Abstract
The marine shrimp Litopenaeus vannamei were used as an active biomonitoring organism to assess the bioavailability and impact of metal contaminants in seven study sites along the Maluan Bay of China. Metal concentrations in the hepatopancreas of shrimps were determined in conjunction with four biomarkers responses after a 7 day in situ cage exposures. The results showed that contaminant tissue burdens at the deployment sites were greater than those of the reference site, and antioxidant enzyme activities were strongly inhibited compared to those of reference organisms. Variations in these biomarker responses were correlated significantly (p < 0.05 or p < 0.01) with the specific metal pollutants at the study sites, but no significant correlations existed between catalase activity responses and the metal contaminants. This suggests the presence of undetermined contaminants or other exposure routes that may be responsible for the decreased catalase activity. Multivariate analysis revealed a causal relationship between contaminants at each deployment site and the biochemical “response” of the caged shrimps at these sites and demonstrated the presence of two contaminant “hot” spots. This investigation suggested that the incorporation of chemical data on trace metal concentrations with the analysis of antioxidant enzymatic activities in caged shrimps can be a useful tool for the identification of causal toxic contaminants in complex mixtures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid industrialization and intense urbanization in China, coastal and estuarine areas are impacted by complex mixtures of various contaminants, especially metals, which affect marine wildlife and degrade the function of marine ecosystems (Wang et al. 2011). Recent laboratory studies have indicated that the reduced water quality from metal pollutants in Maluan Bay (China) could have a negative impact on the indigenous saltwater crustacean Moina monogolica Daday (Wang et al. 2009). Assessment of individual pollutants using ecotoxicity tests under controlled laboratory conditions can quantify the effects of each contaminant, but is often less useful for predicting the impact of complex mixtures of pollutants on local biota (Galloway et al. 2002; Bervoets et al. 2005). In contrast to laboratory tests, field toxicity tests using caged resident indicator species allow more realistic exposure conditions and thus provide more accurate contaminant-related effects on the potential impact of complex mixtures of toxic pollutants on organism health, thus presenting numerous advantages over laboratory toxicity tests (Burton et al. 2005).
Biochemical determinations used as biomarkers can provide more sensitive and biologically relevant information and allow inherent capability to detect the early signs of biological responses to various xenobiotics (Faria et al. 2009). Consequently in recent investigations, biomarker responses were strongly recommended to characterize effects of contaminants to organisms (Hagger et al. 2009).
The marine shrimp Litopenaeus vannamei is a globally important ecologically relevant species in commercial fisheries along its natural range from northern Mexico to northern Peru and is today the principle crustacean species farmed worldwide (Keating et al. 2007). Due to its sensitivity against metal pollutants and their ability to bioaccumulate metals, L. vannamei is an ideal animal for studying the impairment caused by the effects of metals. They are abundant in coastal areas and have been successfully used as an indicator organism for monitoring metal polluted environments (Wu et al. 2008).
In the present investigation, the shrimp L. vannamei enclosed inside cages were deployed in situ at seven sites within Maluan Bay. After 7 days field exposure, multiple biomarkers together with contaminant burdens in hepatopancreas were analysed. The biomarkers selected included antioxidant enzyme activities superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) to evaluate possible oxidative stress induced by metal pollutants. In addition activity of the phase two biotransformation enzyme glutathione S-transferase (GST) was measured to assess possible deleterious effects on the shrimp’s detoxification pathways when exposed to field conditions.
The aims of the present study were as follows: (1) to characterize the biochemical responses of shrimps exposed to spatial variation of metal concentrations in Maluan Bay and to assess the water quality by integrating multiple biomarker responses to contaminant concentrations; (2) to distinguish xenobiotics that are significantly associated with specific biological responses and identify potentially harmful substances through multivariate analysis. To our knowledge this is the first in situ bioassay reporting biomarker responses of the shrimp L. vannamei when used as a biomonitoring organism under field conditions.
Materials and methods
Study sites
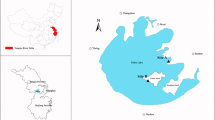
An overview of the Maluan Bay (24°32′47″N, 118°00′38″E) is given in Fig. 1, which is situated in the northwest of Xiamen in the province of Fujian, southeast China. Seven study sites along the suspected pollution gradient were selected. Site ML3, regarded as the most polluted area of the seven sites and located close to the sewage outflow of the highly industrialized area in Xinlin, characterized by metal contamination from heavy industrial activity such as metallurgy, coking plant and plating factory, having in the past discharged trace metals directly in the nearshore environment. Site ML7, was in the mouth of the Maluan Bay and close to the West Sea of Xiamen, which was located in harbor zones with variable degree of ship activity and level of metallic pollution as a result of more than a century of intense harbor activity in this area. Sites of ML2, ML4, ML5 and ML6 were not directly associated with potential sources of pollution but were located near ML3 and ML7. In contrast, ML1 was relatively far from known local sources of industrial or urban pollution and is in a less polluted area. Furthermore, it was noteworthy that the Maluan Bay receives daily tidal seawater from the West Sea although the seawall created in 1960s alters the geological and hydrological dynamics and restricts the seawater flow and renewal. The choice of study sites was made to encompass the decreasing trend of contamination from the inner to outer areas and the deployment sites are indicated.
