Abstract
The bioaccumulation and harmful effects of microcystins (MCs) and the activity of peroxidase (POD) and superoxide dismutase (SOD) were examined in the apple (Malus pumila) exposed in vitro with the crude extract of toxic cyanobacterial blooms from Dianchi Lake in southwestern China. The results showed that the growth and proliferation of M. pumila shoots in vitro decreased markedly after exposure to microcystins above 0.3 μg/ml. Recovered microcystins determined by enzyme-linked immunosorbent assay (ELISA) in M. pumila shoot cultures increased with exposure time and concentration. After 14 days exposure to the concentration of 3 μg/ml microcystins, M. pumila shoot cultures accumulated microcystins up to a concentration of 510.23 ± 141.10 ng MC-LR equiv/g FW (fresh weight), equivalent to an accumulation rate of 36.45 ng/g day. POD activity was significantly increased after 7 days exposure to 3 μg/ml microcystins. After 14 days of exposure, microcystins caused POD to increase significantly at the concentration of 0.3 and 3 μg/ml. The activity of SOD was not affected by microcystins at concentrations up to 3 μg/ml on 7 days. After 14 days exposure to microcystins, SOD activity increased significantly at the concentration of 0.3 and 3 μg/ml in M. pumila shoot cultures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water blooms of cyanobacteria (blue-green algae) can present a public safety hazard because of the potential release of hepatotoxins such as microcystins into water bodies (Codd et al. 1989; Aranda-Rodriguez et al. 2003). The reported incidences of animal and human exposure to microcystins (Carmichael and Falconer 1993; Bell and Codd 1994; Ueno et al. 1996; Jochimsen et al. 1998) emphasize the need for a better understanding of the toxic mechanism of these compounds.
The toxic mechanism of microcystins is the inhibition of protein phosphatases 1 and 2A (PP1 and PP2A) in animal and plant cells (MacKintosh et al. 1990; Dawson 1998; Kurki-Helasmo and Meriluoto 1998). In the last few years, DNA damage induced by microcystins was also documented (Rao et al. 1998; Žegura et al. 2003; Brzuzan et al. 2009). Rao et al. (1998) demonstrated that both cell-free extract of Microcystis aeruginosa and purified toxin microcystin-LR (MC-LR) induced significant DNA fragmentation in mouse cells, and the activities of some enzymes (not PP1 and PP2A) such as lactate dehydrogenase (LDH) and alkaline phosphatase (ALP) changed significantly following microcystin poisoning. Recent studies suggest that oxidative stress may play a significant role in the pathogenesis of microcystin toxicity in animals and humans (Ding et al. 2001; Žegura et al. 2003; Kujbida et al. 2008).
Most of the investigations into the effects of microcystins on plants indicate that exposure to microcystins may affect the productivity of crop plants irrigated with contaminated water. Kós et al. (1995) reported that a microcystin (probably MC-LR) and crude extracts of toxic cyanobacteria were able to inhibit the growth of mustard seedlings. The phytotoxic effects of microcystins on the growth and development of potato (Solanum tuberosum) and runner beans (Phaseolus vulgaris) were also investigated (McElhiney et al. 2001). At present, some evidence indicates that oxidative stress might be involved in the toxicity of microcystins on plants. Chen et al. (2004) reported that the activity of peroxidase (POD) and superoxide dismutase (SOD) changed in rape (Brassica napus) and rice (Oryza sativa) seedlings following exposure to microcystins. The oxidative stress induced by microcystins in the aquatic macrophyte Ceratophyllum demersum (Pflugmacher 2004) and tobacco cells (Yin et al. 2005) was also reported. However, the information about microcystins-induced oxidative stress in plants is still very limited, especially for the mixture of several microcystin variants which occurs in natural toxic cyanobacteria contaminated water.
Dianchi Lake is situated in Yunnan province of China. In the last 20 years the occurrence of toxic freshwater blooms of cyanobacteria has been frequently reported. We have isolated the bloom-forming cyanobacteria M. aeruginosa from the eutrophic lake of Dianchi, and it has been confirmed that the cyanobacteria can produce hepatotoxin (Chen et al. 2004). Apple (Malus pumila) is one of the main fruit trees in China, and may be irrigated with toxic cyanobacteria-contaminated water, however, there is no report to study the effects of microcystins on apples.
