Abstract
Two studies were carried out to examine the impact of maternal fipronil exposure on embryonic and offspring development. In the first study, breeding female zebra finches were orally dosed with single sublethal levels of fipronil (1, 5, and 10 mg/kg body weight) to determine behavioural and developmental consequences on chicks following maternal pesticide exposure. Significant levels of fipronil and fipronil-sulfone residues were detected in eggs laid by females in all dosed groups, however, these were undetectable in eggs laid 13 days after treatment. The level of sulfone detected in eggs was consistently higher than that of the parent fipronil compound. Of the seven eggs laid in the treatment groups, only one (14%) chick hatched and this was from the lowest dose group. This chick was severely underdeveloped at 10 days of age in comparison to control chicks and fiproles were detected in brain, liver, and adipose tissues collected following euthanasia of this individual. In contrast, there was 100% hatchability of control group eggs and all chicks fledged nests on schedule. In the second study, domestic chicken eggs were injected with 5.5, 17.5, and 37.5 mg/kg egg weight of fipronil directly into the yolk sac on day 12 of incubation. Treatment did not affect hatching success, however, behavioural and developmental abnormalities were observed in hatchlings from the highest dose group. These chicks also demonstrated reduced feeding rates, as indicated by reduced body mass at 48 h period post hatch. Both fipronil and fipronil-sulfone residues were detected in brain and liver tissue of hatchlings at all pesticide dose levels tested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fipronil is a new generation, broad-spectrum pesticide commonly used to control locust outbreaks in a number of countries including Australia and others within Africa. Seasonal conditions that promote increases in locust populations, such as rainfall and consequent plant growth, often stimulate breeding activities for a variety of terrestrial vertebrates including birds and lizards. This unfortunately results in locust-control spray events often being coincident with peak breeding periods for many non-target animals. In Australia, over 100 different bird species are known to frequent areas that occasionally receive aerial application of fipronil for locust control (Szabo et al. 2009). While little is known of the effects of fipronil on free-living birds there are many studies that have examined the impact of other pesticides on avian reproduction and development (DeWitt 1955; Fernie et al. 2009; Lundholm 1997; Wiemeyer and Porter 1970). Results from these studies have demonstrated the importance of understanding the effects of pesticides on reproduction for better predicting their effects on avian populations.
Despite the widespread use and obvious exposure risks of fipronil, there is very little known about the impact of fipronil exposure in breeding birds. Insectivorous birds are particularly susceptible as fipronil-contaminated locusts can remain alive for 7–10 days (EPA 2001) and feeding on these and sympatric insects, provides a major route of pesticide exposure. Granivorous birds are also at high risk of exposure as fipronil and its metabolites are detectable in seeds after fipronil spraying events (JMPR 2001), suggesting that ingestion of contaminated seeds is a likely route of exposure (Szabo 2005). Contact of dermal and plumage surfaces to chemical residues on vegetation and soil are also potential routes of exposure for non-target birds. Residue on plumage and nesting material may result in indirect exposure of eggs to the pesticide, while direct exposure can occur in areas of spray operations (Hoffman et al. 2003).
Fipronil acts by targeting gamma-amino butyric acid (GABA) receptors (Hainzl et al. 1998). These receptors are prevalent throughout the central nervous system of both vertebrates and insects, however, fipronil has a much higher affinity for insect than for vertebrate GABA receptors hence its effectiveness as an insecticide. When fipronil was reviewed in the United States in 1994, the USEPA approved its registration for use with the provision that studies examining its chronic effects on avian reproductive be undertaken on two species: bobwhite quail (Colinus virginianus), a highly fipronil-sensitive species, and mallard duck (Anas platyrhynchos), a relatively low fipronil-sensitive species (Bryceland 1994a, b). Both studies found no adverse reproductive effects on either species. However, these were non peer-reviewed reports submitted for pesticide registration purposes, therefore were subject to confidentiality requirements and lack the details needed to scrutinize their methods and results. Following this, the joint meeting on pesticide residues (JMPR 1997, 2001) published two toxicology assessment reports reviewing unpublished data and research on fipronil. The only avian study reviewed was a fipronil dosing study on laying hens (Gallus domesticus); the highest levels of fipronil residues were reportedly detected in the yolk fraction of eggs laid by dosed hens (JMPR 1997, 2001), consistent with fipronil’s lipophilic nature. A study by Russ (2005) also found detectable levels of fipronil residue in the yolk of eggs laid by breeding female zebra (Taeniopygia guttata) finch administered with a single oral dose of fipronil (1.13 × 10−1 mg/kg). However, the study measured total fiproles (fipronil and its derivatives) and did not distinguish between levels of the parent fipronil compound and its metabolites.