Schematic overview of the exposure/study sites located in Maluan Bay, showing source and destination site and main geographical characterization. For the locations of the cages and geographic orientation of deployment sites, see the co-ordinates inserted rectangular highlighted in central table and the map of China or Xiamen. Deployment sites are marked with black circles, and watercourses influencing locations such as waste waters from Xinlin industrial area and the introduction of tidal seawater in the west sea are highlighted. Black arrows indicate tidal flow directions and dashed black arrows indicate pollution gradients (see Discussion)
Experimental organisms and field exposure
Shrimps L. vannamei were obtained from the aquaculture farm located at a clean site from the coast of Xiamen, which was also considered a reference site with no pollution point sources identified (Fig. 1). All specimens used in this study were of a similar size, length and at the same stage of reproduction (mature; carapace width about 9.7 ± 0.2 cm). Organisms were transported to the deployment sites in cool polystyrene boxes. Care was taken to minimize shaking during transport.
The field experiments were conducted at mean water temperatures of 18 ± 2°C in November. Random groups of shrimps were exposed at the seven deployment sites containing two cages at each site so as to provide duplicate assays and 10 individuals in each cage at a depth of 30–50 cm below the water surface. Cages consisted of polyvinylchloride boxes (30 × 25 × 20 cm) with rectangular window (10 × 10 cm) at each side, covered with 150 μm nylon mesh to create regular flow and circulation of water inside the cage and ensure that the conditions inside the cage were the same as the ambient water. Furthermore, their upper surfaces of the cages were transparent to enable light to enter. The cage design allowed organisms to be directly exposed to the contaminants under natural environmental conditions. The cages were deployed at locations illustrated in Fig. 1. Physicochemical parameters or characteristics of water quality conditions including pH, conductivity, salinity, dissolved oxygen, and chlorophyll-α in the water column were directly measured in situ using a portable YSI 6600V2 meter (YSI, Yellow Spring, OH, USA) at each deployment site during the field exposure periods.
Biochemical determinations
Following 7 days of exposure, cages were collected and then transported in cooler boxes to the laboratory in fifteen minutes. Surviving animals were counted and dead animal were discarded. Three replicates (pooled sample of two individuals) of L. vannamei were taken randomly from both cages at each site. Shrimps were dissected and their hepatopancreas were removed for biomarker determination. Hepatopancreas tissues were weighed and then homogenized using a glass tube with a Teflon pestle in ice cold homogenization buffer: phosphate (100 mM) buffer solution (PBS) containing 150 mM KCl and 1 mM EDTA, pH 7.4 at 4°C at a 1:5 weight:volume ratio. After homogenization, samples were centrifuged (10,000×g, 30 min, 4°C) and the supernatant either immediately used for determination of total protein content or stored in cryotubes at −80°C for enzyme analysis. In order to measure metal concentrations and enzyme activities in the hepatopancreas of L. vannamei at the reference site, the hepatopancreas samples were simultaneously dissected out from the shrimps, then freeze dried and ground for metal analysis, or immediately homogenized, shock frozen in liquid nitrogen and stored at −80°C for enzyme analysis. Preliminary studies were performed in order to optimize the methodology involving the use of L. vannamei hepatopancreas, and to assure that the enzyme assays were carried out within the linear range of calibration standards, enzyme concentration versus reaction rate, and time versus reaction rate.
Superoxide dismutase (EC 1.15.11) activity was determined by measuring the degree of inhibition of cytochrome c reduction at 550 nm by the superoxide anion generated by the xanthine oxidase/hypoxanthine reaction according to McCord and Fridovich (1969). Catalase (EC 1.11.1.6) activities were assayed as described in Aebi (1974). The variations of absorbance at 240 nm, caused by the dismutation of hydrogen peroxide, were measured as a function of time (extinction coefficient 40 M−1 cm−1). The methodology used for determination of GPx (EC 1.11.1.9) activity was adapted from Livingstone et al. (1992). GPx activities were measured spectrophotometrically at 340 nm every 2 min for 10 min, using 1 mM cumene hydroperoxide as substrate. Glutathione S-transferase (EC 2.5.1.18) activities were measured spectrophotometry at 340 nm, by following conjugation of the acceptor substrate 1-chloro-2,4-dinitrobenzene (CDNB) with reduced glutathione (Habig et al. 1974).
