accessory sex glands, the female’s uterus, and the number of matured ovary follicles in tested females. At both doses, copper administration led to lower sperm count and caused sperm head anomalies. The higher dose compromised sperm tail membrane integrity, viability and motility. No effect of copper on morphological development was observed in males, and only the lower dose increased testes weight. In females the higher dose had a negative effect on morphological development, and the lower dose increased uterus weight. No effect of copper on ovarian follicle number was found. For the first time, the morphology of the most typical ovarian follicles of the bank vole is presented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollution of the natural environment may lead to irreversible changes in biodiversity, especially in developed countries (Franssen 2009; Das and Khangarot 2011; Davidson et al. 2011; Helmfrid et al. 2012; Satta et al. 2012; Ficken and Byrne 2013). Both an excess and an insufficiency of trace elements can affect an organism’s homeostasis (Linder 2001; Stern 2010). The liver and in some instances the kidney are primary targets of repeated-dose toxicity (Hébert et al. 1993; Aburto et al. 2001). Their cells can store significant amounts of metal ions without any damage to the organism (Linder 2001). In extreme cases excessive intake of elements leads to cell and organ damage and disruption of their functions (Evans and Abraham 1973; Amiard-Triqut et al. 1986; Nikolov et al. 2010).
Copper of both natural and anthropogenic origin is present in natural ecosystems (Spatari et al. 2002). The main artificial sources are mining, metal ore smelting, brass production, galvanization, burning of fossil fuels and excessive use of plant protectants that contain metals (Alloway et al. 1999; Sheffield et al. 2001). Plants growing on contaminated soil may accumulate substantial concentrations of different metals and then be ingested by herbivores (Wijnhoven et al. 2007).
Copper, one of the physiological trace elements, can affect an organism’s homeostasis, especially because it is an important oxidative stress factor (Galhardi et al. 2004) and as a divalent metal can bind with ERα receptors (Martin et al. 2003). Copper is also an integral part of several enzymes such as ferroxidase, cytochrome oxidase and superoxide dismutase (Friberg et al. 1986). The estimated minimum requirement for both immature and adult mice is 6 mg Cu/kg diet (Committee on Animal Nutrition SoLAN 1995) and for rats from 5–8 to 12 mg/kg diet (Lyubimov et al. 2004). In rats, testis weight was lower, spermatozoa became less mobile and the counts of damaged and dead sperm increased after intraperitoneal injection of high concentrations of copper (Lyubimov et al. 2004). Impala males (Aepyceros melampus) with high copper levels in plasma had high amounts of spermatozoa showing vacuolization in the neck region (Ackerman et al. 1999). As compared to normozospermic men, Cu in semen plasma was higher in men with reproductive dysfunctions (Eidi et al. 2010). Copper treatment caused prolificacy in sheep (Murawski et al. 2006). All these findings suggest that copper can interfere with endocrine homeostasis and in consequence may induce male reproductive system disorders and lower the quality and quantity of sperm cells.
Our knowledge of the impact of increased copper doses on physiological processes in wild-living rodents, including reproduction, is limited. At the same time, reduction of rodent population density is widely observed in industrial districts (Kataev et al. 1994; Dmowski et al. 1998; Sheffield et al. 2001). There are no data concerning whether the decrease is due to increased mortality or to altered reproductive ability. We used the bank vole (Myodes glareolus) as a model species in research addressing that question.
Our department’s bank vole breeding program uses stock originating from wild-caught animals. The bank vole is a forest species living mostly in mixed forests with rich undergrowth, thickets, meadows and forest gaps. While foraging they often wander into fields where they can be exposed to metal-contaminated plants and water. In areas near heavy metal smelters, leaves of garden sorrel (Rumex acetosa) accumulate copper at an average level of 601 mg/kg dry matter and sometimes spiking up to even 1102 mg/kg dry matter (Tang et al. 1999). The average amount of copper in various plants from polluted areas is about 150 ppm (Kabata-Pendias and Pendias 1999).
We investigated the effects of copper on small rodent’s reproductive abilities by administering doses corresponding to different environmental levels of contamination: a basal dose (Cu I: 150 mg), elevated dose (Cu II: 600 mg) and control dose (C: 0 mg). The working hypothesis was that we would find a copper-mediated decline in the reproductive abilities of both female and male bank voles.
To determine the impact of copper on bank vole male’s reproductive abilities we compared their body weight and the weight of testes and accessory sex glands between experimental groups. We also examined epididymal sperm quantity and quality, and analyzed the histological morphology of testes obtained from the three experimental groups. To determine the impact of copper on bank vole female’s reproductive abilities we compared their body weight and uterus weight between treatment groups, and made histological slides of ovaries to determine the number and type of matured follicles. We also determined the average size and depict the morphology of matured follicles, which has not been done previously in this species.