Yolk lipids are the primary source of energy and nutrients for the developing embryo (Speake et al. 1998), a process requiring substantial amounts of energy to fuel metabolism and growth. Thus, any contaminants incorporated into the yolk have opportunity to be absorbed by the developing embryo. Because of the known adverse developmental affects of fipronil on vertebrates (Beggel et al. 2010; Stehr et al. 2006), maternal transfer of fipronil residues into yolks has potentially devastating effects on chicks. We have addressed this question through two experiments. We first examined the maternal transfer of both fipronil and its major metabolite (fipronil-sulfone) from exposed female zebra finches into eggs and hatchlings and its consequences on hatching success. Zebra finches are an ideal model as the species naturally co-occurs with locust-control operations in Australia and breed well in captivity over a large part of the year. In the second experiment, we injected known amounts of fipronil directly into the yolk of fertilized eggs to examine dose effects of this pesticide on chick development. Domestic chickens were chosen for these in-ovo studies as there is a great deal known about normal embryonic and post-natal development of the chick, making it easier to assess any teratogenic effects of fipronil.
Materials and methods
Chemicals and preparation of test substances
Fipronil (C12H4Cl2F6N4OS or (±)-5-amino-1-(2,6-dichloro-α,α,α-trifluoro-p-tolyl)-4-trifluoromethylsulphinylpyrazole-3-carbonitrile), CAS No. 120068-37-3, of 97% purity was obtained from Sigma-Aldrich, Pty. Ltd., Australia (Fipronil PESTANAL®). Fresh test solutions were prepared on each day of dosing. Due to the low water solubility of technical grade fipronil (Tingle et al. 2003), the fipronil solution was prepared using a minimum amount of acetone solvent (approx. 60 μl acetone per 20 mg fipronil). Acetone was chosen as the solvent due to the high solubility of fipronil in acetone (545.9 g l−1; BASF 2005). The solution was then diluted in canola oil to the required dose concentration. Canola oil is the vehicle used in common fipronil formulations for locust-control (e.g. Adonis®, BASF 2003). Control solutions consisted of acetone and canola oil only.
Maternal transfer study
Adult zebra finches (Taeniopygia gutatta) were obtained from a breeding colony at the University of Wollongong (Wollongong, NSW, Australia). Four outdoor aviaries at the University of Wollongong were set up for the three treatment groups and one control group, with each housing 5 breeding pairs (n = 20 females). Commercial seed mix (Finch Mix, Avigrain, Berkeley Vale, NSW, Australia), tap water and grit were provided ad libitum. Breeding pair formation was induced by the introduction of covered nest baskets and nesting material. Nests were checked daily to monitor dates of egg-laying, and all eggs laid prior to dosing were removed. Once all four breeding groups had started laying eggs, females were captured and weighed (mean ± SE, zebra finch weight of 12.6 ± 0.4 g) and all previously laid eggs removed. Females within a given cage were administered either fipronil or control solution as a single oral dose via gavage. Fipronil treatment doses were 1, 5, and 10 mg/kg body weight and were known to be sublethal to adult zebra finch (Kitulagodage unpublished data, Kitulagodage et al. 2008). Dosing volumes followed OECD acute oral toxicity testing guideline recommendations of limiting maximum liquid dose volumes to 2 ml/100 g body weight (OECD 2003). Females were immediately returned to their respective aviaries and closely monitored for the next 3 h, then checked regularly over the duration of the study. Once females recommenced laying, alternate eggs were collected for analysis of fipronil and metabolite residue levels; the remaining eggs were allowed to hatch. Behavioural observations were made of chicks in control and treatment nests up to 10 days after hatching and the chicks were then euthanised to collect brain, liver, and adipose tissue for residue analysis.
