Abstract
Despite ubiquity of polycyclic aromatic hydrocarbons (PAHs) in the tropical environments, little information is available concerning responses of tropical fish to PAHs and associated toxicity. In the present study, effects of five PAHs containing two to four aromatic rings on hepatic CYP1A dependent ethoxyresorufin O-deethylase (EROD), glutathione S-transferase (GST) and serum sorbitol dehydrogenase (SDH) activities in Nile tilapia, a potential fish species for biomonitoring pollution in tropical waters, were evaluated. Results showed that EROD activities were induced by the PAHs containing four aromatic rings (pyrene and chrysene) in a dose dependent manner. However PAHs with two to three aromatic rings (naphthalene, phenanthrene and fluoranthene) caused no effect or inhibition of EROD activities depending on the dose and the duration. Fluoranthene was the most potent inhibitor. SDH results demonstrated that high doses of fluoranthene induced hepatic damage. GST activity was induced by the lowest dose of phenanthrene, fluoranthene and chrysene but high doses had no effect. The results indicate that induction of EROD enzyme in Nile tilapia is a useful biomarker of exposure to PAHs such as pyrene and chrysene. However EROD inhibiting PAHs such as fluoranthene in the natural environment may modulate the EROD inducing potential of other PAHs thereby influencing PAH exposure assessments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a group of organic pollutants with two or more fused aromatic rings. They are widespread environmental contaminants as a result of both petrogenic and pyrogenic sources. The occurrence is largely due to fossil fuel burning, motor vehicles, burning waste and oil refining (McElroy et al. 1989). PAHs have received increased attention in recent years as some of these compounds are highly carcinogenic or mutagenic. US Environmental Protection Agency has identified sixteen PAHs under the group “priority pollutants”. Hence exposure assessments of PAHs in the ecosystems are important especially in the developing world as the presence of these pollutants poses a serious threat to environmental health (Srogi 2007). Use of biomarkers in fish is considered as a cost effective strategy to obtain information on the state of the aquatic environment and the effect of pollutants on living resources. Biomarkers measured at the molecular or cellular level in fish have been proposed as sensitive “early warning” tools for biological effect measurements in environmental quality assessments (van der Oost et al. 2003).
Among the enzyme biomarkers, the measurement of a phase I biotransformation enzyme, Cytochrome P4501A (CYP1A) dependent ethoxyresorufin O-deethylase (EROD) in fish has become a promising tool for detecting aquatic contaminations of a variety of highly toxic organic pollutants such as PAHs and coplanar polychlorinated biphenyls (PCBs) (Whyte et al. 2000; van der Oost et al. 2003). Induction of CYP1A activity has been used to infer effects such as cancer related liver lesions and reproductive impairments in fish (Whyte et al. 2000). Numerous in vitro studies with mammalian and piscine cell cultures and a few in vivo studies with some fish species indicate that inducibility of EROD varies with the type of PAH (Till et al. 1999; Whyte et al. 2000; Basu et al. 2001; Behrens et al. 2001; Willett et al. 2001; Bosveld et al. 2002; Billard et al. 2004; Lu et al. 2009). Most of these studies have focused on high molecular weight PAHs with five- or more fused rings. It is less clear how PAHs containing two to four fused rings affect fish. Glutathione S-transferases (GST) which catalyze the conjugation of glutathione with xenobiotics such as PAHs in Phase II biotransformation process seem to play important roles in both detoxification and bioactivation reactions (George 1994). GSTs in some species of fish have been used as a biomarker for aquatic biomonitoring (van der Oost et al. 2003) but little information is available on modulation of GST activities by PAHs especially by low molecular weight PAHs.