The objective of this study was to investigate the toxic mechanism of microcystins in apples in vitro; thus, the crude extract of toxic cyanobacteria was used to ascertain the level of microcystins required to inhibit the growth of M. pumila shoots and the following oxidative stress in apples growing in medium containing the toxins. Exposed apple cultures were then extracted to recover microcystins and the bioaccumulation of microcystins was assessed using a commercially available ELISA kit.
Materials and methods
Plant material
Sterile apple tree shoots (Malus pumila, ‘Chan Fu II’ variety) were provided by our laboratory at Nanjing University, and maintained on the media described below until required for assays.
Reagents and animals
Standard MC-LR, -RR, and -YR were purchased from Calbiochem (Bad Soden, Germany). M. aeruginosa bloom material was collected from Dianchi Lake, Kunming in southwestern China, in August 2001, and was lyophilized and stored at −20°C prior to use. Anti-microcystin-LR monoclonal antibody was kindly provided by Prof. Yoshio Ueno, Science University of Tokyo, Japan, and ELISA kit by Prof. Lirong Song, Institute of Hydrobiology, The Chinese Academy of Sciences. All other reagents, obtained from various commercial sources, were of analytical or higher grades.
Four-week-old male ICR mice, weighing 21–25 g, were obtained from the Animal Unit of Qinglongshan (Nanjing, China; Permit number SCXK SU 2002-0018) and maintained on laboratory stock diet with tap water ad libitum.
Preparation of crude aqueous extract of toxic cyanobacteria
Freeze-dried cyanobacteria were suspended in distilled water, freeze-thawed eight times and then centrifuged at 20,000g for 30 min. The supernatants were pooled and kept at −20°C until further use.
Characterization and quantification of microcystins from the extract
The toxicity of the extract was assessed by intraperitoneal injection mouse bioassay (Rao et al. 1994). Four-week-old male ICR mice were used to determine the 50% lethal dose (LD50) by a dose–response experiment. Determination was carried out using five dose levels of the extract with groups of 12 animals for each dose level.
The isolation, characterization and quantification of microcystins were performed according to the methods used previously (Oudra et al. 2001; Chen et al. 2004). Briefly, after the extract was purified by passing through Sep-pak C18 cartridges (Waters), high-performance liquid chromatography with diode array detection (HPLC–DAD) was performed for the detection of toxin composition, and ELISA was used for the determination of total microcystins and the results were expressed as microcystin-LR equivalents (MC-LR equiv).
On the basis of concentrations able to cause 50% inhibition of antibodies in a competitive ELISA, the cross-reactivity of the antibody was demonstrated to be 100% for microcystin-LR, 109% for microcystin-RR, 44% for microcystin-YR, 26% for microcystin-LA, 51% for [D-Asp3] microcystin-LR, 48% for [Dha7] microcystin-LR, and 20% for nodularin.
Microtiter plates (Costar, USA) were coated with MAB (4.0 μg/ml) and incubated overnight at 4°C, and then blocked with 170 μl of blocking buffer containing 0.5% (w/v) gelatin in phosphate-buffered saline (PBS) for 2 h in the model 237 microplate incubator (Bio-rad, USA) at 37°C or overnight at 4°C. 70 μl of various concentrations of MC-LR was pre-incubated at 37°C for 30 min, and an equal volume of biotinylated MC-MAB (25 ng/ml) was then added to the coated wells for 30 min. Plates were washed thoroughly with PBS-T three times with a model 1,575 immuno-wash apparatus (Bio-rad, USA). HRP-streptavidin (sigma) diluted by 1:10000 with dilution buffer (PBS containing 0.5% (w/v) gelatin and 0.05% (v/v) Tween 20) was added and incubated for 30 min at 37°C. The enzyme reaction was started by adding the substrate solution (0.1 M sodium acetate buffer (pH 5.0) containing 100 μg/ml of TMBZ and 0.005% (v/v) H2O2) and stopped with 1 M H2SO4. The absorbance at 450 nm was measured with a model 550 microtiter plate reader (Bio-Rad, USA).