In-ovo exposure study
Fertile domestic chicken eggs (Gallus domesticus) were obtained on the day laid from The University of Sydney’s Poultry Research Unit (Camden, NSW, Australia). Eggs were placed in Octagon 40 self-rotating incubators (Brinsea Products Ltd, Somerset, UK), held at the University of Wollongong animal housing facilities in a room with a light: dark photoperiod of 12:12 h, including 15 min simulated dawn/dusk intervals using dimmed lighting. Incubators were set at 37.4°C and monitored daily to ensure humidity was maintained at levels that resulted in the optimal egg weight (water) loss of 14% over the 21 day incubation period (Rahn et al. 1974). In addition to being weighed, eggs were candled every third day to determine embryo viability; infertile eggs and early dead embryos were discarded once identified.
After nine days of incubation, 38 eggs containing viable embryos were split into five groups (three pesticide-treated groups, one vehicle-treated control group and one untreated control group). Fipronil treatment doses were 5.5, 17.5, and 37.5 mg/kg egg weight (based on pilot study doses of fipronil up to 1 mg/kg showing no effect); the vehicle consisted of acetone and canola oil only. We simulated maternal transfer of fipronil or vehicle-control solution by direct injection into the yolk on day 12 of incubation using a 50 μl Hamilton glass syringe with a 26-gauge needle. The dosing volume of 0.36 ml solution/kg egg weight was adjusted accordingly for each egg. A hole at the injection site was made manually through the shell using a 1 mm drill bit after candling to locate the position of the yolk sac and to avoid injecting into the developing embryo, while a polystyrene foam stopper was fitted onto the needle to enable injection at the same position in the yolk sac for each egg. Once injected, holes were sealed with melted paraffin wax and eggs then returned to the incubator. The vehicle-control group eggs were injected in the same manner with the acetone and canola oil control solution, whereas eggs in the control group were injection free. Eggs were then candled and weighed every second day and any eggs containing dead embryos were removed from the incubator once detected. Hatchlings were weighed as close to time of hatching as possible then placed in brooder boxes with heat-lamps. Commercial feed (chick starter feed, PETstock, Fairy Meadow, NSW, Australia) and tap water was provided ad libitum. Behavioural and physical observations including ability to right itself, pecking response to food, and general gait and stance of each hatchling were observed within 24 h post hatch. Chicks were euthanised at 48 h post hatch and their brain and liver tissues collected for analysis of fipronil and metabolite residue levels.
All experiments were conducted in accordance with the National Health and Medical Research Council Guidelines for research involving native animals, as approved by the University of Wollongong Animal Ethics Committee (ethics approval number: AE03/26).
Assay methods
Analysis for residue levels of fipronil and the sulfone metabolite (MB 46136) of fipronil was conducted at AgriSolutions Pty. Ltd. (Brisbane, Queensland, Australia). Zebra finch eggs, zebra finch hatchling tissue samples and domestic chicken hatchling tissue samples were homogenised, extracted in hexane and then filtered and liquid-liquid partitioned with acetonitrile. Residues were then isolated, and purified extracts brought up in acetonitrile, and passed through a 0.45 μm polytetrafluoroethylene filter prior to quantitative analysis via gas chromatography mass spectrophotometry (GC/MS). The level of parent fipronil and sulfone for each sample were expressed as mg fiproles per kg of tissue sample (AgriSolutions 2006).
Statistical analysis
Comparative fipronil and fipronil-sulfone residue levels in brain, and liver tissue collected from in-ovo treated chicks 48 h after hatching for each treatment group were analysed using a t-test. The effect of time between, and within, treatment groups on measured chick weights were examined using one-way analysis of variance (1-way ANOVA) with Tukey’s post-hoc tests. Analysis was performed using GraphPad Prism software (Version 5.02, GraphPad Software Inc., CA, USA).