Despite ubiquity of PAHs in the tropical environments, little information is available concerning responses of tropical fish to PAHs and associated toxicity. Nile tilapia (Oreochromis niloticus) which is a widespread food fish species in tropical countries has been suggested as a test species for biomonitoring of aquatic pollution in tropical environments (Gold-Bouchot et al. 2006; Pathiratne et al. 2009). Objective of this study was to compare the effects of commonly occurring five selected PAHs containing two to four fused aromatic rings viz. naphthalene, phenanthrene, fluoranthene, pyrene and chrysene on in vivo hepatic EROD and GST activities in Nile tilapia and to assess their suitability for assessments of exposure to these PAHs in the natural environments. In addition serum SDH levels were measured as an indicator of hepatic health condition.
Materials and methods
Fish and PAH treatments
Nile tilapias were obtained from a fish breeding station, National Aquaculture Development Authority, Sri Lanka. Fish were allowed to acclimate to laboratory conditions in fiberglass tanks filled with continuously aerated aged tap water under natural photoperiod for several weeks prior to the experiments. Half of the water in each tank was exchanged with aged tap water every 5–6 days. The fish were daily fed with commercial fish food pellets (Prima, Sri Lanka) at 1% of the body weight. During the acclimation period, temperature, pH and dissolved oxygen concentration in water in the tanks ranged from 28–30°C, 6.8–7.4 and 3.9-5.2 mg/l, respectively.
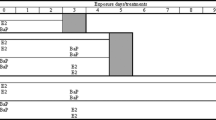
Groups of acclimated Nile tilapias [85–105 g body weight (bw)] were treated with single intraperitoneal injection of naphthalene (20 mg/kg bw) or phenanthrene (1, 5, 20 mg/kg bw) or fluoranthene (1, 5, 20 mg/kg bw) or pyrene (1, 5, 20 mg/kg bw) or chrysene (1, 5, 20 mg/kg bw) dissolved in corn oil (1 ml/kg bw) or corn oil only (control groups). These doses were selected based on the studies carried out earlier with other fish species (Whyte et al. 2000). After the treatments, the fish were maintained in aerated aged tap water in glass tanks (5–6 fish per 70 l of continuously aerated aged tap water) under natural photoperiod until they are used for biomarker studies. Fish were not fed after the treatment. PAH treated groups were tested with separate control groups at different time periods. PAH doses administered intraperitoneally to the Nile tilapia in the present study were designed to ensure a biological response was provoked and were not intended to reflect the conditions likely to be found in the natural environment. In the natural environment, PAH can enter fish via water, sediment or food (Lee and Anderson 2005). However it is likely that similar biological response would result from exposure of fish to these PAHs in the environment.
Tissue preparation
One day and three days after the PAH treatments, control fish and the fish treated with different doses of selected PAHs (n = 5–6 fish per group) were anesthetized using benzocaine and body weights and total lengths were recorded for the determination of condition factor. Blood was collected from the fish by severing the caudal vein. Liver weights were recorded to determine the liver somatic index. Liver tissue from each fish was stored frozen at −80°C for preparation of microsomes and cytosol.
Blood serum was prepared by centrifuging at 5,000×g at 4°C for 10 min. Microsomal and cytosolic fractions of liver tissues were prepared by differential centrifugation. Liver tissues were excised and a 20% homogenate (w/v) was prepared in ice-cold 100 mM KH2PO4/K2HPO4 buffer pH 7.6 containing 0.25 M sucrose, 15% (v/v) glycerol using the Ultra-Turrax T25 tissue homogenizer (IKA Labortechnik, Germany) at medium speed for 30 s. The homogenate was centrifuged at 12,000×g for 30 min at 4°C and the resultant supernatant was further centrifuged at 104,000×g at 4°C for 90 min using the ultra speed centrifuge (Sorvall Ultra 80) to sediment the microsomes. Aliquots of the 104,000×g supernatant (cytosol) were removed from the floating fatty layer for immediate GST assay. The microsomal pellets were resuspended in 100 mM KH2PO4/K2HPO4 buffer pH 7.6 containing 1.15% KCl and 20% (v/v) glycerol and were stored at −80°C for EROD assay. All preparation steps of the enzyme sources were carried out on ice.