All enzyme activities were normalized with the protein concentrations of the supernatant, determined at 595 nm according to Bradford (1976) using the bovine serum albumin as the standard and reported in katal (kat) per milligram of protein (kat mg protein−1), where 1 kat is the conversion of 1 mol of substrate per second. The total protein content and all enzymatic measurements were performed in triplicate for each sample.
Metal concentration determination
Water samples for metal analysis were stored in HCl washed PVC bottles from approximately 30 cm below the water surface and refrigerated to 4°C immediately after sampling from each study sites at the beginning and end of the field experiments. Chemical analyses were conducted within 24 h of water collection. For dissolved metals analyses, water samples were filtered first through a pre-weighed 0.45 μm membrane filters (Xinya membrane, Millipore), then acidified with nitric acid (pH < 1) and directly analyzed using an Agilent 7500 inductively coupled plasma mass spectrometer (ICP-MS). Calibration standards and a reagent blank were analyzed with every 10 samples to monitor signal drift. In every instance, the signal typically changed by 3–5% throughout an analytical run. Additionally, rhenium was used as an internal standard to correct for any non-spectral interference. Metal concentrations in water samples are reported as μg/l.
For metals in suspended solids (SS) analysis, the particulate fractions in the membrane retained by the filter were dried in an air oven at 90°C for 24 h, weighted to allow the mass of SS to be determined by the difference from the pre-weighting 0.45 μm filter and then digested in a closed system using a nitric acid digestion procedure as described by Eaton et al. (1999). Blanks were prepared from ultrapure water carried through all the above procedures.
The hepatopancreas of two L. vannamei from each cage were dissected, pooled, and freeze-dried for metal analysis. Aliquots of samples were pulverized by pestle and mortar, mixed with 5 ml of nitric acid (Suprapur), placed in CEM Teflon bombs (CEM Corp., USA), capped loosely, and left overnight to digest at room temperature. Within each digestion series, appropriate blanks with no shrimp tissues were also subjected to the same procedure to account for background contamination levels and to validate the entire process. The next day, the bombs were sealed tightly after adding 1 ml hydrogen peroxide (Suprapur) and digested with microwave heating for 10 min under high pressure in a microwave digestion system (Mars Xpress, USA). After cooling, digests were diluted and then transferred quantitatively to acid-washed 25-ml volumetric flasks with 2% HNO3. Analytical quality control was achieved using certified reference material provided by the National Institute of Metrology in China, which produced 96–103% recoveries of the spiked metals and certificate materials and agreed with certified values.
Metal concentrations (Cd, Pb, Ni, Cr, Co, Cu, Zn, Mn, As and Se) in digests of SS and shrimp hepatopancreas were also analyzed by ICP-MS (Agilent 7500), which were calculated on a dry weight basis and expressed as μg/g, μg/kg dry weight, respectively.
Data and statistical analysis
Data sets for all study sites were tested for homogeneity of variance using Bartlett’s test and transformed when necessary. Normality was examined using Shapiro–Wilk’s test if needed. One-way analysis of variance (ANOVA) was used to evaluate the variance of the metal concentrations in solution, the SS and the shrimp’s hepatopancreas at the study sites. Student–Newman–Keuls multiple-range tests were used to determine any significant differences in the means at a 0.05 significant level. Significant differences between biomarker responses at the deployment sites and those from the reference site were also determined by one-way ANOVA. Post hoc testing with Student–Newman–Keuls multiple-range tests was used to determine which site means differed significantly.
Significant correlations between biomarker responses and metal concentrations were examined using a Pearson’s correlation analysis. This approach was used to determine if matrices were positively or negatively related in order to identify the potential causal agents of toxicity to the shrimp. The level of significance was set at *p < 0.05 or **0.01. One of the aims of the present study was to determine the relationship between the metal contaminants at the study sites, the metal uptake in the shrimp hepatopancreas and biomarker response of the caged shrimp. We therefore performed a multivariate analysis approach of factor analysis (FA) using the principal component analysis (PCA) as the extraction procedure (Tabachnick and Fidell 1996) applied to the original set of variables and performed on the correlation matrix. The variables were standardized through parallel pairs to ensure their equal treatment. All tests were performed with the SPSS/PC+ statistical package (SPSS software for Windows).
