Abstract
The impact of diazinon spraying in an agricultural tropical soil through the evaluation of both the habitat and retention functions of the soil system was never reported. To fill this gap, five times the recommended dose of a commercial diazinon formulation was sprayed in an agricultural area of Costa Rica, and dilution gradients of the sprayed soil were prepared in the laboratory. Avoidance and reproduction tests with soil organisms (Eisenia andrei, Enchytraeus crypticus and Folsomia candida) to evaluate losses in terrestrial habitat function, and growth and reproduction tests with aquatic organisms (Chlorella vulgaris and Daphnia magna, respectively) to evaluate the retention function of soil were performed. Results demonstrated that regarding habitat function, F. candida reproduction was the most sensitive endpoint (EC50 = 0.288 mg a.i./kg), followed by avoidance behaviour of E. andrei (EC20 = 1.75 mg a.i./kg). F. candida avoidance and the reproduction of E. andrei and E. crypticus were not affected by diazinon. The toxicity tests with aquatic organisms showed that the soil retention function was insufficient to prevent effects of diazinon either on microalgae growth (EC50 ≤ 0.742 mg/L and EC20 ≤ 0.223 mg/L) and on the reproduction of the cladoceran (EC50 ≤ 0.00771 mg/L and EC20 ≤ 0.00646 mg/L). Results suggested that diazinon exerted toxic effects even at the dilution corresponding to the recommended dose, fact which makes its misuse an issue of environmental concern. Furthermore, the present study highlighted the importance and complementary nature of the assessment of both habitat and retention functions to an ecological risk assessment in tropical systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agricultural practices have deleterious consequences for soil and aquatic systems, and are usually associated with the degradation of natural resources (e.g., soil carbon and nutrients in water). In temperate regions, after the conversion of natural ecosystems into agricultural use, soil organic matter losses usually occur in the first 25 years, whereas in tropical soils such losses may occur in the first 5 years after cultivation (Matson et al. 1997). This fact highlights the importance of the local environmental conditions (including properties of the local soils) on the noxious effects of pesticides in natural systems and, consequently, the need for an evaluation of the impact of pesticide applications in tropical agricultural systems integrating both the soil and water components. Recently, a guideline for the ecotoxicological characterization of soils for landfill covering, green areas, parks and recreation areas, and horticulture and agriculture areas, recommended the evaluation of both the habitat and retention functions of the soil (ISO 2003a). According to the latter international standard, the soil habitat function is the ability of soil to serve as habitat for soil living organisms, and the soil retention function is the ability of soil to adsorb contaminants preventing their mobilization into aquatic systems. Whereas the habitat function should be evaluated by means of tests on soils with soil organisms, tests on aqueous soil extracts (i.e., eluates) with aquatic organisms are recommended to evaluate the soil retention function.
Pineapple plantation farming is an example of a horticultural crop industry with an increased development in the last decades, which contributed to the large increase of pesticide applications over the last years. According to the Food and Agriculture Organization (FAO) of the United Nations (Anon 2002), the pineapple world production more than tripled during the last 30 years. Actually, pineapple production is the most important tropical fruit crop in the world after banana and citrus. For instance, in 2009, Costa Rica was the third country in the world with the highest annual pineapple production (1,870,121 t; FAOSTAT 2011). One of the pesticides used on pineapple plantations is the organophosphate diazinon (Rodríguez and Jordán 1993). This insecticide acts on the activity of the acetylcholinesterase enzyme which is involved in the transmission of neural impulses (NPTN 1998). Diazinon is highly toxic to birds, fish and aquatic insects (USEPA 1986), has an average half-life of 40 days in soils and 4 days in foliage at 25 °C (Wauchope et al. 1992), and a water solubility of 40 mg/L at 20 °C (Kidd and James 1991; see Table 1 for details on Diazinon physical and chemical properties). Due to its hazard to agricultural workers and wildlife species, the use of diazinon is restricted in countries such as the US (USEPA 2004) and Australia (APVMA 2003), although it remains a widely used pesticide worldwide.
The toxic effects of diazinon to non-target organism have been reported for several groups of aquatic organisms, such as cladocerans (Fernández-Casalderrey et al. 1994; Sánchez et al. 2000; Bailey et al. 2001; Jemec et al. 2007), decapods (Sucahyo et al. 2008), amphipods (Smith et al. 2007), chironomids (Schuler et al. 2005), oligochaetes (Ankley and Collyard 1995), ploimas (Fernández-Casalderrey et al. 1992), microalgae (Butler et al. 1975; Doggett and Rhodes 1991), bivalves (Bouldin et al. 2007), and fish (Bisson and Hontela 2002), and also for soil organisms, including earthworms (Booth and O’Halloran 2001; Leland et al. 2001), isopods (Vink and Van Straalen 1999), bacteria (Higgins and Hohn 2008), and fungi (Baicu 1982). Its effects on birds’ behaviour (Brasel et al. 2007), mammals (Gokcimen et al. 2007) and along the food chain (risk to passerine birds through earthworms) have also been evaluated (Stephenson et al. 1997). However, to date, none of the assessments of the toxic effects posed by diazinon addressed the impact of its spraying on agricultural soils, through the evaluation of both the habitat and retention functions of the soil system. Aiming to fill this gap, the ecotoxicological characterization of a soil sprayed with diazinon under a real scenario of tropical conditions was performed in the present study. To fully attain this purpose, the study was conducted according to internationally adopted guidelines (ISO 2003a), by carrying out the recommended sublethal toxicity tests on soils (with three species of soil organisms) and on eluates (with two species of aquatic organisms), to evaluate the habitat and retention functions, respectively.
Materials and methods
Test organisms and culture conditions
Soil habitat function tests were performed using the oligochaetes Eisnenia andrei Bouché (Lumbricidae) and Enchytraeus crypticus Westheide & Graefe (Enchytraeidae) and the collembolan Folsomia candida Willem (Isotomidae) as test species. Soil retention function was evaluated using the cladoceran Daphnia magna Straus (Daphniidae) and the green microalgae Chlorella vulgaris Beijerink (Chlorellaceae). Although E. crypticus and D. magna were never observed in Costa Rica, all test species used are recommended for standard toxicity testing and are used worldwide for soil and water quality assessments and monitoring (e.g., OECD 1984a, b; EC 1992; ISO 1996, 1999, 2003b).
All soil test species originated from laboratory cultures kept between 23 and 27 °C under a 16:8 h light:dark photoperiod. The earthworm E. andrei and the collembolan F. candida were both maintained as described by Natal-da-Luz et al. (2009). Whereas the earthworms used for testing were more than 1 month old with a well-developed clitellum and a fresh weight (average ± standard deviation [SD], n = 200) of 341 ± 28.6 mg, the collembolans were 10–12 days old from synchronized cultures. The pot worm E. crypticus was kept in plastic boxes with a natural agricultural soil previously defaunated through more than two freeze–thaw cycles (48 h at −20 °C followed by 48 h at 25 °C). The water content of this soil was adjusted once a week in order to maintain a moisture content of 40–50 % of its water-holding capacity. Cultures were fed every 2 days with ca. 2 g of wetted ground rolled oats (Frutocal company, Lisbon, Portugal) not cooked. Only organisms with a well-developed clitellum and apparently healthy were used in the tests.