Materials and methods
Animals
The bank voles (M. glareolus Schreber, 1780) came from the laboratory colony of the Institute of Environmental Sciences, Jagiellonian University, Krakow. The original stock was obtained in 1976 from the Mammal Research Institute of the Polish Academy of Sciences (Białowieża) and is maintained as an outbred stock colony according to the system described by Green (1966). Briefly, each generation consists of at least 22 breeding pairs; the male and female of each mating pair do not share parents or grandparents. This breeding system ensures the heterogeneity of the colony. The animals are housed in polyethylene cages (40 × 25 × 15 cm) under a 14 h photoperiod (7 am–9 pm light, 9 pm–7 am dark) at 21 ± 1 °C and 60 % humidity. Wood shavings are provided as bedding material, changed once a week. Standard pelleted chow for laboratory rodents (Labofeed H, Kcynia) and liquid in the form of copper solutions are available ad libitum.
For the study, at 19–20 days of age the weanlings were separated from their parents, and at 4 weeks of age 3–5 individuals were placed in same-sex cages. Then both females and males were randomly divided into three experimental groups, each consisting of 12 males and 12 females. The groups were treated with different solutions for 12 weeks.
Experimental groups
Cu I—(150 ppm dose): copper sulphate(II) 5 hydrate (CuSO4·5H2O) AR purity grade (POCH) at concentration of 150 mg Cu2+/l (75 mg Cu2+/kg body mass/day).
Cu II—(600 ppm dose): copper sulphate(II) 5 hydrate (CuSO4·5H2O) AR purity grade (POCH) at concentration of 600 mg Cu2+/l (300 mg Cu2+/kg body mass/day).
C—(0 ppm dose): deionized water (control).
Males
Body and organ weight
After cervical dislocation, males were weighed, after which paired testes, seminal vesicles and coagulation glands were dissected out and weighed (the latter two together).
Epididymal sperm evaluation
Preparation of epididymal sperm suspension
After applying gentle pressure to each cauda epididymis with forceps, allowing epididymal sperm to pass to the vasa deferentia, the content of the latter was suspended in 100 µl M2 medium (Sigma-Aldrich, Germany) and allowed to disperse for two minutes.
Epididymal sperm concentration
A 1:20 dilution of epididymal sperm suspension with M2 medium was prepared, and the number of live sperm cells in 100 squares of a hemocytometer (Bűrker chamber) was counted under a light microscope at 400x. A coverslip was placed on the sample to restrict spermatozoa movement. The average of two sperm counts was taken as the sperm concentration estimate, as described by Styrna and Krzanowska (1995) Kruczek and Styrna (2009).
Epididymal sperm motility
Spermatozoa motility was assessed in a hemocytometer. The percentage of motile sperm (i.e. sperm showing progressive movement) among 200 counted spermatozoa from each male was recorded (Seed et al. 1996).
Epididymal sperm tail membrane integrity—water test
The integrity of the epididymal sperm tail membrane was determined by the hypoosmotic swelling test. The procedure was the same as used for mice and bank voles (Styrna and Krzanowska 1995; Styrna et al. 2003; Kruczek and Styrna 2009): 20 µl epididymal sperm suspension (as described in “Preparation of epididymal sperm suspension” section) was mixed with 120 µl distilled water on a clean glass slide. Then the mixture was gently covered with a coverslip and incubated for 5 min at 37 °C before being examined. The percentage of spermatozoa showing swelling among 200 counted spermatozoa from each male was recorded.
Epididymal sperm viability—eosin-Y test
The test reflects the structural and morphological integrity of the sperm membrane (Walczak et al. 1994; Styrna and Krzanowska 1995). To assess sperm viability, 20 µl epididymal sperm suspension (as described in “Preparation of epididymal sperm suspension” section) was mixed with 20 µl 0.2 % eosin-Y, incubated for 10 min at 37 °C and smeared on a slide. The percentage of spermatozoa with unstained sperm heads (viable spermatozoa) among 200 counted spermatozoa from each male was recorded.
Epididymal spermatozoa with a cytoplasmic droplet
In this procedure, 20 µl epididymal sperm suspension (as described in “Preparation of epididymal sperm suspension” section) was transferred to a slide and gently covered with a coverslip. The percentage of spermatozoa with a cytoplasmic droplet among 200 counted spermatozoa showing progressive movement was recorded from each male (Kruczek and Styrna 2009).
Epididymal sperm morphology
For morphological examination, a small drop of epididymal sperm suspension was smeared on a slide, air-dried, fixed in acetic alcohol (absolute alcohol:glacial acetic acid, 3:1) and stained with Papanicolau to determine the proportions of different sperm head anomalies.
