Abstract
Amphipod crustaceans have been widely used as invertebrate models in ecotoxicology due to their importance in the food chain. However, few studies have evaluated the genotoxic effects of pollutants in this model using the comet assay. The main obstacle to using amphipods in the comet assay is the difficulty in obtaining enough blood cells from a single individual. In this study, we evaluated the genotoxic effects of the water-soluble fraction (WSF) of heavy oil on the brackish/freshwater amphipod Quadrivisio aff. lutzi, which is common in the coastal lagoons of southeastern Brazil, using hemocytes obtained from single amphipods (without pooling) after optimizing hemolymph extraction. The comet assay revealed significantly higher DNA damage levels (2- to 6-fold higher) in treated amphipods compared to untreated ones with a sublethal concentration of 17.6 % of the WSF within 72 h of treatment. Two independent experiments confirmed an “up and down” pattern of DNA damage, measured as the % of DNA contained in the tail of the comets. Elevations in DNA damage levels were observed at the 6 and 48 h time points, while very low levels of DNA damage were observed at the 24 and 72 h time points. Furthermore, the comet assay revealed gender variability in the levels of DNA damage after short-term exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 2010, Brazil produced a mean of 2.1 million barrels of oil per day, with an estimated 14.2-billion-barrel reserve of total oil [Agência Nacional de Petróleo (ANP) ANP 2011]. On the southeastern coast of Brazil, two states, Espírito Santo and Rio de Janeiro, collectively produced 670 million barrels of oil in 2010 (ANP 2011).
Petroleum is composed of hydrocarbons, of which 15–60 % are paraffins, 30–60 % are naphthenes and 3–30 % are aromatic compounds (Petroleum 2009). Asphaltics make up the remaining percentage. Heavy oils [14–19° API (American Petroleum Institute gravity)] are composed of high molecular weight hydrocarbons that typically contain more than 15 carbon atoms, have high viscosities, high amounts of asphaltenes, sulfur and metallic components, such as nickel and vanadium (Trevisan et al. 2009), and lower levels of evaporation than light oils (Mackay and McAuliffe 1988). The Jubarte heavy oil (17° API) (Pellegrini et al. 2008) used in this study originated from the Jubarte field. The Jubarte field was discovered in 2001 and is located in the northern part of the Brazilian Campos Basin, which is located approximately 80 km offshore of the coast of the state of Espírito Santo (Pellegrini et al. 2008). The scales that were found in the pump stages that were used to extract the heavy oil have been shown to have a composition of 86 % barium sulfate, 12 % strontium sulfate and 2 % iron oxidant. Heavy oils constitute 26 % of the proven Brazilian oil volumes (Trevisan et al. 2009). Recent reserve discoveries from the Brazilian Campos Basin and the development of proper production techniques are projected to increase these volumes in the near future (Trevisan et al. 2009).
High levels of oil production, which includes extraction, transport and handling, contribute to a higher probability of spill events. Spill events are known to cause devastating consequences to the ecosystem and living resources (Katsumiti et al. 2009). After an oil spillage in seawater, spreading, drifting, evaporation, dissolution, photolysis, biodegradation and the formation of oil-in-water and water-in-oil emulsions occur (Daling et al. 1990). At least two of these processes, dissolution and dispersion of hydrocarbons in the water, combined with volatilization and degradation reactions, are responsible for the toxicity of water-soluble fractions (WSF) of oil (Mackay and McAuliffe 1988).
The severity of the adverse biological effects of oil spills is critically dependent on the exposure suffered by the organisms in question (for example, the concentration and duration of exposure to oil) (Mackay and McAuliffe 1988). Water bodies that have been contaminated with crude oil are characterized by lower dissolved oxygen concentrations and higher biological oxygen demands than uncontaminated waters (Baden 1982), which has negative consequences for natural resources, affecting the growth, reproduction and survival of local organisms (Baden 1982; Nwabueze and Agbogidi 2010). Nevertheless, the toxic effects of the WSFs of oil appear to be less severe for organisms in the open sea, in which dissolved hydrocarbons are present at concentrations of less than 1 ppm and appear not to be toxic during short-term exposure (see Mackay and McAuliffe 1988). However, aquatic organisms inhabiting small enclosed or semi-enclosed water bodies, such as coastal lagoons that fluctuate in size, may be more susceptible to the impact of oil spillage through river effluents, infiltrates from underground layers, and marine coastal water effluents when barriers are naturally broken or opened by local authorities.
On average, DNA damage (e.g., DNA strand breaks and DNA adducts) and oxidative stress have proven to be 35- to 50-fold more sensitive than whole-organism responses (i.e., survival, reproduction and growth) as biomarkers for evaluating oil toxicity in marine species (Smit et al. 2009). The comet assay (single cell electrophoresis assay), which ecotoxicological relevance was highlighted by Jha (2008), has been widely accepted as a simple, sensitive, rapid tool for assessing DNA damage in individual eukaryotic cells and has been shown to have an increasing number of applications in genetic toxicology (Jha 2004; Kumaravel and Jha 2006). The alkaline version of the comet assay, which is the most commonly used method, detects the broadest spectrum of primary DNA lesions that are related to the mutagenesis processes, including alkali-labile sites, oxidative base damage, double-stranded DNA breaks, and single-stranded DNA breaks, which are associated with incomplete excision repair (Kumaravel and Jha 2006; Hartmann and Speit 2009). When compared with other methods that evaluate DNA damage, the alkaline comet assay proved to be more sensitive than the other assays, including the chromosomal aberration, sister chromatic exchange, alkaline elution, micronucleus (Jha 2008; Dhawan et al. 2009), alkaline unwinding, and DNA adduct assays (Smit et al. 2009).
Invertebrate species have been widely used in genotoxicological studies that use the comet assay (see Mitchelmore and Chipman 1998; Lee and Steinert 2003; Jha 2004, 2008). Although amphipod crustaceans have been used for more than a decade for ecotoxicological evaluations (e.g., Ingersoll et al. 2002; Wang and Zauke 2004; Anderson et al. 2004; Bertoletti 2011), only recently have amphipods been evaluated using the single cell alkaline electrophoresis assay (Lacaze et al. 2010, 2011a, b).
In addition to the important role that they play in aquatic ecosystems, all of the advantages that amphipods possess (they are sensitive, they develop directly, they are small and abundant, and are easy to collect and maintain in the laboratory) led us to select the amphipod Quadrivisio aff. lutzi (Shoemaker 1933) for use as a model organism in our study. This species has been found to inhabit the coastal lagoons of fresh and brackish waters throughout Brazil from the states of Alagoas to São Paulo (Leite et al. 1980). In the region in which our study was conducted (north of the state of Rio de Janeiro), these amphipods are abundant and easy to find either associated with macrophytes (Henriques-de-Oliveira et al. 2007) or green filamentous algae of the genus Cladophora and Rhizoclonium, or under macrophyte debris in polluted or unpolluted coastal lagoons. The aim of the present study was to evaluate the genotoxic effects of the WSF of heavy oil in the brackish/freshwater amphipod Q. aff. lutzi and to evaluate their potential use as a biological indicator for ecotoxicological studies in the high oil-producing Campus Basin within the state of Rio de Janeiro.