The microalgae C. vulgaris was maintained in nonaxenic batch cultures between 21 and 23 °C under a 16:8 light:dark regime (100 μE/m2/s), in cotton-stoppered 1,000-mL Erlenmeyer flasks filled approximately until half with the growth medium recommended by Environment Canada (EC 1992). To obtain the algal inoculum to start new cultures, an aliquot of an exponentially growing culture was harvested, centrifuged (8 min at 3,000×g; Clay Adams Compact II Model 420225 centrifuge, Becton Dichinson, Sparks, MD, USA) and resuspended in algal growth medium. Cultures of D. magna were maintained at 23–26 °C under a 14:10 h light:dark regime (100 μE/m2/s), in reconstituted hard water (ASTM 2002), hereafter referred to as ASTM water, supplemented with selenium (2 μg/L) and vitamin B12 (2 μg/L). Cultures (25 and 12 daphnids/L up to the first brood and from there onwards, respectively) were fed daily 3 × 105 cells/mL of a mixture of Pseudokirchneriella subcapitata (Koršhikov) and C. vulgaris (on a 3:1 ratio). All organisms used in tests were third to fifth brood neonates (6–24 h old).
Experimental design
To simulate a real scenario of pesticide application, a commercial formulation of diazinon (Piñorel 60EC, 600 g a.i./L) was sprayed in an agricultural field with a typical soil from a region in Costa Rica where pineapple plantations are largely abundant and unpredictable heavy rainfalls often occur (Limón province, 10º 06′ N, 83º 36′ W). The soil of the selected area was free of pesticide and fertilizer applications for more than 5 years. Table 2 presents the following soil physical and chemical characteristics, measured immediately after field collection: pH (1 M KCl; 1:6, v:v), water-holding capacity (ISO 1996), organic matter content (loss on ignition at 500 °C for 6 h), density (water volume increase after dry pre-weighed soil addition), and soil texture (LNEC 1970). The area selected was divided into two units with 5 m2 each and with a buffer distance of 2 m from each other. One of the units was sprayed solely with water (control unit) and the other unit with five times the average recommended dose of diazinon for pineapple plantations in Costa Rica, which is 2.4 kg a.i./ha (Chaverri 2002; NRA 2002; test unit sprayed with 12 kg a.i./ha). This high dose of diazinon, hereafter referred to as the 100 % dose, was selected to simulate worst-case scenarios since the amounts of pesticide applied by local farmers often largely exceed the recommended dose (Castillo et al. 1997). Spraying was performed using a 16-L portable atomizer with water for the control unit and test solution for the contaminated one. In both cases, reference water collected from a nearby reference stream was used (pH, conductivity and hardness of 8.3, 64 μS/cm and 26 mg CaCO3/L, respectively).
To avoid cross contamination, the control unit was first sprayed and the top 50-mm soil layer was collected 30 min later. Afterwards, the test unit was sprayed and the soil collection was performed in the same way (30 min was considered sufficient to allow pesticide incorporation into the soil). It was assumed that the soil had an average density of 1.5 g/cm3 and that diazinon would not penetrate the soil to a depth higher than 50 mm, which means that the amount of insecticide applied to the test unit corresponded to a nominal concentration of 16 mg a.i./kg dry weight (DW).
Habitat function
Soil gradients
Both control and diazinon field-sprayed soils were collected and immediately transported to the laboratory, air dried (for 8 h), defaunated (by means of two freeze–thawing cycles), and sieved (5 mm). The diazinon-sprayed soil was diluted with the control soil (uncontaminated soil) to prepare the following gradient of the diazinon-contaminated field soil (each dilution was prepared in a single batch): 100, 70, 45, 30, 20, and 0 % (Table 3). This range of concentrations intended to simulate different application doses of diazinon (e.g., the 20 % dilution represented the recommended dose). Because the quantity of the field diazinon-sprayed soil was not enough to perform all reproduction tests planned to extensively evaluate the soil habitat function, a diazinon laboratory-spiked soil (at the same field nominal dose of 16 mg a.i./kg DW) was used to prepare a second dilution gradient of 100, 20, and 0 % of diazinon. In the laboratory, control soil was spiked with a stock solution of diazinon to achieve a concentration of 16 mg of diazinon/kg of soil (DW equivalent) and this soil was diluted with control soil, following the same procedures as above. This second diazinon soil gradient was only used to perform the E. andrei and E. crypticus reproduction tests (Table 3). For both dilution gradients, soil moisture was adjusted before the toxicity tests. Nominal soil diazinon concentrations of each dilution gradient were 16, 11, 7.2, 4.8, 3.2, and 0 mg/kg and 16, 3.2, and 0 mg/kg, respectively.
Toxicity tests with soil organisms
All avoidance and reproduction tests were conducted at a temperature of 23–27 °C, under a photoperiod of 16:8 h light:dark. The soil pH and the moisture content were always measured at the beginning and at the end of each test. Details on the procedures adopted for the toxicity tests with soil organisms are presented in Table 4. In the avoidance tests with E. andrei and F. candida (ISO 2007a, b), plastic containers were used as replicates and each was divided into two equal sections. During the test each replicate was covered with a transparent lid with a few pierced holes to allow aeration. At the end of the tests, a divider was inserted in the midline of each replicate. The number of worms in each section was immediately determined and the individuals found in the midline of the test container were counted as 0.5 independently of the body part found at each side. For collembola counting, the content of each of the sections was transferred to a small vessel. Individual contents of each section were flooded with water and the organisms floating at the water surface were counted after the addition of few drops of blue ink and gentle stirring. Missing collembolans were considered dead, since dead organisms decompose fast. In two-section treatment replicates, the control soil (0 % diazinon) was tested against each of the prepared soil dilutions. To verify whether the distribution of the individuals was random in both sections of the replicate when soil contamination was not an experimental factor (Yeardley et al. 1996; Hund-Rinke and Wiechering 2001; Natal-da-Luz et al. 2009), dual-control tests with the control soil were also performed.
In the reproduction tests with E. andrei and F. candida, the test vessels consisted of plastic containers covered with transparent lids with a few pierced holes to allow aeration, whereas glass vessels covered with opaque non-perforated lids were used for the test with E. crypticus. In all reproduction tests, replicates were weighed at the beginning of the test and weekly to correct for water losses, times at which test containers were opened for a few seconds to allow aeration. Individuals of E. andrei were pre-weighed, acclimated in control soil for 48 h and exposed for 28 days to the concentration gradient. After the 28-days exposure, adults were retrieved and re-weighed to determine biomass changes, whereas at the end of the test (56 days) the number of juveniles per replicate was determined using a water bath (ISO 1996). At the end of the tests with E. crypticus (28 days), each replicate was filled with ethanol (at 70 %) up to a depth of 4 cm plus 200–300 μL of Bengal red (1 % solution in ethanol), gently stirred, left to rest overnight, washed through a sieve (0.25 mm) with tap water, and transferred to a Petri dish to count juveniles under a binocular microscope. At the end of the collembola test (28 days), each replicate was flooded with water and blue ink. The water surface was photographed by a digital camera and surviving adults and juveniles were counted using the Image Tool software for Windows (version 3.00; Wilcox et al. 2002).