Head anomalies were classed as follows (Kruczek and Styrna 2009):
Normal—Sperm with proper head morphology;
Class 1—lack of the top part of the hook and anomalies in the base of the head;
Class 2—lack of the hook as well as serious anomalies in the proximal part of sperm head; possible changes in the base of the head.
Spermatogenic index
Isolated testis were fixed in formalin, dehydrated in an ethanol series, infiltrated and embedded in paraffin, cut to 7 µm thickness, then stained using hematoxylin-eosin and classified by light microscopy for functional state according to the spermatogenic index (Kruczek 1986). The spermatogenic index (values from 5 to 0) gives a measure of seminiferous epithelium activity, with 5 representing complete spermatogenesis with abundant sperm production and 0 being the presence of only Sertoli cells and spermatogonia; values from 1 to 4 represent incremental changes in the spermatogenesis process. The spermatogenic index was determined from 20 seminiferous tubules situated in the center of the testicular cross section.
Females
Body and organ weight
After cervical dislocation, females were weighed, after which the uterus was dissected out and weighed.
Assessment of ovarian follicles
Isolated ovaries were fixed in aqueous Boinea’s solution, dehydrated in an ethanol series, infiltrated and embedded in paraffin, cut to 6 µm thickness, then stained with hematoxylin-eosin and analyzed by light microscopy to classify the different stages. Follicles in ovaries from females of all experimental groups were classified as type 6 (diameter 355.06–417.99 µm), type 7 (diameter 526.58–594.67 µm) or type 8 (diameter 715.78–867.39 µm) according to Pedersen and Peters (1968). Photographs of the most typical follicles in the control group (Fig. 1) were taken by using Nikon Eclipse 80i microscope. Follicle diameter was determined using ImageJ software. The sum of each type of follicle in an individual was recorded.
The researchers who made the counts, examined sperm and testis tissue and classified the ovaries were blind to the experimental group during those procedures.
Statistical analysis
To obtain linear dependence, angular transformation was applied to percentage results. Means were compared by one-way ANOVA and the significance of differences was determined by the post hoc Tukey test. All results are presented as mean ± SE, and p < 0.05 is taken to indicate significance. For clarity of presentation the bars in the figures reflect percentages. All calculations were done using Statistica PL ver. 10.0.
Ethical standards
The experimental procedures for this study were approved by the Regional Committee on Animal Experimentation in Krakow (Protocol No. 36/2009) acting in compliance with the European Communities Council Directive of 24 Nov. 1986 (86/609/EEC).
Results
Effect of copper on male’s reproductive traits
Organometric parameters
The results summarized in Table 1 show that copper did not affect the male’s body weight or accessory sex gland weight but did affect testes weight. Testis weight was highest in males treated with 150 ppm copper (Cu I), significantly higher than in control males given water (C) and in males treated with 600 ppm copper (Cu II) (F(2,33) = 4.36, p < 0.05).
Epididimal sperm evaluation
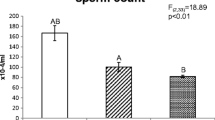
As shown in Fig. 1, the sperm count was highest in males from the control group (C), significantly higher than in males from groups Cu I and Cu II (F(2,33) = 23.80, p < 0.01). There were also significant differences in sperm counts between Cu I and Cu II. The counts were lowest in males exposed to the highest copper concentration (Cu II) (Fig. 1).
Figure 2 shows that the percentage of swollen sperm cells was highest in males exposed to 600 ppm, the highest copper concentration (F(2,33) = 10.69, p < 0.01). There were no significant differences in the percentage of swollen sperm cells between males treated with water and those administered 150 ppm copper. Males exposed to 600 ppm copper showed the lowest percentages of viable (F(2,33) = 8.44, p < 0.01) and motile (F(2,33) = 12.60, p < 0.01) sperm cells. There were no differences in the percentages of viable and motile sperm cells between the control males and those given 150 ppm copper. There were no significant differences in the number of immature sperm cells with droplet between the treatment groups (F(2,33) = 0.02, p–NS) (Fig. 2).
The proportion of abnormal sperm heads was lowest in the control males, and increased significantly with the copper concentration (Table 2) (F(2,33) = 74.36, p < 0.05). There were significant differences in the proportion of both classes of abnormal sperm heads between all the treatment groups.
Spermatogenic index
The spermatogenic index decreased under exposure to copper (Table 2). It was highest in control males; in that group it was significantly higher than in males exposed to 150 ppm and 600 ppm copper (F(2,33) = 188.27, p < 0.01). The index was significantly higher in the males of group Cu I than in those of Cu II (Table 2).