Materials and methods
Sample collection and acclimatization conditions
Adult amphipods of the Q. aff. lutzi species were collected from one of the many lagoons in the North Fluminense region of the state of Rio de Janeiro, Brazil, and transported live to the laboratory. In a pilot experiment, the amphipods were kept in 2.5 L glass flasks. Each flask contained 50–60 amphipods in filtered freshwater at 24 °C and was subject to constant aeration. The amphipods were fed with commercial food (20 mg of Spirulina and 4 mg of vitamin complex per flask). The water was exchanged every 48 h and the amphipods were maintained under these conditions for a 5 day acclimatization period before the bioassays were performed. A second experiment was performed to confirm the results that were obtained in the pilot experiment. The second experiment was conducted after optimizing the maintenance conditions of the amphipods. The amphipods were maintained in 600 mL flasks without aeration in water that had been collected from the same lagoon in which they were collected. The water had been filtered and sterilized using an autoclave. The amphipods were maintained at 24.5 ± 0.5 °C in an incubator with a 12 h photoperiod. The amphipods had ad libitum access to autoclaved and dried aquatic macrophyte debris that had been obtained from the site where the amphipods were collected. These conditions allowed for the long-term survival and reproduction of the amphipods and the development of first juveniles. During the acclimatization period, the water was partially exchanged (50 %) every 2 days and the pellets at the bottom were removed by suction with a pipette. The water in these lagoons is brownish due to the high concentration of humic substances that are generated mainly through the decomposition of aquatic macrophytes and surrounding terrestrial vegetation (Farjalla et al. 2002). The salinity of the water used for the bioassays was 0.3 ppt. It contained 9.10 mg L−1 of dissolved oxygen, had 109 % saturation and had a conductivity of 673 μS.
Preparation of the water-soluble fraction of petroleum
The WSF was obtained from heavy crude oil (Trevisan et al. 2009) (17° API) that originated from the Jubarte field and was supplied by the Brazilian oil Company, PETROBRAS. The fraction was prepared by following the methods outlined by Martínez-Jerónimo et al. (2005), with modifications. One part (50 g) of crude oil was added to nine parts (450 mL) of filtered freshwater (1:9, w/v) and homogenized in the dark using a magnetic stirrer for 24 h at 500 rpm in a 1 L flask. The mixture was then transferred to a separation funnel, in which the layers were allowed to separate for two hours. Subsequently, the WSF layer was collected. This WSF was considered to be 100 % WSF. This fraction was sent to two different certification laboratories [CQA (Centro de Qualidade Analítica) Laboratórios, Campinas, SP and INNOLAP do Brasil, Rio de Janeiro, RJ, Brazil] in order to determine the composition of hydrocarbons and the levels of barium, iron, strontium and sulfates. Although the 100 % WSF showed a brownish turbidity, both laboratories obtained similar results for the analysis of hydrocarbons, which revealed undetectable levels of a wide variety of hydrocarbons, as shown in Table 1. Benzene and sulfates were the most representative compounds in the 100 % WSF.
Lethal concentration
A preliminary bioassay was performed in order to determine the lethal concentration of the WSF in which 50 % of the amphipod Q. aff. lutzi population died (LC50). Fifty-five amphipods per flask were treated in replicate with the following WSF concentrations: 0 % (negative control), 6.25, 12.5, 25, 50 and 100 % for a 72 h period. All flasks were covered in order to protect them from light. During the bioassay, the amphipods were not fed and the water was not exchanged. The survival of the amphipods was counted for each treatment group and replicate. Significant differences in mortality among the control and WSF-treated amphipods were assessed using an ANOVA, with p < 0.05 set as the significance level after verifying normality. In this bioassay, the data were not adjusted to the Probit model (Finney 1964) and the raw data showed a better fit to a linear function than the transformed data did. Therefore, the LC50 was obtained directly from the regression of the percentage of cumulative mortality as a function of the % of the WSF. A second bioassay was conducted in order to confirm the results of the preliminary bioassay after optimizing the rearing conditions for the amphipods. This bioassay was adjusted to the probit model. Therefore, it was used to determine the LC5O. Statistical analyses were performed using the statistical package STATISTICA, v. 7.0 (StatSoft Inc.).
The comet assay
A genotoxic bioassay was performed in order to evaluate genotoxicity with the comet assay. Sixty Q. aff. lutzi amphipods per flask in two replicates were exposed to either 0 % WSF (negative control) or 17.6 % WSF (treated) during a 72 h period under the same conditions used in the preliminary bioassay. The treatment solutions at the desired concentrations were prepared from the 100 % WSF by diluting it to a concentration that was equal to the LC50/AF, in which the application factor (AF) (see Martínez-Jerónimo et al. 2005) resulted in a value of 2.43, and the LC50 (42.8 %) was obtained in the preliminary bioassay. This concentration was obtained after the reconstitution of 440 mL of 100 % WSF to 2.5 L. Ten amphipods from each replicate group were removed at 6, 24, 48 and 72 h and subjected to the comet assay procedure. In the second experiment, 40 females and 40 males were counted and separated by observation under a stereoscope microscope of the gnathopods of the second pair of pereopods. In Quadrivisio spp., as described by Stebbing (1907), the males are larger in size than the females. In addition, the second male gnathopods are very large, while the female ones are small and are constructed differently (not triangular). In the second experiment, a minimum of 3 and a maximum of 7 amphipods of each gender were collected at each time point (6, 24, 48 and 72 h) for hemolymph extraction, after which point the comet assay was conducted. The amphipods were first poured over ice until they became completely unconscious. Then, using an insulin syringe (0.30 mm bore size and 8 mm needle length) with the aid of a stereoscope microscope, approximately 2–3 μL of hemolymph was obtained by inserting the needle ventrally along the lateral inner side of the third epimeral plate of the pleosome, directing the needle to the dorsal angle of the inflexion of the body, avoiding the intestine. Hemocyte viability was assessed using a Neubauer hemocytometer counting chamber. One microliter of the extracted hemolymph was mixed with 5 μL of lagoon water and was sterilized by passing it through the syringe-driven filter unit of a 0.22 μm cellulose acetate membrane (Lave). The mixture was then mixed with 6 μL of a 0.4 % trypan blue (TP) (Inlab) solution that had been diluted in the same sterilized water and added to the chamber. In the first genotoxic bioassay, the amphipods were sacrificed prior to extracting the hemolymph, which lead to the extraction of very low quantities of hemocytes. Instead, pouring the amphipods over ice significantly improved the success and quantity of hemolymph extraction in the second genotoxic