Retention function
Eluates
All soil eluates to perform the tests with the aquatic organisms, were first prepared from each of the six soil dilutions (100, 70, 45, 30, 20, and 0 %; Table 3) according to the German standard method to determine leachability by water (DIN 1984). A mixture of soil and water (1:10 ratio, 100 g/1000 mL, based on DW soil) was shaken in a magnetic stirrer for 24 h, centrifuged at room temperature and the supernatant collected and stored at 4 °C in the dark until use (within 48 h and 1 week of preparation, for the microalgae and cladoceran tests, respectively). Waters used to prepare the eluates were the media used for culturing (but without vitamins and selenium in the case of the ASTM water), as they were also used in the toxicity tests as control media. Eluates for the microalgae test were prepared in 250-mL Erlenmeyer flasks and were centrifuged at 3,000×g for 30 min (Clay Adams Compact II Model 420225 centrifuge). For the cladoceran test, eluates were prepared in 2-L Erlenmeyer flasks and centrifugation was at 1,500×g for 15 min (Beckman Model TJ6 Tabletop centrifuge, Bechman Instruments, Palo Alto, CA, USA). The differences during centrifugation were a compromise between the volume of the mixture to centrifuge and the need to have an eluate with a low level of total suspended solids (TSS) for the microalgae test. Measurements of TSS followed standard methods (Arnold 1995) and were 55 and 110 mg/L for the microalgae and cladoceran control eluates (0 %), respectively.
Toxicity tests with aquatic organisms
A 96-h C. vulgaris growth test was carried out on all six eluates following the OECD (1984a) and EC (1992) guidelines. The test was conducted in 4.5-mL glass vials (1.2-cm diameter), in an orbital shaker (80 rpm; Flat rotator Model DSR-2100A, Digisystem Laboratory Instruments, Hsi Chih City, Taipei Hsien, Taiwan), at 22–24 °C, and under the same light conditions as those used for culturing. Three 2-mL replicate cultures of each eluate and six of the control algal medium were set up and inoculated with a 20 μL algal inoculum, so that the initial cell concentration was 104 cells/mL. At the end of the 96-h exposure, the mean specific growth rate per day was estimated. Final cell densities were counted on well-mixed aliquots of each replicate under a microscope (400× magnification), using a Neubauer chamber (American Optical, Buffalo, NY, USA). Values of pH (Corning pH meter 220, Corning Incorporated Life Sciences, Lowell, MA, USA) and conductivity (WTW 330i conductivity meter, Wissenschaftlich Technische Werkstätten, Weilheim, Germany), measured in all eluates and in the control at the start of the test, were comparable across treatments; pH ranged from 6.70 to 7.53 and conductivity from 128 to 247 μS/cm.
A reproduction test with D. magna was conducted according to the OECD (1984b) guidelines for a 14-day reproduction test, which is the test duration required for the development of at least three broods at 20 °C. In accordance with the work of Gersich and Milazzo (1990) who proposed the shortening of the classic D. magna reproduction test from 21 days at 20 °C to 14 days at 25 °C, it was possible to reduce the duration of a three-brood test from 14 to 12 days by performing it at 23–26 °C instead of at 20 °C. Since the eluate from the 20 % soil dilution was expected to be highly toxic to D. magna, a 48-h lethal test following the OECD (1984b) guidelines was first performed on the following dilutions of the 20 % eluate, using ASTM water as dilution medium: 100, 50.0, 25.0, 12.5, 6.25, and 3.13 %, i.e., on eluates corresponding to 20.0, 10.0, 5.00, 2.50, 1.25, and 0.625 % of the diazinon dose applied in the field (100 % dose). From hereafter all nominal eluate dilutions will refer to the latter dose. The lethal test was incubated under the same conditions as the reproduction test. Given that the 48-h LC50 (and 95 % confidence interval) for diazinon was 1.77 % (1.52–2.08) (calculated using the software PriProbit 1.63; Sakuma 1998), the reproduction test was performed on 2.50, 1.66, 1.10, 0.740, and 0.500 % of eluate (Table 3) and on 2.50 % of eluate from the control soil, to test for the influence of TSS. Ten replicates were set up for each eluate and for the control (ASTM water), each with 30 mL of test solution and one organism. During testing, feeding regime, medium renewal frequency and incubation conditions were similar to those used for culturing. After the 12-day exposure period, fecundity was determined as the total number of neonates released per female. Values of pH, conductivity and dissolved oxygen (WTW Oxi 325 oxygen meter) were measured in three replicates of all eluates and in the control, in new and old medium, at all medium renewals. Comparable values were found across all treatments, ranging between 7.68 and 8.36, 531 and 601 μS/cm, and 6.4 and 9.3 mg/L, respectively.
Chemical analysis
Diazinon concentrations in soil were measured for the concentration gradient prepared from the field-sprayed soil (0, 20, 30, 45, 70, and 100 %); soil samples were taken immediately after preparation and frozen (at −20 °C) until analysis. For the soil dilution gradient prepared from the laboratory spiked soil (0, 20, and 100 %), it was assumed that these soil dilutions had diazinon concentrations similar to those measured in the same dilutions prepared from the field-sprayed soil. This assumption was based on the fact that field spraying was performed very close to the soil surface (to minimize pesticide losses by volatilization and/or spray-drift), a 30-min period was left for pesticide incorporation into the soil and a layer corresponding to 50 mm depth of soil was collected (depth at which most pesticide is expected to be retained). In all eluates used in testing, diazinon concentrations were estimated assuming that all detected diazinon was eluted from the soils (i.e., the soil retention function for diazinon was null, which represents the worst-case scenario for aquatic organisms). Measurements of real diazinon concentrations in eluates were not considered mandatory to fulfil the objective of the present study for which tests on eluates were designed—to assess whether the soil retention function for different doses of diazinon field spraying was protective for aquatic organisms (relatively to the risks posed by diazinon spraying to soil organisms), and not to estimate concentration–response curves between diazinon and microalgae growth or cladoceran reproduction.
Quantifications of diazinon in soil were performed by gas chromatography (Varian 3500, Varian Scientific Instruments, Walnut Creek, CA, USA) using a flame photometric detector equipped with a phosphorus mode assembly (FPD-P) and confirmed by gas chromatography-mass spectrometry (Shimadzu GCMS QP5000, Tokyo, Japan) and capillary columns (25 m BPX-35 fused silica column 0.25 or 0.32 μm, SGE, Austin, TX, USA). All solvents used in the analysis were of residue analysis grade and obtained from J.T. Baker (Phillipsburg, NJ, USA; dichloromethane) or Merck (Darmstadt, Germany; acetone and cyclohexane). The diazinon standard (98 % purity) was obtained from Dr. Ehrenstorfer (Augsburg, Germany). Quantification limits and recoveries were between 6 and 20 μg/kg DW and 70 and 90 %, respectively. For each soil, diazinon was extracted from 25 g of a homogenized air-dried sample, in a 250-mL centrifuge tube, in three steps (15 min each): first with acetone (50 mL), second with dichloromethane (50 mL) and finally with acetone–dichloromethane (50 mL; 1:1 ratio), using an ultrasonic bath. A 15-mL saturated sodium chloride solution (analytical grade from Merck) was added and the separated organic phase was dried and concentrated to 4 mL on a rotary evaporator (Büchi Labortechnik AG, Flawil, Switzerland) and further to 0.1 mL with a gentle stream of nitrogen. This extract was transferred to acetone–cyclohexane (1:9 ratio) to a final volume of 2 mL.