Enzyme assays
Serum SDH activity was determined spectrophotometrically at 30°C as described by Gerlach (1983) using D-fructose using a computer controlled recording spectrophotometer (GBC Cintra 10e, Australia) using a thermostated cuvette holder as kinetic assays. GST in freshly prepared liver cytosol was measured at 30°C spectrophotometrically following the conjugation of glutathione at 340 nm using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate (Habig et al. 1974). EROD activity in the liver microsomes was determined fluorometrically at 30°C using ethoxyresorufin as described by Burke and Mayer (1974) using a computer controlled fluorescence spectrophotometer with scanning facilities (Varian Cary Eclipse fluorescence spectrophotometer, Australia). All enzyme assays were run in triplicates. Proteins present in the liver microsomes and cytosol were determined according to the method of Lowry et al. (1951) with bovine serum albumin as the standard.
Statistical analysis
The biomarker responses of the fish exposed to naphthalene were compared using Student’s t test (P ≤ 0.05). The data relevant to other PAH exposures were analysed separately by one way analysis of variance (ANOVA, P < 0.05). Where differences were significant, multiple comparisons were carried out by Tukey’s test as appropriate. As some of the measured values for serum SDH activities were zero, log transformed data [log10 (X + 1)] were used in the statistical analysis of data and antilog of means and 95% confidence interval are presented as SDH activities (Zar 1999).
Results
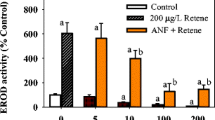
All fish survived after the PAH treatments. No significant differences between the fish treated with PAHs and the comparable control fish were obtained in relation to the condition factor or liver somatic index (results not shown). Hepatic EROD activities in Nile tilapia after PAH treatments are presented in Fig. 1. The results revealed that naphthalene, phenanthrene and fluoranthene treatments caused no significant effect or significant inhibition of hepatic EROD activity (63–84% loss) depending on the dose and the post treatment duration. EROD activities of the fish treated with naphthalene (20 mg/kg bw) were not significantly different from those of the comparable controls at 1 day post treatment whereas a significant inhibition (66% loss from control activity) was evident after 3 days. Fish treated with the highest dose of phenanthrene (20 mg/kg bw) also caused a significant inhibition of EROD activity (60–63% loss compared to control activity) at 1 day and 3 days after the treatment. Fluoranthene was more potent in inhibiting EROD activity, as the lowest dose (1 mg/kg bw) also caused a significant inhibition (77% loss from control activity at 3 days post treatment) in addition to the inhibition caused by higher doses (74–84% loss from control activity at 1 day and 3 days post treatment). In contrast, significant induction of EROD activities in the fish treated with pyrene and chrysene (160–240 and 180–402% of control activity, respectively) was observed in a dose dependent manner. The fish treated with the lowest dose of chrysene (1 mg/kg bw) demonstrated the highest induction of EROD activity at 1 day post treatment.
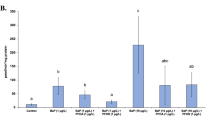
Hepatic GST activities of the fish following PAH administration are presented in Fig. 2. The fish treated with the lowest dose (1 mg/kg bw) of phenanthrene, fluoranthene and chrysene caused a significant increase in the GST activity (130, 140–144, and 153% of control activity, respectively). However GST activities were not affected significantly by the high doses (5 and 20 mg/kg bw) of the PAH treatments. Pyrene treatment had no significant effect on GST activities of the fish.
Serum SDH activities in the fish following PAH treatments are presented in Table 1. The SDH activity was elevated in the fish treated with the high doses of fluoranthene (5 and 20 mg/kg bw after 3 days) in comparison to the controls. The SDH activities in the sera of other PAH treated fish were not significantly different from the respective controls.