bioassay, in which hundreds of hemocytes per individual were easily extracted. The amphipods were sacrificed immediately after the hemolymph extraction, before they recovered consciousness. The alkaline comet assay was performed according to the procedure described by Singh et al. (1988) and Rojas et al. (1999), with modifications. Two microliters of hemolymph were mixed with 20 μL of phosphate buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 and 2 mM KH2PO4, pH 7.4), which was then mixed with 75 μL of warm 0.5 % low melting agarose (LMA). The mixture was then poured on a slide that had been precoated with 1 mL of 0.5 % normal agarose (NA) and covered with a coverslip. After 10 min at 4–7 °C in a refrigerator, the coverslip was removed and another 100 μL of LMA was added and was once again covered with a coverslip. After spending an additional 10 min at a cool temperature, the coverslip was removed and the slides with the samples were submerged in fresh lysis buffer [2.5 M NaCl, 10 mM Tris, 100 mM EDTA, 1 % SDS, 1 % Triton-X-100 and 10 % DMSO (dimethyl sulfoxide, Merck)] for 20 min. The lysis period was reduced for practical purposes because a high number of samples needed to pass through the same chamber. Several previous tests with different lysis incubation times indicated that for the amphipod hemocytes, there is no need to maintain them for longer periods of time. After the lysis period in a cool environment, the slides were rinsed once with distilled water and were then submerged in fresh denaturing buffer (300 mM NaOH and 1 mM EDTA, pH > 13) for another 20 min in the same chamber where electrophoresis takes place. Following this period, electrophoresis was conducted at 331 V and 26 mA for 20 min. Following electrophoresis, the slides were rinsed with distilled water and each slide was covered with a few milliliters of neutralization buffer (0.4 M Tris, pH 7.5) for 5 min. The excess neutralization buffer was then removed by inversion and absolute ethanol was added to cover the slides. The ethanol was left until it had completely evaporated, after which point analysis of the comets was conducted. The slides were analyzed using an epifluorescent Olympus Microscope after they had been stained with the fluorescent DNA-specific stain DAPI (4′,6′-diamidino-2-phenylindole hydrochloride) (Merck). For each slide, a maximum of 100 comets were analyzed and photographed for further analysis. Ghost or cloudy comets (those with complete DNA migration) were not counted and are usually excluded from comet measure analyses (see Kumaravel and Jha 2006) because they may represent necrotic or apoptotic cells (Lovell 2009). The efficiency of the first comet assay procedure was evaluated using a positive control, in which DNA damage was evaluated by visual identification of different levels of damage. Prior to performing the comet assay, the positive control was prepared by incubating a cellular suspension from six amphipods in 50 and 100 μM hydrogen peroxide (H2O2) for two periods of time (20 min and 1 h) following the method outlined by Wilson et al. (1998). Another positive control was performed together with the second genotoxic bioassay under the optimized conditions specified previously to evaluate the consistency of the results that had been obtained in the first bioassay. The second positive control used was 10 mg L−1 of methyl methanesulfonate (MMS) (Acros Organics), which had been previously used to evaluate amphipod toxicity by Lacaze et al. (2011a). Ten males and ten females were used for the positive control assay. Males and females were maintained in separate 600 mL flasks. A negative control was performed together with the positive control (10 mg L−1 MMS), and the amphipods were maintained for 72 h. The percentage of DNA in the tail of the comet (% tail DNA) was used to evaluate DNA damage. This measure is the recommended endpoint because the % tail DNA has been strongly correlated with known gamma radiation-induced DNA damage levels (Kumaravel and Jha 2006).
Statistical analysis
The % tail DNA was determined using the free software Comet Score™ Free Comet Scoring Software, v. 1.5 (TriTek Corporation). In the first bioassay, the cutoff tool of the software was maintained at 100 %. For the second bioassay, the cutoff tool was adjusted so that all the extension of the comet should measure 100 % of the DNA. This adjustment gave more accurate measurements. For each amphipod, the mean and median of the % tail DNA of all comets analyzed were calculated. For each treatment and category (% WSF, time and gender), the mean value of the means and the mean value of the medians was calculated. Because the variable (% tail DNA) had a non-normal distribution, a nonparametric two-sided Mann–Whitney U-test (M–W, Zadj = Adjusted Z) was used, as recommended by Lovell (2009), with p < 0.05 set as the level of significance. This test of significance was used for all possible comparisons between the means obtained from the mean % tail DNA and for all possible comparisons between the means obtained from the medians. The Kruskal–Wallis test showed similar results to the M–W test and was also used. The induction factor (IF) was calculated for the % tail DNA obtained for the amphipod Q. aff. lutzi following the formulas outlined by Štrut et al. (2011). This index is obtained by dividing the mean value obtained in one treatment by the value obtained in the respective negative control.
Results
Lethal concentration
In the first survival bioassay, mortality rates were high within the first 48 h of treatment with either 50 or 100 % WSF. Treatment with low concentrations of the WSF of heavy oil (6.25, 12.5 and 25 %) resulted in mortality rates that were not significantly different from those in the negative control group (F = 1.55; p = 0.344). The 50 and 100 % WSF-treated groups were not significantly different from one another (F = 3.59; p = 0.198), but differed significantly from the control group and the groups that had been treated with lower concentrations of the WSF, which explained the significant overall differences that were found among the treatment groups (F = 13.83; p = 0.003). The relationship between the percentage of cumulative mortality (y) and the percentage of the WSF (x) was represented by a positive and significant (p = 0.0114, r = 0.6992) linear regression function (y = 29.6401 + 0.4759 x), in which 49 % of the variance in cumulative mortality was explained by the percentage of the WSF (r 2 = 0.4889). The LC50 of the WSF of heavy oil after 48 h for the Q. aff. lutzi amphipod, which was derived from the graphical extrapolation of this linear regression, was 42.8 %. In the second 72 h-survival experiment, in which the maintenance conditions of the amphipods had been optimized, no mortality was observed for the groups that had been treated with 0, 6.25, 12.5 and 25 % WSF, but mortality in the groups that had been treated with 50 and 100 % of the WSF was very high (75 and 100 % mortality, respectively) (Fig. 1). For this experiment, the data were fit to the probit model using the equation y = −7.396 + 7.2592x, where y corresponds to the probit and x corresponds to the log (% WSF). The linear regression was significant (p = 0.0407; r 2 = 0.7994) and revealed a high correlation between the parameters (r = 0.8941). Extrapolation from the probit value of five determined an LC50 = 51 % WSF for a 72 h assay (Fig. 1).