Data analysis
Avoidance and reproduction tests with soil organisms
Statistical analyses were performed considering the diazinon concentrations measured in the soil dilutions (100, 70, 45, 30, 20, and 0 %) prepared from the diazinon field-sprayed soil. For avoidance tests, Spearman correlation was used to evaluate the association between survival and actual diazinon concentrations in contaminated soil. An avoidance behaviour, in the two-section avoidance tests, was considered when the number of organisms found in the section with contaminated soil was significantly lower than that found in the control section (p ≤ 0.05), whereas in the dual-control tests it was when the number of organisms found in each of the two soil sections was significantly different (p ≤ 0.05) (Natal-da-Luz et al. 2009). These differences were evaluated by the one- and two-tailed Fisher’s exact test (Zar 1999), respectively. Prior to the statistical analysis, the number of dead or missing organisms in each combination was distributed by both sections of each replicate. The soil dilutions that provoked 50 (EC50) and 20 % (EC20) of avoidance behaviour and the respective 95 % confidence limits (CL) were computed using the Priprobit 1.63 software (Sakuma 1998; http://bru.gmprc.ksu.edu/proj/priprobit/download.asp), with the probit transformation of the proportion of avoiders and the log transformation of the actual concentrations.
For chronic tests, differences in reproduction and biomass (earthworms only) were evaluated by one-way analysis of variance (ANOVA) followed by Dunnett’s tests, to test for significant differences between the control and the diazinon contaminated soils. When the assumptions of normality (Kolmogorov–Smirnov test for p > 0.05) and homoscedasticity (Levene’s test for p > 0.05) were not fulfilled, the Kruskal–Wallis test was used followed by a Fisher LSD on ranks. The EC50 and EC20 values were calculated by non-linear regressions using, for each data, the best fitted model (EC 2004).
Growth and reproduction tests with aquatic organisms
Eluate diazinon concentrations used for statistical analysis were estimated based on soil actual concentrations and assuming that the soil retention function for diazinon was null, as above mentioned. For the microalgae test, estimates were based on the 100, 70, 45, 30, 20, and 0 % soil dilutions, whereas for the reproduction test, all estimates were based on the 20 % soil dilution.
Population growth and reproduction responses of C. vulgaris and D. magna, respectively, were analysed to determine differences between contaminated eluates and control, as detailed above for reproduction tests with soil organisms. Prior to the latter analysis, the standard and eluate controls were compared through a two-tailed Student t test, to verify whether the eluate per se (e.g., TSS, colour) influenced the organism responses. Since the eluate control was found not to affect the measured responses, all subsequent analyses were performed with this treatment (see Results section). The logistic model was used to estimate EC50 and EC20 values and respective 95 % CL (EC 2004). Given that the latter toxicity parameters were based on a worst-case scenario of contamination (null retention function for diazinon), they are here presented as equal to or possibly lower than the estimated value. These estimates were based on nominal diazinon concentrations in eluates, which were estimated from actual soil concentrations. Such approach allows evaluating the toxic risks that diazinon field-spraying poses to aquatic organisms. Statistical analyses for all growth and reproduction data were performed using the STATISTICA 7.0 software (StatSoft).
Results
To facilitate direct comparisons among the results of the different tests performed in the present study, all results in figures are presented (in %) in function of the range of nominal diazinon dilutions prepared from the 100 % dose sprayed at the field site.
Chemical analysis
The mean (±SD) actual diazinon concentrations in the 100, 70, 45, 30, 20, and 0 % gradient prepared from the Piñorel field-sprayed soil were 7.9 ± 0.8, 4.1 ± 0.4, 3.1 ± 0.0, 1.7 ± 0.0, 0.9 ± 0.07, and < 0.02 mg/kg DW, respectively. These levels showed that 30–50 % of the total sprayed diazinon remained in the soil at the moment of its collection. Based on such measurements, and, as above stated, assuming that all diazinon was eluted from the soils, diazinon concentrations in the eluates prepared from the soil dilutions 100, 70, 45, 30, 20, and 0 % for the microalgae growth test were 0.79, 0.41, 0.31, 0.17, 0.090, and <0.002 mg/L, respectively, whereas those in the eluates prepared from the 20 % soil dilution for the cladoceran reproduction test (2.50, 1.66, 1.10, 0.740, 0.500, and 0 %) were 0.011, 0.0075, 0.0050, 0.0033, 0.0023, and 0 mg/L. Following a similar approach, the D. magna 48-h LC50 (and 95 % confidence interval) for diazinon was 0.00797 mg/L (0.00684–0.00936).
Habitat function
Avoidance tests
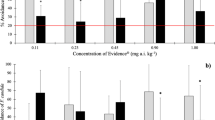
No mortality was observed in all tested combinations with E. andrei. In the dual-control test, the distribution found in both sections was not significantly different. In the two-sections test (control vs. contaminated soil) significant avoidance (p ≤ 0.05) was detected at the 30, 70, and 100 % diazinon dilutions (1.7, 4.1 and 7.9 mg diazinon/kg, respectively; Fig. 1) and the EC20 and EC50 values were 1.75 and >7.9 mg diazinon/kg, respectively (Table 5). The mean (±SD, n = 5) survival of F. candida was 92.0 ± 4.5 % in the dual-control test and ranged between 87.0 ± 11.0 and 88.0 ± 2.7 % in the two-sections test, but was not correlated with the diazinon concentration (R s = 0.196, p = 0.299). As for earthworms, the dual-control test with F. candida showed no significant differences in the distribution of the organisms. Avoidance was detected only at the 70 % diazinon dilution (4.1 mg diazinon/kg; Fig. 1), and thus the EC50 and EC20 values could not be determined (Table 5).
Mean (n = 5) percentage of avoidance ([No control − No test]/[No control + No test] × 100) in the avoidance tests with Eisenia andrei and Folsomia candida towards soil sprayed with diazinon (20 % dilution corresponds to the recommended diazinon dose for pineapple plantations in Costa Rica). The soil dilutions 100, 70, 45, 30, 20, and 0 % (w/w) correspond to the actual soil diazinon concentrations 7.9 ± 0.8, 4.1 ± 0.4, 3.1 ± 0.0, 1.7 ± 0.0, 0.9 ± 0.0, and <0.02 mg/kg, respectively. Error bars indicate +1 standard deviation; * denotes significantly (p ≤ 0.05) higher percentage of organisms in the control section than in the test section
Reproduction tests
The validity criteria defined for the reproduction tests (ISO 1996, 1999, 2003b) were fulfilled. In the tests with the oligochaetes E. andrei and E. crypticus, the reproduction was not affected by diazinon (Kolmogorov–Smirnov test, p > 0.20 and p > 0.20; Levene’s test, p = 0.31 and p = 0.28, respectively) (Fig. 2). The biomass change (% of the initial weight) of E. andrei after a 28-day exposure to diazinon was not significantly different from that of the control (data not shown; Kolmogorov–Smirnov test, p > 0.20; Levene’s test, p = 0.63). The reproduction of F. candida was significantly affected by diazinon (Kolmogorov–Smirnov test, p < 0.01; Levene’s test, p = 0.002) at soil dilutions ≥20 % (≥0.9 mg diazinon/kg; Fig. 3), with EC50 and EC20 values of 0.288 and 0.097 mg/kg, respectively (Table 5).