Results
Metal contaminant concentrations in water column and hepatopancreas of shrimps
The metal contaminant concentrations of water samples in the dissolved forms and SS from the seven deployment sites of Maluan Bay are shown in Table 1. The results indicate that the chemical composition of samples collected contain complex mixtures of metal contaminants that varied among the study sites. Although various metal contaminants were found at ML1, water samples from this site were predominately characterized by high dissolved concentrations of Ni, Co and As. In comparison, water sampled from ML7 was characterized with having high dissolved metal and metalloid concentrations (Cd, Pb, Zn and Se) and also some elevated metals (Cu and Se) in the SS. Samples from ML2 and ML5 were characterized by high dissolved Cu and Cr respectively, although no significant difference of these two metal concentrations were observed among deployment sites. Compared to other deployment sites, the ML4 and ML6 sites were not found to be highly contaminated. As for the metal contamination in SS, ML3 site was characterized by high concentrations of metals, particularly with Cd, Pb, Ni, Cr, Co and Zn.
Metal and metalloid concentrations (Cd, Co, Pb, Cr, Ni, Mn, Cu, Zn, As and Se) accumulated in the hepatopancreas of L. vannamei were determined after 7 days of in situ cage exposure (Fig. 2). High and variable metal concentrations were observed, particularly for Cd, Pb, Cr, Ni, Mn and As, which were significantly greater than those collected at the reference site (p < 0.05), although the metal and metalloid concentrations in shrimp from the reference site were also highly variable. It was noteworthy that the hepatopancreas of shrimp had the highest copper concentrations other than zinc and no remarkable changes of zinc content in shrimps were observed after 7 days field exposure at all deployment sites (p > 0.05). Furthermore, as for the other metal and metalloid concentrations in the hepatopancreas of shrimps, cobalt concentrations from ML1 site, copper concentrations from ML3 and ML7 sites, zinc concentrations from ML1, ML3, ML4 and ML7 sites, together with selenium from ML1, ML2 and ML7 sites were relatively similar, which were slightly lower than those from reference site, although no significant difference were obtained (p > 0.05). The cadmium concentration was highest at ML3, occurring 3.83 fold higher than the values of the reference site. This was due to high concentrations of Cd in SS. The maximum metal concentrations of Ni, Co and Cr were present in ML5, occurring 4.57, 3.49 and 3.14 fold the values of the reference site, respectively. These metal concentrations of water column samples at ML5 were not the highest recorded, which indicated that metal accumulation or bioavailability depends not only on the metal concentrations in water columns, but on other factors such as environmental and physiological parameters, which influence both chemical metal speciation and metal uptake by the organisms.
Metal contents (μg/kg dry weight, except ng/kg dry weight for Se) in the hepatopancreas of L. vannamei after 7 days in situ cage exposure from different deployment sites in Maluan Bay and those of the reference site (RS). Data bars for identical metal tissue burden with the same letter (A, B, C, D or E) are not significantly different (p < 0.05, Student–Newman–Keuls multiple-range tests)
Physicochemical parameters monitored during the field exposure showed that amounts of dissolved oxygen ranged between 6.5 and 7.8 mg/l, pH and salinity had a range of 7.6–7.8 and 20.0–22.0, respectively, and temperature fluctuated from 16 to 20°C, which indicated that water quality conditions in all deployment sites were relatively constant.
Biomarker responses
During the 7-day in situ exposure, low mortality (survival rate >90%) in individuals of the shrimps was observed in all deployment sites, which indicated that the shrimps were in reasonable health or good physiological status and the conditions of these organisms were feasible for field caging experiments.
The results of biomarker responses determined in hepatopancreas of field-exposed L. vannamei were presented in Fig. 3, which revealed the responses of enzymatic activity showing large inhibitory effects, in some cases significantly, among deployment sites.
Biomarker responses of SOD, CAT, GPx and GST activities in hepatopancreas of shrimps after 7 days in situ exposure at deployment sites along the coast of Maluan Bay. Bars indicate the biomarker responses (mean values ± standard deviation). Means for identical biomarker sharing the same letters (A, B, C, D, E, F or G) are not significantly different (p < 0.05, Student–Newman–Keuls multiple-range tests)
Compared to the reference site, SOD activities in the hepatopancreas of shrimps were significantly inhibited after 7 days exposure at all deployment sites. Significant inhibition of SOD was observed between ML2, ML4, ML7 and the reference site. No significant difference was found between ML2, ML4 and ML7. The SOD activities of sites ML1, ML3, ML5 and ML6 were also found to be significantly different from each other. The SOD activity from the ML3 site showed the highest repression, 36% of the value from the reference site.
Biomarkers of CAT and GPx activities exhibited a similar pattern of spatial variation, both of which were inhibited at all deployment sites, with the ML2 site producing the greatest inhibitory effects compared to the reference site. However, CAT activity had the strongest discriminating power showing the largest range of variation among deployment sites.
With the exception of ML7, GST activities in the hepatopancreas of L. vannamei were also significantly decreased at all deployment sites compared to the reference site. The ML5 site showed the highest decrease with 3.23% of GST activity compared to the GST activity in the hepatopancreas of shrimp deployed at the reference site.