Genotoxicity
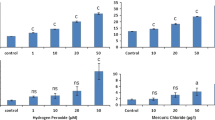
The viability of the hemocytes (Fig. 2) was estimated to be 94.2 ± 2.5 %. The hemocytes which were evaluated in this study, in accordance with the study conducted by Steele and MacPherson (1981), should be any of the circulating hemocytes named granulocytes, adipohemocytes or plasmatocytes that show maximum cellular and nuclear diameters of 11.75 and 6.6 μm, respectively. The other circulating hemocytes, which are characterized by much larger nuclei, were rarely found and were not included in the analyses. The positive control that was used in the first genotoxic bioassay exhibited complete destruction of hemocyte nuclei at 50 and 100 μM H2O2 after 1 h. However, after 20 min, it was possible to observe primarily the D–F stages as represented in Fig. 2 (data not shown). The positive control that was used in the second genotoxic bioassay (10 mg L−1 of MMS), measured by the % tail DNA, induced significantly higher damage than that observed in the controls (from means, Zadj = −3.551, p = 0.00009; from medians, Zadj = −3.429, p = 0.00015) (Fig. 3). When analyzing by gender, both females (from means, p = 0.0317; from medians, p = 0.0472) and males (from means, p = 0.0159; from medians, p = 0.0158) displayed significant differences between the negative control and MMS-treated individuals. Males displayed higher levels of DNA damage compared to their respective controls than females, but this effect was only detectable at different levels of significance after a parametric ANOVA test had been applied (females from means, p = 0.0203 and from medians, p = 0.0370; males from means, p = 0.0033 and from medians, p = 0.0008). Normality was observed in the means of the means (K–S d = 0.1580, p > 0.20) and in the means of the medians (K–S d = 0.1781, p > 0.20) using the Kolmorov–Smirnov test.
a–f) The hemocyte nuclei (comet) obtained from one Q. aff. lutzi amphipod were analyzed using the alkaline comet assay after treatment with 17.6 % WSF of heavy oil. The white bar corresponds to 4 μm. a Without DNA damage; b, c low to intermediate DNA damage levels; d, e intermediate to high DNA damage levels; f complete damage, also termed ghost or cloudy comets. g Hemocytes distributed in one of the cells of the hemocytometer Neubauer chamber during viability determination. Hemocytes showed an approximate size of 11.8 μm
The DNA damage levels observed in all individuals (top panels) and in male and female Q. aff. lutzi amphipods (bottom panels) that had been treated with 10 mg L−1 of MMS for 72 h compared with untreated amphipods (negative control). DNA damage was analyzed by the mean calculated from the mean % tail DNA/amphipod and the mean calculated from the median % tail DNA/amphipod. According to a non-parametric test: *Significant difference from respective control at p < 0.05; ***Significant difference from respective control at p < 0.001. According to parametric factorial ANOVA: +Significant difference from respective control at p < 0.05; ++Significant at p < 0.01; +++Significant at p < 0.001. The vertical bars represent the standard errors of the means
In the first genotoxic bioassay, 79 amphipods were analyzed out of a total of 160, which included the negative controls and the WSF-treated amphipods. The mean levels of DNA damage measured as the % tail DNA of the comets was significantly higher in the WSF-treated units compared with the negative controls (Table 2). When the analyses were performed by gender, no differences in DNA damage were found between the male and female negative controls. However, the WSF-treated males exhibited higher levels of DNA damage compared with the WSF-treated females (Table 2) when evaluated by comparison with their respective controls. The treated males and females exhibited significantly higher levels of % tail DNA compared with their corresponding negative controls. This result was found for calculations made from the mean values of both the means and the medians (Table 2). The differences between the WSF-treated males and females were significant only after the first 6 h period when the means of the medians were compared (Zadj = 2.209, p = 0.0275). When analyzing all amphipods over time, DNA damage was evident after the first 6 h period of treatment, which was demonstrated by significant differences compared with the controls (Table 2). No differences were found in the controls over time. However, an “up and down” variation in DNA damage over time was observed in the treated amphipods, in which 6 and 48 h exposures caused similarly high levels of damage (p ≥ 0.8018), whereas 24 and 72 h exposures caused similarly low values (p ≥ 0.7750), which were both significantly lower than the 6 h-treated units (p ≤ 0.0499). Although low DNA damage levels were observed in the 24- and 72 h-treated amphipods, their levels were also significantly different from their respective controls when the mean of the medians were evaluated (Table 2). When analyses were performed by gender over time, only the males exhibited significant differences between the treated and control units after 6 (from means: Zadj = 1.982, p = 0.0474; from medians: Zadj = −2.209, p = 0.0275) and 24 h exposures (from means: Zadj = −2.191, p = 0.0303; from medians: −2.027, p = 0.0427). The females exhibited significant differences between the treated and control units after a 48 h treatment when analyzed by the mean of the medians (Zadj = −2.193, p = 00318). In the first genotoxic bioassay, the IF obtained indicated that 17.6 % WSF of the heavy oil caused DNA damage levels in the Q. aff. lutzi amphipod that were 2- to 4-fold higher than those observed in amphipods that were not exposed to petroleum and that males exhibited higher DNA damage levels compared with females when treated with the WSF.
In the second genotoxic bioassay, which was conducted with the aim of verifying whether the patterns observed in the first bioassay would consistently repeat, a total of 52 amphipods were evaluated. This bioassay also showed significantly higher DNA damage in the WSF-treated individuals (from means: 35.9 ± 0.8 %, p = 0.0059; from medians: 33.0 ± 6.1, p = 0.0057) than in the controls (from means: 19.1 ± 0.7 %; from medians: 5.2 ± 1.0) (Fig. 4). When analyzing by gender, the females, which were maintained separately from the males, showed higher DNA damage levels in response to the WSF than males. Females showed significantly higher levels of DNA damage in the WSF than their respective controls (from means: p = 0.011); from medians: p = 0.008). Male differences were small between the control and treated individuals and were only detected using an ANOVA after verifying normality in the distribution of the means of the individual mean values (p = 0.040) (Fig. 4). When analyzing the levels of DNA damage over time in the second genotoxic bioassay, the same variations observed in the first bioassay, significantly higher levels of damage at 6 and 48 h than their respective controls (from means: p = 0.0143 and p = 0.0034, respectively; from medians: p = 0.0143 and p = 0.0028, respectively) and no differences from their respective controls at 24 and 72 h, were observed (Fig. 5). When analyzing by gender, the cyclical response was also evident in males and females, but the significance was lower, or in some cases only detected by the more sensitive parametric ANOVA (Fig. 5), due to a reduction in the number of cases while separating genders in the analysis. For the 6 h-evaluation, only the parametric ANOVA detected significant differences between the control and treated females (from means: p = 0.003; from medians: p = 0.0004). ANOVA did not represent an appropriate test for the females because their means do not adjust to the assumptions of normality. For the 48 h-evaluation in females, the non-parametric test was able to detect significant differences (from means and medians: p = 0.0253), and in the male mean of the means, which conform to normality, significance was demonstrated at the 6- and 48 h-evaluations by the parametric ANOVA (6 h: p = 0.0019; 48 h: p = 0.00002) (Fig. 5). The IF obtained in the second genotoxic bioassay (Fig. 6) confirmed most of the observations made in the first bioassay. Treatment with 17.6 % WSF of heavy oil induced DNA damage in the Q. aff. lutzi amphipod and a cellular response during the first 72 h. Amphipods that had been treated with the WSF displayed DNA damage levels that were 2- (measured from the means) to 6-fold (measured from the medians) higher compared with those observed in amphipods that had not been exposed to petroleum. The IF also confirmed the “up and down” variation that was observed over time in the first bioassay, with high levels of damage in the first 6 h and at 48 h and the subsequent low levels of damage that were equivalent to untreated individuals at the 24 and 72 h periods. The IF of the second bioassay did not support the observation that males exhibited higher DNA damage levels compared with females when treated with the WSF, as observed in the first genotoxic bioassay. In this bioassay, the means of the medians revealed that, in general, the females showed higher levels of damage than the males. Finally, the IF corroborated that the mean of the medians revealed higher DNA damage levels than the mean of the means (Fig. 6).