Discussion
EROD activity which measures the induction ability of the CYP1A enzymes responsible for catalyzing biotransformation of certain xenobiotics is used as a biomarker for monitoring exposure of fish to anthropogenic organic contaminants with a planar configuration such as PAHs and polychlorinated biphenyls (Whyte et al. 2000; van der Oost et al. 2003). CYP1A induction is initiated by the binding of the inducer molecule with the aryl hydrocarbon receptor (AhR). Hence these organic compounds are called as AhR agonists (Whyte et al. 2000; Timme-Laragy et al. 2007). In vitro studies with mammalian and piscine cell cultures and a few in vivo studies with some fish species have compared the EROD induction potency of different PAHs (Till et al. 1999; Fent and Batscher 2000; Whyte et al. 2000; Basu et al. 2001; Behrens et al. 2001; Willett et al. 2001; Bosveld et al. 2002; Billard et al. 2004; Lu et al. 2009). In general PAHs with five- or more rings are good CYP1A inducers but inconsistent information is available on EROD induction potential of PAHs containing two to four fused rings. Of the five PAHs tested in the present study, pyrene or chrysene treatments resulted in significant induction of hepatic EROD activity in Nile tilapia where as naphthalene, phenanthrene and fluoranthene failed to cause an induction of this enzyme. Bosveld et al. (2002) tested EROD inducing potency of 10 PAHs in the H411E rat hepatoma in vitro bioassay which included four PAHs tested in the present study. They found no or a very weak response by naphthalene, consistently no response by phenanthrene, weak response at the highest doses by fluoranthene where as strong induction response by chrysene. In a study with fish hepatoma cell line PLHC-1, no induction was observed with naphthalene, low induction was found with fluoranthene and phenanthrene and a concentration related induction of EROD by several PAHs including chrysene and pyrene (Fent and Batscher 2000). Behrens et al. (2001) compared the capacity of nine PAHs to induce CYP1A in primary rainbow trout hepatocytes and liver cell line RTLW1 and found anthracene, phenanthrene, fluoranthene and pyrene are non-inducing PAHs. However, a recent in vivo study demonstrated significant induction of EROD activity in golden grey mullet (Liza aurata) following phenanthrene exposure (Oliveira et al. 2007). Hence CYP1A induction potential and efficacy of PAHs seem to vary with several factors including the fish species and route of exposure. In the present study, the tested PAHs with 4 fused aromatic rings (pyrene and chrysene) caused dose dependent induction of hepatic EROD activity in Nile tilapia. EROD activity in this fish was induced by 1.6- to 2.4-folds following pyrene treatment. However, a previous study on in vivo EROD induction by pyrene in this fish species has recorded a 18-fold increase in hepatic EROD activity at 3 day after single injection (20 mg/kg body weight, ip) of pyrene (Zapata-Perez et al. 2002). In the present study we found that chrysene (a potential carcinogen) was more effective (up to nearly fourfold induction) than pyrene as an inducer of EROD activity in Nile tilapia. However induction potencies of four ring PAHs are much lower when compared with the response of this fish to a high molecular weight carcinogenic PAH, 3 methylcholanthrene (3MC), a prototypical PAH inducer of CYP1A (Pathiratne and George 1998). A 40-fold induction in hepatic EROD activity in Nile tilapia was observed following 3 days post treatment of single ip injection (20 mg/kg body weight) of 3MC (Pathiratne and George 1998). The present in vivo study with Nile tilapia also found no response or significant inhibition of EROD by naphthalene (2 fused aromatic rings), phenanthrene (3 fused aromatic rings) and fluoranthene (4 rings but only 3 fused aromatic rings) depending on the dose and duration of post treatment. A notable inactivation of EROD activity was obtained with high doses of fluoranthene (up to 84% loss). These results suggest that PAHs with 2 or 3 aromatic ring structures in general do not meet the structural requirements to bind the AhR which is considered as the initial step in the CYP1A induction process in the cells. Hence low molecular weight PAHs are not potent inducers of CYP1A. We also found that some of them could act as potential inhibitors of CYP1A. However, no clear cut explanation was found for the EROD inhibitory potential of these three PAHs. Nevertheless, serum SDH activity in the fish treated with high doses of fluoranthene (5 and 20 mg/kg bw, after 3 days) indicates liver damage. Hence a notable inhibition of EROD activity following exposure to high doses of fluoranthene may be associated with cytotoxicity.