Microcystins exposure
M. pumila shoots were established on solid MS medium (Murashige and Skoog 1962) supplemented with 1.0 mg/l 6-benzyladenine (BA) and 0.5 mg/l α-naphthaleneacetic acid (NAA). The crude aqueous extract of toxic cyanobacteria was filter sterilized and added to the sterilized medium to give a range of doses of the extract (equivalent to 0, 0.03, 0.3 and 3 μg MC-LR/ml). M. pumila shoot cultures were maintained at 25 ± 2°C under a 12-h photoperiod provided by cool white fluorescent lamps at 50 μmol/m2 s as measured at culture level. After 7 and 14 days of culture, M. pumila shoots were sampled triply for each treatment for the examination of accumulation of microcystins and enzymes.
Determination of microcystins in exposed apple tissues using ELISA
In order to study the accumulation of microcystins, 0.5 g of M. pumila shoots was ground to a slurry with a mortar and pestle with 2 ml of distilled water, and the slurry centrifuged at 20,000g for 2 min. The supernatants were purified by passing through Sep-pak C18 cartridges (Waters) and total microcystins were measured by immunoassay according to the aforementioned method.
Enzyme activity determination
0.5 g of M. pumila shoots was ground to a slurry with a mortar and pestle with 4 ml of phosphate buffer (pH 7.0) containing 1% (w/v) of insoluble polyvinylpyrrolidone (PVPP) under external cooling condition (ice bath). The homogenates were centrifuged at 14,000g at 4°C for 10 min, and the supernatants were kept at 4°C prior to use for SOD and POD assay.
SOD activity was determined by the ferricytochrome-c assay method using xanthine/xanthine oxidase as the source of superoxide radicals (McCord and Fridovich 1969). The activity of SOD was expressed in terms of protein content of tissue extracts. Protein content was determined by the method of Lowry (Lowry et al. 1951), slightly modified by Peterson (1977), using bovine serum albumin as the standard.
POD activity was determined by measuring the rate of increase in absorbance at 460 nm (∆OD460) of a mixture containing 4 ml of 0.1 M acetate buffer (pH 5.0), 2 ml of 0.25% 2-methoxyphenol, 400 μl of enzyme extract or distilled water for control and 200 μl of 0.75% hydrogen peroxide (total reaction volume = 6.6 ml; Chen et al. 2004).
Statistical analysis
Results are expressed as mean ± SD. Significant differences between data sets were detected by one-way analysis of variance (ANOVA). A confidence level of <0.05 was considered significant. Correlation coefficients (r) were calculated by linear regression analyses of correlations between microcystins accumulation and enzyme activity.
Results
Characterization and quantification of microcystins from M. aeruginosa bloom in Dianchi Lake
In order to study the effects of microcystins on apples, the crude aqueous extract of the toxic bloom from Dianchi Lake was used. For ascertaining the toxicity of the crude extract, it was subjected to the mouse bioassay test. Intraperitoneal injection (i.p.) of crude aqueous extract to test mice caused death of the animals with a median LD50 of 83 mg/kg body weight. Reversed-phase HPLC analysis showed that the crude extract had at least three microcystin variants: microcystin-RR, -LR and -YR, in the proportion of 62, 35 and 3%, respectively (Fig. 1). The three microcystin variants in the crude extract accounted for 89% of total microcystins determined by ELISA. The total microcystins level of the toxic bloom sample was 0.4 μg of microcystin-LR equivalents per mg dry wt of bloom.
Accumulation of microcystins in exposed M. pumila shoots using ELISA
After 2 months exposure to microcystins, the amplification coefficients of in vitro M. pumila shoots showed a gradual decrease with the concentration of microcystins; there were significant differences in amplification coefficients between the treatments and the control (P < 0.05 for 0.03 μg MCs/ml, P < 0.01 for 0.3 and 3 μg MCs/ml; Table 1). When the concentration of exposure microcystins was above 0.3 μg/ml, the growth of apple explants was noticeably inhibited and M. pumila shoots were abnormal (Fig. 2).
By ELISA total microcystins concentration in the extracts of exposed and control M. pumila shoots was determined. The results (Table 2) showed that the concentration of determined microcystins in M. pumila shoots varied with the exposure concentration, and was greater after 2-week exposure than 1-week exposure except M. pumila shoots treated at a concentration of 0.03 μg/ml. Average total microcystins concentration was greatest after exposure to 3 μg/ml microcystins, and was 8- and 14-fold greater than 0.3 and 0.03 μg/ml treatments on 7 days, respectively. On 14 days, the total recovered microcystins concentration of M. pumila shoots exposed to 3 μg/ml microcystins reached more than 510 ng MC-LR equiv/g FW and was 12- and 35-fold greater than 0.3 and 0.03 μg/ml, respectively. No microcystin was detected in the extracts of control M. pumila shoots (Table 2).