Results
Maternal transfer of fipronil
Fipronil and sulfone residues were detected in eggs from females in all three fipronil treatment groups, however, only in eggs that were laid between 2 and 13 days post-treatment. The level of sulfone detected was consistently higher than that of fipronil in all eggs sampled and tended to persist longer (Table 1). At the lowest dose of 1 mg/kg, fipronil was only detected in eggs laid on day 2 post-treatment (0.01 mg/kg). Sulfone levels appeared to reach peak levels between 4 to 6 days post-dosing in all pesticide-treatment groups. No residue of either fipronil or sulfone was detected in eggs laid in the control group.
Of the seven eggs of the pesticide-treatment group remaining in the nests, only one chick (14%) hatched, this was from the 1 mg/kg treatment group, compared with 100% hatch rate of control eggs (12 eggs). The one chick to hatch among the pesticide-treatment group appeared severely underdeveloped at 10 days of age. Its eyes were just opening and its legs were splayed with no demonstrated righting reflex. The hatchling was unable to remain erect and vocalisations were abnormal and muted. No feathers had emerged on its body and all primary feathers were still in sheaths approximately 4 mm long. Ventral feather tracks were visible, but not dorsal or head feather tracks. It weighed only 7.0 g when euthanased, but 0.83 g of this mass was seed that was packed in its crop. In contrast, hatchlings from the control group weighed 8.4 ± 0.4 g at 9 days old, had little or no seed stored in their crop, displayed strong begging behaviour, were very vocal, and able to right themselves. Furthermore, their body feathers had begun emerged both ventrally and dorsally, and primary feathers were unsheathed with one hatchlings measured to be approximately 9 mm long.
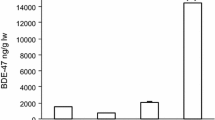
Tissue samples from the 10 day old pesticide-exposed chick contained fipronil in brain, liver and fat tissues, while sulfone was detectable only in brain (Fig. 1). No fiproles were detected in tissues of any control hatchlings (n = 3).
In-ovo exposure study
Of the 40 eggs incubated, two proved to be infertile prior to treatment (fertility rate of 95%). There was no difference in in-ovo mortality post-treatment (Table 2); only one chick died during the hatching process after pipping and this was from the 5.5 mg/kg group.
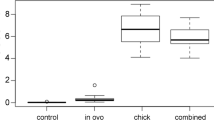
Of the 28 chicks that successfully hatched, behavioural and developmental abnormalities were only observed in the highest dose group (37.5 mg/kg). Observed signs included unsteadiness and resting on haunches. These chicks appeared to be uninterested in food provided in comparison to control chicks that ate considerable amounts of feed. This reduced feeding behaviour was reflected by loss in body mass over the 48 h period post hatch (Fig. 2); measured chick weights differed significantly across the four treatment groups at 48 h after hatching, F 4, 15 = 4.258; P = 0.0168. Tukeys post-hoc comparisons of the treatment groups indicate that body mass observed in the 37.5 mg/kg egg weight group chicks at 48 h post hatch was significantly lower than those observed in control group chicks (P < 0.05). One chick from this highest treatment group was found dead 48 h post hatch and weighed 29.3 g compared to its weight of 36.2 g immediately after hatching, necropsy revealed no abnormal findings, however. Loss in body mass was also observed within the 5.5 mg/kg treatment group with measured chick weights differing significantly across the three time points, F 2, 11 = 8.409; P = 0.0061. Tukeys post-hoc comparison of the time points indicate chick weights measured both 24 and 48 h after hatching were significantly lower than those measured immediately after hatching within the 5.5 mg/kg group (P < 0.05 and P < 0.01, respectively). There was no significant difference in chick weights within the 17.5 or 37.5 mg/kg groups, or between any of the treatment groups either immediately after hatching or at 24 h post hatch.