Mean (n = 4) 56 and 28-days reproduction (juveniles/replicate) of Eisenia andrei and Enchytraeus crypticus (n = 5), respectively, in soils spiked with diazinon (20 % dilution corresponds to the recommended diazinon dose for pineapple plantations in Costa Rica). The soil dilutions 100, 20, and 0 % (w/w) correspond to the soil diazinon concentrations 7.9 ± 0.8, 0.9 ± 0.0, and < 0.02 mg/kg (for estimations of diazinon concentrations see Materials and methods). Error bars indicate +1 standard deviation
Mean (n = 5) 28-days reproduction (juveniles/replicate) of Folsomia candida in soils sprayed with diazinon (20 % dilution corresponds to the recommended diazinon dose for pineapple plantations in Costa Rica). See Fig. 1 for actual soil diazinon concentrations. Error bars indicate +1 standard deviation; * denotes significantly different (p ≤ 0.05) compared with the control (0 %)
Retention function
The validity criteria defined for both the microalgae (OECD 1984a; EC 1992) and cladoceran tests (OECD 1984b) were fulfilled. For the C. vulgaris test, no significant differences in growth were observed between the control treatments, whereas the reproduction of D. magna in the eluate control was significantly higher than in the standard control, demonstrating that the eluate control had no detrimental effects on the organisms. Significant differences in the growth of the microalgae were observed between the eluate control and the diazinon-contaminated eluate dilutions as of the 45 % dilution (0.31 mg diazinon/L; Fig. 4). The EC50 value for diazinon was ≤0.742 mg/L and the EC20 was ≤0.223 mg/L (Table 5). The reproduction of D. magna was also significantly affected by eluates from diazinon contaminated soils as of the 1.66 % dilution (0.0747 mg diazinon/L; Fig. 5), with EC50 and EC20 values ≤0.00771 and ≤0.00646 mg/L, respectively (Table 5).
Mean (n = 3) 96-hours growth rate of Chlorella vulgaris in eluates prepared from diazinon-field sprayed soils (20 % dilution corresponds to the recommended diazinon dose for pineapple plantations in Costa Rica). The eluate diazinon concentrations of soil dilutions 100, 70, 45, 30, 20, and 0 % (w/w) were 0.79, 0.41, 0.31, 0.17, 0.090, and <0.002 mg/kg (for estimations of diazinon concentrations see Materials and methods). Error bars indicate +1 standard deviation; * denotes significantly different (p ≤ 0.05) compared with the control (0 %)
Mean (n = 10) 12-days reproduction of Daphnia magna in eluates prepared from diazinon-field sprayed soils (2.5 % dilution corresponds to 12.5 % of the recommended diazinon dose for pineapple plantations in Costa Rica). The diazinon concentrations of the eluate dilutions 2.50, 1.66, 1.10, 0.740, 0.500, and 0 % (v/v) were 0.0113, 0.00747, 0.00495, 0.00333, 0.00225, and 0 mg/kg (for estimations of diazinon concentrations see Materials and methods). Error bars indicate +1 standard deviation; * denotes significantly different (p ≤ 0.05) compared with the control (0 %)
Discussion
The diazinon recommended dose for pineapple plantations in Costa Rica will correspond to a concentration of 3.2 mg diazinon/kg of soil considering that the pesticide remains in the top 5-cm soil layer. In the present study, the chemical analysis results showed that, in the soil dilutions prepared from the diazinon field-sprayed soil with five times the recommended dose diazinon levels corresponding to the recommended dose were reached in the 45 % instead of the 20 % dilution. In spite of that, the 100 % soil dilution still simulated a pesticide overuse scenario since it corresponded to an actual diazinon concentration more than two times the recommended dose (7.9 mg/kg). However, according to previous studies (Adhya et al. 1987; Smith et al. 2006), a smaller difference between nominal and actual soil diazinon concentrations would be expectable in the present study due to the levels of pH, OM and clay contents of the soil (pH = 5.4, OM = 11.3 %, and clay content = 14 %). It is likely, therefore, that the difference between actual and nominal concentrations of diazinon found in soil dilutions was related to pesticide losses that are usual in field applications (e.g., by air dispersion, leaching to a depth higher than 5 cm) and, in the present study, additional laboratory manipulations.
In the present study, the results obtained in the toxicity tests with soil organisms (habitat function) showed that, from all tests performed, only the reproductive output of F. candida was considerably affected by diazinon contamination, even though no toxic effects were observed on the Collembola avoidance. Also, diazinon was suggested to affetct the avoidance behaviour of earthworms, with an EC50 value higher than the highest tested concentration (>7.9 mg diazinon/kg).
To date, the avoidance behaviour of earthworms against diazinon was studied also for L. terrestris and Aporrectodea caliginosa (Slimak 1997; Hodge et al. 2000). The avoidance behaviour of E. andrei detected in the present study appears to be more sensitive than that of L. terrestris (Slimak 1997), and apparently was in the same order of magnitude of the avoidance behaviour of A. caliginosa (Hodge et al. 2000). The apparent higher sensitivity of E. andrei compared to that of L. terrestris, might be due to differences in physiology and behavioural habits (e.g., feeding selectivity) of the test species, as L. terrestris is an anecic earthworm and E. andrei an epigeic one, and due to differences between the substrates used in both studies, which might have influenced diazinon adsorption and, consequently, its availability to the test organisms conditioning their avoidance behaviour.
Regarding the non-significant weight changes of E. andrei found in the present study when exposed to diazinon, a higher sensitivity was found by Leland et al. (2001) who observed weight losses in E. fetida when exposed to a soil amended with 10 % of pre-composted diazinon residues (corresponding to 0.7 and 5.8 mg diazinon/kg soil DW) for 13 days. However, in that study, earthworms might have used the contaminated organic residue directly as food, which may explain the reduction of earthworm biomass at low diazinon concentrations. Other studies exposing E. fetida to other organophosphates (similar mode of action of diazinon; Espinoza-Navarro and Bustos-Obreg 2005; Bustos-Obreg and Goicochea 2002) have reported decreases in worms body weight and reproductive output at concentrations considerably higher (≥80 mg malathion/kg and ≥444 mg commercial parathion/kg) than those of diazinon in the present study. Also, Booth and O’Halloran (2001) reported detrimental effects on the reproduction of A. caliginosa in a silt loam natural soil (3.8 % OM and pH 6.5–7.0) spiked with 12 mg diazinon/kg DW (nominal concentrations), suggesting the latter species to be more sensitive than E. andrei for which no toxic effects on reproduction were found at nominal diazinon concentrations ≤16 mg diazinon/kg in the present study. However, it has to be taken into account the specific feeding selectivity typical from endogeic species like A. caliginosa (different from that of epigeic species like E. andrei), and the differences between physical and chemical properties of the substrates used in the latter and the present study that may be at the origin of the reported sensitivity differences.