Effect of copper on female’s reproductive traits
Organometric parameters
As shown in Table 1, female body weight was significantly higher in the control group than in those exposed to 600 ppm copper (F(2,33) = 5.04, p < 0.05). To correct for significant differences in female body weight we analyzed relative uterus weight: it was highest in females treated with 150 ppm copper and was significantly higher than in control females (F(2,33) = 3.36, p < 0.05). There were no significant differences in relative uterus weight between Cu I and Cu II females, that is, between those exposed to 150 ppm and 600 ppm copper. Nor were there differences in relative uterus weight between control females and those exposed to 600 ppm (Table 1).
Ovarian follicles
There were no differences in the number of ovarian follicles (Fig. 3) (types 6, 7, 8), between the experimental groups (F(2,33) = 0.38, p—NS) (Table 3).
Discussion
In this study we examined the effects of copper exposure on the reproductive abilities of bank voles (M. glareolus) of both sexes. We analyzed morphological development as well as changes in reproductive traits as indicators of the potential effects of copper toxicity.
Our knowledge of the impact of increased copper concentrations on morphological development in rodents is limited. Stern (2010) suggested that the activity of copper is dose-dependent. For the reproductive system our data give partial confirmation of this: increased testes weight in males and increased uterus weight in females at the lower dose (150 ppm) of copper, with no such effects at the higher dose (600 ppm). The basis of dose-dependent copper activity may lie in the endocrine metabolism of copper ions, especially in estrogens. In research using human breast cancer cell line MCF-7, Martin et al. (2003) suggested that divalent metals like copper activate ERα through the formation of a complex within the hormone-binding domain of the estrogen receptor. One of the main functions of estrogens is to promote the growth and differentiation of female sexual organs and other tissues related to reproduction (Clark et al. 1992). Estrogen is also thought to have a regulatory role in male reproductive processes; estrogens such as estradiol (E2) modify cell function in rodents by binding to the high-affinity estrogen receptor ERα in Leydig cells (Sierens et al. 2005). The absence of ERα was reported to have adverse effects on spermatogenesis and stereidogenesis (Akingbemi 2005). ER activation by copper may also explain the differences in testes and uterus weight in our study; the lower dose (150 ppm) of copper we used presumably saturated the ERα receptors and in consequence accelerated testis and uterus growth, while the higher dose (600 ppm) may have been too toxic to exert the same effect.
In this study we observed no change in accessory sex gland weight in males after administration of copper. In contraception experiments on mature male albino rats (Ahsan et al. 1976) found no change in gland weight after implanting copper wires intravasally, and noted decapitation of most of the spermatozoa as one outcome of the procedure. In immature Wistar rats, (Chattopadhyay et al. 2005) found that accessory sex organ weight significantly declined under treatment with 2000 ppm copper and that it increased in the 1000 ppm treatment reflecting an imbalance in the levels of hormones involved in stereidogenesis. Their research used doses several times higher than in our work and delivered them intraperitonally, perhaps explaining the discrepancy between their results and ours.
We found body weight loss in females treated with 600 ppm copper solution. In rats and mice, doses of 4,000–16,000 ppm cupric sulfate delivered in feed are hepatic and renal toxicants and induce anemia, and are correlated with decreases of body weight in both sexes (Hébert et al. 1993). We found no body weight changes in bank vole males or females under both doses employed. Other work suggests that high dietary copper elevates food intake and promotes weight gain by increasing neuropeptide Y (NPY) (Pau et al. 1989).
The bank vole is a small rodent with a promiscuous mating system. In such a system the quality and quantity of sperm cells is critical to reproductive success. In polluted areas the level of environmental contamination by copper should be a limiting factor in male’s reproductive success. Experiments in vitro or on laboratory animals have suggested that excess copper is highly toxic to the male reproductive system (Roychoudhury et al. 2008; Eidi et al. 2010). In our experiments copper reduced the quantity and quality of sperm cells and affected seminiferous epithelium development. Copper can cause oxidative stress (Galhardi et al. 2004), which in the reproductive system is thought to affect the fertilizing abilities of sperm (Aydemir et al. 2006). Ng and Liu (1990) suggest that copper exerts a direct toxic effect on steroid-producing cells in the adrenal gland and testes. The drop in the sperm cell counts in our study might be an effect of testosterone overload. Testosterone in higher quantities suppresses sperm production as well as secretion of hypothalamus hormones involved in stereidogenesis (Matsumoto et al. 1986). In rats, accumulation of Cu2+ in the adrenal glands produced a biphasic response in the concentration of the mitochondrial cytochrome P-450, reflecting disordered steroidogenesis (Veltman and Maines 1986). Oster and Salgo (1977) suggested that copper is involved in suppression of spermatogenesis in mammals but did not offer a morphology-based or chemistry-based explanation.