Measured activity of POD and SOD during exposure to microcystins
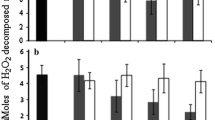
Compared with controls, apple plants after 7 days of exposure to 3 μg/ml microcystins exhibited a significant increase in POD (P < 0.05). No significant difference in POD activity was found between the control and the plants exposed to 0.03 and 0.3 μg/ml microcystins (Fig. 3). After 14 days of exposure to microcystins, POD activity at the concentration of 0.3 and 3 μg/ml was significantly higher than that of the control (P < 0.01). POD of in vitro apple exposed to 0.03 μg/ml microcystins was also elevated, although not significantly (Fig. 3).
In contrast, after 7 days exposure to microcystins SOD activity of in vitro apple did not differ significantly among treatments. However, the activity of SOD was increased after 14 days of exposure, and the activity at the concentration of 0.3 and 3 μg/ml was significantly higher (P < 0.01) than that of the control (Fig. 4).
By correlation analysis (Table 3), the activities of POD and SOD in exposed M. pumila shoots were found to be significantly and positively correlated with tissue concentrations of recovered microcystins on 7 and 14 days, respectively. The results suggest that microcystins from crude aqueous extract of toxic cyanobacteria cause an oxidative stress in apples.
Discussion
The majority of investigations into the effects of cyanobacterial toxins on plants to date have focused on individual microcystin variants (Abe et al. 1996; McElhiney et al. 2001; Máthé et al. 2009). However, there is significant difference in the toxicities of MC-RR, -LR, and -LF on the growth of mustard (Synapis alba) seedlings (McElhiney et al. 2001). Generally, naturally occurring microcystin is also a mixture of several variants, for example, in toxic cyanobacteria contaminated water intended for irrigation use. In order to study the effects of toxin mixture on plants, the crude extract from natural bloom with mixture of MC-RR, -LR and -YR was used in the present study. To a great extent, the situation of this bioassay was representative of the exposure experienced by plants in the environment. Cousins et al. (1996) demonstrated that the aseptic technique could minimize the risk of toxin degradation by bacteria. Accordingly, the plant tissue culture assay used in this study ensured that the symptoms observed in treated M. pumila shoot cultures were attributable to the toxins although the sterile condition was not representative of the exposure experienced by plants in the environment.
Effects of microcystins on terrestrial plants
The exposure of terrestrial plants to microcystins via irrigation water taken from a source which has experienced a bloom has far reaching consequences for both economic and health reasons (Codd et al. 1999; McElhiney et al. 2001). Growth inhibition of seedlings of a variety of terrestrial plants by cyanotoxins (Kós et al. 1995; Kurki-Helasmo and Meriluoto 1998; McElhiney et al. 2001; Hamvas et al. 2002; Chen et al. 2004; Järvenpää et al. 2007), and the inhibition of protein phosphatases (Siegel et al. 1990; Kurki-Helasmo and Meriluoto 1998) and photosynthesis in leaves of terrestrial plants (Abe et al. 1996) have been reported. This study revealed that microcystins concentrations of 0.3 and 3 μg/ml have toxic effects on the growth of M. pumila shoots (Fig. 2) and in vitro propagation of apples (Table 1). The microcystin concentrations that cause adverse effects in the present study are similar to those reported for several other terrestrial plants (McElhiney et al. 2001; Chen et al. 2004). The growth and development of rape (B. napus) seedlings is inhibited at microcystins concentrations of 0.12–3 μg/ml (Chen et al. 2004). Microcystins-LR, -RR, and -LF inhibited the growth of S. alba seedlings, with GI50 (50% growth inhibition) values of 1.9, 1.6 and 7.7 μg/ml, respectively (McElhiney et al. 2001). The growth of potato shoot explants exposed to microcystin-LR concentrations of 0.5–5 μg/ml is completely inhibited (McElhiney et al. 2001). Although these cyanotoxin concentrations exhibiting noticeably adverse effect on terrestrial plants can not well represent the toxin levels examined in lake water (Lawton et al. 1995) and reservoir water (Cousins et al. 1996) during cyanobacterial blooms, the microcystin concentration in soil may grow high under some circumstances as microcystins are considered quite resistant to degradation (Harada 1996). Further, as high as 1.3–1.8 μg/ml of microcystins can be found in a lake treated with an algicide (Jones and Orr 1994).