The DNA damage levels (as an average across all time points) observed in all individuals (top panels) and in male and female Q. aff. lutzi amphipods (bottom panels) that had been treated with 17.6 % WSF compared with untreated amphipods (negative control) that were assayed in the second experiment. DNA damage was analyzed using the mean calculated from the mean % tail DNA/amphipod and the mean calculated from the median % tail DNA/amphipod. According to a non-parametric test: *Significant difference from respective control at p < 0.05. According to a parametric factorial ANOVA: +Significant difference from respective control at p < 0.05. The vertical bars represent the standard errors of the means
The DNA damage levels observed at different time points in Q. aff. lutzi amphipods that had been treated with 17.6 % WSF of heavy oil compared with untreated amphipods (negative control) that were assayed in the second experiment (top panels). The DNA damage levels observed at different time points in male and female Q. aff. lutzi amphipods that had been treated with 17.6 % WSF of heavy oil compared with untreated amphipods (negative control) (bottom panels). DNA damage was analyzed by the mean calculated from the mean % tail DNA/amphipod and the mean calculated from the median % tail DNA/amphipod. According to a non-parametric test: *Significant difference from respective control at p < 0.05; **Significant at p < 0.01. According to a parametric factorial ANOVA: +Significant difference from respective control at p < 0.05; ++Significant at p < 0.01; +++Significant at p < 0.001. The vertical bars represent the 95 % confidence intervals
The DNA damage levels (as an average across all time points) observed in the second bioassay in Q. aff. lutzi amphipods that had been treated with 17.6 % WSF of heavy oil and were compared with untreated amphipods (negative control). DNA damage is presented as the induction factor (DNA damage in treated amphipods divided by the DNA damage in untreated amphipods). The medians revealed higher DNA damage levels than those revealed by the means. Levels of DNA damage that were at least 6-fold higher than the values observed in amphipods that were not exposed to petroleum were revealed after 6 and 48 h in amphipods that had been exposed to petroleum
Discussion
DNA damage levels, measured as strand breakage, may occur as a direct effect of exogenous agents or as a secondary effect of pollutants, and are moderated by endogenous activities that are related to DNA repair (Mitchelmore and Chipman 1998). Polycyclic aromatic hydrocarbons (PAH) are in a class of chemicals that require metabolic activation before DNA damage can occur (Lee and Steinert 2003). Their metabolites, such as quinones and other aromatic nitro compounds, may act by stimulating the metabolic generation of reactive oxygen species, which can damage DNA, as has previously been observed in mollusks and fishes (Lee and Steinert 2003). PAH are also known to accumulate at high concentrations in the tissues of invertebrates at the bottom of the food chain, which exceed the rates of metabolism and elimination, thus inducing acute toxicity (Jha 2004). The hydrocarbon composition in oil WSF is a function of the water/oil Q-proportion, and at Q-values lower than ten (such as the one used in this study) benzene has been found to represent the most soluble hydrocarbon (97 %), followed by cyclohexane (3 %) and PAH (0.3 %) (Mackay and McAuliffe 1988). Although the levels of most components in the 100 % WSF were not detected, we would expect that the acute response of the Q. aff. lutzi amphipod treated with 17.6 % WSF (9:1) would be in great proportion because of the effects of benzene, as predicted by Mackay and McAuliffe (1988) and confirmed with the elevated value found in the 100 % WSF (see Table 1). Further studies may test the accumulation of these components in amphipod tissue that has been exposed to petroleum. Closed small water bodies, such as coastal lagoons, are expected to have lower Q-values than open seawater. Therefore, their living organisms would be exposed to higher concentrations of hydrocarbons in oil-spillage events, which increases the need to study the effects of oil metabolites in brackish and freshwater organisms.
Lethal concentrations
Both dose-dependent bioassays, which aimed to determine the LC50 during a short period of time, consistently showed that exposure to 50 or 100 % of the WSF was highly toxic to the Q. aff. lutzi amphipod. The first bioassay did not allow us to determine the NOEC (chronic non-observed-effect) for the amphipod due to the mortality observed in the controls and at low levels of the WSF (6.25, 12.5 and 25 %). For the second dose-dependent bioassay, the rearing conditions were optimized for the amphipods and no mortality was observed in the amphipods that had been treated with 0–25 % WSF. Therefore, for the tested concentrations, 25 % WSF represents the NOEC concentration during a 72 h-period. Surprisingly, toxicity reached high levels with 50 % WSF, which was observed in both bioassays. The LOEC and NOEC are valuable parameters for hazard/risk assessment (Seitz et al. 2008; Smit et al. 2009). To determine the LOEC (lowest-observed-effect concentration) for the Q. aff. lutzi amphipod it will be necessary to use a range of concentrations between 25 and 50 % WSF. In the first dose-dependent bioassay, the LC50 after 48 h was 42.8 % WSF. In the second assay, which was extended to 72 h, the time period of the genotoxic test revealed an LC50 value that was equal to 51 % WSF for 72 h. Doses for genotoxic analyses by the comet assay must be low enough to rule out cytotoxicity, which leads to DNA fragmentation due to necrosis and apoptosis (Steinert 1996; Morley et al. 2006). The concentration used in this study (17.6 % WSF of oil, obtained from LC50/AF; AF = 2.4–2.9) for the Q. aff. lutzi amphipod) was within the recommended values for chronic and genotoxic assays (Martínez-Jerónimo et al. 2005) and close to the values that were used by these authors in Daphnia in order to test the chronic effects of exposure to the WSF of oil.