Xu et al. (1985) suggested that the major cause of infertility after injection of copper into the rat caput epididymis was a direct inhibitory effect of copper on and that damage to the seminiferous and epididymal epithelium may have contributed. Weak motility is often accompanied by sperm head anomalies, which are attributed to factors that co-act on both spermatozoa motility and morphology (Ma et al. 2006). Our results clearly show an increase in abnormal sperm head morphology with the increase in copper concentration. Schmid et al. (2013) identified differences in element concentrations between sperm and seminal plasma; the higher copper concentration in semen was quantitatively associated with poorer semen quality and increased frequency of genomic sperm defects. The significantly lower proportion of sperm cells with proper tail membrane structure (not swollen) in our experiment might be explained by copper’s oxidative action, which may have contributed to the degradation of membrane structure (Galhardi et al. 2004). Infertile men have higher levels of copper and reactive oxygen species in their seminal plasma than fertile men do (Aydemir et al. 2006), but this potentially oxidation-related disruption did not alter the sperm maturation process in bank vole males.
Knowledge of the effect of a copper excess in females is limited but a number of studies show the effect of a copper deficiency in females (Howell and Hall 1969; Keen et al. 2003). In female rats, physiological systems protect against oxidative stress under copper deficiency, and it is suggested that this is due to the protective action of estrogens (Bureau et al. 2003). Fevold et al. (1936) first suggested that ovulation could be induced by intravenous injection of copper salts in rats. Later, Hazum (1983) demonstrated in vitro that copper ions stimulate GnRH and LH in immature rats, and this was confirmed by Kochman et al. (1997) and Michaluk and Kochman (2007). Those reports suggest that copper might also affect ovarian development, but we found no changes in ovarian follicle morphology. Work by Kozlowski et al. (1990) indicates that copper does not play a role in ovulation processes in rats. In laboratory research on rabbits (Hiroi et al. 1965; Suzuki et al. 1965, 1972; Tsou et al. 1977) and ewes (Murawski et al. 2006), administration of copper salts led to ovulation through an effect on the hypothalamus, possibly suggesting changes in ovarian morphology. It seems that the doses of copper available to rodents in natural ecosystems do not harm the female’s reproductive ability, or that the females’ detoxification system effectively removes the excess of this trace element.
In this work, doses of copper corresponding to levels found in the environment clearly compromised the reproductive abilities of bank vole males, but the effect on females was not so evident. Levengood and Heske (2008) did not detect any changes in the reproductive activity of small mammals from areas contaminated with copper and zinc. Nevertheless, copper does accumulate in different tissues (Appleton et al. 2000; Martiniakova et al. 2010, 2011, 2012) and can cause DNA damage (Yamashita et al. 1998; Linder 2001; De Olivera et al. 2012). In view of this, in further studies we will examine the impact of copper intake on offspring, and specifically its accumulation in different tissues and its effect on sexual maturation.
References
Aburto EM, Cribb A, Fuentealba IC, Ikede BO, Kibenge FSB, Markham F (2001) The failure of selenium supplementation to prevent copper-induced liver damage in Fischer 344 rats. Can J Vet Res 65:104–110
Ackerman DJ, Reinecke AJ, Els HJ, Grobler DG, Reinecke SA (1999) Sperm abnormalities associated with high copper levels in impala (Aepyceros melampus) in the Kruger National Park, South Africa. Ecotoxicol Environ Saf 43:261–266
Ahsan RK, Farooq A, Kapur MM, Laumas KR (1976) Effect of intravasal copper on the fertility of rats. J Reprod Fertil 48:271–274
Akingbemi B (2005) Estrogen regulation of testicular function. Reprod Biol Endocrinol 3(1):51
Alloway BJ, Ayres DC, Kłosowicz S (1999) Chemiczne podstawy zanieczyszczania środowiska. Wydaw. Naukowe PWN, Warszawa
Amiard-Triqut C, Berthet B, Metayer C, Amiard JC (1986) Contribution to the ecotoxicological study of cadmium, copper and zinc in the mussel Mytilus edulisII. Experimental study. Mar Biol 92:7–13