Causal relationships between biomarker responses and metal concentrations
The relationships between metal concentrations and enzyme activities in the tissues of the shrimps were investigated using a Pearson’s correlation coefficient matrix (Table 2). A negative correlation between SOD activity and Cd burdens in hepatopancreas of L. vannamei was observed (p < 0.05). Furthermore, GPx activities were negatively correlated with soluble Cu concentrations (p < 0.05). Whereas, GST activities were positively correlated with the soluble Pb (p < 0.01) and Zn (p < 0.05) concentrations, as well as the As concentrations in SS (p < 0.05) and negatively correlated with Cd burdens in tissues of shrimps (p < 0.05). No significant correlation was observed between CAT activity and metal concentrations in water samples or hepatopancreas of shrimps.
In order to diagnose the causal relationship between metal exposure and effects observed in Maluan Bay, multivariate analysis approaches of FA using the PCA extraction procedure was undertaken. As a whole, the FA/PCA to 34 variables (10 metal concentrations in dissolved states, SS and hepatopancrea tissues, respectively, 4 biomarker responses) for the seven deployment sites were applied, which indicated that the 34 variables could be summarized in three factors accounting for 78.86% of the original dataset variance (Table 3). Only variables whose coefficient was ≥0.3 were considered components of the factors, a value which approximates Comrey’s cutoff for a reasonable association between an original variable and a factor (Comrey 1973). These three factors provide a more concise description than the original data set and can be described according to the dominant group associations as follows:
The first principal factor, #1, accounted for 31.37% of the original variance. The negative values of factor 1 (F1−) were associated with the SOD, CAT and GST enzymatic activities, Cd, Ni and Co in hepatopancreas of L. vannamei, Ni, Co, Cr, Zn and Mn concentrations in SS, as well as soluble Cd and Cr concentrations in water columns. Most of these relationships agreed with Pearson correlations (Table 2). The second factor 2, accounted for 24.38% of the original variance. The positive values of this factor (F2+) combined the CAT and GPx activities as antioxidant biomarkers to exposure to soluble Cu, Ni, Co and As in water columns, and Pb, Cr, As and Mn in tissues of shrimps. These relations were supported by Pearson analysis. The third factor (F3) accounted for 23.11% of the original variance. This factor represents the GST and CAT activity associated with soluble Pb and Zn concentrations in water column, together with the Cd, Pb, Cu, As and Se in SS. A Pearson analysis broadly agreed with the FA/PCA.
The study sites are characterized by complex mixtures of metal contaminants. A graphical representation of factor scores provided by the FA/PCA and prevailed in each study site was presented in Fig. 4, indicating the most significant relationships among variables in each deployment site.
For factor 1, values indicated the inhibition of SOD, CAT and GST activities and exhibited a positive score in ML3 and ML5 exposure cases. The inhibition of these biomarker responses is associated with the presence of metals (Ni, Co, Cr, Zn and Mn). Indeed, these sites are characterized by high concentrations of metals, in particular Ni, Co, Zn and Mn in the SS, and Cr in both the soluble filtrate and in the SS of the water samples. Factor 2 grouped variables with positive and negative coefficients and suggested a negative score in the sites of ML1, ML2 and ML3, as well as a positive score in the sites of ML4, ML5, ML6 and ML7. Finally, factor 3 showed a positive score in ML7 site and in a lesser extent, in ML3 site.
Discussion
Mean metal concentrations in water column measured in this investigation were compared against the primary and secondary criteria of Marine Seawater Quality (GB 3097-97) issued by China Ministry of Environmental Protection and State Oceanic Administration (CMEP-SOA 1998) to protect habitats for fishery and aquaculture. The data indicated that most metal concentrations across deployment sites are in keeping with the current status as low-level contaminated sites (Table 1), although the criteria were limited to the dissolved metal concentrations. Perhaps as a result of mixing and dilution due to strong tidal effects (Fig. 1), the metal concentrations did not show a complete trend towards either increasing or decreasing at particular sites (Table 1). However, site-specific or spatial differences were observed for various metal contaminants, indicating the existence of a pollution gradient and the complex hydrological dynamics of pollutant circulation and deposition in Maluan Bay. Furthermore, the highest amounts of Cd, Pb, Ni, Cr, Co and Zn in the SS of ML3 site demonstrated that these metal contaminants are strongly associated with human activities such as point source loadings of contaminants into the Maluan Bay, because this site is situated in a highly contaminated environment near the effluent outfall of Xinlin industrial area and continuously receives industrial wastewater discharge (Fig. 1).