DNA damage and genotoxicity
DNA damage levels vary greatly among different experiments, making them difficult to compare. Even using the same endpoint, the use of different software or adjustments may produce different levels of damage. DNA damage levels observed in the negative controls of the Q. aff. lutzi amphipod (obtained from the medians) were consistent with values found in negative controls of Gammarus fossarum amphipod (Lacaze et al. 2010) and fish cell lines (Štrut et al. 2011). High DNA damage in the negative controls has been considered a feature of DNA packaging and background alkali-labile sites (Mitchelmore and Chipman 1998). In the first bioassay, the highest mean values of DNA damage found in the amphipods that had been treated with 17.6 % WSF were between 9.5–14 % tail DNA. Those values were within the range of DNA damage found in units of in vitro bioassays with fish cell lines that had been exposed to 0.1–10 μM of benzo(a)pyrene (B(a)P) (Štrut et al. 2011). The DNA damage levels were not lower than 2-fold of the controls. A maximal value was observed after the 48 h treatment that was 4-fold higher than the DNA damage found in the controls. This DNA damage level was also similar to those found when fish cell lines were exposed to 0.63–20 mg L−1 of methanol- and cyclohexane-extracted oil refinery sediments (Štrut et al. 2011). When studying the aqueous fractions (oil/water proportions 1:500 and 2:500) of the Prestige oil spillage in the M. galloprovincialis mussel, Pérez-Cadahía et al. (2004) found low DNA damage levels (3.2–6.2 % tail DNA). The DNA damage levels found in the second genotoxic bioassay of Q. aff. lutzi showed higher values of DNA damage (30–40 %), which were most likely due to technical adjustments of the comet measurement. Taban et al. (2004) studying DNA damage levels in coelomocytes of sea urchins and hemocytes of Mytilus edulis after 4–5 weeks exposed to 0.06–0.25 mg L−1 of water-dissolved crude oil observed DNA damage levels of 13–20 % tail DNA (higher than the values obtained in the first bioassay for the amphipod, but lower than the values obtained for the second one). Therefore, the DNA damage values found in the WSF-treated amphipods in the second bioassay were more consistent with studies that showed higher values of DNA damage (ex. Siu et al. 2004; Nigro et al. 2002). Most studies that exhibited high DNA damage levels in treated individuals also showed higher levels in the controls, as was also verified between the first and second bioassay of Q. aff. lutzi, providing evidence that differences among different studies may be in part related to technical adjustments rather than differences between species. This situation justifies the need to compare results using indexes such as the IF, which represent the levels of DNA damage in treated individuals as the fold of damage observed in the controls. In the literature, routes of exposure to hydrocarbons, target cells, taxa, pollutant composition and concentrations have been cited to explain the large differences in DNA damage that have been found among studies, which makes reaching and comparing conclusions even more difficult. For example, in contrast to our study and previous studies, Lacaze et al. (2011a) did not find differences in spermatic cells of Gammarus fossarum between amphipods that had been treated with 2.5 mg L−1 B(a)P and untreated amphipods after five days of exposure. Amphipod spermatic cells were found to be more sensitive to some toxic agents than hemocytes and oocytes by Lacaze et al. (2010, 2011b), which illustrated the importance of following DNA mutations over generations. However, the contrasting results observed after hydrocarbon exposure that had been evaluated on different amphipod cell types suggested that the use of hemocytes for assessing DNA damage from hydrocarbon-derived metabolites may be more appropriate than male germinative cells. Hemocytes have been considered suitable cells for assessing genotoxicity in invertebrates because hemocytes play an important physiological role in immune defense, phagocytosis, and the transport, excretion (Iwanaga and Lee 2005) and detoxification of xenobiotics (Siu et al. 2004).
Time-dependent response
Variations in DNA damage with time followed an “up-and-down” pattern in the WSF-treated amphipods, which was confirmed in the second genotoxic bioassay (Fig. 5). A similar pattern was observed by Morley et al. (2006) in human skin cell lines after treating them with UVA during a 25 h period. In the amphipod, we observed two events of elevated DNA damage after 6 and 48 h, and two events of reduced DNA damage after 24 and 72 h. Hydrocarbon components of petroleum act on DNA after their metabolites have been produced and released in the organisms. Therefore, a delay in the DNA damage response (6 h response) is expected compared to a UVA-induced response (1 h response, see Morley et al. 2006). Nevertheless, the “up-and-down” response observed in both studies suggests that intrinsic general cellular mechanisms are present in both vertebrates and invertebrates that are detectable at least during a short period of time. Morley et al. (2006), using a specific assay to detect DNA repair, necrotic and apoptotic cells, explained how the variations over time found in UVA-exposed human skin cells may be attributed to these events, especially when the UVA exposure is close to the lethal dose. We believe that the variations in DNA damage over time may be a function of DNA repair. Although, DNA repair activity has been evaluated to be low in aquatic organisms (Espina and Weis 1995) and very little is known about DNA repair efficiency in different wild organisms (Jha 2004, 2008). Morley et al. (2006) also observed that apoptosis may interfere with genotoxicity-induced DNA fragmentation depending on the time when the cells are assayed and on the progress of apoptosis. These authors concluded that only early apoptosis is detected using alkaline comet assay image analysis, which detected fragments of 10–100 kb, whereas subsequent apoptotic DNA fragments of 200–2,000 bp could not be detected using this assay. A cellular response to DNA damage is the checkpoint, which arrests or slows down cell cycle progression and provides time for adequate DNA repair to occur prior to the initiation of the next cell cycle phase (Al-Hakim et al. 2010). Data on micronucleus formation in mussels suggest that the mitotic rates of invertebrate hemocyte cells may range from 48 to 72 h (Siu et al. 2004). DNA repair may explain the reduced DNA damage levels observed after 24 and 72 h of treatment with the WSF in Q. aff. lutzi amphipods after the onset of DNA damaged detected at 6 h. The arrest of the cell cycle, together with continued exposure to the pollutant, which will still produce DNA fragmentation in cells, may explain the subsequent increase in DNA damage levels that was observed at 48 h. In addition, time-dependent variations in the DNA damage levels and the delay in time in having an immediate response may also be attributed to a threshold that has been suggested for mussels, which is contingent upon the accumulation of toxicants exceeding a certain level (Siu et al. 2004). Steinert (1996) also found a decline in DNA damage in the Mytilus edulis mussel between 18 and 30 h after treating it with moderate doses of B(a)P. We believe that our results were not biased by cytotoxic damage (necrosis and apoptosis) because of the low dose of the WSF that was used (17.6 % WSF, which should contains approximately 2.5 mg L−1 of benzene in accordance with the data of Table 1) and due to our exclusion of all ghost and cloudy-shaped comets from the analyses.
Gender-related responses
Gender-related responses in pollutant-exposed aquatic organisms have been poorly explored. Compared with males, female flounders were found to develop a significantly higher proportion of neoplastic liver lesions as a response to organic pollution (Koehler 2004). Lacaze et al. (2010, 2011b), who studied Gammarus fossarum germinative cells, found that spermatic cells have a higher DNA damage response to toxic agents than oocytes, which is believed to be due to an inability of sperm to repair DNA.
In the first bioassay, in which the females and males were maintained together, WSF-treated males exhibited higher DNA damage responses compared to WSF-treated females (Table 2). In the second bioassay, where males and females were maintained separately, this pattern was not confirmed, and WSF-treated females showed higher levels of DNA damage than males (Fig. 4). Therefore, they exhibited highly variable responses, which were most likely more related to their individual physiological conditions and not to their gender, as was previously expected after the first bioassay. The second bioassay showed similar DNA damage responses over time in males and females that had been exposed to petroleum, following more or less the general “up and down” pattern observed.