Appleton J, Lee KM, Sawicka Kapusta K, Damek M, Cooke M (2000) The heavy metal content of the teeth of the bank vole (Clethrionomys glareolus) as an exposure marker of environmental pollution in Poland. Environ Pollut 110:441–449
Aydemir B, Kiziler AR, Onaran I, Alici B, Ozkara H, Akyolcu MC (2006) Impact of Cu and Fe concentrations on oxidative damage in male infertility. Biol Trace Elem Res 112:193–204
Bureau I, Gueux E, Mazur A, Rock E, Roussel AM, Rayssiguier Y (2003) Female rats are protected against oxidative stress during copper deficiency. J Am Coll Nutr 22:239–246
Chattopadhyay A, Sarkar M, Biswas NM (2005) Dose-dependent effect of copper chloride on male reproductive function in immature rats. KUMJ 3:392–400
Clark JH, Schrader WT, O’Malley BW (1992) Mechanism of action of steroid hormone. In: Wilson JD, Foster DW (eds) Textbook of Endocrinology. WB Saunders Company, New York, pp 35–90
Committee on Animal Nutrition SoLAN (1995) Nutrient Requirements of Laboratory Animals. Nutrient Requirements of Laboratory Animals National Academy Press, Washington
Das S, Khangarot BS (2011) Bioaccumulation of copper and toxic effects on feeding, growth, fecundity and development of pond snail Lymnaea luteola L. J Hazard Mater 185:295–305. doi:10.1016/j.jhazmat.2010.09.033
Davidson EA, David MB, Galloway JN, Goodale CL, Haeuber R, Harrison JA, Howarth RW, Jaynes DB, Lowrance RR, Thomas Nolan B, Peel JL, Pinder RW, Porter E, Snyder CS, Townsend AR, Ward MH (2011) Excess nitrogen in the U.S. environment: trends, risks, and solutions. Issues Ecol 15:1–16
De Olivera JV, Boufleur LA, Dos Santos CE, Dias JF, Squeff CH, Silva GR, Ianistcki M, Benvegnu VC, Da Silva J (2012) Occupational genotoxicity among copper smelters. Toxicol Ind Health 28(9):789–795. doi:10.1177/0748233711422735
Dmowski K, Kozakiewicz A, Kozakiewicz M (1998) Small mammal populations and community under conditions of extremely high thallium contamination in the environment. Ecotoxicol Environ Saf 41:2–7
Eidi M, Eidi A, Pouyan O, Shahmohammadi P, Fazaeli R, Bahar M (2010) Seminal plasma levels of copper and its relationship with seminal parameters. Iran J Reprod Med 8:60–65
Evans JL, Abraham PA (1973) Anemia, iron storage and ceruloplasmin in copper nutrition in the growing rat. J Nutr 103:196–201
Fevold H, Hisaw FL, Greep R (1936) Augmentation of the gonad-stimulating action of pituitary extracts by inorganic substances, particularly copper salts. Am J Physiol 117:68–74
Ficken KLG, Byrne PG (2013) Heavy metal pollution negatively correlates with anuran species richness and distribution in south-eastern Australia. Austral Ecol 38:523–533. doi:10.1111/j.1442-9993.2012.02443.x
Franssen CM (2009) The effects of heavy metal mine drainage on population size structure, reproduction, and condition of western mosquitofish, Gambusia affinis. Arch Environ Contam Toxicol 57:145–156. doi:10.1007/s00244-008-9244-0
Friberg L, Nordberg GF, Vouk VB (1986) Handbook on the Toxicology of Metals: specific Metals. Elsevier, New York
Galhardi CM, Diniz YS, Faine LA, Rodrigues HG, Burneiko RC, Ribas BO, Novelli EL (2004) Toxicity of copper intake: lipid profile, oxidative stress and susceptibility to renal dysfunction. Food Chem Toxicol 42:2053–2060. doi:10.1016/j.fct.2004.07.020
Green E (1966) Breeding systems. Biology of laboratory mouse. McGraw-Hill Book Company, New York
Hazum E (1983) Copper and thiol regulation of gonadotropin releasing hormone binding and luteinizing hormone release. Biochem Biophys Res Commun 112:306–312
Hébert CD, Elwell MR, Travlos GS, Fitz CJ, Bucher JR (1993) Subchronic toxicity of cupric sulfate administered in drinking water and feed to rats and mice. Toxicol Sci 21:461–475
Helmfrid I, Berglund M, Lofman O, Wingren G (2012) Health effects and exposure to polychlorinated biphenyls (PCBs) and metals in a contaminated community. Environ Int 44:53–58. doi:10.1016/j.envint.2012.01.009
Hiroi M, Sugita S, Suzuki M (1965) Ovulation induced by implantation of cupric sulfate into the brain of the rabbit. Endocrinology 77:963–967
Howell JM, Hall GA (1969) Histological observations on foetal resorption in copper-deficient rats. Br J Nutr 23:47–50