Body mass of domestic chicken hatchling (represented as means ± SE) after in-ovo exposure to fipronil compared with control groups. Treatment group chicks were exposed to a single dose of fipronil via injection in-ovo at 5.5, 17.5 or 37.5 mg/kg egg weight. Measurements were taken immediately after hatching, at 24 and 48 h after hatching. Asterisks indicate statistically significant differences; * indicate P < 0.05, ** indicate P < 0.01
Fipronil and its sulfone metabolite were the only detectable residues in brain and liver tissue collected 48 h after hatching from the 5.5, 17.5, and 37.5 mg/kg treatment group chicks (Table 3). No fiproles were detected in control hatchlings. Fipronil-sulfone concentrations were significantly greater than those of fipronil in both brain (P = 0.0003) and liver tissue (P < 0.0001) for all fipronil in-ovo treated hatchlings. Comparing the two tissue types, concentrations detected in liver tissue were significantly higher than those in brain tissue for both fipronil (P = 0.0061) and fipronil-sulfone (P < 0.001).
Discussion
This is the first study to show unequivocally that both fipronil and its sulfone metabolite (fipronil-sulfone) are maternally transferred into eggs laid by fipronil-exposed females. This study also demonstrates that once fipronil is deposited in the avian eggs, it is absorbed by the developing embryo, and along with the fipronil-sulfone, accumulates in the tissue of the hatchlings. The higher levels of fipronil-sulfone detected in zebra finch eggs compared with fipronil residue levels is consistent with other studies examining post-exposure residue content in fipronil-exposed animals (JMPR 2001). Metabolic fate studies show that fipronil-sulfone formation occurs rapidly, being detectable within 1 h after fipronil exposure in mice (Hainzl et al. 1998) and within the first 8 h in fipronil-treated avian species (Kitulagodage et al. unpublished data).
The lack of detectable fipronil or fipronil-sulfone residue in zebra finch eggs laid on the day immediately after dosing is likely due to those eggs being formed prior to dosing. The peak levels of sulfones found in eggs laid 4–6 days after dosing is consistent with the temporal pattern of yolk formation in ovulating birds, resulting in a higher proportion of yolk being exposed to maternal fipronil and sulfone in later laid eggs (Astheimer and Grau 1985).
The most striking result was the significant reduction in hatching success among female zebra finches exposed to fipronil (14% compared to 100% in controls). The doses that were administered were known to be sublethal from previous studies (Kitulagodage unpublished data) and none of the females dosed with fipronil showed any adverse effects or signs of toxicity.
The lowest fipronil dose used in this current study was 1 mg/kg, which for an adult zebra finch weighing 12 g is equates to ingestion of 0.12 mg of fipronil. One sample of native grass seeds collected after locust-control spray operations was found to contain 0.839 mg/kg fipronil residue 5 days after application (Szabo 2005). Relating this field data to our study dose, in order to obtain a 1 mg/kg dose of fipronil, a 12 g zebra finch would have to eat 14 g of contaminated seed. Zebra finch have been reported to consume their own body weight in seed over a 48 h period, and can collect this amount daily during the breeding season when they are feeding their chicks (Zann 1996). The scenario of a zebra finch obtaining a 1 mg/kg dose of fipronil in locust-control areas is therefore feasible depending on whether the fipronil residue had accumulated in the seed or in the husk, as finches remove the husks from the seed prior to eating.
The standard rate of fipronil application for controlling locusts in Australia is currently 0.9 g of active ingredient per 300 ml per hectare (APLC, pers. comm.). Given the known exposure of zebra finches to locust-control pesticides in Australia (Fildes et al. 2006), further study of the dose sensitivity of reproductive output in zebra finches and sympatric species to fipronil is warranted.
In the domestic chicken in-ovo study, the effect of drilling through the shell and injecting a solution had no effect on embryo survival rates. Although no clinical signs of toxicity were observed in any of the hatchlings, only the groups treated with fipronil showed significant body mass declines after hatching, with one of the chicks that died 48 h post hatching losing approximately 19% of its body weight. Loss in body mass resulting from fipronil exposure has been demonstrated in studies on adult king quail (Coturnix chinensis) and bobwhite quail (Kitulagodage et al. unpublished data) and reduced feeding rates were observed in these species. No clear signs of reduced feeding behaviour were reportable in the chicken hatchlings as food consumption was not quantified.