The use of median % tail DNA/amphipod
The use of the median % tail DNA was introduced by Duez et al. (2003) and was cited recently as a good parameter for comet analysis by Browne (2009) and Lovell (2009). In the Q. aff. lutzi amphipod, the means calculated from the mean % tail DNA/amphipod and the means calculated from the median % tail DNA/amphipod produced relatively similar results. However, the mean of the medians of the % tail DNA/amphipod enhanced the differences between the groups in the analysis, which was demonstrated by the IF values (Fig. 6). The mean of the % tail DNA of an individual may increase if few comets with high levels of DNA damage are found, which would not greatly affect the median value for the individual. This situation was clearly observed in the amphipods from the control units (see Fig. 4). Therefore, this is an important advantage of the medians over the mean values of individual amphipod % tail DNA. When a sensitive test of significance, such as the parametric ANOVA, is required (e.g., when there is a small sample size), the means of the % tail DNA fit the assumptions of normality more often than medians, as was observed for the male means of the % tail DNA of the Q. aff. lutzi amphipod. This represents an advantage of means over the medians.
Our results for the Q. aff. lutzi amphipod, in combination with recent studies by Lacaze et al. (Lacaze et al. 2010, 2011a, b) for freshwater amphipods, show high sensitivity and, therefore, provide solid evidence for evaluating pollutant effects using the comet assay. Our study represents the first report on the effects of the WSF of a heavy oil on a brackish/freshwater amphipod as measured using the comet assay and in which hemocytes of single gender-identified amphipods were evaluated.
Conclusion
The WSF of heavy oil at a level of 17.6 % (1:9, w/v) is genotoxic to brackish/freshwater Q. aff. lutzi amphipods. These studies have demonstrated DNA damage levels that were 2- to 6-fold higher than the levels observed in amphipods that were not exposed to petroleum. Following 72 h of treatment, the DNA damage levels varied in the WSF-treated amphipods. An “up-and-down” pattern was exhibited, in which toxicity led to high levels of DNA damage at 6 and 48 h of treatment. The low DNA damage levels that were observed after 24 and 72 h of treatment can most likely be explained by DNA repair. Gender variations in the DNA damage response were observed after short-term petroleum exposure, which emphasizes the need to more deeply investigate invertebrate gender-related responses in future studies. We also concluded that it is possible to evaluate pollutant genotoxicity in the hemocytes of individual amphipods using the comet assay and that the Q. aff. lutzi amphipod may be a good model for evaluating toxicity. Finally, the mean DNA damage generated from the median % tail DNA of the comets/amphipod exposed larger differences between treated and untreated amphipods and between genders, providing evidence that supports the use of the median % tail DNA/individual as a parameter for evaluating DNA damage using the comet assay for small invertebrates.
References
Al-Hakim A, Escribano-Diaz C, Landry M-C, O’Donnell L, Panier S, Szilard RK, Durocher D (2010) The ubiquitous role of ubiquitin in the DNA damage response. DNA Repair 9:1229–1240
Anderson BS, Hunt JW, Phillips BM, Nicely PA, Tjeerdema RS, Martin M (2004) A comparison of in situ and laboratory toxicity tests with the estuarine amphipod Eohaustorius estuarius. Arch Environ Contam Toxicol 46:52–60
ANP (2011) Anuário Estatístico 2011—Anuário Estatístico Brasileiro de Petróleo, Gas Natural e Biocombustíveis. http://www.anp.gov.br/?pg=56346#Se_01. Accessed 21 Feb 2012
Baden SP (1982) Oxygen consumption rate of shrimp exposed to crude oil extract. Mar Pollut Bull 13:230–233
Bertoletti E (2011) A escolha do anfípodo bentônico para ensaios ecotoxicológicos com sedimentos marinhos. J Braz Soc Ecotoxicol 6:1–7
Browne M (2009) Imaging and image analysis in the comet assay. In: Dhawan A, Anderson D (eds) The comet assay in toxicology. RSC Publishing, Cambridge, pp 343–390
Daling S, Brandvik J, Macakay D, Johansen Ø (1990) Characterization of crude oils for environmental purposes. Oil Chem Pollut 7:199–224
Dhawan A, Bajpayee M, Parmar D (2009) The comet assay: a versatile tool for assessing DNA damage. In: Dhawan A, Anderson D (eds) The comet assay in toxicology. RSC Publishing, Cambridge, pp 3–53
Duez P, Dehon G, Kumps A, Dubois J (2003) Statistics of the comet assay: a key to discriminate between genotoxic effects. Mutagenesis 18:159–166
Espina NG, Weis P (1995) DNA repair in fish from polluted estuaries. Mar Environ Res 39:309–312
Farjalla VF, Faria BM, Esteves FA (2002) The relationship between DOC and planktonic bacteria in tropical coastal lagoons. Arch Hydrobiol 156:97–119
Finney DJ (1964) Probit analysis: a statistical treatment of the sigmoid response curve, 2nd edn. Cambridge University Press, London
Hartmann A, Speit G (2009) Comet Assay-Protocols and testing strategies. In: Dhawan A, Anderson D (eds) The comet assay in toxicology. RSC Publishing, Cambridge, pp 373–389
Henriques-de-Oliveira C, Batista DF, Nessimian JL (2007) Sewage input effects on the macroinvertebrate community associated to Typha domingensis Pers in a coastal lagoon in southern Brazil. Braz J Biol 67:73–80
Ingersoll CG, MacDonald DD, Brumbaugh WG, Johnson BT, Kemble NE, Kunz JL, May TW, Wang N, Smith JR, Sparks DW, Ireland DS (2002) Toxicity assessment of sediments from the Grand Calumet River and Indiana Harbor Canal in Northwestern Indianan, USA. Arch Environ Contam Toxicol 43:156–167
Iwanaga S, Lee BL (2005) Recent advances in the innate immunity of invertebrate animals. J Biochem Mol Biol 38:128–150
Jha AN (2004) Genotoxicological studies in aquatic organisms: an overview. Mutat Res 552:1–17
Jha AN (2008) Ecotoxicological applications and significance of the comet assay. Mutagenesis 23:207–221
Katsumiti A, Domingos FXV, Azevedo M, da Silva MD, Damian RC, Almeida MIM, Silva de Assis HC, Cestari MM, Randi MAF, Oliveira Ribeiro CA, Freire CA (2009) An assessment of acute biomarker responses in the demersal catfish Cathorops spixii after the Vicuña oil spill in a harbour estuarine area in Southern Brazil. Environ Monit Assess 152:209–222
Koehler A (2004) The gender-specific risk to liver toxicity and cancer of flounder (Platichthys flesus (L.)) at the German Wadden Sea coast. Aquat Toxicol 70:257–276