As PAHs are ubiquitous environmental pollutants and some are AhR agonists, CYP1A dependent EROD has been used as a biomarker of exposure. The results of the present study support the use of hepatic EROD enzyme in Nile tilapia as a biomarker of exposure to EROD inducing PAHs such as pyrene and chrysene. But PAHs exist in the environment in complex mixtures which may contain AhR agonists as well as AhR antagonists that may compound biomarker results. A recent review on PAH ecotoxicology in marine ecosystems, emphasizes that there is a need for increased research efforts to clarify biological effects of two and three ring PAHs and PAH mixtures (Hylland 2006). Some studies show that there are both synergistic and antagonistic interactions between low and high molecular weight PAHs. Basu et al. (2001) found that there was a synergistic interaction between benzo(a)pyrene, a carcinogenic PAH and fluoranthene in juvenile rainbow trout (Oncorhynchus mykiss). Willet et al. (2001) found that in Fundulus heteroclitus, fluoranthene alone treatment had no significant effect on in vivo EROD activity but EROD activities in fish which received benzo(a)pyrene and fluoranthene co treatment were lower compared to fish treated with benzo(a)pyrene alone. They suggested that fluoranthene may inhibit EROD activity by down regulating the CYP1A protein. In our study we found not only fluoranthene but also phenanthrene and naphthalene are not capable of inducing CYP1A dependent EROD activities in a tropical fish, Nile tilapia. They may act as potential inhibitors of CYP1A. As PAHs with 2–3 rings could be present as co-contaminants in the natural environment with the CYP1A inducers, exposure of fish to CYP1A inhibiting PAHs may modulate the EROD inducing potential of other PAHs and their subsequent metabolism thereby influencing PAH exposure assessments. In a review of literature on the significance of cytochrome P450 system responses in marine wildlife following oil spills, Lee and Anderson (2005) have emphasized that most of the PAHs found in crude oil (dominantly 2–3 ring PAHs) are not strong inducers of cytochrome P450.
Glutathione S-transferases which are a multigenic family of mainly soluble enzymes, catalyze the conjugation of electrophilic compounds with glutathione in phase II biotransformation of xenobiotics such as PAHs. A critical role of GSTs is defence against oxidative damage and peroxide products of DNA and lipids (George 1994). They play a major role in protecting the cells from electrophilic compounds such as epoxides which are reactive intermediates produced during bioactivation of toxic organic contaminants such as PAHs mainly through CYP1A dependent phase I biotransformations. Hence toxicities and other adverse effects of PAHs may be reduced by induction of GST enzymes by PAHs. The usefulness of measuring GST in fish liver as a biomarker of exposure to xenobiotics has been discussed previously (van der Oost et al. 2003). Elevation of hepatic GST activity has been reported in several studies after exposure of fish to high molecular weight PAHs but most studies did not show significant alterations (van der Oost et al. 2003). Little or no information is available on modulation of GST activities in fish by PAHs containing two to four fused rings. In a recent study, a hepatic GST activity has been induced in eel, Anguilla anguilla, after 2 and 46 h waterborne naphthalene exposure (Teles et al. 2003). A more recent study carried out by Lu et al. (2009) demonstrated that GST induction potential of five PAHs in gold fish, Carassius auratus, increased in the order fluoranthene < fluorine < benzo(h)fluoranthene < benzo(g,h,i) perylene < indeno(1,2,3-cd)-pyrene. In the present study, only the lowest dose (1 mg/kg bw) of phenanthrene, fluoranthene and chrysene resulted in induction (up to 1.5-fold) of hepatic total GST activities (measured with the CDNB as the substrate) in Nile tilapia where as pyrene had no significant effect on this enzyme. Hence extent of modulation of hepatic GST activity in this fish seems to be low compared with the effect of these compounds on CYP1A dependent EROD. In an earlier study too, no significant effect on hepatic GST activity in Nile tilapia was observed following 3 days post treatment of single ip injection (20 mg/kg bw) of 3MC, a high molecular weight PAH where as 40-fold induction of EROD activity was detected following the treatment (Pathiratne and George 1998).