Kabata-Pendias A, Pendias H (1999) Biogeochemia pierwiastków śladowych wyd. drugie zmienione. Wydawnictwo Naukowe PWN, Warszawa
Kataev GD, Suomela J, Palokangas P (1994) Densities of microtine rodents along a pollution gradient from a copper-nickel smelter. Oecologia 97:491–498
Keen CL, Hanna LA, Lanoue L, Uriu-Adams JY, Rucker RB, Clegg MS (2003) Developmental consequences of trace mineral deficiencies in rodents: acute and long-term effects. J Nutr 133:1477S–1480S
Kochman K, Gajewska A, Kochman H, Kozlowski H, Masiukiewicz E, Rzeszotarska B (1997) Binding of Cu2 + , Zn2 + , and Ni2 + -GnRH complexes with the rat pituitary receptor. J Inorg Biochem 65:277–279
Kozłowski H, Masiukiewicz E, Potargowicz E, Rzeszotarska B, Walczewska-Sumorok A (1990) Ovulation-inducing activity of luliberin (LHRH) complexed by copper(II), nickel(II), and zinc(II) ions. J Inorg Biochem 40:121–125
Kruczek M (1986) Seasonal effects on sexual maturation of male bank voles (Clethrionomys glareolus). J Reprod Fertil 76:83–89
Kruczek M, Styrna J (2009) Semen quantity and quality correlate with bank vole males’ social status. Behav Process 82:279–285. doi:10.1016/j.beproc.2009.07.009
Levengood JM, Heske EJ (2008) Heavy metal exposure, reproductive activity, and demographic patterns in white-footed mice (Peromyscus leucopus) inhabiting a contaminated floodplain wetland. Sci Total Environ 389(2–3):320–328. doi:10.1016/j.scitotenv.2007.08.050
Linder MC (2001) Copper and genomic stability in mammals. Mutat Res/Fundam Mol Mech Mutagen 475:141–152. doi:10.1016/S0027-5107(01)00076-8
Lyubimov AV, Smith JA, Rousselle SD, Mercieca MD, Tomaszewski JE, Smith AC, Levine BS (2004) The effects of tetrathiomolybdate (TTM, NSC-714598) and copper supplementation on fertility and early embryonic development in rats. Reprod Toxicol 19:223–233
Ma YH, Liu RZ, Xu ZG, Zhang HG, Li Z (2006) Relationship between sperm motility parameters and sperm morphology. Zhonghua Nan Ke Xue 12:590–593
Martin MB, Reiter R, Pham T, Avellanet YR, Camara J, Lahm M, Pentecost E, Pratap K, Gilmore BA, Divekar S, Dagata RS, Bull JL, Stoica A (2003) Estrogen-like activity of metals in MCF-7 breast cancer cells. Endocrinology 144:2425–2436. doi:10.1210/en.2002-221054
Martiniakova M, Omelka R, Grosskopf B, Jancova A (2010) Yellow-necked mice (Apodemus flavicollis) and bank voles (Myodes glareolus) as zoomonitors of environmental contamination at a polluted area in Slovakia. Acta Vet Scand 52:58. doi:10.1186/1751-0147-52-58
Martiniakova M, Omelka R, Jancova A, Stawarz R, Formicki G (2011) Concentrations of selected heavy metals in bones and femoral bone structure of bank (Myodes glareolus) and common (Microtus arvalis) voles from different polluted biotopes in Slovakia. Arch Environ Contam Toxicol 60:524–532. doi:10.1007/s00244-010-9545-y
Martiniakova M, Omelka R, Jancova A, Formicki G, Stawarz R, Bauerova M (2012) Accumulation of risk elements in kidney, liver, testis, uterus and bone of free-living wild rodents from a polluted area in Slovakia. J Environ Sci Health A 47:1202–1206
Matsumoto AM, Karpas AE, Bremner WJ (1986) Chronic human chorionic gonadotropin administration in normal men: evidence that follicle-stimulating hormone is necessary for the maintenance of quantitatively normal spermatogenesis in man. J Clin Endocrinol Metab 62:1184–1192
Michaluk A, Kochman K (2007) Involvement of copper in female reproduction. Reprod Biol 7:193–205
Murawski M, Bydłoń G, Sawicka-Kapusta K, Wierzchoś E, Zakrzewska M, Włodarczyk S, Molik E, Zieba D (2006) The effect of long term exposure to copper on physiological condition and reproduction of sheep. Reprod Biol 6(Suppl 1):201–206
Ng TB, Liu WK (1990) Toxic effect of heavy metals on cells isolated from the rat adrenal and testis. In Vitro Cell Dev Biol 26:24–28
Nikolov IG, Joki N, Vicca S, Patey N, Auchère D, Benchitrit J, Flinois JP, Ziol M, Beaune P, Drüeke TB, Lacour B (2010) Tissue accumulation of lanthanum as compared to aluminum in rats with chronic renal failure—Possible harmful effects after long-term exposure. Nephron 115:e112–e121
Oster G, Salgo MP (1977) Copper in mammalian reproduction. Ady Pharmacol Chemother 14:327–409
Pau KYF, Khorram O, Kaynard AH, Spies HG (1989) Simultaneous induction of neuropeptide Y and gonadotropin-releasing hormone release in the rabbit hypothalamus. Neuroendocrinology 49:197–201