Kumaravel TS, Jha AN (2006) Reliable comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat Res 605:7–16
Lacaze E, Geffard O, Bony S, Devaux A (2010) Genotoxicity assessment in the amphipod Gammarus fossarum by use of the alkaline comet assay. Mutat Res Genet Toxicol Environ Mutagen 700:32–38
Lacaze E, Devaux A, Jubeaux G, Mons R, Gardette M, Bony S, Garric J, Geffard O (2011a) DNA damage in Gammarus fossarum sperm as a biomarker of genotoxic pressure: intrinsic variability and reference level. Sci Total Environ 409:3230–3236
Lacaze E, Devaux A, Mons R, Bony S, Garric J, Geffard A, Geffard O (2011b) DNA damage in caged Gammarus fossarum amphipods: a tool for freshwater genotoxicity assessment. Environ Pollut 159:1682–1691
Lee RF, Steinert S (2003) Use of the single cell gel electrophoresis/comet assay for detecting DNA damage in aquatic (marine and freshwater) animals. Mutat Res 544:43–64
Leite FP, Tararam AS, Wakabara Y (1980) Composição e distribuição da fauna de gammaridea na região da enseada da Fortaleza—Ubatuba, Estado de São Paulo. Bolm Inst Oceanogr 29:297–299
Lovell DP (2009) Statistical analysis of comet assay data. In: Dhawan A, Anderson D (eds) The comet assay in toxicology. RSC Publishing, Cambridge, pp 424–450
Mackay D, McAuliffe CD (1988) Fate of hydrocarbons discharged at sea. Oil & Chem Pollut 5:1–20
Martínez-Jerónimo F, Villaseñor R, Ríos G, Espinosa-Chavez F (2005) Toxicity of crude oil water- soluble fraction and Kaolin-absorbed crude oil on Daphnia magna (Crustacea, Anomopoda). Arch Environ Contam Toxicol 48:444–449
Mitchelmore CL, Chipman JK (1998) DNA breakage in aquatic organisms and the potential value of the comet assay in environmental monitoring. Mutat Res 399:135–147
Morley N, Rapp A, Dittmar H, Salter L, Gould D, Greulich KO, Curnow A (2006) UVA-induced apoptosis studied by the new apo/necro-comet assay which distinguishes viable, apoptotic and necrotic cells. Mutagenesis 21:105–114
Nigro M, Frenzilli G, Scarcelli V, Gorbi S, Regoli F (2002) Induction of DNA strand breakage and apoptosis in the eel Anguilla anguilla. Mar Environ Res 54:517–520
Nwabueze AA, Agbogidi OM (2010) Impact of water soluble fractions of crude oil on growth performance of the catfish Heterobranchus bidorsalis. ARPN J Agric Biol Sci 5:43–46
Pellegrini M, Colodette G, Martinez I, Neves L (2008) Jubarte field production enhanced with wellbore ESP. Offshore 68, Brazil, 6 pp. http://www.offshore-mag.com/index/article-display/334453/articles/offshore/volume-68/issue-7/brazil/jubarte-field-production-enhanced-with-wellbore-esp.html. Accessed 2 Aug 2011
Pérez-Cadahía B, Laffon B, Pásaro E, Méndez J (2004) Evaluation of PAH bioaccumulation and DNA damage in mussels (Mytilus galloprovincialis) exposed to spilled Prestige crude oil. Comp Biochem Physiol C 138:453–460
Petroleum (2009) Information on petroleum and crude oil. http://www.petroleum.co.uk/. Accessed 26 July 2011
Rojas E, Lopez MC, Valverde M (1999) Single cell gel electrophoresis assay: methodology and applications. J Chromatogr B 722:225–254
Seitz N, Böttcher M, Keiter S, Kosmehl T, Manz W, Holllert H, Braunbeck T (2008) A novel statistical approach for the evaluation of comet assay data. Mutat Res 652:38–45
Shoemaker CR (1933) Amphipoda from Florida and the West Indies. Am Mus Novit 598:1–24
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Siu WHL, Cao J, Jack RW, Wu RSS, Richardson BJ, Xu L, Lam PKS (2004) Application of the comet assay and micronucleus assays to the detection of B[a]P genotoxicity in haemocytes of the green-lipped mussel (Perna viridis). Aquat Toxicol 66:381–392
Smit MGD, Bechmann RK, Hendriks AJ, Skadsheim A, Larsen BK, Baussant T, Bamber S, Sanni S (2009) Relating biomarkers to whole-organism effects using species sensitivity distributions: a pilot study for marine species exposed to oil. Environ Toxicol Chem 28:1104–1109
Stebbing TRR (1907) The Fauna of brackish ponds at port canning, lower Bengal. Part V. Definition of a new genus of Amphipoda, and description of the typical species. Rec Indian Mus 1:159–162
Steele VJ, MacPherson BR (1981) Morphological features of sessile and circulating hemocytes in the cephalon of Gammarus setosus dementieva (Crustacea: Amphipoda) by light and electron microscopy. J Morphol 170:253–269
Steinert SA (1996) Contribution of apoptosis to observed DNA damage in mussel cells. Mar Environ Res 42:253–259
Štrut M, Traven L, Štambuk A, Kralj S, Žaja M, Mićović V, Klobučar G (2011) Genotoxicity of marine sediments in the fish hepatoma cell lines PLHC-1 as assessed by the comet assay. Toxicol In Vitro 25:308–314
Taban IC, Bechmann RK, Torgrimsen S, Baussant T, Sanni S (2004) Detection of DNA damage in mussels and sea urchins exposed to crude oil using comet assay. Mar Environ Res 58:701–705
Trevisan OV, França FA, Clarissa AO, Lisboa AC (2009) Elements of a heavy oil technology development program. Terrae 6:21–29
Wang X, Zauke G-P (2004) Size-dependent bioaccumulation of metals in the amphipod Gammarus zaddachi (Sexton, 1912) from the River Hunte (Germany) and its relationship to the permeable body surface area. Hydrobiologia 515:11–28
Wilson JT, Pascoe PL, Parry JM, Dixon DR (1998) Evaluation of the comet assay as a method for the detection of DNA damage in the cells of a marine invertebrate, Mytilus edulis L. (Mollusca: Pelecypoda). Mutat Res 399:87–95
Acknowledgments
The authors are grateful for the financial support that was provided by FINEP/PETROBRAS S/A, Research Fund No. 01.06.1191.00, and to FUNEMAC for three IC Grants. We are thankful to Cristiana Serejo for the taxonomic identification of the amphipods and to Lisia Gestinari for the taxonomic identification of the algae to which the amphipods were associated. We are also particularly grateful to two anonymous reviewers for their valuable suggestions and comments which have improved the manuscript considerably.
Conflicts of interest
The experiments outlined in the present work comply with current Brazilian laws. The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weber, L., Carvalho, L., Sá, N. et al. Genotoxic effects of the water-soluble fraction of heavy oil in the brackish/freshwater amphipod Quadrivisio aff. lutzi (Gammaridea) as assessed using the comet assay. Ecotoxicology 22, 642–655 (2013). https://doi.org/10.1007/s10646-013-1055-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-013-1055-z