The relative levels of fipronil and fipronil-sulfone residue detected in the brain and liver tissue of chicken hatchlings is consistent with the pattern seen in zebra finch eggs, with sulfone levels being significantly higher than fipronil levels across all treatment groups. These results do differ to the zebra finch hatchling tissue samples, however, as fipronil levels were higher than sulfone levels in the brain, and sulfone residue was not detected at all in the liver and adipose tissue. It is important to note though that this was based on measurements for one individual.
Comparing tissue types, both fipronil and fipronil-sulfone appear to accumulate at higher levels in liver as opposed to brain of the chicken hatchlings in all fipronil treatment groups and in the surviving chick from the fipronil-dosed female zebra finches. The highest residue levels in the zebra finch chick, however, were detected in the adipose tissue. Although pesticide residues were not determined in adipose from the chicken hatchlings, fipronil-dosing studies in rats, goats (JMPR 2001), adult zebra finches and bobwhite quail (Kitulagodage et al. unpublished data) have also documented greater amounts of fiproles in body lipids compared to either brain, or liver. Because adipose tissues are typically mobilized during periods of reduced food availability and, in females, when synthesizing egg yolk, there is great potential for fipronil to exert its toxic effects long after the time of initial exposure.
One conspicuous difference between the species we studied was the much greater sensitivity of zebra finch chicks to fipronil content in yolk compared to chickens. This may be due, in part, to differences in the stage of embryo development when first encountering fipronil. The zebra finch embryos of fipronil-exposed mothers were metabolizing yolk containing this pesticide and its metabolites from very early embryogenesis. By contrast, the chicken embryos were nearly 60% developed when their yolk was injected on day 12 with fipronil. Another consideration is that injected pesticide would be located predominantly in a discrete location of the yolk, whereas maternal transfer would result in an even mix of the pesticide throughout each yolk layer deposited in accord with circulating levels of fiproles at the time of yolk synthesis. Resolution of ontogenetic and phylogenetic effects on fipronil sensitivity of developing avian embryos is thus of paramount importance when considering risk effects of this agricultural pesticide that are pertinent to breeding bird populations.
References
AgriSolutions (2006) Analysis of fipronil and metabolites in tissue by GC/MS. Certificate of analysis. Method no AAM-FIP-09. AgriSolutions, Australia
Astheimer LB, Grau CR (1985) The timing and energetic consequences of egg formation in the Adélie penguin. Condor 87:256–268
BASF (2003) Material safety data sheet: fipronil. BASF Australia Ltd, Noble Park, VIC, Australia
BASF (2005) Fipronil: worldwide technical bulletin. BASF Agricultural Products, NC
Beggel S, Werner I, Connon RE, Geist JP (2010) Sublethal toxicity of commercial insecticide formulations and their active ingredients to larval fathead minnow (Pimephales promelas). Sci Total Environ 408:3169–3175
Bryceland AC (1994a) M&B 46030 technical: toxicity and reproduction study in bobwhite quail. Rhone-Poulenc Ag Company, Research Triangle Park Data evaluation record EPA MRID no. 429186-22
Bryceland AC (1994b) M&B 46030 technical: toxicity and reproduction study in mallard ducks. Rhone-Poulenc Ag Company, Research Triangle Park Data evaluation record EPA MRID no. 429186-23
OECD (2003) OECD guideline for the testing of chemicals: Acute oral toxicity up-and-down procedure. Guideline 425. Organisation for Economic Co-operation and Development, Environment Directorate. Paris, France
DeWitt JB (1955) Effects of chlorinated hydrocarbon insecticides upon quail and pheasants. Agric Food Chem 3(8):672–676