After 7 days in situ exposure, most metal concentrations in hepatopancreas of L. vannamei were much higher than the reference site (p < 0.05, Fig. 2), which indicated that the hepatopancreas have a strong ability to accumulate metals. Therefore, the hepatopancreas appeared to be an effective organ for use as an indicator for xenobiotic exposure especially for metal contaminants. This is in agreement with other research revealing that the metal accumulation of L. vannamei is tissue-specific and the hepatopancreas accumulates metal concentrations more than other tissues (Keating et al. 2007). However, zinc concentrations in the hepatopancreas from all deployment sites were in the same range as those reported for the reference site. One possible reason is that the shrimp have developed mechanisms to regulate accumulation of zinc as essential metal or the organisms taken from the aquaculture farm had a high background concentration of zinc (Wang et al. 2010). Surprisingly, zinc concentrations were much lower than copper concentrations in the hepatopancreas of L. vannamei, which is different from the relationships between zinc and copper concentrations in tissues of other organisms such as bivalves. A possible reason is that the shrimp use copper as the oxygen-binding element for their respiratory pigment (Neff 2002). Furthermore, metal contaminants largely accumulated in biological tissues of cultivated marine animals, may not only cause direct toxicity to the organisms, but may be transferred through the food web and thus represent a risk to predatory species or even pose a problem to health of human populations, since shrimps are increasingly used as food. Clearly, further investigations of exposure beyond 7 days are needed to establish reliable environmental indices for the quality assessment of the coastal environment and for management purposes.
Exposure to xenobiotics, especially toxic pollutants, may have the potential to cause oxidative stress in aquatic organisms through free radical and reactive oxygen species (ROS) mechanisms, which produce an imbalance between endogenous and exogenous ROS (Valavanidis et al. 2006). In the present study, after 7 days in situ exposure, responses of enzymatic systems were strongly inhibited compared to those of reference organisms (Fig. 3) and the shrimp showed signs of oxidative damage. This not only confirmed the sensitivity of antioxidant biomarkers to metal contaminants as early signals of the contaminants presence, but suggested the failure of antioxidant defenses to remove exogenous ROS produced by redox cycling metals either by being inhibited by those contaminants or from being overwhelmed by an excess ROS. This is apparent especially for the activities of CAT and GPx in ML2 site (Fig. 3). Many metals including Cd, Ni and Pb, etc. may produce oxidative stress by indirectly depleting glutathione levels or via metal-induced displacement of redox metal ions (Barata et al. 2005). Therefore, there was a strong potential for measured metals along the deployment sites to increase ROS production and cause oxidative tissue damage in L. vannamei, and for antioxidant enzymes to respond to the increased metal exposure. Indeed, relevant correlation analysis in Tables 2 and 3 denoted causal relationships.
The uptake of pollutants by aquatic organisms under field conditions can occur from various routes, such as through exposure to soluble water concentration, suspended particulate matter, and food sources which depend on the particular dietary and feeding behavior of the aquatic organisms. Therefore, body concentrations of contaminants could be the result of internal uptake as well as of passive adsorption, but in any case are the combined stressors such as contaminants and environmental factors influencing the mechanism of accumulation and the efficiency of antioxidant system (Bocchetti et al. 2004). In L. vannamei, the hepatopancrea is the major tissue for metal accumulation and plays important roles in several metabolic processes in crustaceans such as protein degradation (Paez-Osuna and Tron-Mayen 1996). So there is a general consensus that alteration in the metabolism is intrinsically related to bioaccumulation of metals in this organ (Wu et al. 2008). In the present investigation, strong inhibition in biomarker responses related to metabolism, detoxification and possible toxicity were observed (Fig. 3), and the enzymatic activities of SOD and GST in the assays were directly correlated with tissue burdens of cadmium (Table 2). Metal pollutants in the hepatopancreas produced the alteration to functions of the protein catabolism, reflecting deleterious effects experienced by the organisms in exposure conditions. Our results are in agreement with those of Giarratano et al. (2007), who reported scenarios for metal exposure of various species of crustaceans such as amphipods and other shrimps, indicating that this in situ assay was robust and consistent across different environmental conditions and studies.
Enzymatic activities can be used as fast and specific biological responses to identify contaminants causing toxic effects in organisms. The relationship between contaminant toxicity, free radical processes and defensive responses of free radical scavenging system are thought to be crucial (Bocchetti et al. 2004). For metal contaminants, CAT constitute one of the best prognostic biomarkers to monitor biological effects in exposed biota, but in this study, inhibition patterns of CAT in hepatopancreas were not related to metal concentrations determined in the three matrices, which precluded the identification of a single causal relationship. The reason may be that xenobiotics from West Sea but not measured in the present study affect those activities, because the harbor waters present a complex mixture of contaminants, which may affect free radical processes and, hence, may alter free radical scavenging systems, such as antioxidant enzyme activities (Livingstone 2001). Furthermore, biomarker responses to contaminants not only depend on the degree of pollution and its composition, but on their exposure routes not limited to dissolved and particulate sources, which indirectly influence their effects (Maria et al. 2009).