Effects of microcystins on antioxidant enzymes of plants
Plants possess the antioxidative enzymes, such as POD, SOD, catalase (CAT), and glutathione peroxidase (GPX), to scavenge reactive oxygen species (ROS) and to avoid oxidative damage. Oxygen radicals are generated during plant metabolism, especially in the plants exposed to environmental stresses. Microcystins contaminated water may be one of abiotic stresses for terrestrial plants via irrigation techniques (Chen et al. 2004). It has been shown that oxidative damage involves in the toxicity of microcystins on fish (Cazenave et al. 2006; Prieto et al. 2006), rats (Kujbida et al. 2008), humans (Kujbida et al. 2008), and some plants (Chen et al. 2004; Pflugmacher 2004; Mitrovic et al. 2005; Yin et al. 2005; Huang et al. 2008). This study investigated the activity of POD and SOD in M. pumila shoots after exposure to microcystins for the first time. The results showed that POD and SOD activity of M. pumila shoots was significantly increased after 14 days exposure to 0.3 and 3 μg/ml microcystins (Figs. 3, 4), which agreed with the observed significant increase in POD in B. napus seedlings (Chen et al. 2004), SOD in the aquatic plant C. demersum (Pflugmacher 2004), and POD, SOD, and GPX in tobacco BY-2 cells after 48 h exposure to microcystin-RR (Yin et al. 2005). The microcystins-induced decrease in SOD activity in rice (O. sativa) seedlings noted by Chen et al. (2004) was not confirmed by this study. Similarly, Mitrovic et al. (2005) demonstrated that POD activity of Lemna minor was significantly increased after 5 days exposure to concentrations of 10 and 20 μg/ml microcystin-LR, while POD of Chladophora fracta was not affected by microcystin-LR at concentrations up to 10 μg/ml. It is therefore likely that the response on antioxidant enzymes of plants after exposure to cyanobacterial toxins depends on plant species, microcystin variants, exposure time and time period, etc.
Potential for bioaccumulation of microcystins
The ELISA assay employed in the present study demonstrated that there was a positive relationship between treatment concentrations and bioaccumulation of microcystins in M. pumila shoot cultures (Table 2). Recently, several studies have indicated that microcystins could be recovered from the tissues of exposed terrestrial plants (Kurki-Helasmo and Meriluoto 1998; McElhiney et al. 2001; Chen et al. 2004; Järvenpää et al. 2007) and aquatic plants (Pflugmacher et al. 1998; Chen et al. 2004; Mitrovic et al. 2005). Pflugmacher et al. (1998) revealed that a detoxification mechanism might occur in aquatic plant C. demersum. This suggests that lower level of microcystins in the extract of exposed aquatic plants should be checked than in terrestrial plants, which is in agreement with a previous study in which much lower level of microcystins in the extract of exposed O. sativa seedlings was determined than in B. napus seedlings (Chen et al. 2004). The present study also confirmed the suggestion. In this study, M. pumila shoot cultures accumulated microcystins up to a concentration of 510.23 ± 141.10 ng MC-LR equiv/g FW after 14 days exposure to 3 μg/ml microcystins (Table 2). The concentration of recovered microcystins is close to the concentration of 651.00 ± 78.71 ng MC-LR equiv/g FW taken up by B. napus seedlings after 10 days exposure to the same concentration of microcystins (Chen et al. 2004). The higher concentration of total microcystins recovered in exposed terrestrial plants than aquatic plants suggests that terrestrial plants appear to exhibit more serious toxic effects on human health in the food web by biomagnification or accumulation of microcystins when irrigation practices with water containing microcystins are used. Our future research will concentrate on the possibility of transferring of microcystins into apple fruits and the metabolism of microcystins during the plant growth.
In conclusion, this study provides an evidence for the first time that microcystins are manifested as an oxidative stress in apples and exposure to microcystins via irrigation route poses a threat to the yield and quality of fruit trees.