Conclusion
The potency of five PAHs viz. naphthalene, phenanthrene, fluoranthene, pyrene and chrysene to induce hepatic CYP1A dependent EROD activities in tropical fish, Nile tilapia was dependent on the number of fused aromatic rings of the PAH molecule. Pyrene and chrysene which contain four fused rings are potent inducers of EROD activity in this fish. However naphthalene, phenanthrene and fluoranthene which contain two to three fused aromatic rings, are non-inducers of EROD and could act as inhibitors of EROD activity depending on the dose and the duration. EROD inhibition by high doses of fluoranthene was associated with liver damage as demonstrated by elevated serum SDH enzyme levels. The results indicate that induction of EROD enzyme in Nile tilapia is a useful biomarker of exposure to PAHs such as pyrene and chrysene. However, EROD inhibiting PAHs could be present as co-contaminants in the natural environment with the CYP1A inducers as mixtures. Hence exposure of fish to EROD inhibiting PAHs may modulate the EROD inducing potential of other PAHs and their subsequent metabolism thereby influencing PAH exposure assessments. Therefore further research is needed to investigate inducibility of in vivo EROD activities in Nile tilapia by mixture of PAHs and their interactions. GST activities were not affected by PAH treatments except in few cases where up to 1.5-fold increase was evident in the fish treated with the lowest dose. Hence hepatic total GST activity in Nile tilapia does not seem to be feasible as a biomarker of exposure to PAHs. However hepatic GST in this fish could be included in biomonitoring aquatic pollution as a non specific biomarker as they play important roles in detoxification of toxic organic pollutants.
References
Basu N, Billard S, Fragoso N, Omoike A, Tabash S, Brown S, Hodson P (2001) Ethoxyresorufin-O-deethylase induction in trout exposed to mixtures of polycyclic aromatic hydrocarbons. Environ Toxicol Chem 20:1244–1251
Behrens A, Schirmer K, Bols KNC, Segner H (2001) Polycyclic aromatic hydrocarbons as inducers of cytochrome activity in the rainbow trout liver cell line, RTL-W1 and in primary cultures of rainbow trout hepatocytes. Environ Toxicol Chem 20:632–643
Billard SM, Bols NC, Hodson PV (2004) In vitro and in vivo comparisons of fish specific CYP1A induction relative potency factors for selected polycyclic aromatic hydrocarbons. Ecotoxicol Environ Saf 59:292–299
Bosveld ATC, de Bie PAF, van den Brink NW, Jongepier H, Klomp AV (2002) In vitro EROD induction equivalency factors for the 10 PAHs generally monitored in risk assessment studies in The Netherlands. Chemosphere 49:75–83
Burke MD, Mayer RT (1974) Ethoxyresorufin: direct fluorometric assay of a microsomal O-deethylation which is preferentially inducible by 3-methylcholanthrene. Drug Metab Dispos 2:583–588
Fent K, Batscher R (2000) Cytochrome P4501A induction potencies of polycyclic aromatic hydrocarbons in a fish hepatoma cell line: demonstration of additive interactions. Environ Toxicol Chem 19(8):2047–2058
George SG (1994) Enzymology and molecular biology of phase II xenobiotic conjugating enzymes in fish. In: Malins DC, Ostrander GK (eds) Aquatic toxicology: molecular, biochemical and cellular perspectives. Lewis Publishers, CRC press, Boca Raton, pp 37–85
Gerlach U (1983) Sorbitol dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie, Weinheim, pp 112–117
Gold-Bouchot G, Zapta-Perez O, Rodriguez-Fuentes G, Ceja-Moreno V, Rio-Garcia MD, Chzan-Cocom E (2006) Biomarkers and pollutants in the Nile tilapia, Oreochromis niloticus, in four lakes from San Miguel, Chiapa, Mexico. Int J Environ Pollut 26(123):130–141