In Maluan Bay, the study sites are affected by various low-level contaminants and other confounding factors. The elucidation of the origin of an observed biological alteration in an environment characterized by complex mixtures of metal contaminants is highly difficult due to the wide range of possible causes. Although L. vannamei invariably are exposed to complex mixtures of metal contaminants, only a small number of contaminants may be responsible for the observed toxicity. So in order to diagnose biochemical responses, the causal relationship between variations of biomarkers and field exposure were explored by means of the FA/PCA. In Table 3, negative factor 1 accounted for the relations between inhibition of SOD, CAT and GST activities in hepatopancreas and Ni, Co, Cr, Zn and Mn bound to SS. This relation was dominant in site ML3 and ML5 (Fig. 4), where hepatopancreas of shrimps exhibited SOD, CAT and GST activities significantly lower than those of reference site and water columns in these sites presented the highest Ni, Cr and Zn in SS.
It is important to understand the factors and processes that influence the bioavailability of metal contaminants in deployment sites and how these contaminants can be toxic to biota once they are accumulated. Multivariate analysis using FA/PCA relating metal concentrations in water columns and tissue burdens with responses of antioxidant enzymes was used to integrate this information (metal contamination, contaminants bioavailability and toxicity) into a framework that helped to assess the toxicity of the contaminated waters. The FA/PCA identified the sites of ML3 and ML5 as the highly impacted sites and two contaminant “hot” spots, which accorded with the realistic scenarios, because ML3 site was nearest to the outfall of wastewater discharges from Xinlin industrial areas, and simultaneously besides the sites of ML6 and ML7, perhaps ML5 site was also affected by contaminants from West Sea, which is subjected to metallic pollution as a result of more than a century of intense harbor activity in this area. Nevertheless, the sites of ML2, ML4, ML6, ML7, and especially ML1 were less impacted by the pollution according to the factor 1 score of Fig. 4, which was near or low to zero, reflecting less contamination of these sites and indicating the existence of pollution gradients in Maluan Bay (Figs. 1, 4). Furthermore, based on the score of factor 2 and factor 3 in Fig. 4, it can be inferred that metal contaminants are of great concern in Maluan Bay due to contamination of dissolved Ni and Co (ML2), particulate Cd, Pb, Cr and Co (ML3), particulate Ni (ML5), as well as dissolved Cd, Pb and Zn (ML7). The presence of these metals in deployment sites must be considered potentially significant in the overall environmental quality of the coastal ecosystems, which have been characterized as “producing adverse effects”. Thus, measured responses of caged organisms may reflect those responses occurring in natural populations, and the major environmental problem in this coastal area of Maluan Bay is directly related to the impact of industrial effluents and port facilities.
Conclusions
The present study showed that shrimp L. vannamei is a sensitive species with changes in hepatopancreas enzyme biomarkers an effective indicator of xenobiotic exposure to metal contaminants in Maluan Bay. Biomarker responses were significantly correlated with metal concentrations at deployment sites, thus denoting a clear cause-and–effect relationship and indicating these contaminants are likely to have caused adverse effects from oxidative stress to these organisms. No significant correlation between CAT activity responses and metal contaminants suggested the presence of other xenobiotics not analyzed in this study were causing oxidative damage to the shrimps and/or the importance of other exposure routes. Multivariate analysis allowed the identification of principal contaminants in each study site and demonstrated the potential presence of two contaminant “hot” spots in Maluan Bay. Thus, we recommend the use of biomarker assays in caged organisms and chemical analysis together with FA/PCA as a useful tool to evaluate biological effects and identify the toxic components in complex coastal environment, thus contributing to a more realistic assessment of ecological risks.