According to the available literature, the effect of diazinon on the avoidance behaviour and reproduction of F. candida was never evaluated. Nevertheless, the effect of other organophosphates like chlorpyrifos (similar mode of action of diazinon) reported in other studies at the collembola community level (Frampton and Van den Brink 2007; Fountain et al. 2007) suggests a high toxicity of diazinon to F. candida. In addition, Jager et al. (2007) showed that low concentrations of chlorpyrifos in food (nominal concentration <20 mg chlorpyrifos/kg food) provoke negative effects to F. candida after an exposure longer than 45 days, which agrees with the high sensitivity found for the reproduction of collembola in the present study. The known higher sensitivity of arthropods (including collembola) than oligochaetes to the insecticide diazinon (Frampton et al. 2006, Jänsch et al. 2006) or to the generality of insecticides (Daam et al. 2011) also supports these findings. On the other hand, the absence of collembola avoidance behaviour found in the present study, lead to assume that springtails are not able to detect diazinon in soil at least as much as needed to avoid it at the concentrations tested. However, since the EC50 value estimated for the reproduction of collembolans (0.288 mg diazinon/kg) was considerably lower than the diazinon recommended dose for pineapple plantations (3.2 mg diazinon/kg), the results of the present study suggest that the diazinon recommended dose induces a significant loss of habitat function in soil systems.
Based on the actual diazinon concentrations in the soil dilutions (assuming no retention of diazinon by soil), the diazinon concentrations in the eluates ranged from 0.79 to 0.090 mg/L and 0.011–0.0023 mg/L in the microalgae growth and cladoceran reproduction tests, respectively. However, considering the results obtained by Arienzo et al. (1994), who demonstrated that OM (when >2 %) is the most influential property on diazinon adsorption to soils, it is likely that the 11.3 % of OM content of the soil used in the present study contributed to a high retention function to diazinon. This assumption is also supported in the relationship K d = (K oc) × (%OC)/(100) used by Weber et al. (2004), where K d is the ratio of pesticide sorbed (mg/kg) to pesticide remaining in solution (mg/L), K oc is the pesticide soil adsorption coefficient, and %OC is the soil organic carbon content (%). Considering %OC = 0.58 × OM and K oc = 191, the K d of diazinon for the soil used in the present study was 12.5, which leads to suppose that most likely only 8 % of the diazinon present in the soil was mobilized into the eluates. Based on these assumptions, it is probable that the real diazinon concentrations in the tested eluates were considerably lower than those estimated by assuming a null retention function of the soil toward diazinon. Consequently, the diazinon concentrations that provoked 50 and 20 % of toxic effects on aquatic organisms might be lower than the estimated EC50 and EC20 values, fact which makes the risk of diazinon applications to the adjacent aquatic systems even higher than that suggested by the toxic values estimated in the present study.
Considering the results obtained in the toxicity tests with aquatic organisms (retention function), the spraying of the insecticide diazinon was less toxic to the microalgae C. vulgaris than to the cladoceran D. magna. In effect, invertebrates have been demonstrated to be the most sensitive toward diazinon among all tested groups of organisms (invertebrates, fish, amphibians, and microalgae) (Werner et al. 2002; USEPA 2005). In the present study, for C. vulgaris growth, the 96-h EC50 value was ≤0.742 mg/L, whereas the 96-h EC20 value was ≤0.223 mg/L. To date, the toxicity of this pesticide to C. vulgaris growth, to our knowledge, has never been reported. Nevertheless, the sensitivity of this microalgae species to the tested diazinon concentrations seems to be high compared to the 7-day EC50 value of 6.4 mg/L for Selenastrum capricornutum (USEPA 2005). Also, Butler et al. (1975) and Doggett and Rhodes (1991) reported concentrations of 10–25 mg/L and higher than 20 mg/L of diazinon to inhibit in 50 % the 15-day growth of several strains of green algae (primarily Chlorella sp. strains) and one diatom and the 10-day growth of four strains of green microalgae (Chlorella sp., Selenastrum sp. and Synechococcus sp.), respectively.
In the present study, the 48-h LC50 value for D. magna (1.77 % which corresponds to 0.00797 mg/L) was higher than the 24-h LC50 of 0.0009 mg diazinon/L reported for the same species by Fernández-Casalderrey et al. (1994). On the other hand, the 12-day EC50 and EC20 reproduction values for D. magna (≤0.00771 and ≤0.00646 mg/L, respectively) were about 100 times lower than the 96-h EC50 value for C. vulgaris growth (≤0.742 mg/L). Also, the diazinon toxicity toward D. magna reproduction found in the present study was 12–15 times lower than that found in previous studies, either for D. magna (Fernández-Casalderrey et al. 1994; Sánchez et al. 2000; Jemec et al. 2007) or other invertebrates, such as Ceriodaphnia dubia (Bailey et al. 2001; Werner et al. 2002) and the amphipod Hyalella azteca (Smith et al. 2007). However, whereas in most of the previous studies the toxicity of diazinon was evaluated in its pure form in standard laboratory tests using artificial reconstituted water, in the present study the eluates contained suspended particles that are capable of sequestering diazinon by adsorption, decreasing its availability to the test organisms. In addition, it has to be taken into account that the EC50 and EC20 values calculated in the present experiment for aquatic organisms were based on the worst-case scenario (100 % of soil diazinon mobilized into the eluates). Despite these differences, the results of the present study demonstrated that the diazinon recommended dose is highly toxic for the cladoceran D. magna, and that the aquatic systems adjacent to pineapple plantations sprayed with the recommended diazinon dose are indeed at risk.
Conclusions
In conclusion, the results here obtained suggest that the spraying of diazinon at doses recommended for pineapple plantations in Costa Rica may significantly affect the habitat function of soil and that the soil retention function may be insufficient to prevent marked toxicity to aquatic organisms, especially to cladocerans. These findings highlight the harmfulness of diazinon for soil and aquatic ecosystems, and evidence the usefulness and complementary of the assessment of both habitat and retention functions of soil to an adequate ecological risk assessment in tropical systems where unpredictable heavy rainfalls are frequent. However, since the actual exposure concentrations of diazinon were not measured in the eluates but were estimated based upon measured soil field sprayed concentrations, further research is needed to confirm the toxic effects of diazinon to soil and aquatic systems. Moreover, further testing to evaluate the influence of diazinon spraying in multiple soil types and on multiple invertebrate species would be desirable to draw conclusions at a wider geographic level.