References
Abe T, Lawson T, Weyers JDB, Codd GA (1996) Microcystin-LR inhibits photosynthesis of Phaseolus vulgaris primary leaves: implications for current spray irrigation practice. New Phytol 133:651–658
Aranda-Rodriguez R, Kubwabo C, Benoit FM (2003) Extraction of 15 microcystins and nodularin using immunoaffinity columns. Toxicon 42:587–599
Bell SG, Codd GA (1994) Cyanobacterial toxins and human health. Rev Med Microbiol 5:869–872
Brzuzan P, Woźny M, Ciesielski S, Łuczyński MK, Góra M, Kuźmiński H, Dobosz S (2009) Microcystin-LR induced apoptosis and mRNA expression of p53 and cdkn1a in liver of whitefish (Coregonus lavaretus L.). Toxicon 54:170–183
Carmichael WW, Falconer IR (1993) Diseases related to freshwater blue-green algal toxins, and control measures. In: Falconer IR (ed) Algal toxins in seafood and drinking water. Academic Press, New York, pp 187–209
Cazenave J, Bistoni MA, Pesce SF, Wunderlin DA (2006) Differential detoxification and antioxidant response in diverse organs of Corydoras paleatus experimentally exposed to microcystin-RR. Aquat Toxicol 76:1–12
Chen JZ, Song LR, Dai J, Gan NQ, Liu ZL (2004) Effects of microcystins on the growth and the activity of superoxide dismutase and peroxidase of rape (Brassica napus L.) and rice (Oryza sativa L.). Toxicon 43:393–400
Codd GA, Bell SG, Brooks WP (1989) Cyanobacterial toxins in water. Water Sci Technol 21:1–13
Codd GA, Metcalf JS, Beattie KA (1999) Retention of Microcystis aeruginosa and microcystin by salad lettuce (Lactuca sativa) after spray irrigation with water containing cyanobacteria. Toxicon 37:1181–1185
Cousins IT, Bealing DJ, James HA, Sutton A (1996) Biodegradation of microcystin-LR by indigenous mixed bacterial populations. Water Res 30:481–485
Dawson RM (1998) The toxicology of microcystins. Toxicon 36:953–962
Ding WX, Shen HM, Ong CN (2001) Critical role of reactive oxygen species formation in microcystin-induced cytoskeleton disruption in primary cultured hepatocytes. J Toxicol Environ Health A 64:507–519
Hamvas MM, Mathe C, Molnar E, Vasa G, Grigorsky I, Borbely G (2002) Microcystin-LR alters the growth, anthanocyanin content and single-stranded DNase enzyme activities in Sinapis alba L. seedlings. Aquat Toxicol 61:1–9
Harada KI (1996) Chemistry and detection of microcysitns. In: Watanabe MF, Harada KI, Carmichael WW, Fujiki H (eds) Toxic Microcystis. CRC Press, Boca Raton, pp 103–148
Huang WM, Xing W, Li DH, Liu YD (2008) Microcystin-RR induced apoptosis in tobacco BY-2 suspension cells is mediated by reactive oxygen species and mitochondrial permeability transition pore status. Toxicol In Vitro 22:328–337
Järvenpää S, Lundberg-Niinistö C, Spoof L, Sjövall O, Tyystjärvi E, Meriluoto J (2007) Effects of microcystins on broccoli and mustard, and analysis of accumulated toxin by liquid chromatography—mass spectrometry. Toxicon 49:865–874
Jochimsen EM, Carmichael WW, An JS, Cardo DM, Cookson ST, Holmes CE, Antunes MB, de Melo Filho DA, Lyra TM, Barreto VS, Azevedo SM, Jarvis WR (1998) Liver failure and death after exposure to microcystins at a haemodialysis center in Brazil. New Engl J Med 338:873–878
Jones GJ, Orr PT (1994) Release and degradation of microcystin following algicide treatment of a Microcystis aeruginosa bloom in a recreational lake, as determined by HPLC and protein phosphatase inhibition assay. Water Res 28:871–876
Kós P, Gorzó G, Surányi G, Borbély G (1995) Simple and efficient method for isolation and measurement of cyanobacterial hepatotoxins by plant tests (Sinapis alba L.). Anal Biochem 225:49–53
Kujbida P, Hatanaka E, Campa A, Curi R, Farsky SHP, Pinto E (2008) Analysis of chemokines and reactive oxygen species formation by rat and human neutrophils induced by microcystin-LA, -YR and -LR. Toxicon 51:1274–1280
Kurki-Helasmo K, Meriluoto J (1998) Microcystin uptake inhibits growth and protein phosphatase activity in Mustard (Sinapis alba L.) seedlings. Toxicon 36:1921–1926