References
Aebi H (1974) Methods of enzymatic analysis. In: Bergmayer HU (ed) Catalase. Academic Press, London, pp 671–684
Barata C, Lekumberri I, Vila-Escale M, Prat N, Porte C (2005) Trace metal concentration, antioxidant enzyme activities and susceptibility to oxidative stress in the tricoptera larvae Hydropsyche exocellata from the Llobregat river basin (NE Spain). Aquat Toxicol 74:3–19
Bervoets L, Voets J, Covaci A, Chu SG, Qadah D, Smolders R, Schepens P, Blust R (2005) Use of transplanted zebra mussels (Dreissena polymorpha) to assess the bioavailbility of microcontaminants in Flemish surface waters. Environ Sci Technol 39:1492–1505
Bocchetti R, Fattorini D, Gambi MC, Regoli F (2004) Trace metal concentrations and susceptibility to oxidative stress in the polychaete Sabella spallanzanii (Gmelin) (Sabellidae): potential role of antioxidants in revealing stressful environmental conditions in the Mediterranean. Arch Environ Contam Toxicol 46:353–361
Bradford MM (1976) Rapid and sensitive method for quantification of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72:248–254
Burton GA, Greenberg MS, Rowland CD, Irvine CA, Lavoie DR, Brooker JA, Moore L, Raymer DFN, McWilliam RA (2005) In situ exposures using caged organisms: a multi-compartment approach to detect aquatic toxicity and bioaccumulation. Environ Pollut 134:133–144
CMEP-SOA (China Ministry of Environmental Protection, State Oceanic Administration) (1998) The People’s Republic of China National Standards GB 3097-97 marine seawater quality. China Ministry of Environmental Protection and State Oceanic Administration, Beijing
Comrey AL (1973) A first course in factor analysis. Academic Press, New York
Eaton AD, Clesceri LS, Greenberg AE (1999) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, American Waterworks Association, Water Environment Federation, Washington
Faria M, Carrasco L, Diez S, Riva MC, Bayona JM, Barata C (2009) Multi-biomarker responses in the freshwater mussel Dreissena polymorpha exposed to polychlorobiphenyls and metals. Comp Biochem Physiol C 149:281–288
Galloway TS, Sanger RC, Smith KL, Filimann G, Readman JW, Ford TE, Depledge MH (2002) Rapid assessment of marine pollution using multiple biomarkers and chemical immunoassays. Environ Sci Technol 36:2219–2226
Giarratano E, Comoglio L, Amin O (2007) Heavy metal toxicity in Exosphaeroma gigas (Crustacea, Isopoda) from the coastal zone of Beagle Channel. Ecotoxicol Environ Saf 68:451–462
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hagger JA, Galloway TS, Langston WJ, Jones MB (2009) Application of biomarkers to assess the condition of European marine sites. Environ Pollut 157:2003–2010
Keating J, Delaney M, Meehan-meola D, Warren W, Alcivar A, Alcivar-warren A (2007) Histological fidings, cadmium bioaccumulation, and isolation of expressed sequence tags (ESTs) in cadmium-exposed, specific pathogen-free shrimp, Litopenaeus vannamei postlarvae. J Shellfish Res 26:1225–1237
Livingstone DR (2001) Contaminated-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42:857–864
Livingstone DR, Lips F, Martinez PG, Pipe RK (1992) Antioxidant enzymes in the digestive gland of the common mussel Mytilus edulis. Mar Biol 112:265–276
Maria VL, Santos MA, Bebianno MJ (2009) Biomarkers of damage and protection in Mytilus galloprovincialis cross transplanted in Ria Formosa Lagoon (Portugal). Ecotoxicology 18:1018–1028
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymatic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Neff JM (2002) Bioaccumulation in marine organisms. Elsevier, Oxford
Paez-Osuna F, Tron-Mayen L (1996) Concentration and distribution of heavy metals in tissues of wild and farmed shrimp Penaeus vannamei from the northwest coast of Mexico. Environ Int 22:443–450
Tabachnick BG, Fidell LS (1996) Using multivariate statistics, 3rd edn. Harper Collins, New York
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64:178–189
Wang ZS, Yan CZ, Zhang X (2009) Acute and chronic cadmium toxicity to a saltwater cladoceran Moina monogolica Daday and its relative importance. Ecotoxicology 18:47–54
Wang ZS, Yan CZ, Hyne RV (2010) Effects of dietary cadmium exposure on reproduction of saltwater cladoceran Moina monogolica Daday: implications in water quality criteria. Environ Toxicol Chem 29:365–372
Wang ZS, Yan CZ, Pan QK, Yan YJ (2011) Concentrations of some heavy metals in water, suspended solids, and biota species from Maluan Bay, China and their environmental significance. Environ Monit Assess 175:239–249
Wu JP, Chen HC, Huang DJ (2008) Histopathological and biochemical evidence of hepatopancreatic toxicity caused by cadmium and zinc in the white shrimp, Litopenaeus vannamei. Chemosphere 73:1019–1026
Acknowledgments
This work is financially supported by the project of NSFC (National Scientific Foundation of China) in “Research of ecotoxicity effects and assessment methods based on in situ tests” (Grant No. 20807034/B070704 to Zaosheng Wang). Dr Zaosheng Wang was also supported by the KC Wong Education Foundation, Hong Kong. The authors would be very grateful for the help by Dr Yue Lin in the fishery research institute of Xiamen, who provided sufficient quantities of shrimps for field experiments. The manuscript benefited greatly from the helpful comments of three anonymous reviewers.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Z., Yan, C., Yan, Y. et al. Integrated assessment of biomarker responses in caged shrimps (Litopenaeus vannamei) exposed to complex contaminants from the Maluan Bay of China. Ecotoxicology 21, 869–881 (2012). https://doi.org/10.1007/s10646-011-0849-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0849-0