References
Adhya TK, Wahid PA, Sethunathan N (1987) Persistence and biodegradation of selected organophosphorus insecticides in flooded versus non-flooded soils. Biol Fertil Soils 4:36–40
Ankley GT, Collyard SA (1995) Influence of piperonyl butoxide on the toxicity of organophosphate insecticides to three species of freshwater benthic invertebrates. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 110:149–155
Anon (2002) FAOSTAT (Food and Agriculture Organisation of the United Nations) Database. Rome, Italy
APVMA (2003) The reconsideration of registrations of products containing diazinon and their labels. Part 1 Product cancellations. Review Series 1. R. Report. Australian Pesticides & Veterinary Medicines Authority, Canberra, Australia, pp 1–25
Arienzo M, Crisanto T, Sánchez-Martín MJ, Sánchez-Camazano M (1994) Effect of soil characteristics on adsoprtion and mobility of (14C) Diazinon. J Agric Food Chem 42:1803–1808
Arnold EG (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington, DC
ASTM (2002) Standard guide for conducting acute toxicity tests on test materials with fishes, macroinvertebrates, and amphibians. E 729–96. In: Annual book of ASTM standards, vol. 11.05. American Society for Testing and Material, Philadelphia, PA
Baicu T (1982) Toxicity of some pesticides to Trichoderma viride Pers. Crop Prot 1:349–359
Bailey HC, Elphick JR, Krassoi R, Lowell A (2001) Joint acute toxicity of diazinon and ammonia to Ceriodaphnia dubia. Environ Toxicol Chem 20:2877–2882
Bisson M, Hontela A (2002) Cytotoxic and endocrine-disrupting potential of atrazine, diazinon, endosulfan, and mancozeb in adrenocortical steroidogenic cells of rainbow trout exposed in vitro. Toxicol Appl Pharmacol 180:110–117
Booth LH, O’Halloran K (2001) A comparison of biomarker responses in the earthworm Aporrectodea caliginosa to the organophosphorus insecticides diazinon and chlorpyrifos. Environ Toxicol Chem 20:2494–2502
Bouldin JL, Farris JL, Moore MT, Smith S, Cooper CM (2007) Assessment of diazinon toxicity in sediment and water of constructed wetlands using deployed Corbicula fluminea and laboratory testing. Arch Environ Contam Toxicol 53:174–182
Brasel JM, Collier AC, Pritsos CA (2007) Differential toxic effects of carbofuran and diazinon on time of flight in pigeons (Columba livia): potential for pesticide effects on migration. Toxicol Appl Pharmacol 219:241–246
Bustos-Obreg E, Goicochea RI (2002) Pesticide soil contamination mainly affects earthworm male reproductive parameters. Asian J Androl 4:195–199
Butler GL, Deason TR, O’Kelley JC (1975) The effect of atrazine, 2,4-D, methoxychlor, carbaryl and diazinon on the growth of planktonic algae. Br Phycol J 10:371–376
Castillo LE, De La Cruz E, Ruepert C (1997) Ecotoxicology as pesticides ni tropical aquatic ecosystems of central america. Environ Toxicol Chem 16:41–51
Chaverri F (2002) Importaciones y uso de plaguicidas en Costa Rica: análisis del perriodo 1994–1995. Universidad Nacional. Instituto Regional de Estudios en Sustancias Tóxicas, Herédia, Costa Rica, p 58
Daam MA, Leitão S, Cerejeira MJ, Sousa JP (2011) Comparing the sensitivity of soil invertebrates to pesticides with that of Eisenia andrei. Chemosphere 85:1040–1047
DIN (1984). German standard methods for the examination of water, waste and sludge: sludge and sediments, determination of leachability by water. DIN 38 414–S4. Deutsches Institut für Normung, Berlin, Germany
Doggett SM, Rhodes RG (1991) Effects of a dizinon formulation on unialgal growth rates and phytoplankton diversity. Bull Environ Contam Toxicol 47:36–42
EC (1992) Biological test method: growth inhibition test using the freshwater alga Selenastrum capricornutum. Report EPS 1/RM/25. Environment Canada, Ottawa, ON, Canada
EC (2004) Biological test methods for measuring the survival and reproduction of springtails exposed to contaminants in soil. Reports EPS 1/RM/47. Environment Canada, Ottawa, ON, Canada
Espinoza-Navarro O, Bustos-Obreg E (2005) Effect of malathion on the male reproductive organs of earthworms, Eisenia fetida. Asian J Androl 7:97–101
FAOSTAT (2011) Pineapple production in Costa Rica in 2009. Food and Agriculture Organization of the United Nations. http://faostat.fao.org. Accessed 18 October 2011
Fernández-Casalderrey A, Ferrando MD, Andreu-Moliner E (1992) Filtration and ingestion rates of Brachionus calyciflorus after exposure to endosulfan and diazinon. Comp Biochem Physiol C Comp Pharmacol 103:357–361
Fernández-Casalderrey A, Ferrando MD, Andreu-Moliner E (1994) Effect of sublethal concentrations of pesticides on the feeding behavior of Daphnia magna. Ecotoxicol Environ Saf 27:82–89
Fountain MT, Brown VK, Gange AC, Symondson WOC, Murray PJ (2007) The effects of the insecticide chlorpyrifos on spider and Collembola communities. Pedobiologia 51:147–158
Frampton GK, Van den Brink PJ (2007) Collembola and macroarthropod community responses to carbamate, organophosphate and synthetic pyrethroid insecticides: direct and indirect effects. Environ Poll 147:14–25
Frampton GK, Jänsch S, Scott-Fordsmand JJ, Römbke J, Van den Brink PJ (2006) Effects of pesticides on soil invertebrates in laboratory studies: a review and analysis using species sensitivity distributions. Environ Toxicol Chem 25:2480–2489
Gersich FM, Milazzo DP (1990) Evaluation of a 14-day static renewal toxicity test with Daphnia magna Straus. Arch Environ Contam Toxicol 19:72–76
Gokcimen A, Gulle K, Demirin H, Bayram D, Kocak A, Altuntas I (2007) Effects of diazinon at different doses on rat liver and pancreas tissues. Pest Biochem Physiol 87:103–108
Higgins J, Hohn C (2008) Effects of prevalent freshwater chemical contaminants on in vitro growth of Escherichia coli and Klebsiella pneumoniae. Environ Poll 152:259–266
Hodge S, Webster KM, Booth L, Hepplethwaite V, O’Halloran K (2000) Non-avoidance of organophosphate insecticides by the earthworm Aporrectodea caliginosa (lumbricidae). Soil Biol Biochem 32:425–428
HSDB (2011) Toxnet home: toxicology data network. United States national library of medicine. Diazinon. http://toxnet.nlm.nih.gov. Accessed 4 October 2011
Hund-Rinke K, Wiechering H (2001) Earthworm avoidance test for soil assessment. J Soils Sediments 1:15–20
ISO (1996) Soil quality: effects of pollutants on earthworms (Eisenia fetida)—Part 2: determination of effects on reproduction. ISO 1268-2.2. International Organization for Standardization, Geneva, Switzerland
ISO (1999) Soil quality: inhibition of reproduction of Collembola (Folsomia candida) by soil pollutants. ISO 11269. International Organization for Standardization, Geneva, Switzerland
ISO (2003a) Soil quality: guidance on the ecotoxicological characterization of soils and soil materials. ISO 15799. International Organization for Standardization, Geneva, Switzerland