EPA (2001) Assessment of the impact of insecticide spraying of Australian plague locusts. Environment Protection Agency, Department for Environment and Heritage, Government of South Australia. Adelaide, Australia
Fernie K, Shutt J, Letcher R, Ritchie I, Bird D (2009) Environmentally relevant concentrations of DE-71 and HBCD alter eggshell thickness and reproductive success of American kestrels. Environ Sci Technol 43(6):2124–2130
Fildes KJ, Astheimer LB, Story P, Buttemer WA, Hooper MJ (2006) Cholinesterase response in native birds exposed to fenitrothion during locust-control operations in eastern Australia. Environ Toxicol Chem 25:2964–2970
Hainzl D, Cole LM, Casida JE (1998) Mechanisms for selective toxicity of fipronil insecticide and its sulfone metabolite and desulfinyl photoproduct. Chem Res Toxicol 11:1529–1535
Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr (2003) Handbook of ecotoxicology. Lewis Publishers, Boca Raton
JMPR (1997) Pesticide residues in food 1997 evaluations part II toxicological & environmental: 932 fipronil. Joint meeting on pesticide residues in food and the environment: FAO and WHO. http://www.inchem.org/documents/jmpr/jmpmono/v097pr09.htm. Accessed 20 August 2010
JMPR (2001) Pesticide residues in food: fipronil-2001 evaluations. Part I, FAO plant production and protection paper 171. Joint meeting on pesticide residues: FAO and WHO
Kitulagodage M, Astheimer LB, Buttemer WA (2008) Diacetone alcohol, a dispersant solvent, contributes to acute toxicity of a fipronil-based insecticide in a passerine bird. Ecotoxicol Environ Saf 71:597–600
Lundholm CE (1997) DDE-induced eggshell thinning in birds: Effects of p, p′-DDE on the calcium and prostaglandin metabolism of the eggshell gland. Comp Biochem Physiol C 118(2):113–128
Rahn H, Paganelli CV, Ar A (1974) The avian egg: air-cell gas tension, metabolism, and incubation time. Respir Physiol 22:297–309
Russ M (2005) An investigation of the effects locust pesticides, fenitrothion and fipronil, on avian development using an in-ovo model. Masters thesis, University of Wollongong, NSW, Australia
Speake BK, Murray AMB, Noble RC (1998) Transport and transformation of yolk lipids during development of the avian embryo. Prog Lipid Res 37(1):1–32
Stehr CM, Linbo TL, Incardona JP, Scholz NL (2006) The developmental neurotoxicity of fipronil: notochord degeneration and locomotor defects in zebrafish embryos and larvae. Toxicol Sci 92(1):270–278
Szabo JK (2005) Avian-locust interactions in eastern Australia and the exposure of birds to locust control pesticides. PhD thesis. Texas Tech University, Lubbock
Szabo JK, Davy PJ, Astheimer LB, Hooper MJ (2009) Predicting avian species distributions to evaluate the spatio-temporal overlap with locust control operations in eastern Australia. Ecol Appl 19:2026–2037
Tingle CCD, Rother JA, Dewhurst CF, Lauer S, King WJ (2003) Fipronil: environmental fate, ecotoxicology, and human health concerns. Rev Environ Contam Toxicol 176:1–66
Wiemeyer SN, Porter RD (1970) DDE thins eggshells of captive American kestrels. Nature 227(5259):737–738
Zann RA (1996) The zebra finch: a synthesis of field and laboratory studies. Oxford University Press, Oxford
Acknowledgments
We thank the Australian Research Council, the University of Wollongong Institute for Conservation Biology, and the Australian Plague Locust Commission for supporting this research. We also thank Professor Tom Scott from the University of Sydney’s Poultry Research Unit for provision of fertilized chicken eggs. Thanks are also due to members of the Physiological Ecology lab (University of Wollongong, Australia) for their helpful discussions and providing assistance in caring for birds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kitulagodage, M., Buttemer, W.A. & Astheimer, L.B. Adverse effects of fipronil on avian reproduction and development: maternal transfer of fipronil to eggs in zebra finch Taeniopygia guttata and in ovo exposure in chickens Gallus domesticus . Ecotoxicology 20, 653–660 (2011). https://doi.org/10.1007/s10646-011-0605-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0605-5