Lawton LA, Edwards C, Beattie KA, Pleasance S, Dear GJ, Codd GA (1995) Isolation and characterisation of microcystins from laboratory cultures and environmental samples of Microcystis aeruginosa and from an associated animal toxicosis. Nat Toxins 3:50–57
Lowry OH, Rosebrough NH, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
MacKintosh C, Beattie KA, Klumpp S, Cohen P, Codd GA (1990) Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett 264:187–192
Máthé C, Beyer D, Erdődi F, Serfőző Z, Székvölgyi L, Vasas G, M-Hamvas M, Jámbrik K, Gonda S, Kiss A, Szigeti ZM, Surányi G (2009) Microcystin-LR induces abnormal root development by altering microtubule organization in tissue-cultured common reed (Phragmites australis) plantlets. Aquat Toxicol 92:122–130
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
McElhiney J, Lawton LA, Leifert C (2001) Investigations into the inhibitory effects of microcystins on plant growth, and the toxicity of plant tissues following exposure. Toxicon 39:1411–1420
Mitrovic SM, Allis O, Furey A, James KJ (2005) Bioaccumulation and harmful effects of microcystin-LR in the aquatic plants Lemna minor and Wolffia arrhiza and the filamentous alga Chladophora fracta. Ecotox Environ Safe 61:345–352
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Oudra B, Loudiki M, Sbiyyaa B, Martins R, Vasconcelos V, Namikoshi N (2001) Isolation, characterization and quantification of microcystins (heptapeptides hepatotoxins) in Microcystis aeruginosa dominated bloom of Lalla Takerkoust lakereservoir (Morocco). Toxicon 39:1375–1381
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:570–577
Pflugmacher S (2004) Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat Toxicol 70:169–178
Pflugmacher S, Wiegand C, Beattie KA, Codd GA, Steinberg CEW (1998) Uptake of the cyanobacterial hepatotoxin microcystin-LR by aquatic macrophytes. J Appl Bot 72:228–232
Prieto A, Jos Á, Pichardo S, Moreno I, Cameán AM (2006) Differential oxidative stress responses to microcystins LR and RR in intraperitoneally exposed tilapia fish (Oreochromis sp.). Aquat Toxicol 77:314–321
Rao PVL, Bhattacharaya R, Das Gupta S (1994) Isolation, culture and toxicity of cyanobacterium (blue-green alga) Microcystis aeruginosa from freshwater source in India. B Environ Contam Tox 52:878–885
Rao PVL, Bhattacharaya R, Parida MM, Jana AM, Bhaskar ASB (1998) Freshwater cyanobacterium Microcystis aeruginosa (UTEX 2385) induced DNA damage in vivo and in vitro. Environ Toxicol Phar 5:1–6
Siegel G, MacKintosh C, Stitt M (1990) Sucrose-phosphate synthase is dephosphorylated by protein phosphotase 2A in spinach leaves: evidence from the effects of okadaic acid and microcystin. FEBS Lett 270:198–202
Ueno Y, Nagata S, Tsutsumi T, Hasegawa A, Watanabe MF, Park HD, Chen GC, Chen G, Yu SZ (1996) Detection of microcystins, a blue-green algal hepatotoxin, in drinking water sampled in Haimen and Fusui, endemic areas of primary liver cancer in China, by highly sensitive immunoassay. Carcinogenesis 17:1317–1321
Yin LY, Huang JQ, Huang WM, Li DH, Wang GH, Liu YD (2005) Microcystin- RR-induced accumulation of reactive oxygen species and alteration of antioxidant systems in tobacco BY-2 cells. Toxicon 39:1411–1420
Žegura B, Sedmak B, Filipič M (2003) Microcystin-LR induces oxidative DNA damage in human hepatoma cell line HepG2. Toxicon 41:41–48
Acknowledgments
This research was funded by the National “863” High Science and Technology Project of China (AA-64-10-30) and Major Science and Technology Specific Project of Zhejiang Province (2008C12009). We would like to thank Dr. Nanqin Gan for her assistance in the determination of microcystins.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J., Dai, J., Zhang, H. et al. Bioaccumulation of microcystin and its oxidative stress in the apple (Malus pumila). Ecotoxicology 19, 796–803 (2010). https://doi.org/10.1007/s10646-009-0456-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-009-0456-5