ISO (2003b) Soil quality: effects of pollutants on Enchytraeidae (Enchytraeus sp.)—Determination of effects on reproduction and survival. ISO 16387. International Organization for Standardization, Geneva, Switzerland
ISO (2007a) Soil quality: avoidance test for testing the quality of soils and effects of chemicals on behaviour—Part 1: test with earthworms (Eisenia fetida and Eisenia andrei). ISO 17512–1.2 (Draft). International Organization for Standardization, Geneva, Switzerland
ISO (2007b) Soil quality: avoidance test for testing the quality of soils and effects of chemicals on behaviour—Part 2: test with collembolans (Folsomia candida). ISO 17512–2 (Draft). International Organization for Standardization, Geneva, Switzerland
Jager T, Crommentuijn T, Van Gestel CAM, Kooijman SALM (2007) Chronic exposure to chlorpyrifos reveals two modes of action in the springtails Folsomia candida. Environ Poll 145:452–458
Jänsch S, Frampton GK, Römbke J, Van den Brink PJ, Scott-Fordsmand JJ (2006) Effects of pesticides on soil invertebrates in model ecosystem and field studies: a review and comparison with laboratory toxicity data. Environ Toxicol Chem 9:2490–2501
Jemec A, Tisler T, Drobne D, Sepcić K, Fournier D, Trebse P (2007) Comparative toxicity of imidacloprid, of its commercial liquid formulation and of diazinon to a non-target arthropod, the microcrustacean Daphnia magna. Chemosphere 68:1408–1418
Kidd H, James DR (1991) The agrochemicals handbook, 3rd edn. Royal Society of Chemistry Information Services, Cambridge, UK
Leland JE, Mullins DE, Barry DF (2001) Evaluating environmental hazards of land applying composted diazinon using earthworm bioassays. J Environ Sci Health B 36:821–834
LNEC (1970) Solos—Análise granulométrica por peneiração húmida. LNEC-E 239, Laboratório Nacional de Engenharia Civil, Lisbon, Portugal
Matson PA, Parton WJ, Power AG, Swift M (1997) Agricultural intensification and ecosystem properties. Science 277:504–509
Natal-da-Luz T, Tidona S, Jesus B, Morais PV, Sousa JP (2009) The use of sewage sludge as soil amendment. The need for an ecotoxicological evaluation. J Soils Sediments 9:246–260
NPTN (1998) Diazinon. National Pesticide Telecommunications Network, <http://npic.orst.edu/factsheets/diazinon.pdf.>. Accessed 4 October 2011
NRA (2002) The NRA review of diazinon, vol 1. National Registration Authority for Agricultural and Veterinary Chemicals, Canberra, Australia
OECD (1984a) Algal growth inhibition test. OECD guidelines for testing of chemicals 201. Organization for Economic Cooperation and Development, Paris, France
OECD (1984b) Daphnia sp., acute immobilization test and reproduction test. OECD guidelines for testing of chemicals 202. Organization for Economic Cooperation and Development, Paris, France
Rodríguez G, Jordán P (1993) Guía práctica para el cultivo de la piña. CORDEP, Cochabamba, Bolivia, p 31
Sakuma M (1998) Probit analysis of preference data. Appl Entomol Zool 33:339–347
Sánchez M, Ferrando MD, Sancho E, Andreu E (2000) Physiological perturbations in several generations of Daphnia magna Straus exposed to diazinon. Ecotoxicol Environ Saf 46:87–94
Schuler LJ, Trimble AJ, Belden JB, Lydy MJ (2005) Joint toxicity of triazine herbicides and organophosphate insecticides to the midge Chironomus tentans. Arch Environ Contam Toxicol 49:173–177
Slimak KM (1997) Avoidance response as a sublethal effect of pesticides on Lumbricus terrestris (Oligochaeta). Soil Biol Biochem 29:713–715
Smith LH, Liyanage JA, Watawala RC, Aravinna AGP, Kookana RS (2006) Degradation of the pesticides carbofuran and diazinon in tropical soils from Sri Lanka. CSIRO Land and Water Science Report 67/06, pp 26
Smith S, Lizotte RE, Moore MT (2007) Toxicity assessment of diazinon in a constructed wetland using Hyalella azteca. Bull Environ Contam Toxicol 79:58–61
Stephenson GL, Wren CD, Middelraad ICJ, Warner JE (1997) Exposure of the earthworm, Lumbricus terrestris, to diazinon, and relative risk to passerine birds. Soil Biol Biochem 29:717–720
Sucahyo D, Van Straalen NM, Krave A, Van Gestel CAM (2008) Acute toxicity of pesticides to the tropical freshwater shrimp Caridina laevis. Ecotox Envon Safe 69:421–427
USEPA (1986) Pesticide fact sheet: diazinon. United States Environmental Protection Agency, Washington, DC
USEPA (2004) Economic and environmental benefit analysis of the final effluent limitations guidelines and standards for the concentrated aquatic animal production point source category. Office of water, United States Environmental Protection Agency, Washington, DC
USEPA (2005) Aquatic life ambient water quality criteria: diazinon. Final Report EPA-822-R-05-006. Office of water, United States Environmental Protection Agency, Washington, DC
Vink K, Van Straalen NM (1999) Effects of benomyl and diazinon on isopod-mediated leaf litter decomposition in microcosms. Pedobiologia 43:345–359
Wauchope RD, Buttler TM, Hornsby AG, Augustijn-Beckers PWM, Burt JP (1992) The SCS ARS CES Pesticide properties database for environmental decision-making. Rev Environ Contam Toxicol 123:1–155
Weber JB, Wilkerson GG, Reinhardt CF (2004) Calculating pesticide sorption coefficient (K d ) using selected soil properties. Chemosphere 55:157–166
Werner I, Deanovic A, Hinton DE, Henderson JD, Oliveira GH, Wilson BW, Krueger W, Wallender WW, Oliver MN, Zalom FG (2002) Toxicity of stormwater runoff after dormant spray application of diazinon and esfenvalerate (Asana®) in a french prune orchard, Glenn County, California, USA. Bull Environ Contam Toxicol 68:29–36
Wilcox D, Dove B, McDavid D, Greer D (2002) UTHSCSA ImageTool Version 3.0. Copyright 1995-2002, The University of Texas Health Science Center at San Antonio. http://ddsdx.uthscsa.edu/dig/itdesc.html. Accessed 21 October 2011
Yeardley JRB, Lazorchak JM, Gast LC (1996) The potential of an earthworm avoidance test for evaluation of hazardous waste sites. Environ Toxicol Chem 15:1532–1537
Zar J (1999) Biostatistical analysis. Hall International, London, UK
Acknowledgments
We would like to acknowledge Irene Campos and Johnny Campos for making available the agricultural field and for all field assistance, Fernando Mojica for characterizing the soil texture, Andrea Suárez for supporting in laboratory tests, and the Laboratorio de Estudios Ecotoxicológicos (ECOTOX) team for assistance. This research was partially funded by a postdoctoral grant of M. Moreira-Santos from the Portuguese institution “Fundacão para a Ciência e a Tecnologia” (SFRH/BPD/7196/2001) and by Ciência 2007-FSE and POPH funds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Natal-da-Luz, T., Moreira-Santos, M., Ruepert, C. et al. Ecotoxicological characterization of a tropical soil after diazinon spraying. Ecotoxicology 21, 2163–2176 (2012). https://doi.org/10.1007/s10646-012-0970-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-012-0970-8