Pedersen T, Peters H (1968) Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil 17:555–557
Roychoudhury S, Slivková J, Bulla J, Massányi P (2008) Copper administration alerts fine parameters of spermatozoa motility in vitro. Folia Veterinaria 52:64–68
Satta A, Verdinelli M, Ruiu L, Buffa F, Salis S, Sassu A, Floris I (2012) Combination of beehive matrices analysis and ant biodiversity to study heavy metal pollution impact in a post-mining area (Sardinia, Italy). Environ Sci Pollut Res Int 19:3977–3988. doi:10.1007/s11356-012-0921-1
Schmid TE, Grant PG, Marchetti F, Weldon RH, Eskenazi B, Wyrobek AJ (2013) Elemental composition of human semen is associated with motility and genomic sperm defects among older men. Hum Reprod 28:274–282. doi:10.1093/humrep/des321
Seed J, Chapin RE, Clegg ED, Dostal LA, Foote RH, Hurtt ME, Klinefelter GR, Makris SL, Perreault SD, Schrader S, Seyler D, Sprando R, Treinen KA, Veeramachaneni DNR, Wise LD (1996) Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: a consensus report. Reprod Toxicol 10:237–244
Sheffield SR, Sawicka-Kapusta K, Cohen JB, Rattner BA (2001) Rodentia and lagomorpha. In: Shore RF, Rattner BA (eds) Ecotoxicology of Wild Mammals. Wiley, Chichester, pp 215–314
Sierens JE, Sneddon SF, Collins F, Millar MR, Saunders PT (2005) Estrogens in testis biology. Ann N Y Acad Sci 1061:65–76. doi:10.1196/annals.1336.008
Spatari S, Bertram M, Fuse K, Graedel TE, Rechberger H (2002) The contemporary European copper cycle: 1 year stocks and flows. Ecol Econ 42:27–42
Stern BR (2010) Essentiality and toxicity in copper health risk assessment: overview, update and regulatory considerations. J Toxicol Environ Health A 73:114–127. doi:10.1080/15287390903337100
Styrna J, Krzanowska H (1995) Sperm select penetration test reveals differences in sperm quality in strains with different Y chromosome genotype in mice. Arch Androl 35:111–118
Styrna J, Kilarski W, Krzanowska H (2003) Influence of the CBA genetic background on sperm morphology and fertilization efficiency in mice with a partial Y chromosome deletion. Reproduction 126:579–588
Suzuki M, Watanabe S, Hoshii M (1965) Effect of estrogen on copper-induced ovulation in the rabbit. Endocrinology 76:1205–1207
Suzuki M, Tnemoto Y, Takahashi K (1972) The effect of copper salts on ovulation, especially on hypothalamic ovulatory hormone releasing factor. Tohoku J Exp Med 108:9–18
Tang S, Wilke BM, Huang C (1999) The uptake of copper by plants dominantly growing on copper mining spoils along the Yangtze River, the People’s Republic of China. Plant Soil 209:225–232
Tsou RC, Dailey RA, McLanahan CS, Parent AD, Tindall GT, Neill JD (1977) Luteinizing hormone releasing hormone (LHRH) levels in pituitary stalk plasma during the preovulatory gonadotropin surge of rabbits. Endocrinology 101:534–539
Veltman JC, Maines MD (1986) Regulatory effect of copper on rat adrenal cytochrome P-450 and steroid metabolism. Biochem Pharmacol 35:2903–2909
Walczak R, Strumiłło E, Kula K (1994) Eosin and water tests and results of conventional semen analysis. Ginekol Pol 65:99–102
Wijnhoven S, Leuven RS, van der Velde G, Jungheim G, Koelemij EI, de Vries FT, Eijsackers HJ, Smits AJ (2007) Heavy-metal concentrations in small mammals from a diffusely polluted floodplain: importance of species- and location-specific characteristics. Arch Environ Contam Toxicol 52:603–613. doi:10.1007/s00244-006-0124-1
Xu Y, Xiao FL, Xu N, Qian SZ (1985) Effect of intra-epididymal injection of copper particles on fertility, spermatogenesis, and tissue copper levels in rats. Int J Androl 8:168–174
Yamashita N, Murata M, Inoue S, Burkitt MJ, Milne L, Kawanishi S (1998) α-Tocopherol induces oxidative damage to DNA in the presence of copper(II) ions. Chem Res Toxicol 11:855–862
Acknowledgments
We thank Elżbieta Pochroń for technical assistance. This work was supported by Grants from the Jagiellonian University (DS/MND/WBiNoZ/INoŚ/16/2011, DS/MND/WBiNoZ/INoŚ/22/2013).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miska-Schramm, A., Kruczek, M. & Kapusta, J. Effect of copper exposure on reproductive ability in the bank vole (Myodes glareolus). Ecotoxicology 23, 1546–1554 (2014). https://doi.org/10.1007/s10646-014-1295-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1295-6