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hylland K (2006) Polycyclic aromatic hydrocarbon (PAH) ecotoxicology in marine ecosystems. J Toxicol Environ Health A 69:109–123
Lee RF, Anderson JW (2005) Significance of cytochrome P450 system responses and levels of bile fluorescent aromatic compounds in marine wildlife following oil spills. Mar Pollut Bull 50:705–723
Lowry H, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Lu GH, Wang C, Zhu Z (2009) The dose-response relationships for EROD and GST induced by polyaromatic hydrocarbons in Carassius auratus. Bull Environ Contam Toxicol 82(2):194–199
McElroy AE, Farrington JW, Teal JM (1989) Bioavailability of polycyclic aromatic hydrocarbons in the aquatic environment. In: Varanasi U (ed) Metabolism of polycyclic aromatic hydrocarbons in the aquatic environment. CRC press, Boca Raton, pp 1–40
Oliveira M, Pacheco M, Santos MA (2007) Cytochrome P4501A, genotoxic and stress responses in golden grey mullet (Liza aurata) following short term exposure to phenanthrene. Chemosphere 66:1284–1291
Pathiratne A, George SG (1998) Toxicity of malathion to Nile tilapia, Oreochromis niloticus and modulation by other environmental contaminants. Aquat Toxicol 43:261–271
Pathiratne A, Chandrasekera LWHU, Pathiratne KAS (2009) Use of biomarkers in Nile tilapia (Oreochromis niloticus) to assess the impacts of pollution in Bolgoda Lake, an urban water body in Sri Lanka. Environ Monit Assess 156:361–375
Srogi K (2007) Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: a review. Environ Chem Lett 5:169–195
Teles M, Pacheco M, Santos MA (2003) Angilla anguilla L. liver ethoxyresorufin O-deethylation, glutathione S-transferase, erythrocytic nuclear abnormalities and endocrine responses to naphthalene and β-naphthoflavone. Ecotoxicol Environ Saf 55:98–107
Till M, Riebniger D, Schmitz HJ, Schrenk D (1999) Potency of various polycyclic aromatic hydrocarbons as inducers of CYP1A in rat hepatocyte cultures. Chem Biol Interact 117(2):135–150
Timme-Laragy AR, Cockman CJ, Matson CW, Di Giulio RT (2007) Synergistic induction of AHR regulated genes in developmental toxicity from co-exposure to two model PAHs in zebrafish. Aquat Toxicol 85:241–250
van der Oost R, Beyer J, Vermeulan NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Whyte JJ, Jung RE, Schmitt CJ, Tillitt DE (2000) Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Crit Rev Toxicol 30:347–570
Willett KL, Wassenberg D, Lienesch L, Reichert W, Di Giulio RT (2001) In vivo and in vitro inhibition of CYP1A-dependent activity in Fundulus heteroclitus by the polynuclear aromatic hydrocarbon fluoranthene. Toxicol Appl Pharmacol 177:264–271
Zapata-Perez O, Gold-Bouchot G, Ortega A, Lopez T, Albores A (2002) Effect of pyrene on hepatic cytochrome P450 1A (CYP1A) expression in Nile tilapia, (Oreochromis niloticus). Arch Environ Contam Toxicol 42:477–485
Zar JH (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River, NJ
Acknowledgements
This study was financially supported by a research grant from National Research Council of Sri Lanka (Grant number 05-24). The second author was financially supported by National Science Foundation of Sri Lanka (Grant number RG/2006/EB/07) in the form of a research assistantship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pathiratne, A., Hemachandra, C.K. Modulation of ethoxyresorufin O-deethylase and glutathione S-transferase activities in Nile tilapia (Oreochromis niloticus) by polycyclic aromatic hydrocarbons containing two to four rings: implications in biomonitoring aquatic pollution. Ecotoxicology 19, 1012–1018 (2010). https://doi.org/10.1007/s10646-010-0482-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-010-0482-3